1. Introduction
Coronavirus Disease 2019 (COVID-19) rapidly became a leading cause of death and short and long-term morbidity among people over the age of 45 [
1,
2], posing an unprecedented burden to healthcare systems with worldwide economic consequences and prolonged lockdowns [
3]. Vaccines currently being rolled out are anticipated to significantly modify these trends. While their effectiveness and safety have been proven in recent studies [
4,
5,
6], data in specific groups remain lacking. Generally, people with a previous history of COVID-19 in whom vaccination is currently advised [
7] were excluded from the clinical trials [
4,
5,
6]. Whilst it is accepted that prior infection with COVID-19 induces a natural immunity potentially lasting for at least six months [
8], it is unknown if previous infection may be associated with a greater number of vaccination side effects. Moreover, the safety and reactogenicity of the different types of vaccines (mRNA or viral vector-based) have not been compared head-to-head. This anonymized online survey was conducted to compare the safety profiles of available COVID-19 vaccines and evaluate their side effects in different groups of vaccine recipients.
2. Materials and Methods
This online survey, developed in plain English language and piloted by experts and lay people, captured basic epidemiological data, details on COVID-19 exposure, vaccination history and the incidence and severity of the respective side effects (
Appendix A:
Table A1). More specifically, we enquired about the following symptoms: localized reactions (pain, swelling, tenderness, redness, itching or other), fever, skin rash, shortness of breath, tingling in the mouth, face, body/extremities, swelling in the face or mouth, generalized swelling, anaphylaxis (severe allergic reaction with face swelling and breathlessness), tiredness or fatigue, flu-like illness or any other side effects. It was launched via Google Forms on 3 February 2021 for 11 days and was shared within the institutions of the investigators through professional contacts and social media. The only inclusion criterion was the receipt of the first dose of any COVID-19 vaccine at least seven days prior to survey completion.
The main objectives were to evaluate the differences in the incidence and severity of vaccination side effects among (i) people with versus without previously reported COVID-19 infection and (ii) those who received different vaccine types. Moreover, we explored the differences in self-reported side effects between the first and second vaccine dose among different ethnicities and among those with different preconceptions toward the vaccine. Finally, we explored the impact of the interval between COVID-19 exposure and vaccination and the incidence of side effects.
For our main analysis, a positive COVID-19 history was considered in cases of (a) a self-reported history of symptoms consistent with COVID-19 disease provided that COVID-19 was not excluded by a negative PCR test, (b) a positive COVID-19 PCR test or (c) a positive COVID-19 antigen test. In a sensitivity analysis, a COVID-19 infection was only considered valid if it was confirmed by PCR or antigen testing while patients with an uncertain exposure (clinical history not confirmed by laboratory testing) were excluded.
Between group differences were assessed using chi-squared and Mann–Whitney U tests for dichotomous and continuous variables, respectively, after a Shapiro–Wilk test excluded the normal distribution of the latter. Between group differences in the incidence of side effects are presented as risk ratios (RR) with the respective 95% confidence intervals (CI). Predictors of the incidence and severity of side effects were evaluated in univariate followed by multivariate binomial logistic regression and cumulative link models for ordinal data, respectively. Age, gender, ethnicity, vaccine type, prophylactic analgesia or other medication use prior to vaccination, vaccine preconceptions and prior COVID-19 exposure were evaluated as potential confounding factors. Unless otherwise specified, the analyses were based on side effect profiles from the first dose of the vaccine.
Ethics approval was not necessary for this anonymized survey.
3. Results
Within 11 days, this online survey was completed by 2002 participants (
Table A2,
Figure A1), mostly health professionals of a working age (median: 45, interquartile range [IQR]: 35–50 years). A total of 532 (26.6%) had a history of a previous COVID-19 infection of whom 366 (68.8%) were confirmed by PCR (
n = 273) and/or antigen testing (
n = 162). A COVID-19 infection preceded the first vaccination dose by a median of 87 (IQR: 47–223) days. The majority of respondents were Caucasians (88.3%) mostly from the UK (78.6%) and Greece (16.6%). As anticipated, a prior history of a COVID-19 infection was more prevalent among frontline workers, health professionals and people from the UK where a very high incidence of COVID-19 was documented [
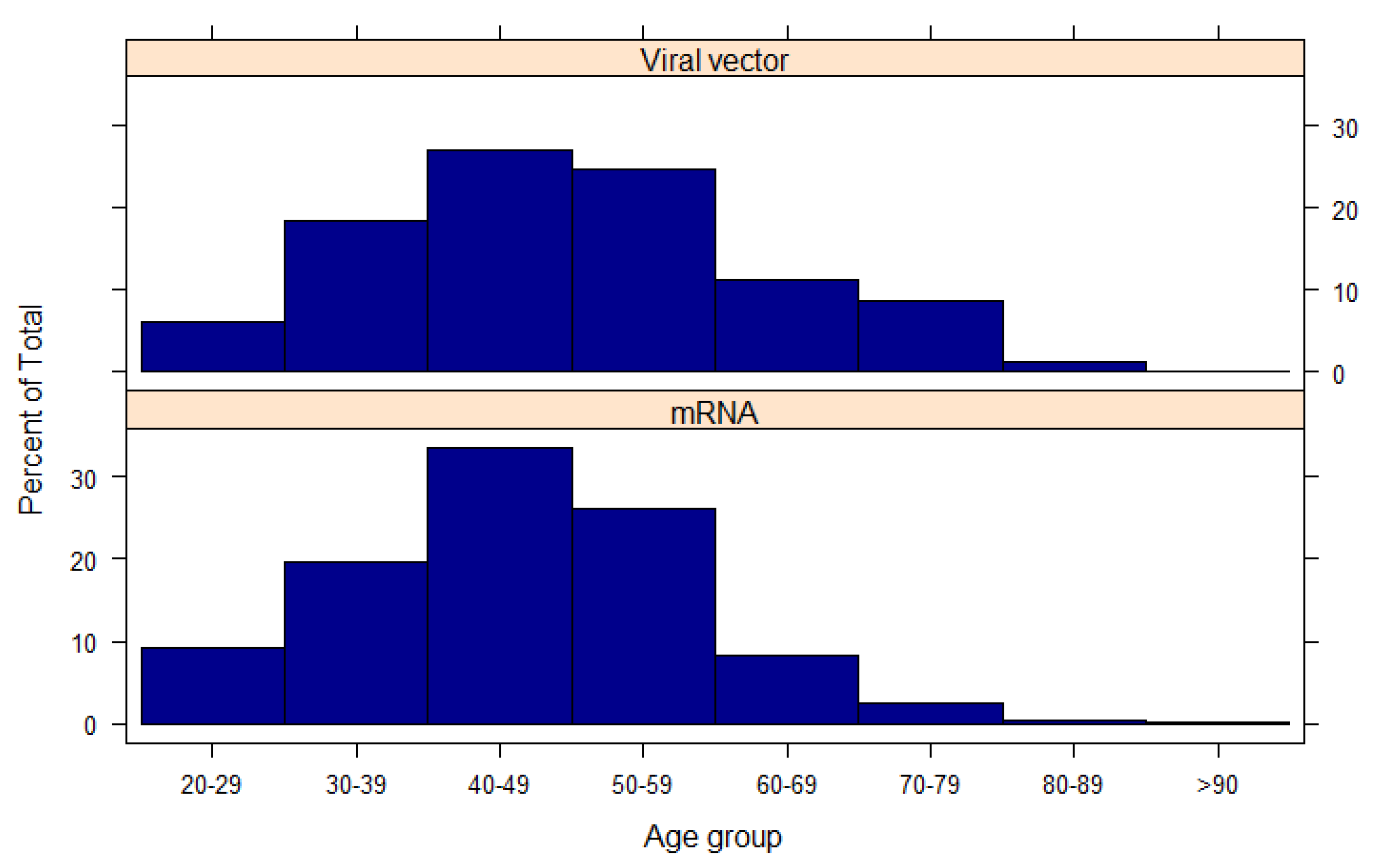
9]. Moreover, recipients of a viral vector-based vaccine (mainly the AstraZeneca vaccine) were relatively older (
Figure A2,
p < 0.001) and were mostly based in the UK (89.7% compared with 76.4% of those that received viral mRNA vaccines,
p < 0.001). Finally, doctors were more likely to have received an mRNA-based vaccine compared with the other groups (
p < 0.001).
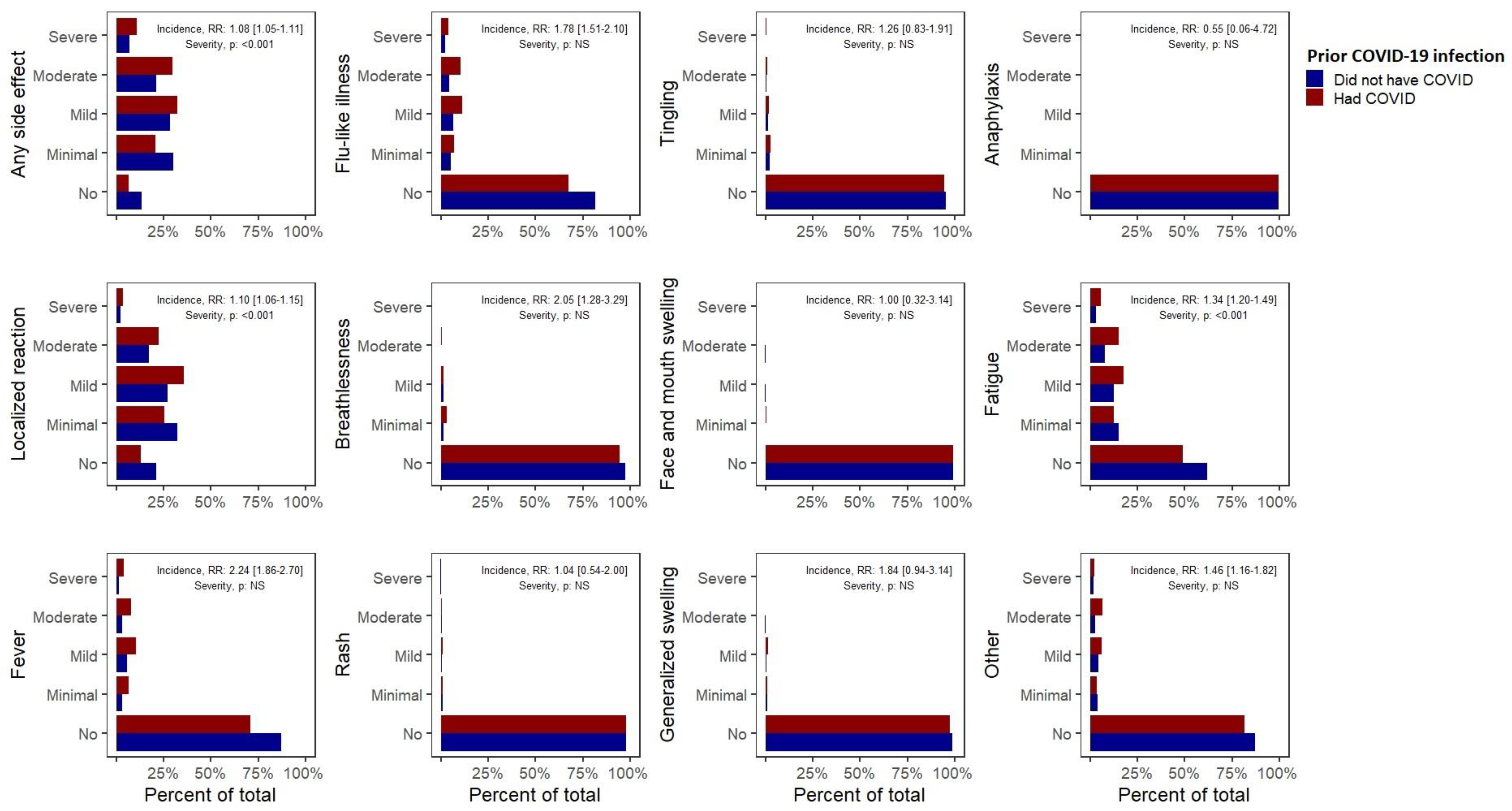
A prior COVID-19 infection was associated with an 8% increase in the risk of having any side effects after the first vaccine dose (RR 1.08, 95% CI (1.05–1.11),
Table 1,
Figure 1). We also observed a significantly increased risk of self-reported fever (2.24 (1.86–2.70)), breathlessness (2.05 (1.28–3.29)), flu-like illness (1.78 (1.51–2.10)), fatigue (1.34 (1.2–1.49)), local reactions (1.10 (1.06–1.15)) and “other” side effects (1.46 (1.16–1.82)). Among those experiencing side effects, a prior COVID-19 infection was associated with an increased severity of any side effect, local side effects or fatigue (
p < 0.001). More importantly, a prior COVID-19 infection was associated with the risk of experiencing a severe side effect requiring hospital care (1.56 (1.14–2.12)). These observations remained significant in multivariate analyses and our sensitivity analysis (
Table A3). A similar increase in the risk of any side effects following the second dose in those with a prior COVID-19 infection was also noted (1.08 (1.05–1.11)), although the lack of significant associations with specific side effects may have resulted from the limited sample included in this analysis.
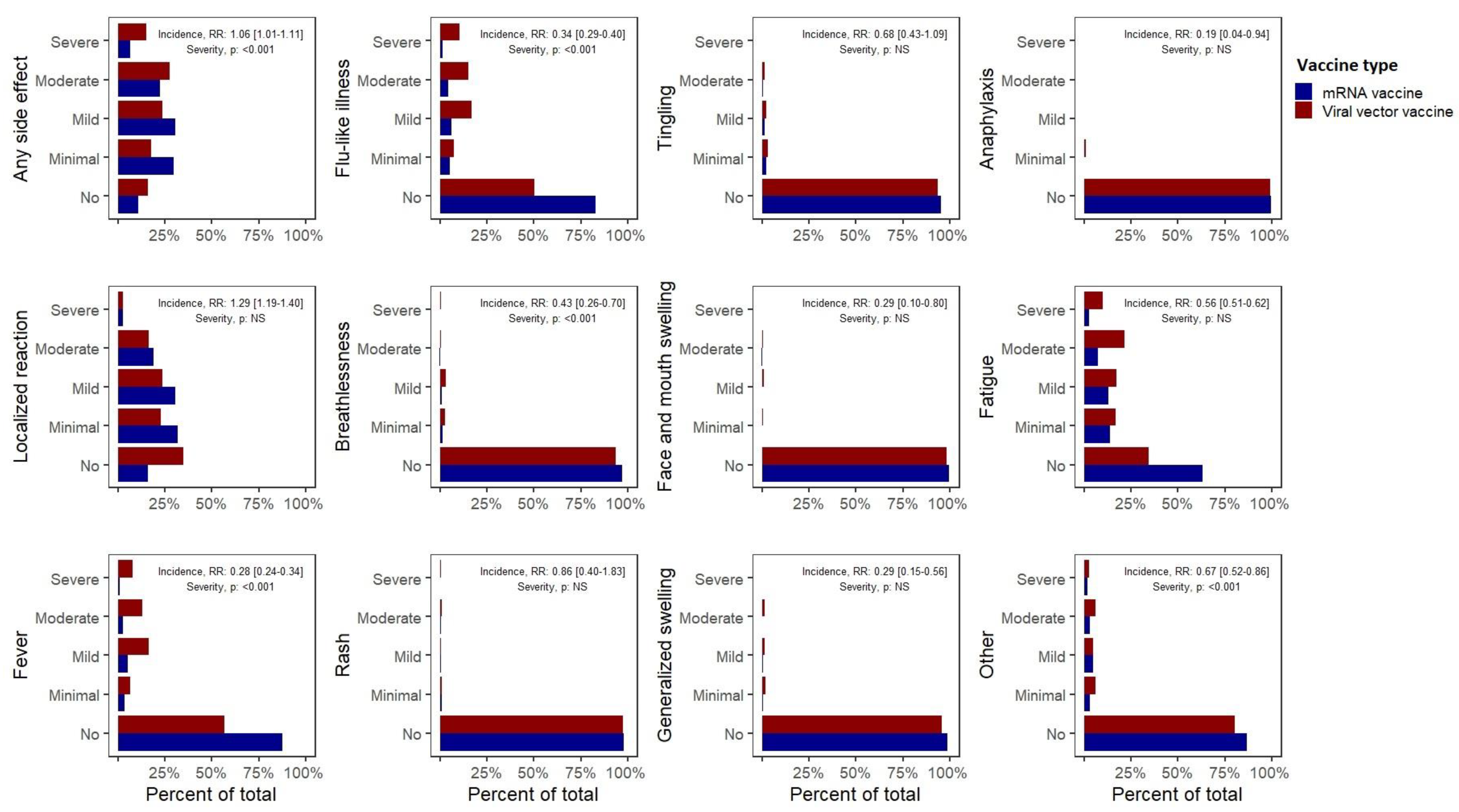
Furthermore, significant differences were observed between the side effect profiles of mRNA versus viral vector vaccines (predominantly Pfizer versus AstraZeneca,
Table 2,
Figure 2). Overall, the recipients of mRNA vaccines reported a higher incidence of any self-reported side effects (1.06 (1.01–1.11)), which were, however, of significantly milder severity compared with those who received viral vector vaccines. While mRNA vaccines were associated with an increased incidence of reported local reactions (1.29 (1.19–1.40)), they were associated with a considerably lower incidence of self-reported systemic side effects including anaphylaxis (0.19 (0.04–0.62)), fever (0.28 (0.24–0.34)), swelling in the face or mouth (0.29 (0.10–0.80)) or generalized swelling (0.29 (0.15–0.56)), flu-like illness (0.34 (0.29–0.40)), breathlessness (0.43 (0.26–0.70)), fatigue (0.56 (0.51–0.62)) or other side effects (0.67 (0.52–0.86)). These observations were corroborated by multivariate analyses. Most importantly, mRNA vaccines were associated with a significantly lower incidence of severe side effects (requiring hospital care, RR 0.42 (0.31–0.58)).
In general, the second dose of the vaccine was associated with a higher incidence of side effects (
Table 3). More specifically, respondents reported experiencing more frequently any side effects (1.04 (1.01–1.07)), skin rash (2.25 (1.4–3.62)), fever (1.72 (1.46–2.02)), flu-like illness (1.67 (1.45–1.91)) and fatigue (1.40 (1.28–1.53)). In addition, a multivariate regression demonstrated that participants who had side effects after the first vaccine dose were at a significantly higher risk of having the same side effects after the second dose. Among those experiencing side effects, the severity did not significantly differ between the two doses. However, the likelihood of having a severe side effect requiring hospital care was significantly decreased (0.58 (0.38–0.88)).
Stratification by ethnicity revealed that white participants reported a lower incidence of fever (0.62 (0.48–0.79)) and flu-like illness (0.78 (0.62–0.97)) compared with the remaining participants (
Table A4). Finally, those reporting a pre-vaccination concern about the safety of the vaccine reported more often tingling (2.23 (1.45–3.42)), breathlessness (1.73 (1.00–2.98)) and fatigue (1.17 (1.03–1.34)) (
Table A5).
Multivariate analyses also revealed a strong negative association between age and the self-reporting of any side effect, local reactions, fever, flu-like illness, rash, tingling, generalized swelling and fatigue (p < 0.01). Finally, a history of allergy was associated with an increased incidence of self-reported breathlessness and rash (p < 0.01). However, as described in the previous paragraphs and tables, most of the associations observed in univariate analyses remained significant in multivariate analyses accounting for these and other potential confounding factors.
4. Discussion
People with a prior COVID-19 exposure were largely excluded from the vaccine trials [
4,
5,
6] and, as a result, the safety and reactogenicity of the vaccines in this population have not been previously fully evaluated. For the first time, this study demonstrated a significant association between a prior COVID-19 infection and a significantly higher incidence and severity of self-reported side effects after a vaccination for COVID-19. Consistently, compared with the first dose of the vaccine, we found an increased incidence and severity of self-reported side effects after the second dose when recipients had been previously exposed to viral antigen, probably because they had already developed an immunity against the antigens. This was supported by recent studies demonstrating that seropositive individuals developed rapid immune responses with higher antibody titres after the first vaccination dose compared with those without a previous COVID-19 infection [
10,
11]. In view of the rapidly accumulating data demonstrating that COVID-19 survivors generally have an adequate natural immunity for at least six months, it may be appropriate to re-evaluate the recommendation for the immediate vaccination of this group. In the meantime, taking into account our findings as well as studies demonstrating higher antibody titres among individuals with a prior COVID-19 infection, it might be appropriate for a note to be included in the vaccine information sheets highlighting that these people are more likely to experience non-serious side effects.
Moreover, this is the first head-to-head real-world comparison of the self-reported safety of viral vector versus mRNA vaccines with the latter associated with a 58% decreased incidence of self-reported severe side effects requiring hospital care. While a greater number of recipients of mRNA vaccines reported at least one (any) side effect, the difference was predominantly driven by the frequent local reactions. The incidence of the systemic side effects evaluated (flu-like illness, pyrexia and fatigue), which are more burdensome to the recipients, was significantly reduced. The recipients of the viral vector-based vaccines were relatively older. However, differences in the incidence of adverse events were confirmed in multivariate analyses accounting for the age of the respondents as a covariate. Moreover, given that older people reported side effects less frequently, a potential bias due to age difference would be expected to favour viral vector-based vaccines. These findings may have an impact on vaccine choice and health policies. The cause of the observed imbalance between the safety profiles of mRNA-based versus viral-vector vaccines was unclear and should be evaluated in future studies.
The main strengths of our study included a large study population that better reflected real-life compared with the populations studied in the clinical trials, the availability of adequate details about the participants and the safety profiles of the vaccines and very limited missing data. The potential bias of respondents is the main limitation of any survey and as this survey was shared though social media, we were not able to estimate the non-response rate. However, the bias of respondents was more likely to affect the absolute incidence of side effects, which we did not evaluate here, rather than the relative incidence and severity across different groups of people. Potential recall bias should also be mentioned although all participants had been vaccinated within 10 weeks prior to completing the survey. As noted, most respondents were from the UK and Greece due to the ability of the investigators to establish contacts quickly to publicise this survey. The UK has also been successful in rolling out COVID-19 vaccines quickly leading to more of those invited being eligible to participate. It is not surprising that the Pfizer vaccine was the most delivered vaccine as it was the first vaccine to be licensed within the UK, with more individuals receiving it in total when the survey was circulated.
In conclusion, this extensive survey of over 2000 recipients of COVID-19 vaccines confirmed the findings of recent randomised controlled trials (RCTs) demonstrating that COVID-19 vaccines are generally safe with limited severe side effects. Moreover, it linked previous COVID-19 illnesses with an increased incidence of vaccination side effects. It also demonstrated that mRNA vaccines caused milder, less frequent systemic side effects but more local reactions. These findings will need to be validated in clinical studies, preferably randomized controlled trials including patients from multiple groups.
Author Contributions
Conceptualization, N.D.B. and M.G.; methodology, A.G.M., M.G., A.U., S.A., R.B. and N.D.B.; Survey distribution A.G.M., M.G., A.U., S.A., R.B., L.P.P., D.P. and N.D.B.; data curation, A.G.M. and N.D.B.; formal analysis, A.G.M. and N.D.B.; writing—original draft preparation, A.G.M. and N.D.B.; writing—review and editing, A.G.M. and N.D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
An ethical review and approval were waived for this anonymous online survey. This was confirmed by the Research & Development Directorate of the Salford Royal NHS Foundation Trust.
Informed Consent Statement
Patient consent was implied by the completion of this online survey. Written consent was waived because this online survey was completely anonymous. This was confirmed by the Research & Development Directorate of the Salford Royal NHS Foundation Trust.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
A.G.M. was supported by the NIHR Manchester Biomedical Research Centre (NIHR Manchester BRC). We are thankful to Matthew Snape (Oxford Vaccine Group, University of Oxford) for his advice regarding vaccine safety and reactogenicity tracking and to the Coronavirus Medical Group Greece for disseminating our survey among Greek health professionals.
Conflicts of Interest
All authors have completed the ICMJE uniform disclosure form. None of the authors has any conflict of interest in relation to this work. A.G.M. reports grants from Boehringer Ingelheim outside the submitted work. R.B. reports contract research on behalf of Public Health England for GlaxoSmithKline, Pfizer and Sanofi Pasteur outside the submitted work. N.D.B. reports personal fees from TEVA Pharma, GSK, AstraZeneca, Boehringer Ingelheim and Chiesi Pharma outside the submitted work.
Appendix A
Table A1.
Definitions of the severity of side effects.
Table A1.
Definitions of the severity of side effects.
| Severity | Definition |
|---|
| Minimal | Negligible impact |
| Mild | No treatment needed |
| Moderate | Needed treatment or advice from healthcare professional outside the hospital |
| Severe | Needed hospital care |
Table A2.
Baseline characteristics of the study participants. Continuous variables are presented as medians (IQR) and categorical as n (%). Between group differences were anticipated and explained by the incidence of COVID-19 in different subgroups. Characteristically, a higher incidence of a prior COVID-19 infection was observed among frontline workers, health professionals and among British people (a very high incidence of COVID-19 was documented in the UK).
Table A2.
Baseline characteristics of the study participants. Continuous variables are presented as medians (IQR) and categorical as n (%). Between group differences were anticipated and explained by the incidence of COVID-19 in different subgroups. Characteristically, a higher incidence of a prior COVID-19 infection was observed among frontline workers, health professionals and among British people (a very high incidence of COVID-19 was documented in the UK).
| Characteristics | Participants with a Prior COVID-19 Infection
(n = 532) | Participants with No Known Prior COVID-19 Infection
(n = 1470) | Missing Data | Between Group Differences
(p-Value) |
|---|
| Gender (Female) | 393 (73.9%) | 1051 (71.5%) | 0.7% | NS |
| Age ≥ 60 (%) * | 56 (10.5%) | 202 (13.7%) | 0.5% | NS |
| Weight (kg) | 75 (64–88) | 74 (64–85) | 4.0% | NS |
| Height (cm) | 168 (163–173) | 168 (162–175) | 2.2% | NS |
| Country | | | 0.6% | <0.001 |
| Europe | | |
| UK | 472 (88.7%) | 1100 (74.8%) |
| Greece | 38 (7.1%) | 294 (20%) |
| Other European countries | 10 (1.9%) | 30 (2.0%) |
| Americas | 5 (0.9%) | 17 (1.2%) |
| Asia | 5 (0.9%) | 17 (1.2%) |
| Africa | 0 (0%) | 1 (0.1%) |
| Ethnicity | | | 1.8% | NS |
| White | 464 (87.2%) | 1303 (88.6%) |
| Asian | 35 (6.6%) | 63 (4.3%) |
| Arab | 21 (3.9%) | 45 (3.1%) |
| Other | 7 (1.3%) | 28 (1.9%) |
| Role | | | 3.2% | <0.001 |
| Doctor | 140 (26.3%) | 486 (33.1%) |
| Nurse | 125 (23.5%) | 188 (12.8%) |
| Other health professional | 161 (30.3%) | 382 (26.0%) |
| Not a health professional | 105 (19.7%) | 401 (27.8%) |
| Frontline workers | 372 (69.9%) | 795 (54.1%) | 0.6% | <0.001 |
| COVID-19 prior to vaccination | | | 0% | |
| Laboratory confirmed exposure | 366 (68.8%) | NA |
| Consistent symptoms, not tested | 166 (31.2%) | NA |
| No known exposure | NA | 1470 (100%) |
| Vaccine type | | | 0.5% | NS |
| Pfizer | 443 (83.3%) | 1230 (83.7%) |
| Oxford AstraZeneca | 80 (15.0%) | 202 (13.7%) |
| Other | 4 (0.8%) | 20 (1.4%) |
| Unknown | 2 (0.4%) | 3 (0.2%) |
| Vaccine preconception | | | 0.8% | NS |
| Positive | 343 (64.5%) | 1027 (69.9%) |
| Neutral | 76 (14.3%) | 174 (11.8%) |
| Negative | 110 (20.7%) | 259 (17.6%) |
| Second vaccine dose received | 114 (21.4%) | 411 (28.0%) | 0% | 0.004 |
| Past medical history | | | 7.7% | |
| Chronic cardiac disease | 9 (1.7%) | 25 (1.7%) | NS |
| Chronic respiratory disease | 74 (13.9%) | 171 (11.6%) | NS |
| Chronic kidney disease | 4 (0.8%) | 9 (0.6%) | NS |
| Chronic liver disease | 1 (0.2%) | 6 (0.4%) | NS |
| Chronic neurological disease | 8 (1.5%) | 17 (1.2%) | NS |
| Active cancer | 1 (0.2%) | 9 (0.6%) | NS |
| Asplenia | 1 (0.2%) | 4 (0.3%) | NS |
| Allergy | 56 (10.5%) | 134 (9.1%) | NS |
| Diabetes | 17 (3.2%) | 49 (3.3%) | NS |
| Hay fever, eczema | 114 (21.4%) | 251 (17.1%) | 0.04 |
| Immunosuppression | 14 (2.6%) | 49 (33.3%) | NS |
| Transplantation history | 0 (0%) | 0 (0%) | NS |
| None | 282 (53.0%) | 825 (56.1%) | NS |
Table A3.
Differences in the incidence and severity of side effects after the first dose of a COVID-19 vaccine among participants who had or did not have a prior self-reported COVID-19 infection. Sensitivity analysis only included participants with a prior COVID-19 infection confirmed with a consistent PCR or antibody test (n = 366) versus those without any suspicion of a prior COVID-19 infection (n = 1470).
Table A3.
Differences in the incidence and severity of side effects after the first dose of a COVID-19 vaccine among participants who had or did not have a prior self-reported COVID-19 infection. Sensitivity analysis only included participants with a prior COVID-19 infection confirmed with a consistent PCR or antibody test (n = 366) versus those without any suspicion of a prior COVID-19 infection (n = 1470).
| Side Effect | Incidence of Side Effects: Risk Ratio (95% CI) | Incidence of Side Effects: Multivariate Logistic Regression, Coefficient (p-Value) | Severity of Side Effects: Univariate Cumulative Risk Models (p-Value) | Severity of Side Effects: Multivariate Cumulative Risk Models (p-Value) |
|---|
| Any side effect | 1.09 (1.05–1.12) | 0.581 (0.015) | <0.001 | 0.004 |
| Localized reaction | 1.11 (1.06–1.16) | 0.411 (0.019) | 0.002 | NS |
| Fever | 2.45 (2.01–3) | 0.902 (<0.001) | NS | NS |
| Flu-like illness | 1.92 (1.61–2.29) | 0.691 (<0.001) | NS | NS |
| Shortness of breath | 2.06 (1.22–3.49) | 0.564 (0.043) | NS | NS |
| Skin rash | 1.38 (0.7–2.71) | NS | NS | NS |
| Tingling | 1.22 (0.75–1.98) | NS | NS | NS |
| Swelling | 0.73 (0.16–3.28) | NS | NS | NS |
| Generalized swelling | 1.72 (0.8–3.73) | NS | NS | NS |
| Anaphylaxis | 0.8 (0.09–6.85) | NS | NS | NS |
| Fatigue or tiredness | 1.39 (1.24–1.56) | 0.459 (<0.001) | <0.001 | 0.002 |
| Other | 1.45 (1.12–1.87) | 0.288 (0.069) | NS | NS |
| | | Worse outcomes associated with a prior COVID-19 infection |
Table A4.
Differences in the incidence and severity of side effects among different ethnicities (white or other).
Table A4.
Differences in the incidence and severity of side effects among different ethnicities (white or other).
| Side Effect | Incidence of Side Effects: Risk Ratio (95% CI) | Incidence of Side Effects: Multivariate Logistic Regression, Coefficient (p-Value) | Severity of Side Effects: Univariate Cumulative Risk Models (p-Value) | Severity of Side Effects: Multivariate Cumulative Risk Models (p-Value) |
|---|
| Any side effect | 1.05 (0.99–1.11) | NS | NS | NS |
| Localized reaction | 1.04 (0.97–1.12) | NS | NS | NS |
| Fever | 0.62 (0.48–0.79) | –0.546 (0.003) | NS | NS |
| Flu-like illness | 0.78 (0.62–0.97) | NS | NS | NS |
| Shortness of breath | 1.16 (0.54–2.5) | NS | NS | NS |
| Skin rash | 0.7 (0.32–1.56) | NS | NS | NS |
| Tingling | 1.69 (0.79–3.61) | NS | NS | NS |
| Swelling | 0.86 (0.2–3.81) | NS | NS | NS |
| Generalized swelling | 0.64 (0.27–1.53) | NS | NS | NS |
| Anaphylaxis | 0.66 (0.08–5.67) | NS | NS | NS |
| Fatigue or tiredness | 0.88 (0.76–1.02) | NS | NS | NS |
| Other | 1.38 (0.94–2.03) | 0.446 (0.049) | NS | NS |
| | | Worse outcomes: non-white ethnicity |
| | | Worse outcomes: white ethnicity |
Table A5.
Differences in the incidence and severity of side effects among people with a different preconception toward the vaccine prior to vaccination and those who were keen to receive the vaccine versus those who were concerned about receiving the vaccine.
Table A5.
Differences in the incidence and severity of side effects among people with a different preconception toward the vaccine prior to vaccination and those who were keen to receive the vaccine versus those who were concerned about receiving the vaccine.
| Side Effect | Incidence of Side Effects: Risk Ratio (95% CI) | Incidence of Side Effects: Multivariate Logistic Regression, Coefficient (p-Value) | Severity of Side Effects: Univariate Cumulative Risk Models (p-Value) | Severity of Side Effects: Multivariate Cumulative Risk Models (p-Value) |
|---|
| Any side effect | 1.01 (0.97–1.06) | NS | <0.001 | 0.025 |
| Localized reaction | 0.99 (0.93–1.05) | NS | 0.002 | NS |
| Fever | 1.19 (0.93–1.53) | NS | 0.009 | NS |
| Flu-like illness | 1.07 (0.86–1.34) | NS | <0.001 | NS |
| Shortness of breath | 1.73 (1.00–2.98) | –0.085 (0.03) | NS | NS |
| Skin rash | 1.25 (0.59–2.65) | NS | NS | NS |
| Tingling | 2.23 (1.45–3.42) | –0.114 (0.001) | NS | NS |
| Swelling | 0.4 (0.05–3.03) | NS | NS | NS |
| Generalized swelling | 0.72 (0.26–2.04) | NS | NS | NS |
| Anaphylaxis | NA | NS | NS | NS |
| Fatigue or tiredness | 1.17 (1.03–1.34) | NS | 0.009 | NS |
| Other | 1.26 (0.96–1.66) | –0.043 (0.045) | NS | NS |
| | | Worse outcomes: concerned |
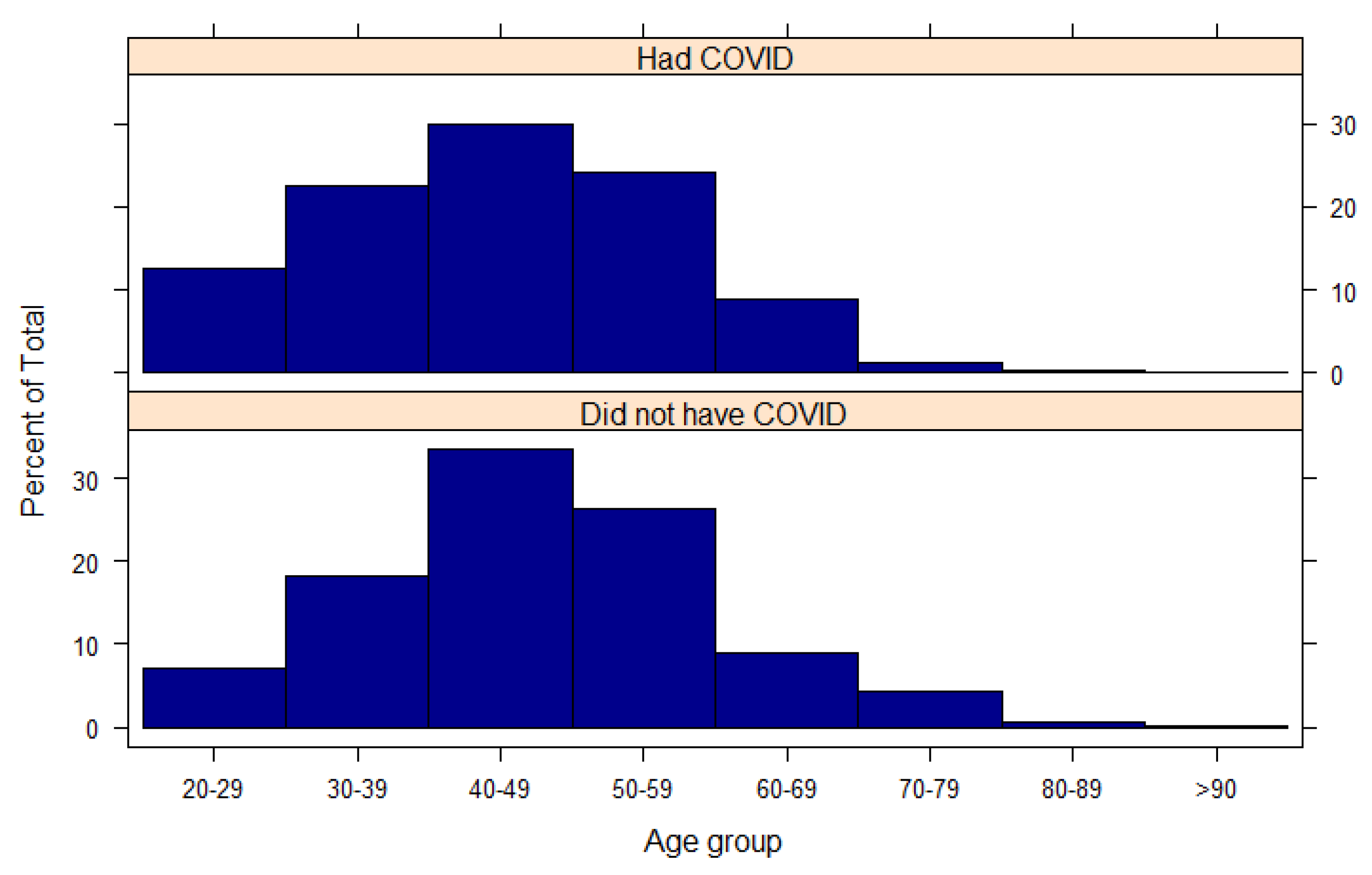
Figure A1.
Age of the participants stratified by whether they had or did not have a previous COVID-19 infection.
Figure A1.
Age of the participants stratified by whether they had or did not have a previous COVID-19 infection.
Figure A2.
Age of the participants stratified by the type of vaccine they received.
Figure A2.
Age of the participants stratified by the type of vaccine they received.
References
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the Leading Cause of Death in the United States. JAMA 2021, 325, 123–124. [Google Scholar] [PubMed]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2020, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Tangcharoensathien, V.; Bassett, M.T.; Meng, Q.; Mills, A. Are overwhelmed health systems an inevitable consequence of covid-19? Experiences from China, Thailand, and New York State. BMJ 2021, 372, n83. [Google Scholar] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Frequently Asked Questions about COVID-19 Vaccination. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html (accessed on 8 February 2021).
- Pradenas, E.; Trinité, B.; Urrea, V.; Marfil, S.; Ávila-Nieto, C.; Rodríguez de la Concepción, M.L.; Tarrés-Freixas, F.; Pérez-Yanes, S.; Rovirosa, C.; Ainsua-Enrich, E.; et al. Stable neutralizing antibody levels six months after mild and severe COVID-19 episode. bioRxiv 2021. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/table (accessed on 14 February 2021).
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Saadat, S.; Tehrani, Z.R.; Logue, J.; Newman, M.; Frieman, M.B.; Harris, A.D.; Sajadi, M.M. Binding and Neutralization Antibody Titers After a Single Vaccine Dose in Health Care Workers Previously Infected With SARS-CoV-2. JAMA 2021. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).