Endocannabinoid System as Therapeutic Target of PTSD: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Quality Assessment
2.4. Data Extraction
2.5. Risk of Bias Assessment
3. Results
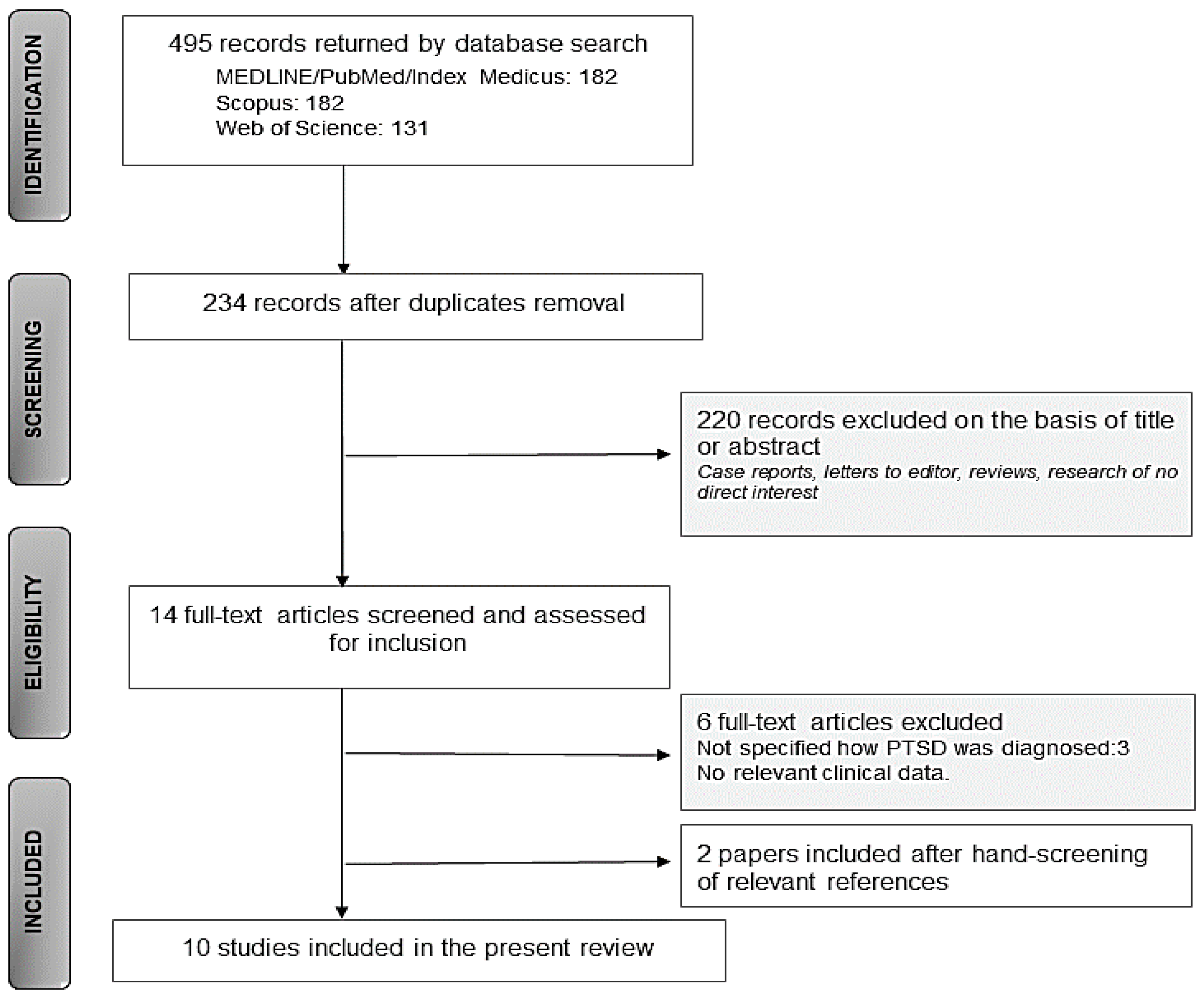
3.1. Search Results
3.2. Content Results
3.3. Quality and Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- American Psychiatric Association. DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Watson, P. PTSD as a Public Mental Health Priority. Curr. Psychiatry Rep. 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Passie, T.; Emrich, H.M.; Karst, M.; Brandt, S.D.; Halpern, J.H. Mitigation of post-traumatic stress symptoms by Cannabis resin: A review of the clinical and neurobiological evidence. Drug Test. Anal. 2012, 4, 649–659. [Google Scholar] [CrossRef]
- Berardi, A.; Schelling, G.; Campolongo, P. The endocannabinoid system and Post Traumatic Stress Disorder (PTSD): From preclinical findings to innovative therapeutic approaches in clinical settings. Pharmacol. Res. 2016, 111, 668–678. [Google Scholar] [CrossRef]
- Kelmendi, B.; Adams, T.G.; Yarnell, S.; Southwick, S.; Abdallah, C.G.; Krystal, J.H. PTSD: From neurobiology to pharmacological treatments. Eur. J. Psychotraumatol. 2016, 7, 31858. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, A.P.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Influence of single and repeated cannabidiol administration on emotional behavior and markers of cell proliferation and neurogenesis in non-stressed mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 27–34. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascioll, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Lu, H.C.; MacKie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Riebe, C.J.; Pamplona, F.; Kamprath, K.; Wotjak, C.T. Fear relief-toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience 2012, 204, 159–185. [Google Scholar] [CrossRef] [PubMed]
- Trezza, V.; Campolongo, P. The endocannabinoid system as a possible target to treat both the cognitive and emotional features of post-traumatic stress disorder. Front. Behav. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- De Bitencourt, R.M.; Pamplona, F.A.; Takahashi, R.N. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: Potential extinction enhancers. Neuropharmacology 2013, 64, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.; Whalen, P.J. The amygdala: Vigilance and emotion. Mol. Psychiatry 2001, 6, 13–34. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef]
- Ganon-Elazar, E.; Akirav, I. Cannabinoids prevent the development of behavioral and endocrine alterations in a rat model of intense stress. Neuropsychopharmacology 2012, 37, 456–466. [Google Scholar] [CrossRef]
- Patel, S.; Roelke, C.T.; Rademacher, D.J.; Cullinan, W.E.; Hillard, C.J. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 2004, 145, 5431–5438. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Gray, J.M.; Wilson, C.D.; Lee, T.T.Y.; Pittman, Q.J.; Deussing, J.M.; Hillard, C.J.; McEwen, B.S.; Schulkin, J.; Karatsoreos, I.N.; Patel, S.; et al. Sustained glucocorticoid exposure recruits cortico-limbic CRH signaling to modulate endocannabinoid function. Psychoneuroendocrinology 2016, 66, 151–158. [Google Scholar] [CrossRef]
- Hidalgo, R.B.; Davidson, J.R.T. Posttraumatic stress disorder: Epidemiology and health-related considerations. J. Clin. Psychiatry 2000, 61, 5–13. [Google Scholar] [PubMed]
- Wynn, G.H. Complementary and Alternative Medicine Approaches in the Treatment of PTSD. Curr. Psychiatry Rep. 2015, 17. [Google Scholar] [CrossRef]
- Robertson, M.; Humphreys, L.; Ray, R. Psychological Treatments for Posttraumatic Stress Disorder: Recommendations for the Clinician Based on a Review of the Literature. J. Psychiatr. Pract. 2004, 10, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Akiki, T.J.; Abdallah, C.G. Are there effective psychopharmacologic treatments for PTSD? J. Clin. Psychiatry 2019, 80, 18ac12473. [Google Scholar] [CrossRef] [PubMed]
- Bernardy, N.C.; Friedman, M.J. Psychopharmacological Strategies in the Management of Posttraumatic Stress Disorder (PTSD): What Have We Learned? Curr. Psychiatry Rep. 2015, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Mendlowicz, M.V.; Marques-Portella, C.; Kinrys, G.; Fontenelle, L.F.; Marmar, C.R.; Figueira, I. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: A systematic review. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 169–180. [Google Scholar] [CrossRef]
- Shin, H.J.; Greenbaum, M.A.; Jain, S.; Rosen, C.S. Associations of psychotherapy dose and SSRI or SNRI refills with mental health outcomes among veterans with PTSD. Psychiatr. Serv. 2014, 65, 1244–1248. [Google Scholar] [CrossRef][Green Version]
- Ronan, P.J.; Wongngamnit, N.; Beresford, T.P. Molecular Mechanisms of Cannabis Signaling in the Brain. In Proceedings of the Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, pp. 123–147. [Google Scholar]
- Sbarski, B.; Akirav, I. Cannabinoids as therapeutics for PTSD. Pharmacol. Ther. 2020, 211, 107551. [Google Scholar] [CrossRef]
- Morena, M.; Patel, S.; Bains, J.S.; Hill, M.N. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 2016, 41, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, A.; Normandin, M.D.; Pietrzak, R.H.; Piomelli, D.; Zheng, M.Q.; Gujarro-Anton, A.; Potenza, M.N.; Bailey, C.R.; Lin, S.F.; Najafzadeh, S.; et al. Elevated brain cannabinoid CB 1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol. Psychiatry 2013, 18, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Cougle, J.R.; Bonn-Miller, M.O.; Vujanovic, A.A.; Zvolensky, M.J.; Hawkins, K.A. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol. Addict. Behav. 2011, 25, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Sakurai, T.; Rainero, I.; Maj, M.C.; Kukkonen, J.P. Orexin receptor multimerization versus functional interactions: Neuropharmacological implications for opioid and cannabinoid signalling and pharmacogenetics. Pharmaceuticals 2017, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.M.; Takahashi, R.N. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. Front. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kamboj, S.K.; Ramadas, M.; Yogan, K.; Gupta, V.; Redman, E.; Curran, H.V.; Morgan, C.J.A. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology 2013, 226, 781–792. [Google Scholar] [CrossRef]
- Uhernik, A.L.; Montoya, Z.T.; Balkissoon, C.D.; Smith, J.P. Learning and memory is modulated by cannabidiol when administered during trace fear-conditioning. Neurobiol. Learn. Mem. 2018, 149, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Berardi, A.; Colucci, P.; Palmery, M.; Trezza, V.; Hill, M.N.; Campolongo, P. Enhancing Endocannabinoid Neurotransmission Augments the Efficacy of Extinction Training and Ameliorates Traumatic Stress-Induced Behavioral Alterations in Rats. Neuropsychopharmacology 2018, 43, 1284–1296. [Google Scholar] [CrossRef]
- Roitman, P.; Mechoulam, R.; Cooper-Kazaz, R.; Shalev, A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin. Drug Investig. 2014, 34, 587–591. [Google Scholar] [CrossRef]
- Fraser, G.A. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci. Ther. 2009, 15, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Jetly, R.; Heber, A.; Fraser, G.; Boisvert, D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015, 51, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Watson, D.; Robinson, J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: A retrospective evaluation. J. Clin. Psychopharmacol. 2014, 34, 559–564. [Google Scholar] [CrossRef]
- Cowling, T.; MacDougall, D. Nabilone for the Treatment of Post-Traumatic Stress Disorder: A Review of Clinical Effectiveness and Guidelines; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019; pp. 1–16. [Google Scholar]
- Ney, L.J.; Matthews, A.; Bruno, R.; Felmingham, K.L. Cannabinoid interventions for PTSD: Where to next? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Wei, Z.; Phang, J.; Laprairie, R.B.; Zhang, Y. Cannabinoids as an Emerging Therapy for Posttraumatic Stress Disorder and Substance Use Disorders. J. Clin. Neurophysiol. 2020, 37, 28–34. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, b2535. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Schünemann, H.J.; Tugwell, P.; Knottnerus, A. GRADE guidelines: A new series of articles in the Journal of Clinical Epidemiology. J. Clin. Epidemiol. 2011, 64, 380–382. [Google Scholar] [CrossRef]
- Higgins, J.P.; Sterne, J.A.; Savovic, J.; Page, M.J.; Hróbjartsson, A.; Boutron, I.; Reeves, B.; Eldridge, S. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst. Rev. 2016, 10, 29–31. [Google Scholar]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2019, 25, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Chan, S.; Blake, A.; Wolt, A.; Zhang, L.; Wan, B.A.; Zaki, P.; Lam, H.; Deangelis, C.; Slaven, M.; et al. Medical cannabis use in military and police veterans diagnosed with post-traumatic stress disorder (Ptsd). J. Pain Manag. 2017, 10, 397–405. [Google Scholar]
- Greer, G.R.; Grob, C.S.; Halberstadt, A.L. PTSD Symptom Reports of Patients Evaluated for the New Mexico Medical Cannabis Program. J. Psychoact. Drugs 2014, 46, 73–77. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Boden, M.T.; Bucossi, M.M.; Babson, K.A. Self-reported cannabis use characteristics, patterns and helpfulness among medical cannabis users. Am. J. Drug Alcohol Abuse 2014, 40, 23–30. [Google Scholar] [CrossRef]
- Reznik, I. Post-traumatic stress disorder and medical cannabis use: A naturalistic observational study. Eur. Neuropsychopharmacol. 2012, 22, S363–S364. [Google Scholar] [CrossRef]
- Rabinak, C.A.; Blanchette, A.; Zabik, N.L.; Peters, C.; Marusak, H.A.; Iadipaolo, A.; Elrahal, F. Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: A preliminary study. Psychopharmacology 2020, 237, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Davis, L.L.; Neylan, T.C.; Raskind, M.A.; Schnurr, P.P.; Stein, M.B.; Vessicchio, J.; Shiner, B.; Gleason, T.D.; Huang, G.D. It Is Time to Address the Crisis in the Pharmacotherapy of Posttraumatic Stress Disorder: A Consensus Statement of the PTSD Psychopharmacology Working Group. Biol. Psychiatry 2017, 82, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.S.; Nogueira Derenusson, G.; Borduqui Ferrari, T.; Wichert-Ana, L.; Duran, F.L.S.; Martin-Santos, R.; Vinícius Simões, M.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB 1 and CB 2 receptor pharmacology of three plant cannabinoids: Δ 9-tetrahydrocannabinol, cannabidiol and Δ 9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Hill, M.N.; Campolongo, P.; Yehuda, R.; Patel, S. Integrating Endocannabinoid Signaling and Cannabinoids into the Biology and Treatment of Posttraumatic Stress Disorder. Neuropsychopharmacology 2018, 43, 80–102. [Google Scholar] [CrossRef] [PubMed]
- Raymundi, A.M.; Da Silva, T.R.; Sohn, J.M.B.; Bertoglio, L.J.; Stern, C.A. Effects of Δ9-tetrahydrocannabinol on aversive memories and anxiety: A review from human studies. BMC Psychiatry 2020, 20. [Google Scholar] [CrossRef]
- Hindocha, C.; Cousijn, J.; Rall, M.; Bloomfield, M.A.P. The Effectiveness of Cannabinoids in the Treatment of Posttraumatic Stress Disorder (PTSD): A Systematic Review. J. Dual Diagn. 2020, 16, 120–139. [Google Scholar] [CrossRef]
- Oropeza, V.C.; Mackie, K.; Van Bockstaele, E.J. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007, 1127, 36–44. [Google Scholar] [CrossRef]
- Friedman, M.J.; Bernardy, N.C. Considering future pharmacotherapy for PTSD. Neurosci. Lett. 2017, 649, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Do Monte, F.H.; Souza, R.R.; Bitencourt, R.M.; Kroon, J.A.; Takahashi, R.N. Infusion of cannabidiol into infralimbic cortex facilitates fear extinction via CB1 receptors. Behav. Brain Res. 2013, 250, 23–27. [Google Scholar] [CrossRef]
- Tull, M.T.; McDermott, M.J.; Gratz, K.L. Marijuana dependence moderates the effect of posttraumatic stress disorder on trauma cue reactivity in substance dependent patients. Drug Alcohol Depend. 2016, 159, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Breivogel, C.S.; Sim-Selley, L.J. Basic neuroanatomy and neuropharmacology of cannabinoids. Int. Rev. Psychiatry 2009, 21, 113–121. [Google Scholar] [CrossRef]
- Gunduz-Cinar, O. The endocannabinoid system in the amygdala and modulation of fear. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105. [Google Scholar] [CrossRef] [PubMed]
- Berardis, D.; Marini, S.; Serroni, N.; Iasevoli, F.; Tomasetti, C.; Bartolomeis, A.; Mazza, M.; Tempesta, D.; Valchera, A.; Fornaro, M.; et al. Targeting the Noradrenergic System in Posttraumatic Stress Disorder: A Systematic Review and Meta-Analysis of Prazosin Trials. Curr. Drug Targets 2015, 16, 1094–1106. [Google Scholar] [CrossRef]
- Chagas, M.H.N.; Crippa, J.A.S.; Zuardi, A.W.; Hallak, J.E.C.; MacHado-De-Sousa, J.P.; Hirotsu, C.; Maia, L.; Tufik, S.; Andersen, M.L. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J. Psychopharmacol. 2013, 27, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.T.; Yi, P.L.; Li, C.L.; Chang, F.C. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology 2012, 62, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E.; Palomero-Rivero, M.; Millán-Aldaco, D.; Mechoulam, R.; Drucker-Colín, R. Effects on sleep and dopamine levels of microdialysis perfusion of cannabidiol into the lateral hypothalamus of rats. Life Sci. 2011, 88, 504–511. [Google Scholar] [CrossRef]
- Carlini, E.A.; Cunha, J.M. Hypnotic and antiepileptic effects of cannabidiol. J. Clin. Pharmacol. 1981, 21. [Google Scholar] [CrossRef]
- Murillo-Rodríguez, E.; Millán-Aldaco, D.; Palomero-Rivero, M.; Mechoulam, R.; Drucker-Colín, R. Cannabidiol, a constituent of Cannabis sativa, modulates sleep in rats. FEBS Lett. 2006, 580, 4337–4345. [Google Scholar] [CrossRef]
- Suraev, A.S.; Marshall, N.S.; Vandrey, R.; McCartney, D.; Benson, M.J.; McGregor, I.S.; Grunstein, R.R.; Hoyos, C.M. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med. Rev. 2020, 53. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E. The role of the CB1 receptor in the regulation of sleep. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Zalai, D.; Chung, S.; Hussain, N.; Shapiro, C. Does cannabinoid really improve sleep? Testing the sleep effects of nabilone in chronic pain patients: A placebo-controlled, randomized, pilot study. Psychother. Psychosom. 2015, 84, 81. [Google Scholar]
- Baron, E.P. Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science. Headache 2018, 58, 1139–1186. [Google Scholar] [CrossRef]
- Sihota, A.; Smith, B.K.; Ahmed, S.; Bell, A.; Blain, A.; Clarke, H.; Cooper, Z.D.; Cyr, C.; Daeninck, P.; Deshpande, A.; et al. Consensus-Based Recommendations for Titrating Cannabinoids and Tapering Opioids for Chronic Pain Control. Int. J. Clin. Pract. 2020, 2020, e13871. [Google Scholar]
- Manz, J.; Hyakutake, M.; Kelly, E. Calling for Openness to the Study of Cannabis Use in Chronic Pelvic Pain. J. Obstet. Gynaecol. Can. 2020. [Google Scholar] [CrossRef]
- Chaves, C.; Bittencourt, P.C.T.; Pelegrini, A. Ingestion of a THC-Rich Cannabis Oil in People with Fibromyalgia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Pain Med. 2020, 21, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Chiappini, S.; Volpe, U.; De Berardis, D.; Latini, R.; Papanti, G.D.; Corkery, J.M. Use of medicinal cannabis and synthetic cannabinoids in post-traumatic stress disorder (PTSD): A systematic review. Medicina 2019, 55, 525. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Babson, K.A.; Vandrey, R. Using cannabis to help you sleep: Heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014, 136, 162–165. [Google Scholar] [CrossRef]
- Earleywine, M.; Bolles, J.R. Marijuana, Expectancies, and Post-Traumatic Stress Symptoms: A Preliminary Investigation. J. Psychoact. Drugs 2014, 46, 171–177. [Google Scholar] [CrossRef]
- Shalev, A.; Liberzon, I.; Marmar, C. Post-Traumatic Stress Disorder. N. Engl. J. Med. 2017, 376, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Shorter, D.; Hsieh, J.; Kosten, T.R. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am. J. Addict. 2015, 24, 705–712. [Google Scholar] [CrossRef]
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “just the facts”; What we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008, 102, 1–18. [Google Scholar] [CrossRef]
- Korem, N.; Zer-Aviv, T.M.; Ganon-Elazar, E.; Abush, H.; Akirav, I. Targeting the endocannabinoid system to treat anxiety-related disorders. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Elliott, L.; Golub, A.; Bennett, A.; Guarino, H. PTSD and Cannabis-Related Coping Among Recent Veterans in New York City. Contemp. Drug Probl. 2015, 42, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Loflin, M.J.; Babson, K.A.; Bonn-Miller, M.O. Cannabinoids as therapeutic for PTSD. Curr. Opin. Psychol. 2017, 14, 78–83. [Google Scholar] [CrossRef] [PubMed]
| Author | Year and Country | Period | Study Design | Study Sample | Treatment | Comparator Group | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| Elms et al. [47] | 2019, US (Colorado) | 8 weeks | Retrospective study Grade: * | N = 11 (8 F, 3 M); mean age 39.91 ± 17.39; DSM-5 diagnosis of PTSD | N = 1: 9 mg/d (mean dose, range 1–16) CBD liquid oil spray; N = 4: 25 mg/d (mean dose, range 25–100) CBD capsule; N = 6: both formulations | None | Changes in PCL-5 score | Decline in PCL-5 mean scores of 21% (from 51.82 to 40.73) in 91% subjects at 4 weeks. Further decrease of 9% (to 37.14) in 75% subjects at 8 weeks. |
| Rabinak et al. [52] | 2019, US (Michigan) | Acute study | Randomized, double-blind, placebo-controlled, between-subjects study Grade: **** | N = 71 (36 F, 35 M) age 20–45; N = 22: DSM-5 diagnosis of PTSD; N = 24: TEC; N = 25: HC | THC 7.5 mg capsule | Placebo | Threat-processing paradigm at fMRI (amygdala, mPFC/rACC activation and functional connectivity) | In the PTSD group THC lowered threat-related bilateral amygdala activity (drug x group interaction left: F(1,65): 5.131, p = 0.027; right: F(1,65) = 4.456, p = 0.039), increased mPFC activation (drug x group interaction F(2,65) = 4.887, p = 0.011), increased mPFC-right amygdala functional coupling (F(1,65) = 8.181, p = 0.006). |
| Smith et al. [48] | 2017, Canada | 2 years, variable treatment duration and time to follow-up | Retrospective study Grade: *** | N = 100 veterans (3 F, 97 M); mean age 43; DSM-5 diagnosis of PTSD | Medical cannabis (THC and CBD at variable percentages), start dose 1 g/d, self-titrated based on clinical response, maximum dose 10 g/d | None | Changes in PTSD-related symptoms, pain, and social impact, scored 1–10 as reported in clinical charts | Decrease of PTSD aggregate symptoms score from 7 to 2.9 (59% reduction, ES 1.5, p < 0.0001), decrease of suicidal thoughts score from 4.1 to 0.9 (77% reduction, ES 1, p < 0.0001), decrease of pain score from 6.6 to 3.4 (48% reduction, ES 1.5), decrease of PTSD social impact score from 6.6 to 2.7 (59% reduction, ES 1.2, p < 0.0001) after treatment. |
| Cameron et al. [40] | 2014, Canada | 43 months | Retrospective study Grade: ** | N = 104 males; mean age 32.7 (range 19–55); 91% of which affected by DSM-IV-TR PTSD | Nabilone, mean initial dose 1.4 mg/d (range 0.5–2), mean final dose 4 mg/d (range 0.5–6), mean length of treatment 11.2 weeks (range 1 day–36 weeks) | None | Changes in PCL-C scores | Significant reduction of PLC-C scores (54.7 ± 13 vs 38.8 ± 7.1, p = 0.001) after treatment, consistent with a reduction from moderate to mild-borderline symptoms. |
| Greer et al. [49] | 2014, US (California) | 30 months | Retrospective study Grade: ** | N = 80 adults; DSM-IV diagnosis of PTSD | Medical cannabis (THC and CBD at variable percentages), start dose 1 g/d, self-titrated based on clinical response, maximum dose 10 g/d | None | Changes in CAPS scores | Decrease in total CAPS score (22.5 ± 16.9 vs 98.8 ± 17.6, p < 0.0001) in subjects using cannabis. Significant reductions in CAPS symptom cluster scores (Cannabis × Cluster: F(2,158) = 39.87, p < 0.0001). |
| Jetly et al. [39] | 2014, Canada | Two periods of 7 weeks separated by a period of 2 weeks | Randomized, double-blind, placebo-controlled crossover study Grade: **** | N = 10 military male personnel; age 18–65; DSM-IV-TR diagnosis of PTSD, current distressing nightmares, and difficulty falling/staying asleep as evaluated by CAPS | Nabilone 0.5 mg (start dose) weekly titrated to a maximum of 3 mg | Placebo | Changes in CAPS Recurrent Distressing Dreams and Difficulty Falling or Staying Asleep items, CGI-C, PTSD Dream Rating Scale, WBQ scores, Sleep Diary | Significant reduction of CAPS Recurring and Distressing Dream Frequency (−1.9 ± 1.3 vs −0.4 ± 1.4, p = 0.05) and Intensity (−1.7 ± 1.3 vs −0.6 ± 1.1, p = 0.06) scores, significant lower CGI-C (1.9 ± 1.1 vs 3.2 ± 1.2, p = 0.05) score, significant increase of WBQ (49.3 ± 21.6 vs 23 ± 17.2, p = 0.04) score after treatment with nabilone. |
| Roitman et al. [37] | 2014, Israel | 3 weeks | Non-randomized, open-label, adjusted doses, study Grade: *** | N = 10 (3 F, 7 M); mean age 52.3 ± 8.3; DSM-IV PTSD diagnosis | TCH oil 0.1 cc (=2.5 mg) bid, after 2 days raised to 0.2 cc (=5 mg) bid | None | Changes in CAPS, CGI, PSQI, NFQ, NES scores | Significant decrease in CAPS arousal (32.3 ± 4.73 vs 24.3 ± 9.11, p < 0.02), CGI-S (6 ± 0.47 vs 4.9 ± 0.99, p < 0.02), NFQ frequency of nightmares (0.81 ± 0.55 vs 0.44 ± 0.41, p < 0.04), NES (32.2 ± 11.29 vs 22.9 ± 8.7, p < 0.002), PSQI (17.20 ± 2.65 vs 13.9 ± 4.48, p < 0.05) scores after treatment with THC. |
| Bonn-Miller et al. [50] | 2013, US (California) | Not specified | Cross-sectional study Grade: ** | N = 217 (26.7% F, 73.3% M); mean age 41.2 ± 14.9 (range 18–74 years); 40 (18.9%) affected by PTSD | Medical cannabis, flexible-dose (mean use: 3 times/d, 9–12 g/week) | None | Subjective help received from medical cannabis as measured by a 5-point-likert scale | Traumatic intrusions predicted cannabis helpfulness (beta 0.22, p < 0.01), as well as the use of cannabis for social anxiety problems (p < 0.004). |
| Reznik [51] | 2012, Israel | 3 years | Naturalistic observational study Grade: ** | N = 167 25 pure PTSD, 43 PTSD + clinical depression, 88 PTSD + chronic pain | Medicinal cannabis (20–25% THC), range 2–3 g/d | None | Changes in CAPS, QOLS, CGI-I scores | Significant improvement in QOLS and pain scores in most cases, with some positive changes in CAPS scores. The majority of improved subjects belonged to comorbidity groups. |
| Fraser [38] | 2009, Canada | 2 years of clinical observation; flexible duration of treatment with nabilone (depending on the clinical response) | Non-randomized, open-label study Grade: *** | N = 47 (27 F, 20 M); mean age 44 ± 9; DSM-IV-TR diagnosis of PTSD, treatment-resistant nightmares | Nabilone 0.5 mg (start dose) before bedtime, then titrated; doses were kept below 6 mg/d | None | Changes in the intensity of PTSD-related nightmares | 34 (72%) subjects experienced total cessation or significant reduction of nightmares. Nabilone discontinuation was successful in 4 (8%) subjects, whilst the others experienced a recurrence of nightmares. |
| References | Overall Risk a | Randomization | Intervention | Missing Data | Outcome Measurement | Reported Results |
|---|---|---|---|---|---|---|
| Rabinak et al. (2019) [52] | +/− | − | − | +/− | − | − |
| Jetly et al. (2014) [39] | +/− | +/− | − | − | − | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steardo, L., Jr.; Carbone, E.A.; Menculini, G.; Moretti, P.; Steardo, L.; Tortorella, A. Endocannabinoid System as Therapeutic Target of PTSD: A Systematic Review. Life 2021, 11, 214. https://doi.org/10.3390/life11030214
Steardo L Jr., Carbone EA, Menculini G, Moretti P, Steardo L, Tortorella A. Endocannabinoid System as Therapeutic Target of PTSD: A Systematic Review. Life. 2021; 11(3):214. https://doi.org/10.3390/life11030214
Chicago/Turabian StyleSteardo, Luca, Jr., Elvira Anna Carbone, Giulia Menculini, Patrizia Moretti, Luca Steardo, and Alfonso Tortorella. 2021. "Endocannabinoid System as Therapeutic Target of PTSD: A Systematic Review" Life 11, no. 3: 214. https://doi.org/10.3390/life11030214
APA StyleSteardo, L., Jr., Carbone, E. A., Menculini, G., Moretti, P., Steardo, L., & Tortorella, A. (2021). Endocannabinoid System as Therapeutic Target of PTSD: A Systematic Review. Life, 11(3), 214. https://doi.org/10.3390/life11030214







