Theranostics in Boron Neutron Capture Therapy
Abstract
1. Introduction: What Is BNCT and Why Is Theranostics Necessary for the Further Development of This Modality?
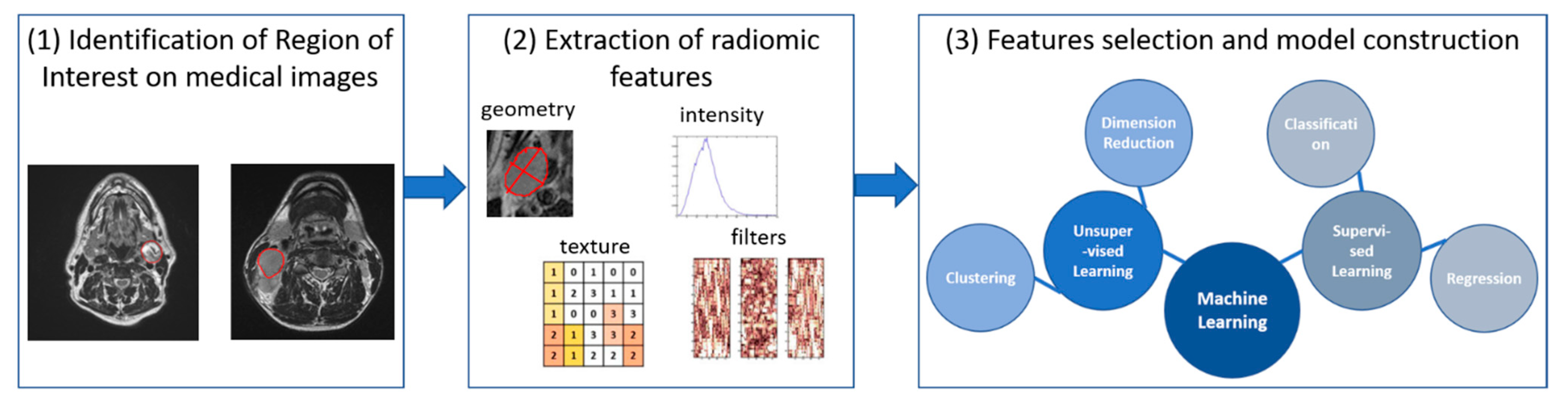
2. Multi-Modal Imaging for Treatment Optimization and Response Evaluation
2.1. Medical Image Registration for BNCT Treatment Planning
2.2. Medical Imaging for BNCT Response Evaluation: Current Use and Future Perspectives
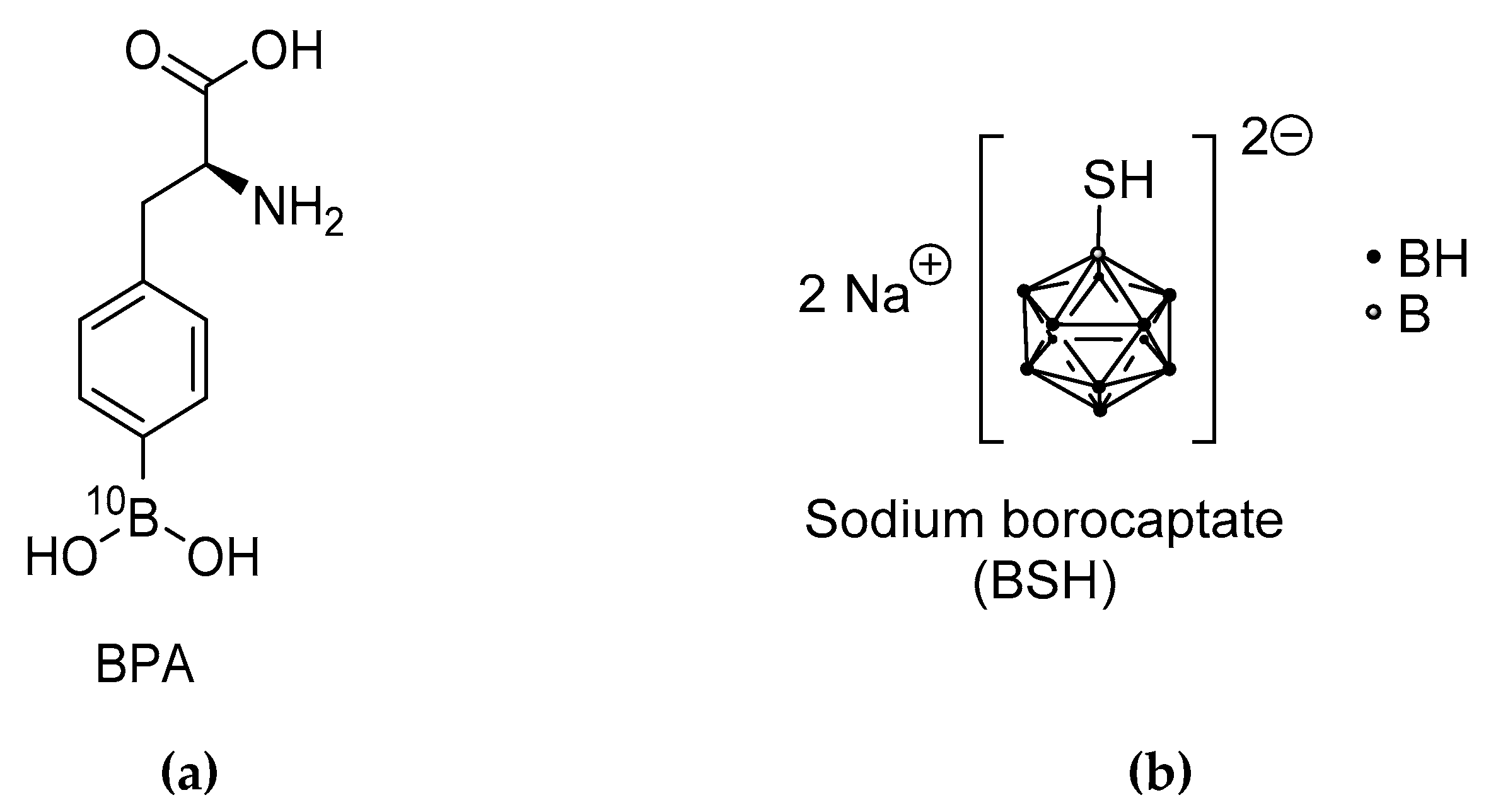
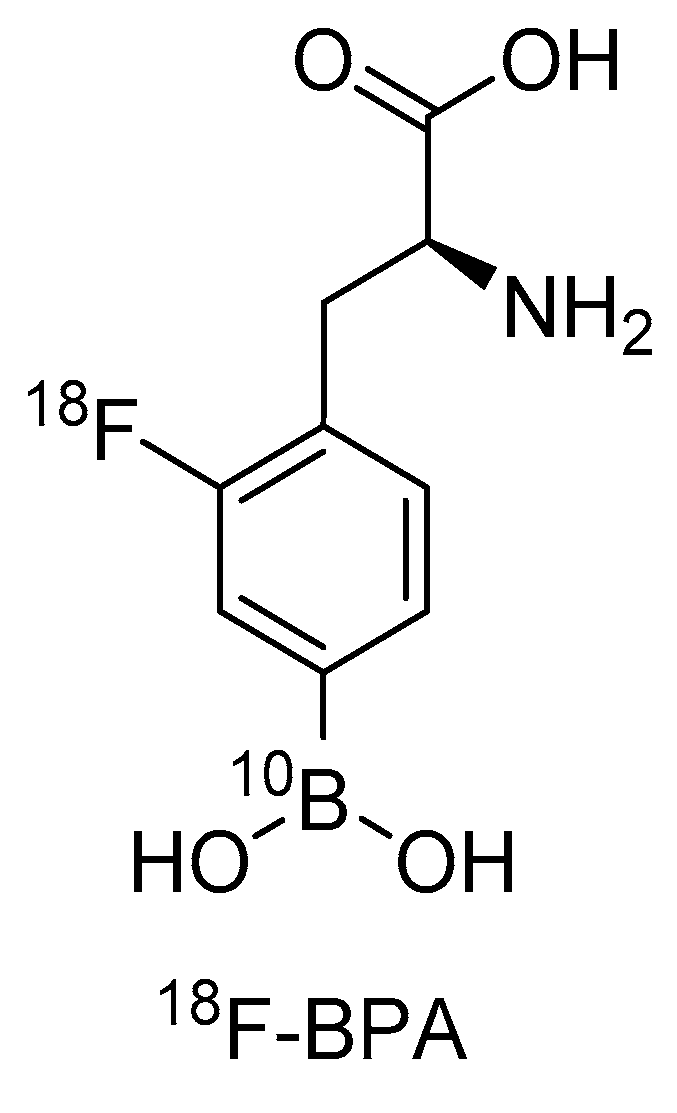
3. BPA and [18F]FBPA: An Existing First Theranostic Approach in BNCT
4. Further Diagnostic Possibilities for Theranostic Approaches in BNCT
5. Molecular Modalities with Theranostic Applications in BNCT
5.1. Small Molecules
5.2. Cell-Penetrating Peptides (CPP) for BNCT
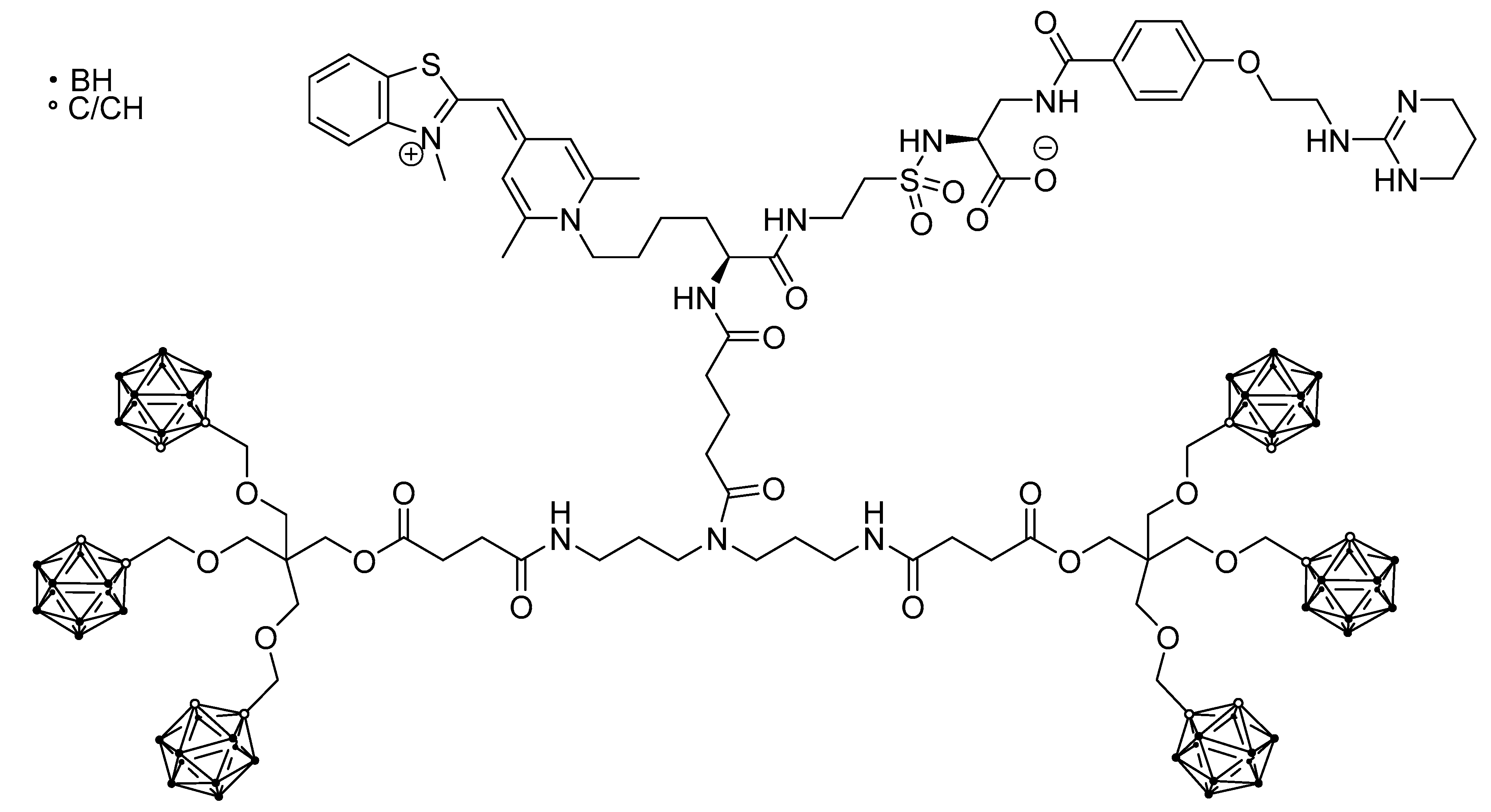
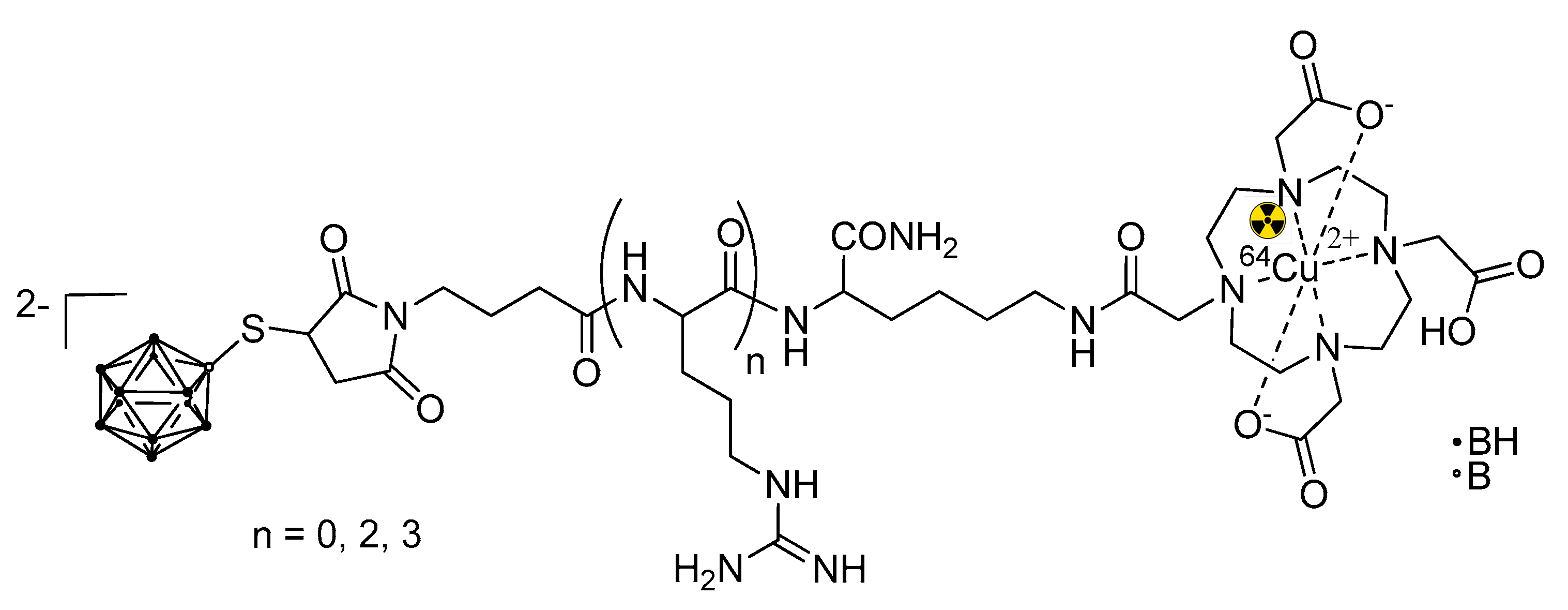
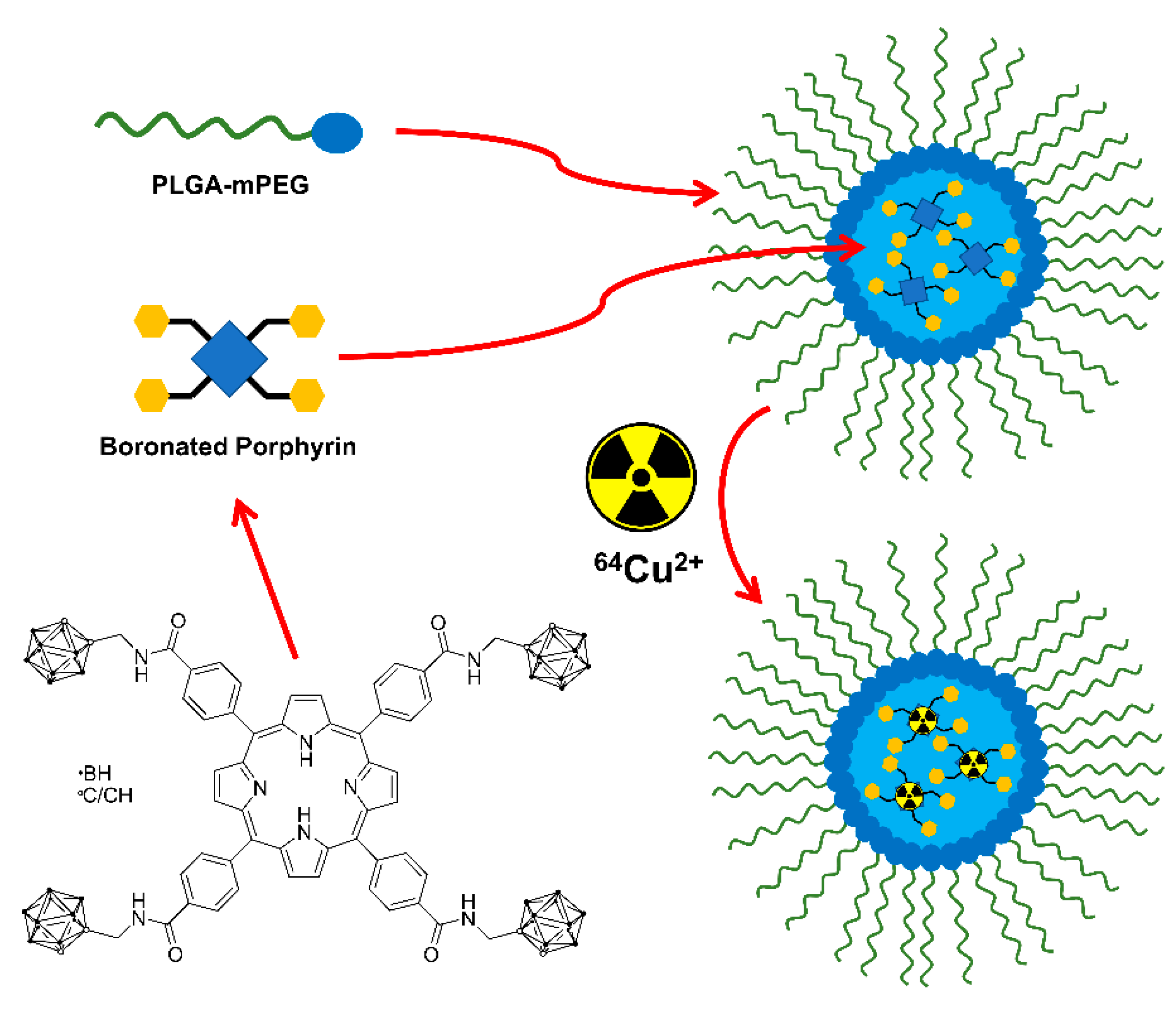
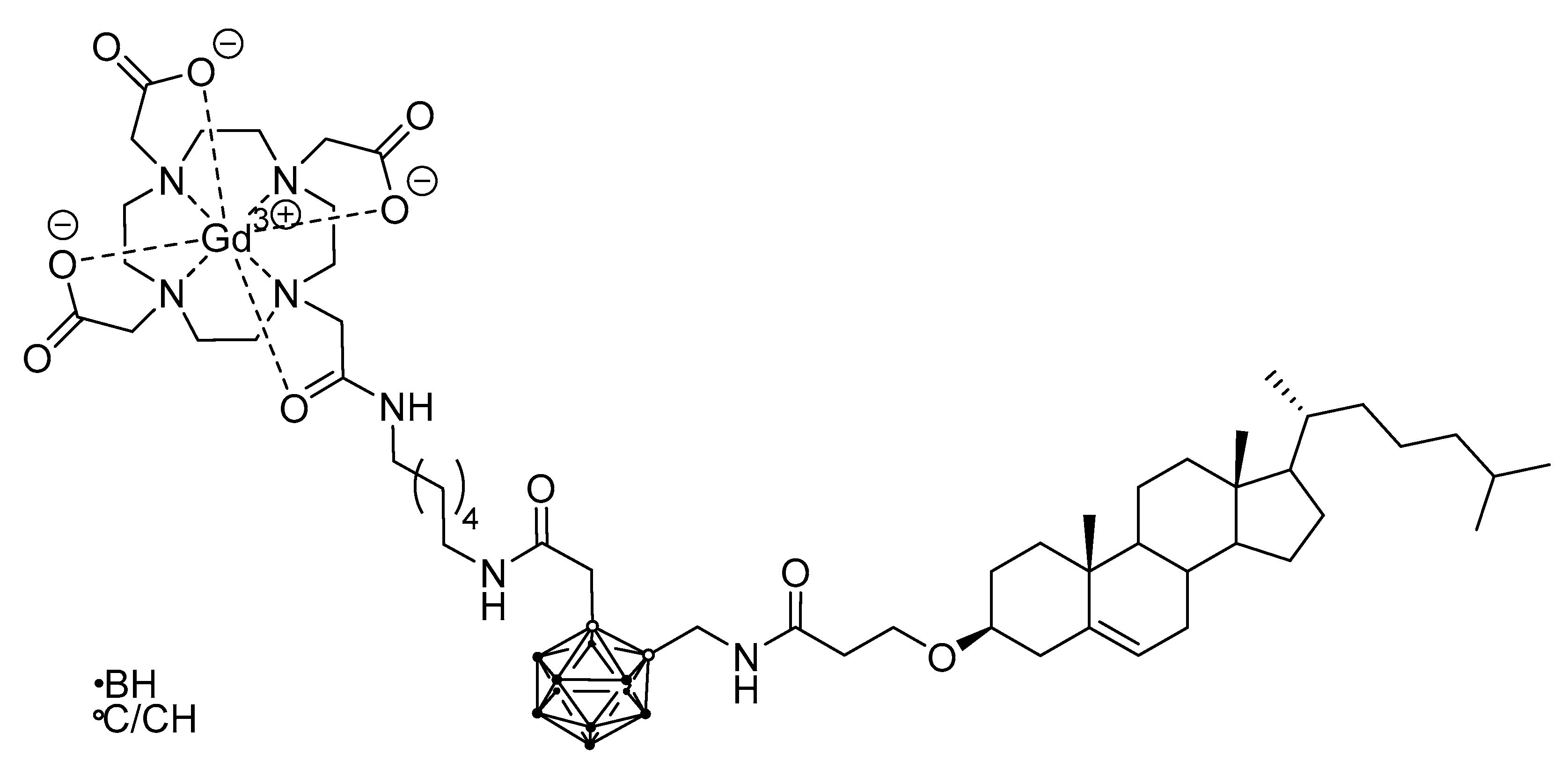
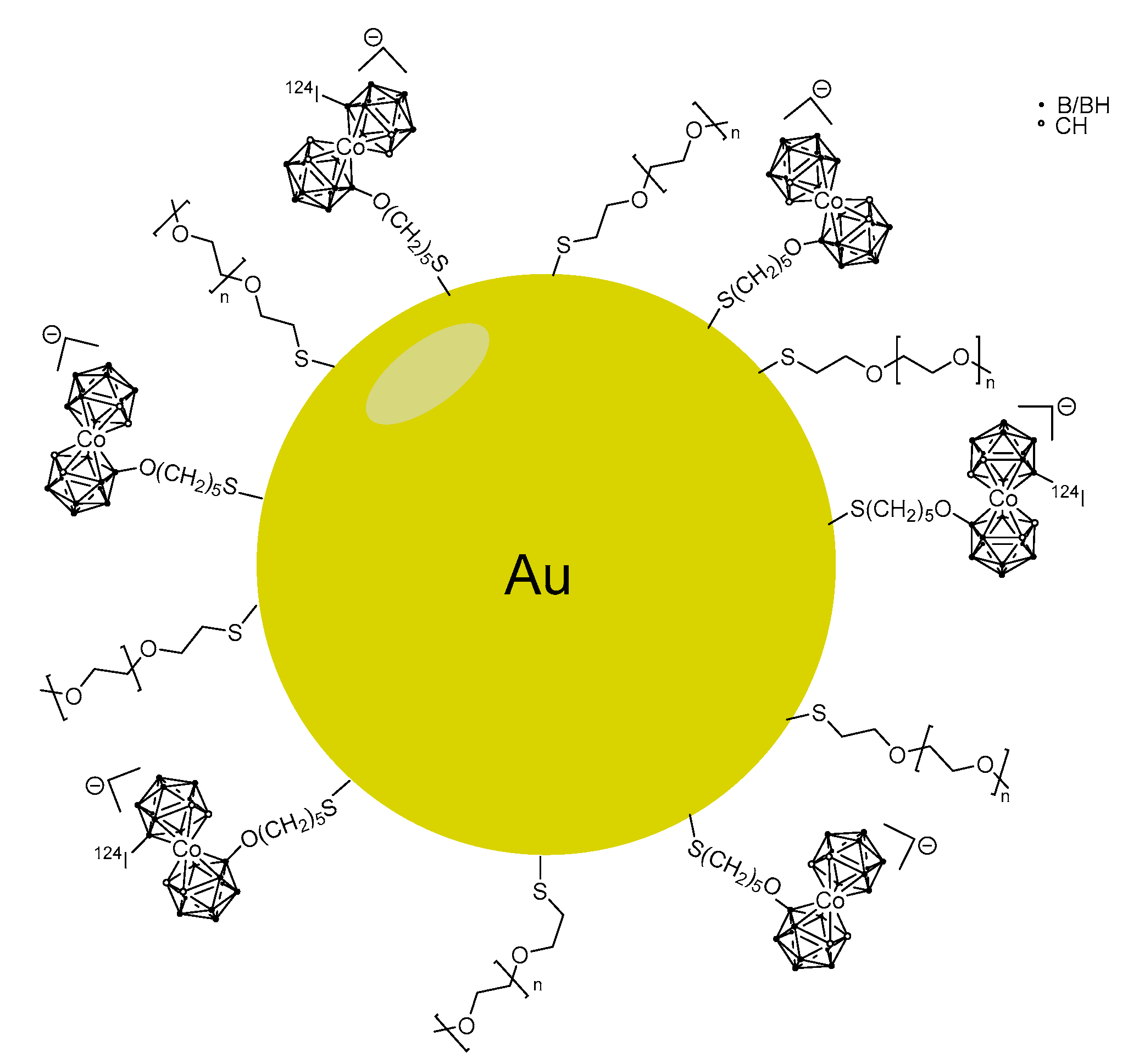
5.3. Larger Boron Vectors: Proteins, Antibodies and Nanoparticles
6. Proteomics for Predicting Outcome
- discovery and validation of biomarkers useful for diagnosis and follow-up of therapy;
- characterization of metabolic pathways involved in the investigated diseases;
- elucidation of molecular mechanism (endotypes) related to disease etiology and clinical stratification.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sauerwein, W.A.G. Principles and roots of neutron capture therapy. In Neutron Capture Therapy: Principles and Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–16. ISBN 9783642313349. [Google Scholar]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 601820. [Google Scholar] [CrossRef] [PubMed]
- Sauerwein, W.; Bet, P.; Wittig, A. Drugs for BNCT: BSH and BPA. In Neutron Capture Therapy. Principles and Application; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 117–160. [Google Scholar]
- Snyder, H.R.; Reedy, A.J.; Lennarj, W.J. Synthesis of aromatic boronic acids. Aldehyde boronic acids and a boronic acid analog of tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Soloway, A.H.; Hatanaka, H.; Davis, M.A. Penetration of brain and brain tumor. VII. Tumor binding sulfhydryl boron compounds. J. Med. Chem. 1967, 10, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Mirzaei, H.R.; Sahebkar, A.; Salehi, R.; Nahand, J.S.; Karimi, E.; Jaafari, M.R.; Mirzaei, H. Boron neutron capture therapy: Moving toward targeted cancer therapy. J. Cancer Res. Ther. 2016, 12, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. (Lond.) 2018, 38, 35. [Google Scholar] [CrossRef]
- Silberstein, E.B. Radioiodine: The classic theranostic agent. Semin. Nucl. Med. 2012, 42, 164–170. [Google Scholar] [CrossRef]
- Levine, R.; Krenning, E.P. Clinical history of the theranostic radionuclide approach to neuroendocrine tumors and other types of cancer: Historical review based on an interview of Eric P. Krenning by Rachel Levine. J. Nucl. Med. 2017, 58, 3S–9S. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, S.; Kortesniemi, M.; Timonen, M.; Reijonen, V.; Kuusela, L.; Uusi-Simola, J.; Salli, E.; Koivunoro, H.; Seppala, T.; Lonnroth, N.; et al. Boron neutron capture therapy (BNCT) in Finland: Technological and physical prospects after 20 years of experiences. Phys. Med. 2013, 29, 233–248. [Google Scholar] [CrossRef]
- Wang, L.W.; Chen, Y.W.; Ho, C.Y.; Hsueh Liu, Y.W.; Chou, F.I.; Liu, Y.H.; Liu, H.M.; Peir, J.J.; Jiang, S.H.; Chang, C.W.; et al. Fractionated boron neutron capture therapy in locally recurrent head and neck cancer: A prospective phase I/II trial. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 396–403. [Google Scholar] [CrossRef]
- Sato, H.; Takata, T.; Sakurai, Y. Influence of field-of-view and section thickness of diagnostic imaging on thermal neutron flux estimation in dose-planning for boron neutron capture therapy. Radiol. Phys. Technol. 2019, 12, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Chen, Y.W.; Chuang, K.S.; Hsu, F.Y.; Chou, F.I.; Hsu, S.M.; Yen, S.H.; Wu, Y.H. The dosimetric impact of shifts in patient positioning during boron neutron capture therapy for brain tumors. Biomed. Res. Int. 2018, 2018, 5826174. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Kageji, T.; Mizobuchi, Y.; Nagahiro, S.; Nakagawa, Y.; Kumada, H. Long-survivors of glioblatoma treated with boron neutron capture therapy (BNCT). Appl. Radiat. Isot. 2011, 69, 1800–1802. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Kawabata, S.; Kajimoto, Y.; Aoki, A.; Yokoyama, K.; Yamada, M.; Kuroiwa, T.; Tsuji, M.; Imahori, Y.; Kirihata, M.; et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: An efficacy study based on findings on neuroimages. J. Neurosurg. 2005, 103, 1000–1009. [Google Scholar] [CrossRef]
- Nakagawa, N.; Akai, F.; Fukawa, N.; Fujita, Y.; Suzuki, M.; Ono, K.; Taneda, M. Early effects of boron neutron capture therapy on rat glioma models. Brain Tumor Pathol. 2007, 24, 7–13. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun. (Lond.) 2018, 38, 36. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.L.; Chou, F.I.; Lin, K.H.; Pan, P.S.; Lee, J.C.; Huang, W.S.; Liu, Y.M.; Chao, Y.; Chen, Y.W. Using salvage Boron Neutron Capture Therapy (BNCT) for recurrent malignant brain tumors in Taiwan. Appl. Radiat. Isot. 2020, 160, 109105. [Google Scholar] [CrossRef] [PubMed]
- Koivunoro, H.; Kankaanranta, L.; Seppala, T.; Haapaniemi, A.; Makitie, A.; Joensuu, H. Boron neutron capture therapy for locally recurrent head and neck squamous cell carcinoma: An analysis of dose response and survival. Radiother. Oncol. 2019, 137, 153–158. [Google Scholar] [CrossRef]
- Kageji, T.; Sogabe, S.; Mizobichi, Y.; Nakajima, K.; Shinji, N.; Nakagawa, Y. Radiation-induced meningiomas after BNCT in patients with malignant glioma. Appl. Radiat. Isot. 2015, 106, 256–259. [Google Scholar] [CrossRef]
- Vos, M.J.; Turowski, B.; Zanella, F.E.; Paquis, P.; Siefert, A.; Hideghety, K.; Haselsberger, K.; Grochulla, F.; Postma, T.J.; Wittig, A.; et al. Radiologic findings in patients treated with boron neutron capture therapy for glioblastoma multiforme within EORTC trial 11961. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 392–399. [Google Scholar] [CrossRef]
- Hiramatsu, R.; Kawabata, S.; Furuse, M.; Miyatake, S.; Kuroiwa, T. Identification of early and distinct glioblastoma response patterns treated by boron neutron capture therapy not predicted by standard radiographic assessment using functional diffusion map. Radiat. Oncol. 2013, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Shiroishi, M.S.; Boxerman, J.L.; Pope, W.B. Physiologic MRI for assessment of response to therapy and prognosis in glioblastoma. Neuro-Oncology 2016, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Breton, E.; Lallemand, D.; Grenier, P.; Cabanis, E.; Laval-Jeantet, M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology 1986, 161, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Marzi, S.; Piludu, F.; Sanguineti, G.; Marucci, L.; Farneti, A.; Terrenato, I.; Pellini, R.; Benevolo, M.; Covello, R.; Vidiri, A. The prediction of the treatment response of cervical nodes using intravoxel incoherent motion diffusion-weighted imaging. Eur. J. Radiol. 2017, 92, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Martinez-Lage, M.; Sakai, Y.; Chawla, S.; Kim, S.G.; Alonso-Basanta, M.; Lustig, R.A.; Brem, S.; Mohan, S.; Wolf, R.L.; et al. Differentiating tumor progression from pseudoprogression in patients with glioblastomas using diffusion tensor imaging and dynamic susceptibility contrast MRI. Am. J. Neuroradiol. 2016, 37, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, J.; Schaarschmidt, B.M.; Sauerwein, W.; Deuschl, C.; Arweiler-Harbeck, D.; Holtmann, L.; Stebner, V.; Umutlu, L.; Antoch, G.; Ruhlmann, V. 18F-FDG PET/MRI vs MRI in patients with recurrent adenoid cystic carcinoma. Head Neck 2019, 41, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Scalco, E.; Rizzo, G. Texture analysis of medical images for radiotherapy applications. Br. J. Radiol. 2017, 90, 20160642. [Google Scholar] [CrossRef]
- Scalco, E.; Marzi, S.; Sanguineti, G.; Vidiri, A.; Rizzo, G. Characterization of cervical lymph-nodes using a multi-parametric and multi-modal approach for an early prediction of tumor response to chemo-radiotherapy. Phys. Med. 2016, 32, 1672–1680. [Google Scholar] [CrossRef]
- Bendel, P. Boron imaging: Localized quantitative detection and imaging of boron by magnetic resonance. In Neutron Capture Therapy: Principles and Applications; Sauerwein, W.A.G., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 2113–2223. [Google Scholar]
- Bendel, P.; Frantz, A.; Zilberstein, J.; Kabalka, G.W.; Salomon, Y. Boron-11 NMR of borocaptate: Relaxation and in vivo detection in melanoma-bearing mice. Magn. Reson. Med. 1998, 39, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Bendel, P.; Sauerwein, W. Optimal detection of the neutron capture therapy agent borocaptate sodium (BSH): A comparison between H-1 and B-10 NMR. Med. Phys. 2001, 28, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Bendel, P.; Wittig, A.; Basilico, F.; Mauri, P.L.; Sauerwein, W. Metabolism of borono-phenylalanine-fructose complex (BPA-fr) and borocaptate sodium (BSH) in cancer patients-results from EORTC trial 11001. J. Pharm. Biomed. Anal. 2010, 51, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Honda, C.; Ichihashi, M.; Obara, H.; Hiratsuka, J.; Fukuda, H.; Karashima, H.; Kobayashi, T.; Kanda, K.; Yoshino, K. Treatment of malignant melanoma by single thermal neutron capture therapy with melanoma-seeking 10B-compound [letter]. Lancet 1989, 2, 388–389. [Google Scholar] [CrossRef]
- Wittig, A.; Sauerwein, W.; Coderre, J.; Coderre, J. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat. Res. 2000, 153, 173–180. [Google Scholar] [CrossRef]
- Ishiwata, K.; Ido, T.; Mejia, A.A.; Ichihashi, M.; Mishima, Y. Synthesis and radiation dosimetry of 4-borono-2-[18F]fluoro-D,L-phenylalanine: A target compound for PET and boron neutron capture therapy. Int. J. Radiat. Appl. Instrum. A 1991, 42, 325–328. [Google Scholar] [CrossRef]
- Kabalka, G.W.; Nichols, T.L.; Smith, G.T.; Miller, L.F.; Khan, M.K.; Busse, P.M. The use of positron emission tomography to develop boron neutron capture therapy treatment plans for metastatic malignant melanoma. J. Neurooncol. 2003, 62, 187–195. [Google Scholar] [CrossRef]
- Imahori, Y.; Ueda, S.; Ohmori, Y.; Kusuki, T.; Ono, K.; Fujii, R.; Ido, T. Fluorine-18-labeled fluoroboronophenylalanine PET in patients with glioma. J. Nucl. Med. 1998, 39, 325–333. [Google Scholar]
- Imahori, Y.; Ueda, S.; Ohmori, Y.; Sakae, K.; Kusuki, T.; Kobayashi, T.; Takagaki, M.; Ono, K.; Ido, T.; Fujii, R. Positron emission tomography-based boron neutron capture therapy using boronophenylalanine for high-grade gliomas: Part II. Clin. Cancer Res. 1998, 4, 1833–1841. [Google Scholar]
- Havu-Aurén, K.; Kiiski, J.; Lehtiö, K.; Eskola, O.; Kulvik, M.; Vuorinen, V.; Oikonen, V.; Vähätalo, J.; Jääskeläinen, J.; Minn, H. Uptake of 4-borono-2-[18F]fluoro-L-phenylalanine in sporadic and neurofibromatosis 2-related schwannoma and meningioma studied with PET. Eur. J. Nucl. Med. Mol. Imaging 2006, 34, 87–94. [Google Scholar] [CrossRef]
- Aihara, T.; Hiratsuka, J.; Morita, N.; Uno, M.; Sakurai, Y.; Maruhashi, A.; Ono, K.; Harada, T. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head Neck 2006, 28, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, Y.; Miyatake, S.; Kimura, Y.; Shimahara, T.; Kawabata, S.; Nagata, K.; Suzuki, M.; Maruhashi, A.; Ono, K.; Shimahara, M. Boron neuron capture therapy using epithermal neutrons for recurrent cancer in the oral cavity and cervical lymph node metastasis. Oncol. Rep. 2007, 18, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Nariai, T.; Ishiwata, K.; Kimura, Y.; Inaji, M.; Momose, T.; Yamamoto, T.; Matsumura, A.; Ishii, K.; Ohno, K. PET pharmacokinetic analysis to estimate boron concentration in tumor and brain as a guide to plan BNCT for malignant cerebral glioma. Appl. Radiat. Isot. 2009, 67, 348–350. [Google Scholar] [CrossRef] [PubMed]
- PMDA. List of Drugs Approved between April 2019 and March 2020. Available online: https://www.pmda.go.jp/files/000235289.pdf (accessed on 17 March 2021).
- Wu, C.Y.; Lin, J.J.; Chang, W.Y.; Hsieh, C.Y.; Wu, C.C.; Chen, H.S.; Hsu, H.J.; Yang, A.S.; Hsu, M.H.; Kuo, W.Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef]
- Pulagam, K.R.; Gona, K.B.; Gomez-Vallejo, V.; Meijer, J.; Zilberfain, C.; Estrela-Lopis, I.; Baz, Z.; Cossio, U.; Llop, J. Gold nanoparticles as boron carriers for boron neutron capture therapy: Synthesis, radiolabelling and in vivo evaluation. Molecules 2019, 24, 3609. [Google Scholar] [CrossRef] [PubMed]
- Feiner, I.V.J.; Pulagam, K.R.; Gómez-Vallejo, V.; Zamacola, K.; Baz, Z.; Caffarel, M.M.; Lawrie, C.H.; Ruiz-de-Angulo, A.; Carril, M.; Llop, J. Therapeutic pretargeting with gold nanoparticles as drug candidates for boron neutron capture therapy. Part. Part. Syst. Charact. 2020, 37, 2000200. [Google Scholar] [CrossRef]
- Wang, S.; Blaha, C.; Santos, R.; Huynh, T.; Hayes, T.R.; Beckford-Vera, D.R.; Blecha, J.E.; Hong, A.S.; Fogarty, M.; Hope, T.A.; et al. Synthesis and initial biological evaluation of boron-containing prostate-specific membrane antigen ligands for treatment of prostate cancer using boron neutron capture therapy. Mol. Pharm. 2019, 16, 3831–3841. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Zhang, Z.; Duan, D.; Zhang, Z.; Liu, H.; Liu, T.; Liu, Z. Tracing boron with fluorescence and PET imaging of boronated porphyrin nanocomplex for imaging guided boron neutron capture therapy. ACS Appl Mater. Interfaces 2018, 10, 43387–43395. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.; Matsui, H. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Timonen, M.; Kankaanranta, L.; Lundbom, N.; Kortesniemi, M.; Seppala, T.; Kouri, M.; Savolainen, S.; Heikkinen, S. Acquisition-weighted MRSI for detection and quantification of BNCT 10B-carrier L-p-boronophenylalanine-fructose complex, a phantom study. J. Radiat. Res. 2009, 50, 435–440. [Google Scholar] [CrossRef][Green Version]
- Porcari, P.; Capuani, S.; D’Amore, E.; Lecce, M.; La Bella, A.; Fasano, F.; Migneco, L.M.; Campanella, R.; Maraviglia, B.; Pastore, F.S. In vivo F-19 MR imaging and spectroscopy for the BNCT optimization. Appl. Radiat. Isot. 2009, 67, S365–S368. [Google Scholar] [CrossRef]
- Timonen, M.; Kankaanranta, L.; Lundbom, N.; Collan, J.; Kangasmaki, A.; Kortesniemi, M.; Hakkinen, A.M.; Lonngren, A.; Karjalainen, S.; Rasilainen, M.; et al. 1H MRS studies in the Finnish boron neutron capture therapy project: Detection of 10B-carrier, L-p-boronophenylalanine-fructose. Eur. J. Radiol. 2005, 56, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.; Kawabata, S.; Yokoyama, K.; Kuroiwa, T.; Michiue, H.; Sakurai, Y.; Kumada, H.; Suzuki, M.; Maruhashi, A.; Kirihata, M.; et al. Survival benefit of Boron neutron capture therapy for recurrent malignant gliomas. J. Neurooncol. 2009, 91, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Icten, O.; Kose, D.A.; Matissek, S.J.; Misurelli, J.A.; Elsawa, S.F.; Hosmane, N.S.; Zumreoglu-Karan, B. Gadolinium borate and iron oxide bioconjugates: Nanocomposites of next generation with multifunctional applications. Mater. Sci. Eng. C 2018, 92, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Alberti, D.; Protti, N.; Toppino, A.; Deagostino, A.; Lanzardo, S.; Bortolussi, S.; Altieri, S.; Voena, C.; Chiarle, R.; Geninatti Crich, S.; et al. A theranostic approach based on the use of a dual boron/Gd agent to improve the efficacy of Boron Neutron Capture Therapy in the lung cancer treatment. Nanomedicine 2015, 11, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Alberti, D.; Deagostino, A.; Toppino, A.; Protti, N.; Bortolussi, S.; Altieri, S.; Aime, S.; Geninatti Crich, S. An innovative therapeutic approach for malignant mesothelioma treatment based on the use of Gd/boron multimodal probes for MRI guided BNCT. J. Control. Release 2018, 280, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kuthala, N.; Vankayala, R.; Li, Y.N.; Chiang, C.S.; Hwang, K.C. Engineering novel targeted boron-10-enriched theranostic nanomedicine to combat against murine brain tumors via MR imaging-guided boron neutron capture therapy. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Kalot, G.; Godard, A.; Busser, B.; Pliquett, J.; Broekgaarden, M.; Motto-Ros, V.; Wegner, K.D.; Resch-Genger, U.; Koster, U.; Denat, F.; et al. Aza-BODIPY: A new vector for enhanced theranostic boron neutron capture therapy applications. Cells 2020, 9, 1953. [Google Scholar] [CrossRef]
- Chandra, S.; Ahmad, T.; Barth, R.F.; Kabalka, G.W. Quantitative evaluation of boron neutron capture therapy (BNCT) drugs for boron delivery and retention at subcellular-scale resolution in human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS). J. Microsc. 2014, 254, 146–156. [Google Scholar] [CrossRef]
- Arlinghaus, H.F.; Fartmann, M.; Kriegeskotte, C.; Dambach, S.; Wittig, A.; Sauerwein, W.; Lipinsky, D. Subcellular imaging of cell cultures and tissue for boron localization with laser-SNMS. Surf. Interface Anal. 2004, 36, 698–701. [Google Scholar] [CrossRef]
- Wittig, A.; Arlinghaus, H.F.; Kriegeskotte, C.; Moss, R.L.; Appelman, K.; Schmid, K.W.; Sauerwein, W.A. Laser postionization secondary neutral mass spectrometry in tissue: A powerful tool for elemental and molecular imaging in the development of targeted drugs. Mol. Cancer Ther. 2008, 7, 1763–1771. [Google Scholar] [CrossRef][Green Version]
- Sancey, L.; Motto-Ros, V.; Busser, B.; Kotb, S.; Benoit, J.M.; Piednoir, A.; Lux, F.; Tillement, O.; Panczer, G.; Yu, J. Laser spectrometry for multi-elemental imaging of biological tissues. Sci. Rep. 2014, 4, 6065. [Google Scholar] [CrossRef] [PubMed]
- Busser, B.; Bonneterre, V.; Sancey, L.; Motto-Ros, V. LIBS imaging is entering the clinic as a new diagnostic tool. Spectroscopy 2020, 35, 17–19. [Google Scholar]
- Busser, B.; Moncayo, S.; Coll, J.; Sancey, L.; Motto-Ros, V. Elemental imaging using laser-induced breakdown spectroscopy: A new and promising approach for biological and medical applications. Coord. Chem. Rev. 2018, 358, 70–79. [Google Scholar] [CrossRef]
- Michel, J.; Balossier, G.; Wittig, A.; Sauerwein, W.; Zierold, K. EELS spectrum-imaging for boron detection in biological cryofixed tissues. Instrum. Sci. Technol. 2005, 33, 631–644. [Google Scholar] [CrossRef]
- Michel, J.; Sauerwein, W.; Wittig, A.; Balossier, G.; Zierold, K. Subcellular localization of boron in cultured melanoma cells by electron energy-loss spectroscopy of freeze-dried cryosections. J. Microsc. 2003, 210, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Bortolussi, S.; Altieri, S. Boron concentration measurement in biological tissues by charged particle spectrometry. Radiat. Environ. Biophys. 2013, 52, 493–503. [Google Scholar] [CrossRef]
- Altieri, S.; Bortolussi, S.; Bruschi, P.; Chiari, P.; Fossati, F.; Stella, S.; Prati, U.; Roveda, L.; Zonta, A.; Zonta, C.; et al. Neutron autoradiography imaging of selective boron uptake in human metastatic tumours. Appl. Radiat. Isot. 2008, 66, 1850–1855. [Google Scholar] [CrossRef]
- Wittig, A.; Michel, J.; Moss, R.L.; Stecher-Rasmussen, F.; Arlinghaus, H.F.; Bendel, P.; Mauri, P.L.; Altieri, S.; Hilger, R.; Salvadori, P.A.; et al. Boron analysis and boron imaging in biological materials for Boron Neutron Capture Therapy (BNCT). Crit. Rev. Oncol. Hematol. 2008, 68, 66–90. [Google Scholar] [CrossRef]
- Turner, J.H. An introduction to the clinical practice of theranostics in oncology. Br. J. Radiol. 2018, 91, 20180440. [Google Scholar] [CrossRef]
- Weissleder, R.; Mahmood, U. Molecular imaging. Radiology 2001, 219, 316–333. [Google Scholar] [CrossRef]
- Kojima, R.; Aubel, D.; Fussenegger, M. Novel theranostic agents for next-generation personalized medicine: Small molecules, nanoparticles, and engineered mammalian cells. Curr. Opin. Chem. Biol. 2015, 28, 29–38. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Ishiwata, K. 4-Borono-2-(18)F-fluoro-L-phenylalanine PET for boron neutron capture therapy-oriented diagnosis: Overview of a quarter century of research. Ann. Nucl. Med. 2019, 33, 223–236. [Google Scholar] [CrossRef]
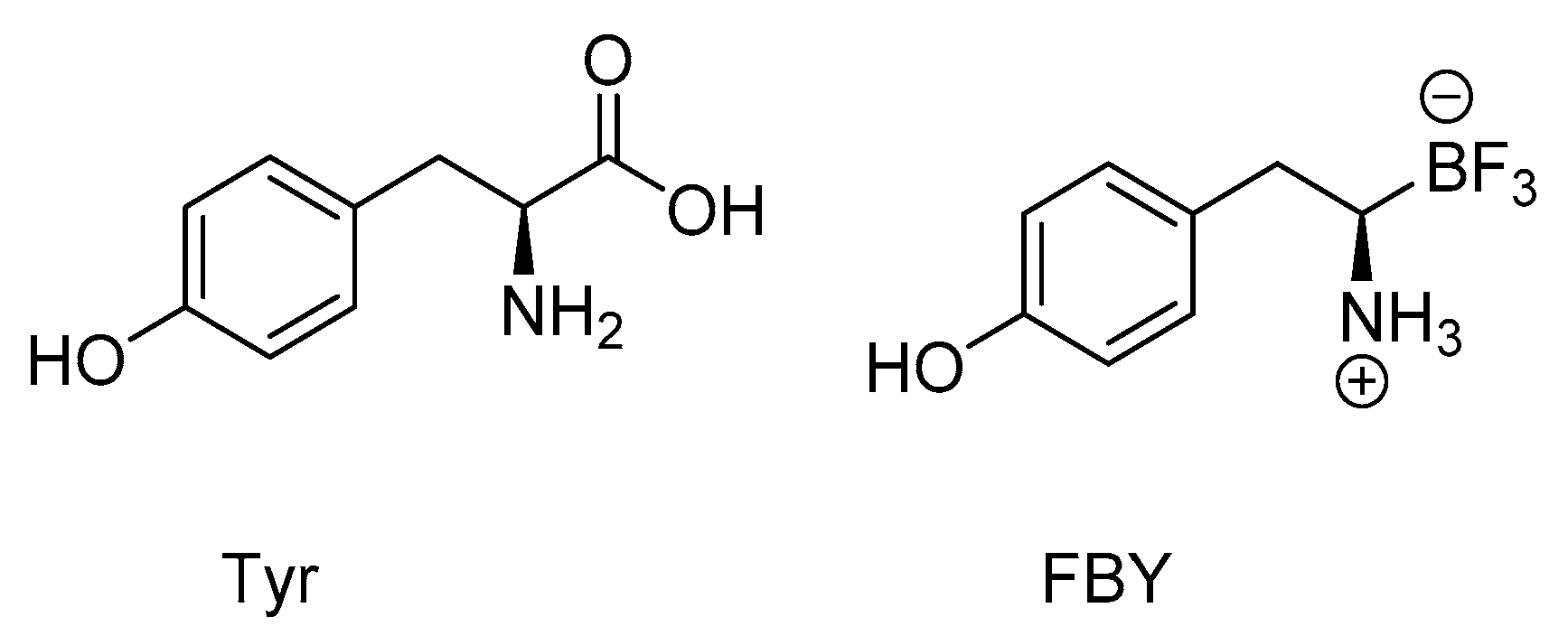
- Li, J.; Shi, Y.; Zhang, Z.; Liu, H.; Lang, L.; Liu, T.; Chen, X.; Liu, Z. A Metabolically stable boron-derived tyrosine serves as a theranostic agent for positron emission tomography guided boron neutron capture therapy. Bioconj. Chem. 2019, 30, 2870–2878. [Google Scholar] [CrossRef]
- Dubey, R.; Kushal, S.; Mollard, A.; Vojtovich, L.; Oh, P.; Levin, M.D.; Schnitzer, J.E.; Zharov, I.; Olenyuk, B.Z. Tumor targeting, trifunctional dendritic wedge. Bioconj. Chem. 2015, 26, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Thierauch, K.-H. Small molecule drugs. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3448–3451. [Google Scholar]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-penetrating peptides in diagnosis and treatment of human diseases: From preclinical research to clinical application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Isono, A.; Tsuji, M.; Sanada, Y.; Matsushita, A.; Masunaga, S.; Hirayama, T.; Nagasawa, H. Design, synthesis, and evaluation of lipopeptide conjugates of mercaptoundecahydrododecaborate for Boron Neutron Capture Therapy. ChemMedChem 2019, 14, 823–832. [Google Scholar] [CrossRef]
- Luderer, M.J.; de la Puente, P.; Azab, A.K. Advancements in tumor targeting strategies for Boron Neutron Capture Therapy. Pharm. Res. 2015, 32, 2824–2836. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Katayama, M.; Hattori, Y.; Ishimura, M.; Inaura, S.; Fujiwara, D.; Takatani-Nakase, T.; Fujii, I.; Futaki, S.; Kirihata, M. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem. Commun. (Camb.) 2019, 55, 13955–13958. [Google Scholar] [CrossRef]
- Tang, G.; Tang, X.; Wang, X. A facile automated synthesis of N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB) for 18F-labeled cell-penetrating peptide as PET tracer. J. Labelled Comp. Radiopharm. 2010, 53, 543–547. [Google Scholar] [CrossRef]
- Fujimura, A.; Yasui, S.; Igawa, K.; Ueda, A.; Watanabe, K.; Hanafusa, T.; Ichikawa, Y.; Yoshihashi, S.; Tsuchida, K.; Kamiya, A.; et al. In vitro studies to define the cell-surface and intracellular targets of polyarginine-conjugated sodium borocaptate as a potential delivery agent for Boron Neutron Capture Therapy. Cells 2020, 9, 2149. [Google Scholar] [CrossRef] [PubMed]
- Burger, A.M.; Hartung, G.; Stehle, G.; Sinn, H.; Fiebig, H.H. Pre-clinical evaluation of a methotrexate-albumin conjugate (MTX-HSA) in human tumor xenografts in vivo. Int. J. Cancer 2001, 92, 718–724. [Google Scholar] [CrossRef]
- Kikuchi, S.; Kanoh, D.; Sato, S.; Sakurai, Y.; Suzuki, M.; Nakamura, H. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumor for neutron capture therapy. J. Control. Release 2016, 237, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Yang, W.; Adams, D.M.; Rotaru, J.H.; Shukla, S.; Sekido, M.; Tjarks, W.; Fenstermaker, R.A.; Ciesielski, M.; Nawrocky, M.M.; et al. Molecular targeting of the epidermal growth factor receptor for neutron capture therapy of gliomas. Cancer Res. 2002, 62, 3159–3166. [Google Scholar] [PubMed]
- Yang, W.; Wu, G.; Barth, R.F.; Swindall, M.R.; Bandyopadhyaya, A.K.; Tjarks, W.; Tordoff, K.; Moeschberger, M.; Sferra, T.J.; Binns, P.J.; et al. Molecular targeting and treatment of composite EGFR and EGFRvIII-positive gliomas using boronated monoclonal antibodies. Clin. Cancer Res. 2008, 14, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Barth, R.F.; Wu, G.; Huo, T.; Tjarks, W.; Ciesielski, M.; Fenstermaker, R.A.; Ross, B.D.; Wikstrand, C.J.; Riley, K.J.; et al. Convection enhanced delivery of boronated EGF as a molecular targeting agent for neutron capture therapy of brain tumors. J. Neurooncol. 2009, 95, 355–365. [Google Scholar] [CrossRef]
- Daum, S.; Magnusson, J.P.; Pes, L.; Garcia Fernandez, J.; Chercheja, S.; Medda, F.; Nollmann, F.I.; Koester, S.D.; Perez Galan, P.; Warnecke, A.; et al. Development of a novel imaging agent for determining albumin uptake in solid tumors. Nucl. Med. Mol. Imaging 2019, 53, 189–198. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Achmad, A.; Hanaoka, H.; Heryanto, Y.D.; Bhattarai, A.; Khongorzul, E.; Shintawati, R.; Kartamihardja, A.A.P.; Kanai, A.; Sugo, Y.; et al. Immuno-PET imaging for non-invasive assessment of cetuximab accumulation in non-small cell lung cancer. BMC Cancer 2019, 19, 1000. [Google Scholar] [CrossRef]
- Alberti, D.; Toppino, A.; Geninatti Crich, S.; Meraldi, C.; Prandi, C.; Protti, N.; Bortolussi, S.; Altieri, S.; Aime, S.; Deagostino, A. Synthesis of a carborane-containing cholesterol derivative and evaluation as a potential dual agent for MRI/BNCT applications. Org. Biomol. Chem. 2014, 12, 2457–2467. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shi, Y.; Du, P.; Zhang, Z.; Liu, T.; Zhang, R.; Liu, Z. On-demand biodegradable boron nitride nanoparticles for treating triple negative breast cancer with Boron Neutron Capture Therapy. ACS Nano 2019, 13, 13843–13852. [Google Scholar] [CrossRef]
- Feiner, I.V.J.; Pulagam, K.R.; Uribe, K.B.; Passannante, R.; Simo, C.; Zamacola, K.; Gomez-Vallejo, V.; Herrero-Alvarez, N.; Cossio, U.; Baz, Z.; et al. Pre-targeting with ultra-small nanoparticles: Boron carbon dots as drug candidates for boron neutron capture therapy. J. Mater. Chem. B 2021, 9, 410–420. [Google Scholar] [CrossRef]
- Barabasi, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Flores-Morales, A.; Iglesias-Gato, D. Quantitative mass spectrometry-based proteomic profiling for precision medicine in prostate cancer. Front. Oncol. 2017, 7, 267. [Google Scholar] [CrossRef]
- Mauri, P.; Scigelova, M. Multidimensional protein identification technology for clinical proteomic analysis. Clin. Chem. Lab. Med. 2009, 47, 636–646. [Google Scholar] [CrossRef]
- Azzalin, A.; Brambilla, F.; Arbustini, E.; Basello, K.; Speciani, A.; Mauri, P.; Bezzi, P.; Magrassi, L. A new pathway promotes adaptation of human glioblastoma cells to glucose starvation. Cells 2020, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- De Palma, A.; Fanelli, G.; Cretella, E.; De Luca, V.; Palladino, R.A.; Panzeri, V.; Roffia, V.; Saliola, M.; Mauri, P.; Filetici, P. Gcn5p and Ubp8p affect protein ubiquitylation and cell proliferation by altering the fermentative/respiratory flux balance in saccharomyces cerevisiae. mBio 2020, 11, e01504-20. [Google Scholar] [CrossRef]
- Sato, A.; Itoh, T.; Imamichi, S.; Kikuhara, S.; Fujimori, H.; Hirai, T.; Saito, S.; Sakurai, Y.; Tanaka, H.; Nakamura, H.; et al. Proteomic analysis of cellular response induced by boron neutron capture reaction in human squamous cell carcinoma SAS cells. Appl. Radiat. Isot. 2015, 106, 213–219. [Google Scholar] [CrossRef]
- Ferrari, E.; Wittig, A.; Basilico, F.; Rossi, R.; De Palma, A.; Di Silvestre, D.; Sauerwein, W.A.G.; Mauri, P.L. Urinary proteomics profiles are useful for detection of cancer biomarkers and changes induced by therapeutic procedures. Molecules 2019, 24, 794. [Google Scholar] [CrossRef]
- Mauri, P.; Basilico, F. Proteomic investigations for Boron Neutron Capture Therapy. In Neutron Capture Therapy—Principles and Applications; Sauerwein, W., Wittig, A., Moss, R., Nakagawa, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 189–200. [Google Scholar]
- Brambilla, F.; Lavatelli, F.; Merlini, G.; Mauri, P. Clinical proteomics for diagnosis and typing of systemic amyloidoses. Proteomics Clin. Appl. 2013, 7, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-radiolabeled silica nanocarriers for targeted detection and treatment of HER2-positive breast cancer. Int. J. Nanomed. 2021, 16, 1943–1960. [Google Scholar] [CrossRef]
- Garraway, L.A.; Verweij, J.; Ballman, K.V. Precision oncology: An overview. J. Clin. Oncol. 2013, 31, 1803–1805. [Google Scholar] [CrossRef]
- Lu, Y.F.; Goldstein, D.B.; Angrist, M.; Cavalleri, G. Personalized medicine and human genetic diversity. Cold Spring Harb Perspect. Med. 2014, 4, a008581. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauerwein, W.A.G.; Sancey, L.; Hey-Hawkins, E.; Kellert, M.; Panza, L.; Imperio, D.; Balcerzyk, M.; Rizzo, G.; Scalco, E.; Herrmann, K.; et al. Theranostics in Boron Neutron Capture Therapy. Life 2021, 11, 330. https://doi.org/10.3390/life11040330
Sauerwein WAG, Sancey L, Hey-Hawkins E, Kellert M, Panza L, Imperio D, Balcerzyk M, Rizzo G, Scalco E, Herrmann K, et al. Theranostics in Boron Neutron Capture Therapy. Life. 2021; 11(4):330. https://doi.org/10.3390/life11040330
Chicago/Turabian StyleSauerwein, Wolfgang A. G., Lucie Sancey, Evamarie Hey-Hawkins, Martin Kellert, Luigi Panza, Daniela Imperio, Marcin Balcerzyk, Giovanna Rizzo, Elisa Scalco, Ken Herrmann, and et al. 2021. "Theranostics in Boron Neutron Capture Therapy" Life 11, no. 4: 330. https://doi.org/10.3390/life11040330
APA StyleSauerwein, W. A. G., Sancey, L., Hey-Hawkins, E., Kellert, M., Panza, L., Imperio, D., Balcerzyk, M., Rizzo, G., Scalco, E., Herrmann, K., Mauri, P., De Palma, A., & Wittig, A. (2021). Theranostics in Boron Neutron Capture Therapy. Life, 11(4), 330. https://doi.org/10.3390/life11040330















