DUAL-tDCS Treatment over the Temporo-Parietal Cortex Enhances Writing Skills: First Evidence from Chronic Post-Stroke Aphasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Clinical Data
2.4. Materials
2.5. Procedure
2.5.1. Transcranial Direct Current Stimulation (tDCS)
2.5.2. Language Treatment
2.5.3. Data Analysis
3. Results
3.1. Accuracy Data
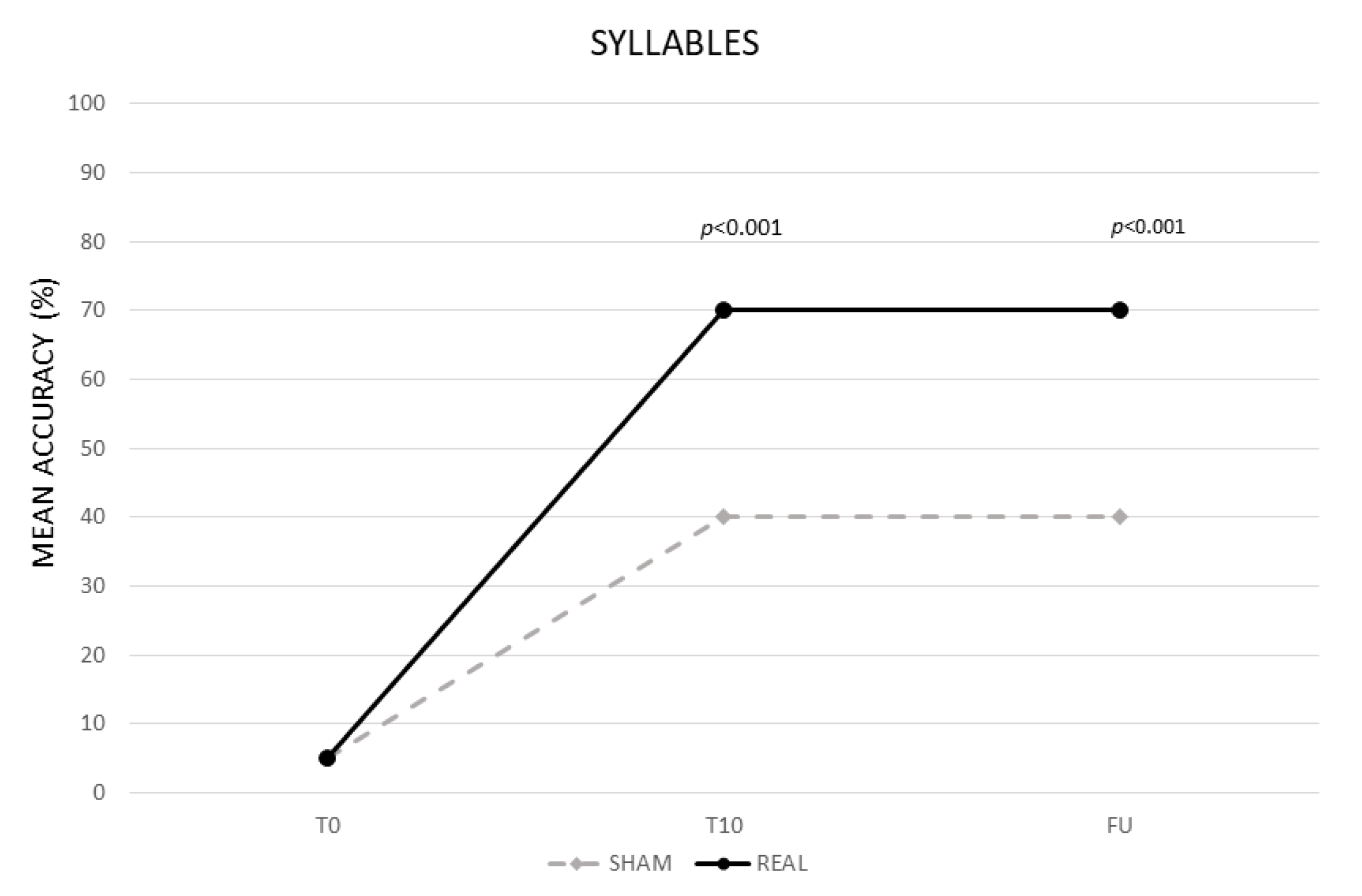
3.1.1. Syllables
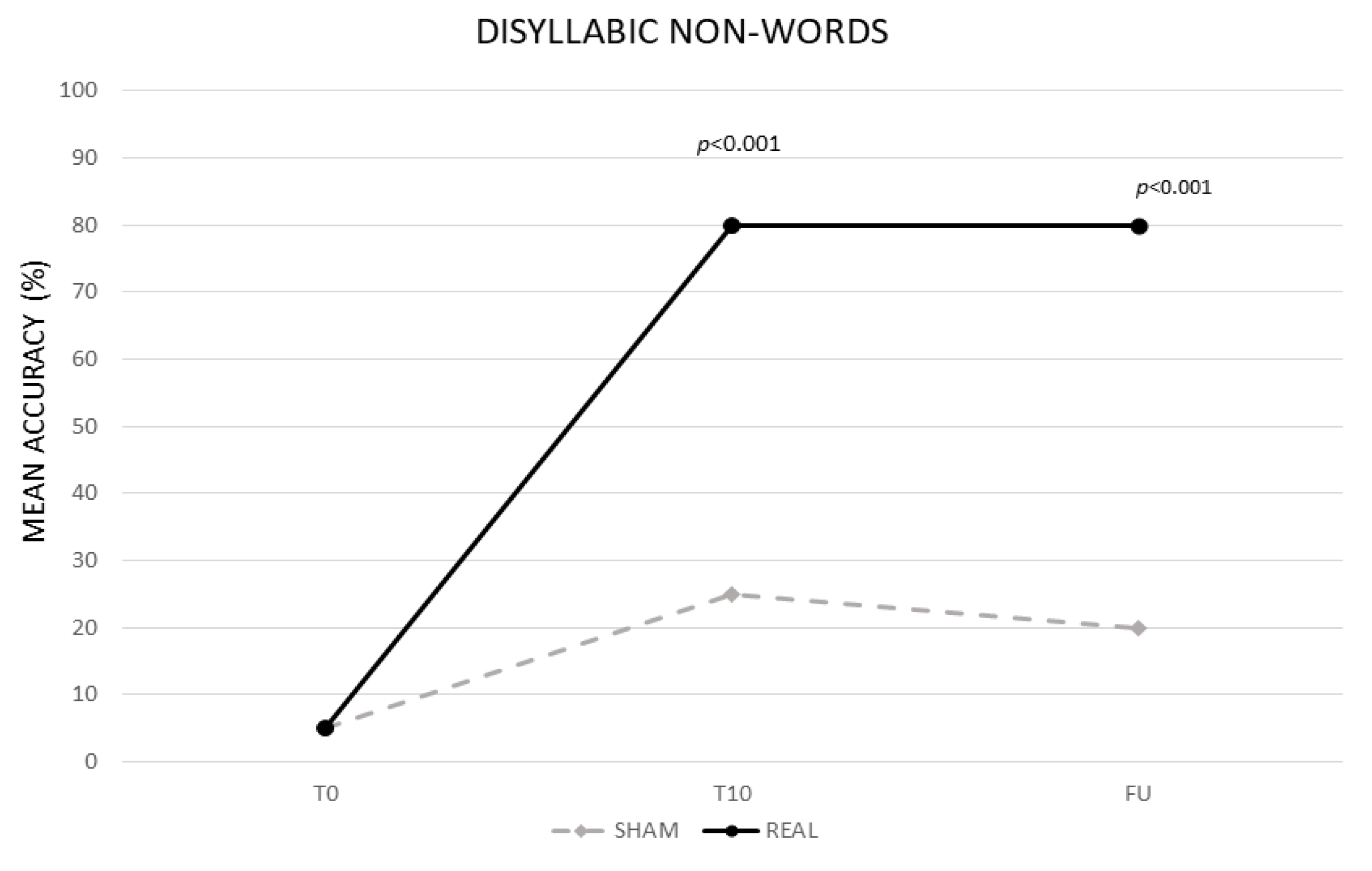
3.1.2. Disyllabic Nonwords
3.1.3. Trisyllabic Nonwords
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, M.; Lyden, P.; Brady, M. VISTA Collaboration. Aphasia and Dysarthria in Acute Stroke: Recovery and Functional Outcome. Int. J. Stroke 2015, 10, 400–406. [Google Scholar] [CrossRef]
- Sinanović, O.; Mrkonjić, Z.; Zukić, S.; Vidović, M.; Imamović, K. Post-stroke language disorders. Acta Clin. Croat. 2011, 50, 79–94. [Google Scholar]
- Thiel, L.; Sage, K.; Conroy, P. Retraining writing for functional purposes: A review of the writing therapy literature. Aphasiology 2015, 29, 423–441. [Google Scholar] [CrossRef]
- Ellis, A.W.; Young, A.W. Human Cognitive Neuropsychology: A Textbook with Readings; Psychology Press: Hove, UK, 2000. [Google Scholar]
- Beeson, P.M.; Hirsch, F.M.; Rewega, M.A. Successful single word writing treatment: Experimental analyses of four cases. Aphasiology 2002, 16, 473–491. [Google Scholar] [CrossRef]
- Kiran, S. Training phoneme to grapheme conversion for patients with written and oral production deficits: A model-based approach. Aphasiology 2005, 19, 53–76. [Google Scholar] [CrossRef]
- Coslett, H.B.; Turkeltaub, P. Acquired Dyslexia. In Neurobiology of Language; Academic Press: Cambridge, MA, USA, 2016; pp. 791–803. [Google Scholar] [CrossRef]
- Angelelli, P.; Marinelli, C.V.; Putzolu, A.; Notarnicola, A.; Iaia, M.; Burani, C. Learning to spell in a language with transparent orthography: Distributional properties of orthography and whole-word lexical processing. Q. J. Exp. Psychol. 2018, 71, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Beauvois, M.F.; Dérouesné, J. Lexical or orthographic agraphia. Brain 1981, 104, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Shallice, T. Phonological agraphia and the lexical route in writing. Brain 1981, 104, 413–429. [Google Scholar] [CrossRef]
- Baxter, D.M.; Warrington, E.K. Category specific phonological dysgraphia. Neuropsychologia 1985, 23, 653–666. [Google Scholar] [CrossRef]
- Harris, M.; Coltheart, M. Language Processing in Children and Adults; Routledge and Kegan Paul: London, UK, 1986. [Google Scholar]
- Patterson, K.E. Lexical but non semantic spelling? Cogn. Neuropsychol. 1986, 3, 341–367. [Google Scholar] [CrossRef]
- Goodman, A.G.; Caramazza, A. Aspects of the spelling process: Evidence from a case of acquired dysgraphia. Lang. Cogn. Process 1986, 1, 263–296. [Google Scholar] [CrossRef]
- Ardila, A.; Rosselli, M.; Ostrosky-solis, F. Agraphia in the Spanish language. Aphasiology 1996, 10, 723–739. [Google Scholar] [CrossRef]
- Luzzi, S.; Bartolini, M.; Coccia, M.; Provinciali, L.; Piccirilli, M.; Snowden, J.S. Surface dysgraphia in a regular orthography: Apostrophe use by an Italian writer. Neurocase 2003, 9, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Bigozzi, L.; Tarchi, C.; Pinto, G. Spelling across Tasks and Levels of Language in a Transparent Orthography. PLoS ONE 2016, 11, e0163033. [Google Scholar] [CrossRef] [PubMed]
- Laiacona, M.; Capitani, E.; Zonca, G.; Scola, I.; Saletta, P.; Luzzatti, C. Integration of lexical and sublexical processing in the spelling of regular words: A multiple single-case study in Italian dysgraphic patients. Cortex 2009, 45, 804–815. [Google Scholar] [CrossRef]
- Roeltgen, D.P.; Sevush, S.; Heilman, K.M. Phonological agraphia: Writing by the lexical-semantic route. Neurology 1983, 33, 755–765. [Google Scholar] [CrossRef]
- Bub, D.; Kertesz, A. Deep agraphia. Brain Lang. 1982, 17, 146–165. [Google Scholar] [CrossRef]
- Beeson, P.M. Remediation of written language. Top Stroke Rehabil. 2004, 11, 37–48. [Google Scholar] [CrossRef]
- Hillis, A.E.; Heidler, J. Contributions and limitations of the cognitive neuropsychological approach to treatment: Illustrations from studies of reading and spelling therapy. Aphasiology 2005, 19, 985–993. [Google Scholar] [CrossRef]
- Johnson, J.P.; Ross, K.; Kiran, S. Multi-step treatment for acquired alexia and agraphia (Part I): Efficacy, generalisation, and identification of beneficial treatment steps. Neuropsychol. Rehabil. 2019, 29, 534–564. [Google Scholar] [CrossRef]
- Cardell, E.A.; Chenery, H.J. A cognitive neuropsychological approach to the assessment and remediation of acquired dysgraphia. Lang. Test. 1999, 16, 353–388. [Google Scholar] [CrossRef]
- Beeson, P.M.; Rewega, M.A.; Vail, S.; Rapcsak, S.Z. Problem-solving approach to agraphia treatment: Interactive use of lexical and sublexical spelling routes. Aphasiology 2000, 14, 551–565. [Google Scholar] [CrossRef]
- Luzzatti, C.; Colombo, C.; Frustaci, M.; Vitolo, F. Rehabilitation of spelling along the sub-word level routine. Neuropsychol. Rehabil. 2000, 10, 249–278. [Google Scholar] [CrossRef]
- Carlomagno, S.; Luzzatti, C. La Riabilitazione dei Disturbi di Scrittura Nei Pazienti Afasici; Masson: Millan, Italy, 1997. [Google Scholar]
- Caravolas, M. Spelling development in alphabetic writing systems: A cross-linguistic perspective. Eur. Psychol. 2004, 9, 3–14. [Google Scholar] [CrossRef]
- Notarnicola, A.; Angelelli, P.; Judica, A.; Zoccolotti, P. Development of spelling skills in a shallow orthography: The case of Italian language. Read Writ. 2012, 25, 1171–1194. [Google Scholar] [CrossRef]
- Marinelli, C.V.; Romani, C.; Burani, C.; Zoccolotti, P. Spelling Acquisition in English and Italian: A Cross-Linguistic Study. Front Psychol. 2015, 8, 1843. [Google Scholar] [CrossRef] [PubMed]
- Marangolo, P. The potential effects of transcranial direct current stimulation (tDCS) on language functioning: Combining neuromodulation and behavioral intervention in aphasia. Neurosci. Lett. 2020, 719, 133329. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Transcranial direct current stimulation—Update 2011. Restor. Neurol. Neurosci. 2011, 29, 463–492. [Google Scholar] [CrossRef]
- Lefaucheur, J.P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, H.J.; Yoon, K.J.; Chang, W.H.; Kim, Y.H. Effects of dual transcranial direct current stimulation for aphasia in chronic stroke patients. Ann. Rehabil. Med. 2013, 37, 603–610. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Cipollari, S.; Campana, S.; Razzano, C.; Di Paola, M.; Koch, G.; Caltagirone, C. Bihemispheric stimulation over left and right inferior frontal region enhances recovery from apraxia of speech in chronic aphasia. Eur. J. Neurosci. 2013, 38, 3370–3377. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Sabatini, U.; De Pasquale, G.; Razzano, C.; Caltagirone, C.; Gili, T. Bilateral Transcranial Direct Current Stimulation Language Treatment Enhances Functional Connectivity in the Left Hemisphere: Preliminary Data from Aphasia. J. Cogn. Neurosci. 2016, 28, 724–738. [Google Scholar] [CrossRef]
- De Aguiar, V.; Bastiaanse, R.; Capasso, R.; Gandolfi, M.; Smania, N.; Rossi, G.; Miceli, G. Can tDCS enhance item-specific effects and generalization after linguistically motivated aphasia therapy for verbs? Front. Behav. Neurosci. 2015, 9, 190. [Google Scholar] [CrossRef]
- Crinion, J.; Price, C.J. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain 2005, 128, 2858–2871. [Google Scholar] [CrossRef]
- Leff, A.; Crinion, J.; Scott, S.; Turkheimer, F.; Howard, D.; Wise, R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann. Neurol. 2002, 51, 553–558. [Google Scholar] [CrossRef]
- Robson, H.; Zahn, R.; Keidel, J.L.; Binney, R.J.; Sage, K.; Lambon Ralph, M.A. The anterior temporal lobes support residual comprehension in Wernicke’s aphasia. Brain 2014, 137 Pt 3, 931–943. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Messing, S.; Norise, C.; Hamilton, R.H. Are networks for residual language function and recovery consistent across aphasic patients? Neurology 2011, 76, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. Contrasting opinions on the role of the right hemisphere in the recovery of language. A critical survey. Aphasiology 2015, 29, 1020–1037. [Google Scholar] [CrossRef]
- Picano, C.; Quadrini, A.; Pisano, F.; Marangolo, P. Adjunctive Approaches to Aphasia Rehabilitation: A Review on Efficacy and Safety. Brain Sci. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- De Tommaso, B.; Piedimonte, A.; Caglio, M.M.; D’Agata, F.; Campagnoli, M.; Orsi, L.; Raimondo, S.; Vighetti, S.; Mortara, P.; Massazza, G.; et al. The rehabilitative effects on written language of a combined language and parietal dual-tDCS treatment in a stroke case. Neuropsychol. Rehabil. 2017, 27, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.J.; Turkeltaub, P.E.; Eden, G.F.; Rapp, B. Examining the central and peripheral processes of written word production through meta-analysis. Front Psychol. 2011, 2, 239. [Google Scholar] [CrossRef] [PubMed]
- Planton, S.; Jucla, M.; Roux, F.E.; Démonet, J.F. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013, 49, 2772–2787. [Google Scholar] [CrossRef] [PubMed]
- DeMarco, A.T.; Wilson, S.M.; Rising, K.; Rapcsak, S.Z.; Beeson, P.M. Neural substrates of sublexical processing for spelling. Brain Lang. 2017, 164, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Wagner, A.D. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia 2003, 41, 304–317. [Google Scholar] [CrossRef]
- Beeson, P.; Rapcsak, S.; Plante, E.; Chargualaf, J.; Chung, A.; Johnson, S.; Trouard, T. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology 2003, 17, 647–665. [Google Scholar] [CrossRef]
- Tsapkini, K.; Rapp, B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex 2010, 46, 185–205. [Google Scholar] [CrossRef][Green Version]
- Rapcsak, S.Z.; Beeson, P.M. Neuroanatomical correlates of spelling and writing. In The Handbook of Adult Language Disorders; Hillis, A.E., Ed.; Psychology Press: Hove, UK, 2015; pp. 87–116. [Google Scholar]
- Turkeltaub, P.E.; Benson, J.; Hamilton, R.H.; Datta, A.; Bikson, M.; Coslett, H.B. Left lateralizing transcranial direct current stimulation improves reading efficiency. Brain Stimul. 2012, 5, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, F.; Varuzza, C.; Rossi, S.; Sdoia, S.; Varvara, P.; Oliveri, M.; Koch, G.; Vicari, S.; Menghini, D. Reading changes in children and adolescents with dyslexia after transcranial direct current stimulation. Neuroreport 2016, 27, 295–300. [Google Scholar] [CrossRef]
- Costanzo, F.; Rossi, S.; Varuzza, C.; Varvara, P.; Vicari, S.; Menghini, D. Long-lasting improvement following tDCS treatment combined with a training for reading in children and adolescents with dyslexia. Neuropsychologia 2019, 130, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cancer, A.; Antonietti, A. tDCS Modulatory Effect on Reading Processes: A Review of Studies on Typical Readers and Individuals with Dyslexia. Front Behav. Neurosci. 2018, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Ludersdorfer, P.; Kronbichler, M.; Wimmer, H. Accessing orthographic representations from speech: The role of left ventral occipitotemporal cortex in spelling. Hum Brain Mapp. 2015, 36, 1393–1406. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Ciurli, P.; Marangolo, P.; Basso, A. Esame del Linguaggio (II versione); Organ. Spec. Firenze: Florence, Italy, 1996; ISBN 9788809403048. [Google Scholar]
- De Renzi, E.; Faglioni, P. Normative data and screening power of a shortened version of the token test. Cortex 1978, 14, 41–49. [Google Scholar] [CrossRef]
- Smith, C.L. Handbook of the International Phonetic Association: A Guide to the Use of the International Phonetic Alphabet 1999. Phonology 2000, 17, 291–295. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Beeson, P.M.; Rising, K.; Kim, E.S.; Rapcsak, S.Z. A treatment sequence for phonological alexia/agraphia. J. Speech Lang Hear Res. 2010, 53, 450–468. [Google Scholar] [CrossRef]
- Galletta, E.E.; Cancelli, A.; Cottone, C.; Simonelli, I.; Tecchio, F.; Bikson, M.; Marangolo, P. Use of Computational Modeling to Inform tDCS Electrode Montages for the Promotion of Language Recovery in Post-stroke Aphasia. Brain Stimul. 2015, 8, 1108–1115. [Google Scholar] [CrossRef]
- Dockery, C.A.; Hueckel-Weng, R.; Birbaumer, N.; Plewnia, C. Enhancement of planning ability by transcranial direct current stimulation. J Neurosci. 2009, 29, 7271–7277. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Cohen Kadosh, R.; Soskic, S.; Iuculano, T.; Kanai, R.; Walsh, V. Modulating neuronal activity produces specific and long-lasting changes in numerical competence. Curr. Biol. 2010, 20, 2016–2020. [Google Scholar] [CrossRef]
- Marangolo, P.; Marinelli, C.V.; Bonifazi, S.; Fiori, V.; Ceravolo, M.G.; Provinciali, L.; Tomaiuolo, F. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav. Brain Res. 2011, 225, 498–504. [Google Scholar] [CrossRef]
- Meinzer, M.; Darkow, R.; Lindenberg, R.; Flöel, A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain 2016, 139 Pt 4, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time- but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cereb. Cortex. 2015, 25, 109–117. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: Potential implications for motor learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, F.; Menghini, D.; Caltagirone, C.; Oliveri, M.; Vicari, S. High frequency rTMS over the left parietal lobule increases non-word reading accuracy. Neuropsychologia 2012, 50, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, F.; Menghini, D.; Caltagirone, C.; Oliveri, M.; Vicari, S. How to improve reading skills in dyslexics: The effect of high frequency rTMS. Neuropsychologia 2013, 51, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Perceval, G.; Martin, A.K.; Copland, D.A.; Laine, M.; Meinzer, M. High-definition tDCS of the temporo-parietal cortex enhances access to newly learned words. Sci. Rep. 2017, 7, 17023. [Google Scholar] [CrossRef]
- Fiori, V.; Coccia, M.; Marinelli, C.V.; Vecchi, V.; Bonifazi, S.; Ceravolo, M.G.; Provinciali, L.; Tomaiuolo, F.; Marangolo, P. Transcranial direct current stimulation improves word retrieval in healthy and nonfluent aphasic subjects. J. Cogn. Neurosci. 2011, 23, 2309–2323. [Google Scholar] [CrossRef]
- Fiori, V.; Nitsche, M.; Iasevoli, L.; Cucuzza, G.; Caltagirone, C.; Marangolo, P. Differential effects of bihemispheric and unihemispheric transcranial direct current stimulation in young and elderly adults in verbal learning. Behav. Brain Res. 2017, 321, 170–175. [Google Scholar] [CrossRef]
- Floel, A.; Rosser, N.; Michka, O.; Knecht, S.; Breitenstein, C. Noninvasive brain stimulation improves language learning. J.Cogn. Neurosci. 2008, 20, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Meinzer, M.; Jähnigen, S.; Copland, D.A.; Darkow, R.; Grittner, U.; Avirame, K.; Rodriguez, A.D.; Lindenberg, R.; Flöel, A. Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex 2014, 50, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Savill, N.; Ashton, J.; Gugliuzza, J.; Poole, C.; Sim, Z.; Ellis, A.W.; Jefferies, E. tDCS to temporoparietal cortex during familiarisation enhances the subsequent phonological coherence of nonwords in immediate serial recall. Cortex 2015, 63, 132–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Price, C.J. A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 2012, 62, 816–847. [Google Scholar] [CrossRef] [PubMed]
- Breitenstein, C.; Jansen, A.; Deppe, M.; Foerster, A.-F.; Sommer, J.; Wolbers, T.; Knecht, S. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage 2005, 25, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Paulesu, E.; Vallar, G.; Berlingeri, M.; Signorini, M.; Vitali, P.; Burani, C.; Perani, D.; Fazio, F. Supercalifragilisticexpialidocious: How the brain learns words never heard before. Neuroimage 2009, 45, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Maloney, E.; Risko, E.F.; O’Malley, S.; Besner, D. Tracking the transition from sublexical to lexical processing: On the creation of orthographic and phonological lexical representations. Q. J. Exp. Psychol. 2009, 62, 858–867. [Google Scholar] [CrossRef]
- Kuo, M.F.; Paulus, W.; Nitsche, M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 2014, 3, 948–960. [Google Scholar] [CrossRef]
- Hamilton, R.H.; Chrysikou, E.G.; Coslett, B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011, 118, 40–50. [Google Scholar] [CrossRef]
- Hartwigsen, G. Adaptive Plasticity in the Healthy Language Network: Implications for Language Recovery after Stroke. Neural. Plast. 2016, 2016, 9674790. [Google Scholar] [CrossRef]
- Sehm, B.; Schäfer, A.; Kipping, J.; Margulies, D.; Conde, V.; Taubert, M.; Villringer, A.; Ragert, P. Dynamic modulation of intrinsic functional connectivity by transcranial direct current stimulation. J. Neurophysiol. 2012, 108, 3253–3263. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S. What is the nature of poststroke language recovery and reorganization? ISRN Neurol. 2012, 2012, 786872. [Google Scholar] [CrossRef] [PubMed]

| p | Sex | Age | Ed. Level | Time Post Onset | Stroke Type | Lesion Side LH | Oral NN | Oral VN | Written NN | Written VN | W R | NWR | W Read | NW Read | WD | NW D | TT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 57 | 13 | 3 years | I | FTI | 7.5 | 10 | 0 | 0 | 35 | 22.5 | 15 | 5 | 0 | 0 | 4 |
| 2 | M | 59 | 13 | 3 years | I | T | 0 | 0 | 0 | 0 | 25 | 30 | 17.5 | 15 | 0 | 0 | 2.5 |
| 3 | M | 53 | 17 | 1 year | I | FTI | 0 | 0 | 0 | 0 | 22.5 | 30 | 12.5 | 15 | 0 | 0 | 6 |
| 4 | F | 65 | 8 | 3 years | I | FTI | 15 | 15 | 0 | 0 | 42.5 | 20 | 20 | 10 | 0 | 0 | 10 |
| 5 | M | 55 | 13 | 4 years | I | T | 15 | 10 | 0 | 0 | 20 | 15 | 15 | 5 | 0 | 0 | 10 |
| 6 | M | 64 | 13 | 1 year | I | FTP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 7 | M | 62 | 17 | 1 year | I | FTI | 7.5 | 0 | 0 | 0 | 40 | 32.5 | 12.5 | 2.5 | 0 | 0 | 10 |
| 8 | M | 63 | 17 | 4 years | I | FTI | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| 9 | F | 55 | 13 | 3 years | I | FTP | 15 | 15 | 0 | 0 | 80 | 35 | 32.5 | 10 | 0 | 0 | 8 |
| 10 | F | 57 | 8 | 1 year | I | T | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 |
| 11 | F | 55 | 13 | 1 year | H | FTP | 15 | 15 | 0 | 0 | 85 | 30 | 15 | 15 | 0 | 0 | 10 |
| 12 | F | 65 | 13 | 2 years | I | FTP | 10 | 15 | 0 | 0 | 15 | 10 | 15 | 15 | 0 | 0 | 10 |
| 13 | F | 58 | 8 | 4 years | I | FTP | 10 | 10 | 0 | 0 | 20 | 20 | 12.5 | 5 | 0 | 0 | 7.5 |
| 14 | F | 65 | 13 | 3 years | I | FTP | 15 | 12.5 | 0 | 0 | 10 | 15 | 15 | 5 | 0 | 0 | 4 |
| p | C | Oral NN | Oral VN | Written NN | Written VN | W R | Ntable R | W Read | NW Read | W Dict | NW Dict | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | T0 | T10 | ||
| Real First | |||||||||||||||||||||
| 1 | R | 7.5 | 80 ^ | 10 | 60 ^ | 0 | 55 ^ | 0 | 40 ^ | 35 | 92.5 ^ | 22.5 | 90 ^ | 15 | 67.5 ^ | 5 | 50 ^ | 0 | 72.5 ^ | 0 | 55 ^ |
| S | 80 | 85 | 60 | 70 | 55 | 55 | 40 | 45 | 92.5 | 90 | 90 | 95 | 67.5 | 70 | 50 | 45 | 72.5 | 75 | 55 | 55 | |
| 3 | R | 0 | 67.5 ^ | 0 | 60 ^ | 0 | 37.5 ^ | 0 | 35 ^ | 22.5 | 85 ^ | 30 | 80 ^ | 12.5 | 42.5 ^ | 15 | 60 ^ | 0 | 50 ^ | 0 | 52.5 ^ |
| S | 67.5 | 80 * | 60 | 80 ** | 37.5 | 35 | 35 | 40 | 85 | 92.5 | 80 | 95 ** | 42.5 | 45 | 60 | 60 | 50 | 55 | 52.5 | 62.5 | |
| 5 | R | 15 | 70 ^ | 10 | 80 ^ | 0 | 42.5 ^ | 0 | 32.5 ^ | 20 | 82.5 ^ | 15 | 80 ^ | 15 | 60 ^ | 5 | 62.5 ^ | 0 | 57.5 ^ | 0 | 67.5 ^ |
| S | 70 | 77.5 | 80 | 85 | 42.5 | 42.5 | 32.5 | 35 | 82.5 | 97.5 ^ | 80 | 80 | 60 | 60 | 62.5 | 62.5 | 57.5 | 57.5 | 67.5 | 52.5 | |
| 7 | R | 7.5 | 55 ^ | 0 | 60 ^ | 0 | 30 ^ | 0 | 27.5 ^ | 40 | 72.5 ^ | 32.5 | 87.5 ^ | 12.5 | 62.5 ^ | 2.5 | 47.5 ^ | 0 | 40 ^ | 0 | 45 ^ |
| S | 55 | 75 ** | 60 | 70 | 30 | 35 | 27.5 | 27.5 | 72.5 | 72.5 | 87.5 | 87.5 | 62.5 | 57.5 | 47.5 | 47.5 | 40 | 40 | 45 | 45 | |
| 9 | R | 15 | 45 ^ | 15 | 65 ^ | 0 | 40 ^ | 0 | 15 ^ | 80 | 100 ^ | 35 | 82.5 ^ | 32.5 | 80 ^ | 10 | 50 ^ | 0 | 50 ^ | 0 | 12.5 ^ |
| S | 45 | 54 | 65 | 70 | 40 | 55 * | 15 | 15 | 100 | 92.5 | 82.5 | 92.5 * | 80 | 80 | 50 | 50 | 50 | 42.5 | 12.5 | 17 | |
| 11 | R | 15 | 60 ^ | 15 | 80 ^ | 0 | 55.5 ^ | 0 | 17.5 ^ | 85 | 95 * | 30 | 92.5 ^ | 15 | 60 ^ | 15 | 77.5 ^ | 0 | 65 ^ | 0 | 20 ^ |
| S | 60 | 60 | 80 | 80 | 55.5 | 60 | 17.5 | 20 | 95 | 97.5 | 92.5 | 95 | 60 | 60 | 77.5 | 82 | 65 | 65 | 20 | 27.5 | |
| 13 | R | 10 | 67.5 ^ | 10 | 75 ^ | 0 | 42.5 ^ | 0 | 30 ^ | 20 | 65 ^ | 20 | 70 ^ | 12.5 | 65 ^ | 5 | 45 ^ | 0 | 55 ^ | 0 | 47.5 ^ |
| S | 67.5 | 65 | 75 | 80 | 42.5 | 50 | 30 | 30 | 65 | 72.5 | 70 | 70 | 65 | 65 | 45 | 50 | 55 | 57.5 | 47.5 | 47 | |
| Sham First | |||||||||||||||||||||
| 2 | S | 0 | 20 ^ | 0 | 5 | 0 | 10 ** | 0 | 5 | 25 | 35 | 30 | 35 | 17.5 | 12.5 | 15 | 15 | 0 | 20 ^ | 0 | 5 |
| R | 20 | 45 ^ | 5 | 10 | 10 | 40 ^ | 5 | 20 ** | 35 | 85 ^ | 35 | 90 ^ | 12.5 | 57.5 ^ | 15 | 57.5 ^ | 20 | 50 ^ | 5 | 42.5 ^ | |
| 4 | S | 15 | 27.5 * | 15 | 30 * | 0 | 5 | 0 | 10 ** | 42.5 | 47.5 | 20 | 20 | 20 | 30 | 10 | 10 | 0 | 25 ^ | 0 | 25 ^ |
| R | 27.5 | 55 ^ | 30 | 62.5 ^ | 5 | 50 ^ | 10 | 35 ^ | 47.5 | 62.5 * | 20 | 62.5 ^ | 30 | 75 ^ | 10 | 62.5 ^ | 25 | 55 ^ | 25 | 67.5 ^ | |
| 6 | S | 0 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 17.5 ^ | 0 | 20 ^ | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 5 |
| R | 2.5 | 15 ** | 0 | 0 | 0 | 10 ** | 0 | 0 | 17.5 | 47.5 ^ | 20 | 65 ^ | 0 | 10 ** | 5 | 15 * | 0 | 0 | 5 | 25 ^ | |
| 8 | S | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| R | 0 | 20 ^ | 0 | 0 | 0 | 15 ^ | 0 | 0 | 5 | 17.5 ** | 5 | 25 ^ | 0 | 12.5 ^ | 0 | 15 ^ | 0 | 0 | 0 | 20 ^ | |
| 10 | S | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 20 ^ | 0 | 20 ^ | 0 | 15 ^ | 0 | 2.5 | 0 | 0 | 0 | 0 |
| R | 0 | 45 ^ | 0 | 35 ^ | 5 | 35 ^ | 0 | 10 ** | 20 | 65 ^ | 20 | 62.5 ^ | 15 | 42.5 ^ | 2.5 | 25 ^ | 0 | 40 ^ | 0 | 35 ^ | |
| 12 | S | 10 | 15 | 15 | 20 | 0 | 10 ** | 0 | 0 | 15 | 20 | 10 | 22.5 * | 15 | 30 * | 15 | 20 | 0 | 25 ^ | 0 | 10 ** |
| R | 15 | 60 ^ | 20 | 65 ^ | 10 | 55 ^ | 0 | 15 ^ | 20 | 72.5 ^ | 22.5 | 75 ^ | 30 | 75 ^ | 20 | 65 ^ | 25 | 70 ^ | 10 | 50 ^ | |
| 14 | S | 15 | 32.5 ** | 12.5 | 20 | 0 | 5 | 0 | 0 | 10 | 17.5 | 15 | 15 | 15 | 27.5 * | 5 | 10 | 0 | 15 ^ | 0 | 5 |
| R | 32.5 | 80 ^ | 20 | 80 ^ | 5 | 45.5 ^ | 0 | 20 ^ | 17.5 | 72.5 ^ | 15 | 65 ^ | 27.5 | 75 ^ | 10 | 35 ^ | 15 | 65 ^ | 5 | 57.5 ^ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisano, F.; Caltagirone, C.; Incoccia, C.; Marangolo, P. DUAL-tDCS Treatment over the Temporo-Parietal Cortex Enhances Writing Skills: First Evidence from Chronic Post-Stroke Aphasia. Life 2021, 11, 343. https://doi.org/10.3390/life11040343
Pisano F, Caltagirone C, Incoccia C, Marangolo P. DUAL-tDCS Treatment over the Temporo-Parietal Cortex Enhances Writing Skills: First Evidence from Chronic Post-Stroke Aphasia. Life. 2021; 11(4):343. https://doi.org/10.3390/life11040343
Chicago/Turabian StylePisano, Francesca, Carlo Caltagirone, Chiara Incoccia, and Paola Marangolo. 2021. "DUAL-tDCS Treatment over the Temporo-Parietal Cortex Enhances Writing Skills: First Evidence from Chronic Post-Stroke Aphasia" Life 11, no. 4: 343. https://doi.org/10.3390/life11040343
APA StylePisano, F., Caltagirone, C., Incoccia, C., & Marangolo, P. (2021). DUAL-tDCS Treatment over the Temporo-Parietal Cortex Enhances Writing Skills: First Evidence from Chronic Post-Stroke Aphasia. Life, 11(4), 343. https://doi.org/10.3390/life11040343







