Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy
Abstract
1. Introduction
2. Gut Dysbiosis in Food Allergy
2.1. Evidence from Human Observational Studies
2.2. Evidence from Animal Models of Food Allergy
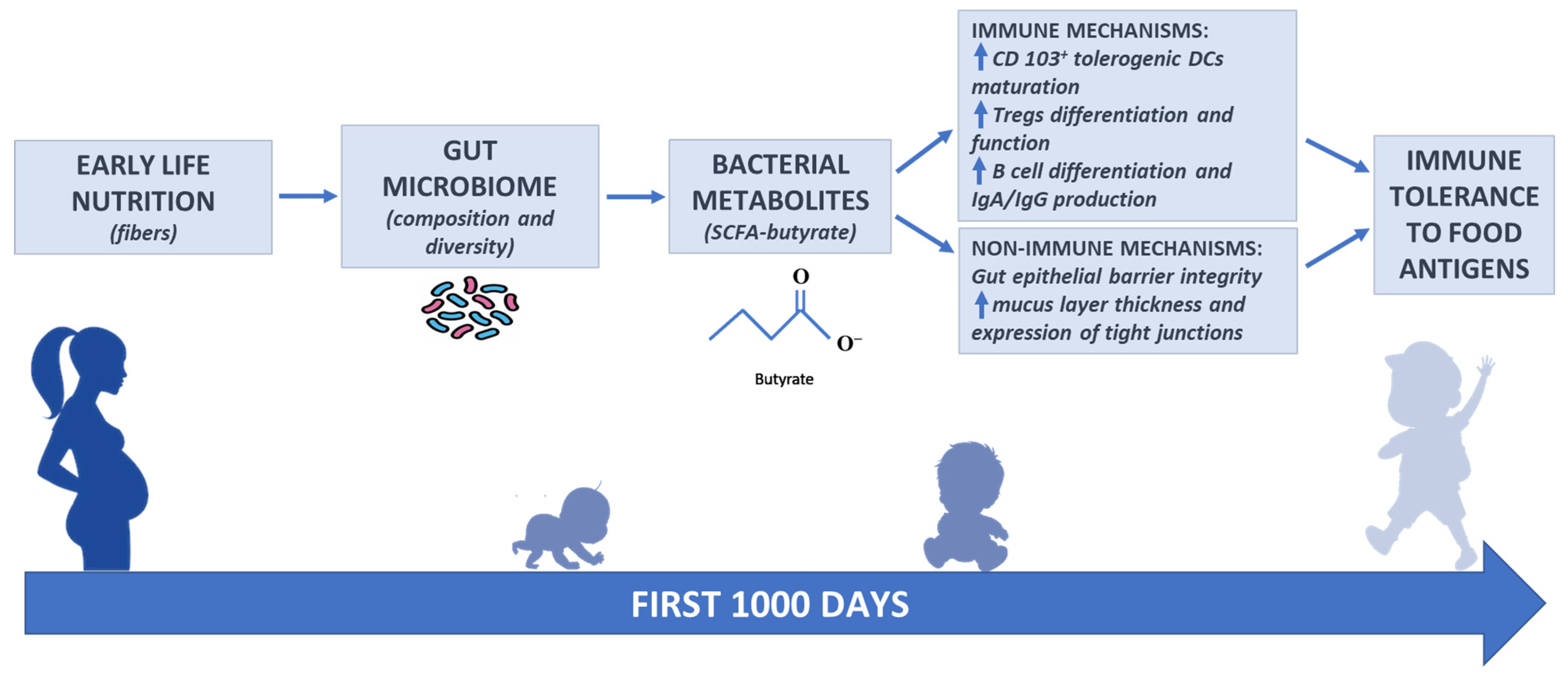
3. Interrelation among Nutrition, Gut Microbiome, and Immune System: The Role of Butyrate
3.1. Fibers and SCFA Butyrate Production by Gut Microbiome
3.2. Reduced Fecal Levels of SCFA Butyrate in Food Allergy: Evidence from Human Observational Studies
3.3. Early Life Nutrition and SCFA Butyrate in Food Allergy
3.3.1. Breastfeeding: Butyrate as Bioactive Human Milk Protective Component against Food Allergy
3.3.2. Food Allergy and Relation to Diet and Microbial Metabolites
3.3.3. Emerging Role of Butyrate in the Active Diet-Therapy in Pediatric Patients with Cow’s Milk Allergy
3.4. Butyrate: Immune and Non-Immune Mechanisms of Action Against Food Allergy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALDH | Aldehyde dehydrogenases |
| eHCF | Extensively hydrolyzed casein formula |
| FoxP3 | Forkhead box P3 |
| GPCRs | G-protein coupled receptors |
| HDAC | Histone deacetylases |
| IECs | Intestinal epithelial cells |
| ILC | Innate lymphoid cells |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| LGG | Lactobacillus rhamnosus GG |
| MUC2 | Mucin2 |
| NLRP3 | NOD-, LRR-, and pyrin domain-containing protein 3 |
| PBMCs | Peripheral blood mononuclear cells |
| RALDH | Retinaldehyde dehydrogenases |
| RORγt | Retinoic acid-related orphan receptor γt |
| SCFAs | Short-chain fatty acids |
| Th | T helper |
| Tregs | Regulatory T cells |
References
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Barcik, W.; Untersmayr, E.; Pali-Schöll, I.; O’Mahony, L.; Frei, R. Influence of Microbiome and Diet on Immune Responses in Food Allergy Models. Drug Discov. Today Dis. Model. 2015, 17–18, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef]
- Luu, M.; Visekruna, A. Short-Chain Fatty Acids: Bacterial Messengers Modulating the Immunometabolism of T Cells. Eur. J. Immunol. 2019, 49, 842–848. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic T Reg Cell Homeostasis. Science 2013, 341, 6145. [Google Scholar] [CrossRef]
- Cait, A.; Cardenas, E.; Dimitriu, P.A.; Amenyogbe, N.; Dai, D. Reduced Genetic Potential for Butyrate Fermentation in the Gut Microbiome of Infants Who Develop Allergic Sensitization. J. Allergy Clin. Immunol. 2019, 144, 1638–1647.e3. [Google Scholar] [CrossRef]
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as Bioactive Human Milk Protective Component against Food Allergy. Allergy 2020, 1–18. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Di Costanzo, M.; Carucci, L.; Canani, R.B.; Biasucci, G. Gut Microbiome Modulation for Preventing and Treating Pediatric Food Allergies. Int. J. Mol. Sci. 2020, 21, 5275. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; Hayglass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant Gut Microbiota and Food Sensitization: Associations in the First Year of Life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Nakayama, J.; Kobayashi, T.; Tanaka, S.; Korenori, Y.; Tateyama, A.; Sakamoto, N.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K. Aberrant Structures of Fecal Bacterial Community in Allergic Infants Profiled by 16S RRNA Gene Pyrosequencing. FEMS Immunol. Med. Microbiol. 2011, 63, 397–406. [Google Scholar] [CrossRef]
- Tsabouri, S.; Priftis, K.N.; Chaliasos, N.; Siamopoulou, A. Modulation of Gut Microbiota Downregulates the Development of Food Allergy in Infancy. Allergol. Immunopathol. 2014, 42, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.; Sicherer, S.; et al. Early-Life Gut Microbiome Composition and Milk Allergy Resolution. J. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Bao, R.; Nadeau, K.C.; Nagler, C.R.; Bao, R.; Hesser, L.A.; He, Z.; Zhou, X.; Nadeau, K.C.; Nagler, C.R. Allergic Twins Fecal Microbiome and Metabolome Differ in Healthy and Food-Allergic Twins. J. Clin. Investig. 2021, 131, e141935. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered Fecal Microbiota Composition Associated with Food Allergy in Infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, K.J.; Kong, M.S.; Chang, H.J.; Huang, J.L. Alterations in the Gut Microbiotas of Children with Food Sensitization in Early Life. Pediatr. Allergy Immunol. 2016, 27, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus Rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Inoue, R.; Sawai, T.; Sawai, C.; Nakatani, M.; Romero-Pérez, G.A.; Ozeki, M.; Nonomura, K.; Tsukahara, T. A Preliminary Study of Gut Dysbiosis in Children with Food Allergy. Biosci. Biotechnol. Biochem. 2017, 81, 2396–2399. [Google Scholar] [CrossRef]
- Kourosh, A.; Luna, R.A.; Balderas, M.; Nance, C.; Anagnostou, A.; Devaraj, S.; Davis, C.M. Fecal Microbiome Signatures Are Different in Food-Allergic Children Compared to Siblings and Healthy Children. Pediatr. Allergy Immunol. 2018, 29, 545–554. [Google Scholar] [CrossRef]
- Fazlollahi, M.; Chun, Y.; Grishin, A.; Wood, R.A.; Burks, A.W.; Dawson, P.; Jones, S.M.; Leung, D.Y.M.; Sampson, H.A.; Sicherer, S.H.; et al. Early-Life Gut Microbiome and Egg Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 1515–1524. [Google Scholar] [CrossRef]
- Savage, J.H.; Lee-Sarwar, K.A.; Sordillo, J.; Bunyavanich, S.; Zhou, Y.; O’Connor, G.; Sandel, M.; Bacharier, L.B.; Zeiger, R.; Sodergren, E.; et al. A Prospective Microbiome-Wide Association Study of Food Sensitization and Food Allergy in Early Childhood. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 145–152. [Google Scholar] [CrossRef]
- Lee, K.H.; Guo, J.; Song, Y.; Ariff, A.; O’sullivan, M.; Hales, B.; Mullins, B.J.; Zhang, G. Dysfunctional Gut Microbiome Networks in Childhood Ige-mediated Food Allergy. Int. J. Mol. Sci. 2021, 22, 2079. [Google Scholar] [CrossRef]
- Goldberg, M.R.; Mor, H.; Magid Neriya, D.; Magzal, F.; Muller, E.; Appel, M.Y.; Nachshon, L.; Borenstein, E.; Tamir, S.; Louzoun, Y.; et al. Microbial Signature in IgE-Mediated Food Allergies. Genome Med. 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Mennini, M.; Fierro, V.; Di Nardo, G.; Pecora, V.; Fiocchi, A. Microbiota in Non-IgE-Mediated Food Allergy. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 323–328. [Google Scholar] [CrossRef]
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by Non-IgE-Mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Guadamuro, L.; Espinosa-Martos, I.; Mancabelli, L.; Jiménez, S.; Molinos-Norniella, C.; Pérez-Solis, D.; Milani, C.; Rodríguez, J.M.; Ventura, M.; et al. Microbiota and Derived Parameters in Fecal Samples of Infants with Non-IgE Cow’s Milk Protein Allergy under a Restricted Diet. Nutrients 2018, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.R.; Mohn, W.W.; McNagny, K.M.; et al. Early Life Antibiotic-Driven Changes in Microbiota Enhance Susceptibility to Allergic Asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef]
- Rodriguez, B.; Prioult, G.; Bibiloni, R.; Nicolis, I.; Mercenier, A.; Butel, M.J.; Waligora-Dupriet, A.J. Germ-Free Status and Altered Caecal Subdominant Microbiota Are Associated with a High Susceptibility to Cow’s Milk Allergy in Mice. FEMS Microbiol. Ecol. 2011, 76, 133–144. [Google Scholar] [CrossRef]
- Hazebrouck, S.; Przybylski-Nicaise, L.; Ah-Leung, S.; Adel-Patient, K.; Corthier, G.; Wal, J.M.; Rabot, S. Allergic Sensitization to Bovine β-Lactoglobulin: Comparison between Germ-Free and Conventional BALB/c Mice. Int. Arch. Allergy Immunol. 2008, 148, 65–72. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.Q.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; Desantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy Infants Harbor Intestinal Bacteria That Protect against Food Allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg Induction by a Rationally Selected Mixture of Clostridia Strains from the Human Microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; Van Seuningen, I.; Renes, I.B. The Regulation of Intestinal Mucin MUC2 Expression by Short-Chain Fatty Acids: Implications for Epithelial Protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- McKenzie, C.; Tan, J.; Macia, L.; Mackay, C.R. The Nutrition-Gut Microbiome-Physiology Axis and Allergic Diseases. Immunol. Rev. 2017, 278, 277–295. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Diversity, Metabolism and Microbial Ecology of Butyrate-Producing Bacteria from the Human Large Intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sandin, A.; Bråbäck, L.; Norin, E.; Björkstén, B. Faecal Short Chain Fatty Acid Pattern and Allergy in Early Childhood. Acta Paediatr. Int. J. Paediatr. 2009, 98, 823–827. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High Levels of Butyrate and Propionate in Early Life Are Associated with Protection against Atopy. Allergy Eur. J. Allergy Clin. Immunol. 2019, 74, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef]
- Mitselou, N.; Hallberg, J.; Stephansson, O.; Almqvist, C.; Melén, E.; Ludvigsson, J.F. Cesarean Delivery, Preterm Birth, and Risk of Food Allergy: Nationwide Swedish Cohort Study of More than 1 Million Children. J. Allergy Clin. Immunol. 2018, 142, 1510–1514.e2. [Google Scholar] [CrossRef]
- Arboleya, S.; Sánchez, B.; Solís, G.; Fernández, N.; Suárez, M.; Hernández-Barranco, A.M.; Milani, C.; Margolles, A.; De Los Reyes-Gavilán, C.G.; Ventura, M.; et al. Impact of Prematurity and Perinatal Antibiotics on the Developing Intestinal Microbiota: A Functional Inference Study. Int. J. Mol. Sci. 2016, 17, 649. [Google Scholar] [CrossRef]
- Silvers, K.M.; Frampton, C.M.; Wickens, K.; Pattemore, P.K.; Ingham, T.; Fishwick, D.; Crane, J.; Town, G.I.; Epton, M.J. Breastfeeding Protects against Current Asthma up to 6 Years of Age. J. Pediatr. 2012, 160, 991–996.e1. [Google Scholar] [CrossRef]
- Zhong, H.; Penders, J.; Shi, Z.; Ren, H.; Cai, K.; Fang, C.; Ding, Q.; Thijs, C.; Blaak, E.E.; Stehouwer, C.D.A.; et al. Impact of Early Events and Lifestyle on the Gut Microbiota and Metabolic Phenotypes in Young School-Age Children. Microbiome 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Jensen-Jarolim, E. Anti-Acid Medication as a Risk Factor for Food Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2011, 66, 469–477. [Google Scholar] [CrossRef]
- Pelak, G.; Wiese, A.M.; Maskarinec, J.M.; Phillips, W.L.; Keim, S.A. Infant Feeding Practices during the First Postnatal Year and Risk of Asthma and Allergic Disease during the First 6 Years of Life. Breastfeed. Med. 2021. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Wesley Burks, A.; Abrams, S.A.; Fuchs, G.J.; Kim, J.H.; Wesley Lindsey, C.; Magge, S.N.; Rome, E.S.; Schwarzenberg, S.J. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Garcia-Larsen, V.; Ierodiakonou, D.; Jarrold, K.; Cunha, S.; Chivinge, J.; Robinson, Z.; Geoghegan, N.; Ruparelia, A.; Devani, P.; Trivella, M.; et al. Diet during Pregnancy and Infancy and Risk of Allergic or Autoimmune Disease: A Systematic Review and Meta-Analysis. PLoS Med. 2018, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.J.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.C.; Hoest, A.; Lack, G.; et al. EAACI Food Allergy and Anaphylaxis Guidelines. Primary Prevention of Food Allergy. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 590–601. [Google Scholar] [CrossRef]
- Grimshaw, K.E.C.; Maskell, J.; Oliver, E.M.; Morris, R.C.G.; Foote, K.D.; Mills, E.N.C.; Margetts, B.M.; Roberts, G. Diet and Food Allergy Development during Infancy: Birth Cohort Study Findings Using Prospective Food Diary Data. J. Allergy Clin. Immunol. 2014, 133, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marcos, L.; Castro-Rodriguez, J.A.; Weinmayr, G.; Panagiotakos, D.B.; Priftis, K.N.; Nagel, G. Influence of Mediterranean Diet on Asthma in Children: A Systematic Review and Meta-Analysis. Pediatr. Allergy Immunol. 2013, 24, 330–338. [Google Scholar] [CrossRef]
- Chatzi, L.; Apostolaki, G.; Bibakis, I.; Skypala, I.; Bibaki-Liakou, V.; Tzanakis, N.; Kogevinas, M.; Cullinan, P. Protective Effect of Fruits, Vegetables and the Mediterranean Diet on Asthma and Allergies among Children in Crete. Thorax 2007, 62, 677–683. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-Level Adherence to a Mediterranean Diet Beneficially Impacts the Gut Microbiota and Associated Metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; García-Marcos, L.; Williams, H.; Keil, U.; Robertson, C.; Nagel, G. Do Fast Foods Cause Asthma, Rhinoconjunctivitis and Eczema? Global Findings from the International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three. Thorax 2013, 68, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Berthon, B.S.; MacDonald-Wicks, L.K.; Gibson, P.G.; Wood, L.G. Investigation of the Association between Dietary Intake, Disease Severity and Airway Inflammation in Asthma. Respirology 2013, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of Cow’s Milk Allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: Espghan Gi Committee Practical Guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Carucci, L.; Nocerino, R.; Paparo, L.; Di Scala, C.; Berni Canani, R. Dietary Prevention of Atopic March in Pediatric Subjects With Cow’s Milk Allergy. Front. Pediatr. 2020, 8, 440. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Coruzzo, A.; Cosenza, L.; Leone, L.; Troncone, R. Effect of Lactobacillus GG on Tolerance Acquisition in Infants with Cow’s Milk Allergy: A Randomized Trial. J. Allergy Clin. Immunol. 2012, 129, 580–582.e5. [Google Scholar] [CrossRef]
- Berni Canani, R.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively Hydrolyzed Casein Formula Containing Lactobacillus Rhamnosus GG Reduces the Occurrence of Other Allergic Manifestations in Children with Cow’s Milk Allergy: 3-Year Randomized Controlled Trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913.e4. [Google Scholar] [CrossRef]
- Vonk, M.M.; Blokhuis, B.R.J.; Diks, M.A.P.; Wagenaar, L.; Smit, J.J.; Pieters, R.H.H.; Garssen, J.; Knippels, L.M.J.; Van Esch, B.C.A.M. Butyrate Enhances Desensitization Induced by Oral Immunotherapy in Cow’s Milk Allergic Mice. Mediat. Inflamm. 2019, 2019, 9062537. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate Modifies Intestinal Barrier Function in IPEC-J2 Cells through a Selective Upregulation of Tight Junction Proteins and Activation of the Akt Signaling Pathway. PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Goverse, G.; Molenaar, R.; Macia, L.; Tan, J.; Erkelens, M.N.; Konijn, T.; Knippenberg, M.; Cook, E.C.L.; Hanekamp, D.; Veldhoen, M.; et al. Diet-Derived Short Chain Fatty Acids Stimulate Intestinal Epithelial Cells to Induce Mucosal Tolerogenic Dendritic Cells. J. Immunol. 2017, 198, 2172–2181. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; Ian McKenzie, C.; Hijikata, A.; Wong, C.; et al. Metabolite-Sensing Receptors GPR43 and GPR109A Facilitate Dietary Fibre-Induced Gut Homeostasis through Regulation of the Inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut Microbiota, Metabolites and Host Immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Sanchez, H.N.; Moroney, J.B.; Gan, H.; Shen, T.; Im, J.L.; Li, T.; Taylor, J.R.; Zan, H.; Casali, P. B Cell-Intrinsic Epigenetic Modulation of Antibody Responses by Dietary Fiber-Derived Short-Chain Fatty Acids. Nat. Commun. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Qie, Y.; Park, J.; Kim, C.H. Gut Microbial Metabolites Fuel Host Antibody Responses. Cell Host Microbe 2016, 20, 202–214. [Google Scholar] [CrossRef]
- Schulthess, J.; Pandey, S.; Capitani, M.; Rue-Albrecht, K.C.; Arnold, I.; Franchini, F.; Chomka, A.; Ilott, N.E.; Johnston, D.G.W.; Pires, E.; et al. The Short Chain Fatty Acid Butyrate Imprints an Antimicrobial Program in Macrophages. Immunity 2019, 50, 432–445.e7. [Google Scholar] [CrossRef] [PubMed]

| Cait et al. 2019 (n = 105) | ↓ Fecal levels of SCFA butyrate (stool samples collected at 3 months and 1 year of age) | ↑ Food allergy/food sensitization at 1 and 3 years | Ref. [10] |
| Roduit et al. 2018 (n = 301) | ↑ Fecal levels of SCFA butyrate (stool samples collected at 1 year of age) | ↓ Food allergy/food sensitization up to 6 years | Ref. [44] |
| Sandin et al. 2009 (n = 139) | ↓ Fecal levels of SCFA butyrate (stool samples collected at 1 and 4 year of age) | ↑ Food allergy/food sensitization at 1 and 4 years | Ref. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Costanzo, M.; De Paulis, N.; Biasucci, G. Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life 2021, 11, 384. https://doi.org/10.3390/life11050384
Di Costanzo M, De Paulis N, Biasucci G. Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life. 2021; 11(5):384. https://doi.org/10.3390/life11050384
Chicago/Turabian StyleDi Costanzo, Margherita, Nicoletta De Paulis, and Giacomo Biasucci. 2021. "Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy" Life 11, no. 5: 384. https://doi.org/10.3390/life11050384
APA StyleDi Costanzo, M., De Paulis, N., & Biasucci, G. (2021). Butyrate: A Link between Early Life Nutrition and Gut Microbiome in the Development of Food Allergy. Life, 11(5), 384. https://doi.org/10.3390/life11050384







