Overview of Immunological Responses and Immunomodulation Properties of Trichuris sp.: Prospects for Better Understanding Human Trichuriasis
Abstract
1. Introduction
2. Basic Immunology Concept against Trichuris trichiura
2.1. Innate Immune System Also Determines the Fate of Infection
2.2. Antibody-Dependent Cell-mediated Cytotoxicity (ADCC): Is It Reliable for Trichuriasis?
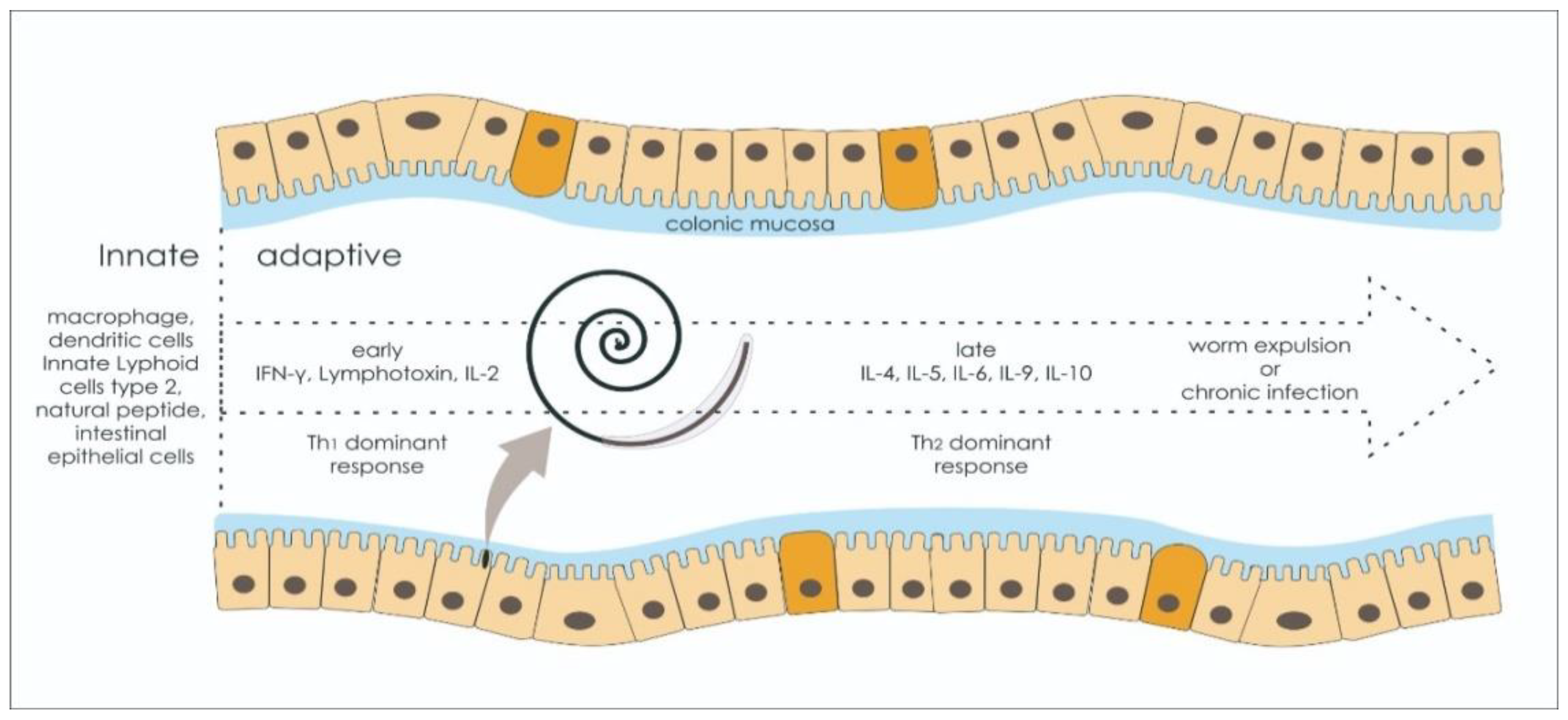
2.3. Adaptive Immune Response: Worm Expulsion versus Chronic Infection
3. Innate Lymphoid Cells: A Paucity Not to Be Ignored
4. Immunomodulation Properties of Trichuris sp. and Clinical Implications: Focus on the Role of Excretory/Secretory (ESPs) and Soluble Products (SPs)
4.1. Secreted Products Modulate Pattern Recognition Receptor (PRR)
4.2. Secreted Product Skewed Innate Immune System
4.3. Secreted Product Produce Deviant Cytokine Response
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garcia, L.S. Classification of Human Parasites, Vectors, and Similar Organisms. Clin. Infect. Dis. 1999, 29, 734–736. [Google Scholar] [CrossRef]
- Horton, J. Helminth-Nematode: Trichuris trichiura. Encycl. Food Saf. 2014, 2, 111–115. [Google Scholar] [CrossRef]
- Bharti, B.; Bharti, S.; Khurana, S. Worm infestation: Diagnosis, treatment and prevention. Indian J. Pediatr. 2018, 85, 1017–1024. [Google Scholar] [CrossRef]
- Vejzagic, N.; Adelfio, R.; Keiser, J.; Kringel, H.; Thamsborg, S.M.; Kapel, C.M. Bacteria-induced egg hatching differs for Trichuris muris and Trichuris suis. Parasit Vectors 2015, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K. Bacteria-induced hatching of Trichuris muris eggs occurs without direct contact between eggs and bacteria. Parasitol. Res. 2016, 115, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Beer, R. Studies on the biology of the life-cycle of Trichuris suis Schrank, 1788. Parasitology 1973, 67, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, H.G. Electron microscopy of the bacillary band and stichosome of Trichuris muris and T. vulpis. J. Parasitol. 1963, 49, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.D.; Cruickshank, S.M.; Starborg, T.; Withers, P.J.; Else, K.J. Characterisation of cuticular inflation development and ultrastructure in Trichuris muris using correlative X-ray computed tomography and electron microscopy. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, A.; Yarrarapu, S.N.S.; Williams, M. Trichuris Trichiura. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507843/ (accessed on 15 November 2020).
- Sunkara, T.; Sharma, S.R.; Ofosu, A. Trichuris trichiura—An Unwelcome Surprise during Colonoscopy. Am. J. Trop. Med. Hyg. 2018, 99, 555–556. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.M.; Reguera-Gomez, M.; Valero, M.A.; Cutillas, C. Differentiation of Trichuris species eggs from non-human primates by geometric morphometric analysis. Int. J. Parasitol. Parasites Wildl. 2020, 12, 214–219. [Google Scholar] [CrossRef]
- World Health Organization. Bench Aids for the Diagnosis of Intestinal Parasites; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Betson, M.; Soe, M.J.; Nejsum, P. Human Trichuriasis: Whipworm Genetics, Phylogeny, Transmission and Future Research Directions. Curr. Trop. Med. Rep. 2015, 2, 209–2017. [Google Scholar] [CrossRef]
- Beer, R.J.S. The relationship between Trichuris trichiura (Linnaeus 1758) of man and Trichuris suis (Schrank 1788) of the pig. Res. Vet. Sci. 1976, 20, 47–54. [Google Scholar] [CrossRef]
- Cutillas, C.; Callejon, R.; de Rojas, M.; Tewes, B.; Ubeda, J.M.; Ariza, C.; Guevara, D.C. Trichuris suis and Trichuris trichiura are different nematode species. Acta Trop. 2009, 111, 299–307. [Google Scholar] [CrossRef]
- Meekums, H.; Hawash, M.B.; Sparks, A.M.; Oviedo, Y.; Sandoval, C.; Chico, M.E.; Stothard, J.R.; Cooper, P.J.; Nejsum, P.; Betson, M. A genetic analysis of Trichuris trichiura and Trichuris suis from Ecuador. Parasites Vectors 2015, 8, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Al-Jubury, A.; Hansen, T.V.; Olsen, A.; Christensen, H.; Thamsborg, S.M.; Nejsum, P. Genetic analysis of Trichuris suis and Trichuris trichiura recovered from humans and pigs in a sympatric setting in Uganda. Vet. Parasitol. 2012, 188, 68–77. [Google Scholar] [CrossRef]
- Rivero, J.; García-Sánchez, Á.M.; Zurita, A.; Cutillas, C.; Callejón, R. Trichuris trichiura isolated from Macaca sylvanus: Morphological, biometrical, and molecular study. BMC Vet. Res. 2020, 16, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Phosuk, I.; Sanpool, O.; Thanchomnang, T.; Sadaow, L.; Rodpai, R.; Anamnart, W.; Janwan, P.; Wijit, A.; Laymanivong, S.; Pa Aung, W.P.; et al. Molecular Identification of Trichuris suis and Trichuris trichiura Eggs in Human Populations from Thailand, Lao PDR, and Myanmar. Am. J. Trop. Med. Hyg. 2018, 98, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Guardone, L.; Deplazes, P.; Macchioni, F.; Magi, M.; Mathis, A. Ribosomal and mitochondrial DNA analysis of Trichuridae nematodes of carnivores and small mammals. Vet. Parasitol. 2013, 197, 364–369. [Google Scholar] [CrossRef]
- Callejón, R.; Halajian, A.; De Rojas, M.; Marrugal, A.; Guevara, D.; Cutillas, C. 16S partial gene mitochondrial DNA and internal transcribed spacers ribosomal DNA as differential markers of Trichuris discolor populations. Vet. Parasitol. 2012, 186, 350–363. [Google Scholar] [CrossRef]
- Roach, T.; Wakelin, D.; Else, K.; Bundy, D. Antigenic cross-reactivity between the human whipworm, Trichuris trichiura, and the mouse trichuroids Trichuris muris and Trichinella spiralis. Parasite Immunol. 1988, 10, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Klementowicz, J.E.; Travis, M.A.; Grencis, R.K. Trichuris muris: A model of gastrointestinal parasite infection. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 815–828. [Google Scholar]
- Eichenberger, R.M.; Talukder, M.H.; Field, M.A.; Wangchuk, P.; Giacomin, P.; Loukas, A.; Sotillo, J. Characterization of Trichuris muris secreted proteins and extracellular vesicles provides new insights into host-parasite communication. J. Extracell. Vesicles 2018, 7, 1428004. [Google Scholar] [CrossRef] [PubMed]
- Leroux, L.P.; Nasr, M.; Valanparambil, R.; Tam, M.; Rosa, B.A.; Siciliani, E.; Hill, D.E.; Zarlenga, D.S.; Jaramillo, M.; Weinstock, J.V.; et al. Analysis of the Trichuris suis excretory/secretory proteins as a function of life cycle stage and their immunomodulatory properties. Sci. Rep. 2018, 8, 15921. [Google Scholar] [CrossRef] [PubMed]
- Lillywhite, J.; Cooper, E.; Needham, C.; Venugopal, S.; Bundy, D.; Bianco, A. Identification and characterization of excreted/secreted products of Trichuris trichiura. Parasite Immunol. 1995, 17, 47–54. [Google Scholar] [CrossRef]
- Leonardi, I.; Gerstgrasser, A.; Schmidt, T.S.; Nicholls, F.; Tewes, B.; Greinwald, R.; von Mering, C.; Rogler, G.; Frey-Wagner, I. Preventive Trichuris suis ova (TSO) treatment protects immunocompetent rabbits from DSS colitis but may be detrimental under conditions of immunosuppression. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Dige, A.; Rasmussen, T.K.; Nejsum, P.; Hagemann-Madsen, R.; Williams, A.R.; Agnholt, J.; Dahlerup, J.F.; Hvas, C.L. Mucosal and systemic immune modulation by Trichuris trichiura in a self-infected individual. Parasite Immunol. 2017, 39, e12394. [Google Scholar] [CrossRef] [PubMed]
- Motran, C.C.; Silvane, L.; Chiapello, L.S.; Theumer, M.G.; Ambrosio, L.F.; Volpini, X.; Celias, D.P.; Cervi, L. Helminth infections: Recognition and Modulation of the immune Response by innate immune Cells. Front. Immunol. 2018, 9, 664. [Google Scholar] [CrossRef]
- Tritten, L.; Tam, M.; Vargas, M.; Jardim, A.; Stevenson, M.M.; Keiser, J.; Geary, T.G. Excretory/secretory products from the gastrointestinal nematode Trichuris muris. Exp. Parasitol. 2017, 178, 30–36. [Google Scholar] [CrossRef]
- Schachter, J.; de Oliveira, D.A.; da Silva, C.M.; de Barros Alencar, A.C.M.; Duarte, M.; da Silva, M.M.P.; Ignácio, A.C.d.P.R.; Lopes-Torres, E.J. Whipworm Infection Promotes Bacterial Invasion, Intestinal Microbiota Imbalance, and Cellular Immunomodulation. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef]
- Else, K.J.; Keiser, J.; Holland, C.V.; Grencis, R.K.; Sattelle, D.B.; Fujiwara, R.T.; Bueno, L.L.; Asaolu, S.O.; Sowemimo, O.A.; Cooper, P.J. Whipworm and roundworm infections. Nat. Rev. Dis. Primers 2020, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Low, C.; Nutman, T. IgE production in human helminth infection. Reciprocal interrelationship between IL-4 and IFN-gamma. J. Immunol. 1993, 150, 1873–1880. [Google Scholar] [PubMed]
- Belhassen-García, M.; Pardo-Lledías, J.; del Villar, L.P.; Muro, A.; Velasco-Tirado, V.; de Castro, A.B.; Vicente, B.; García, M.I.G.; Bellido, J.L.M.; Cordero-Sánchez, M. Relevance of eosinophilia and hyper-IgE in immigrant children. Medicine 2014, 93, e46. [Google Scholar] [CrossRef]
- Lillywhite, J.; Bundy, D.; Didier, J.; Cooper, E.; Bianco, A. Humoral immune responses in human infection with the whipworm Trichuris trichiura. Parasite Immunol. 1991, 13, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Else, K.; Entwistle, G.; Grencis, R. Correlations between worm burden and markers of Th1 and Th2 cell subset induction in an inbred strain of mouse infected with Trichuris muris. Parasite Immunol. 1993, 15, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, N.M.; Else, K.J. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infect. Immun. 2001, 69, 3860–3868. [Google Scholar] [CrossRef][Green Version]
- Houlden, A.; Hayes, K.S.; Bancroft, A.J.; Worthington, J.J.; Wang, P.; Grencis, R.K.; Roberts, I.S. Chronic Trichuris muris infection in C57BL/6 mice causes significant changes in host microbiota and metabolome: Effects reversed by pathogen clearance. PLoS ONE 2015, 10, e0125945. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.B.; Sorobetea, D.; Kiilerich, P.; Ramayo-Caldas, Y.; Estellé, J.; Ma, T.; Madsen, L.; Kristiansen, K.; Svensson-Frej, M. Chronic Trichuris muris infection decreases diversity of the intestinal microbiota and concomitantly increases the abundance of lactobacilli. PLoS ONE 2015, 10, e0125495. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.D.; Betts, C.J.; Else, K.J. Peripheral cytokine responses to Trichuris muris reflect those occurring locally at the site of infection. Infect. Immun. 2000, 68, 1815–1819. [Google Scholar] [CrossRef][Green Version]
- Bancroft, A.J.; Else, K.J.; Humphreys, N.E.; Grencis, R.K. The effect of challenge and trickle Trichuris muris infections on the polarisation of the immune response. Int. J. Parasitol. 2001, 31, 1627–1637. [Google Scholar] [CrossRef]
- Glover, M.; Colombo, S.A.; Thornton, D.J.; Grencis, R.K. Trickle infection and immunity to Trichuris muris. PLoS Pathog. 2019, 15, e1007926. [Google Scholar] [CrossRef]
- Kringel, H.; Iburg, T.; Dawson, H.; Aasted, B.; Roepstorff, A. A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int. J. Parasitol. 2006, 36, 915–924. [Google Scholar] [CrossRef]
- Coackley, G.; Harris, N.L. The Intestinal Epithelium at the Forefront of Host–Helminth Interactions. Trends Parasitol. 2020, 36, 761–772. [Google Scholar] [CrossRef]
- Ogra, P.L.; Mestecky, J.; Lamm, M.E.; Strober, W.; McGhee, J.R.; Bienenstock, J. Handbook of Mucosal Immunology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Cortés, A.; Muñoz-Antoli, C.; Toledo, R.; Esteban, J.G. Th2 and Th1 Responses: Clear and Hidden Sides of Immunity Against Intestinal Helminths. Trends Parasitol. 2017, 33, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deschoolmeester, M.L.; Manku, H.; Else, K.J. The innate immune responses of colonic epithelial cells to Trichuris muris are similar in mouse strains that develop a type 1 or type 2 adaptive immune response. Infect. Immun. 2006, 74, 6280–6286. [Google Scholar] [CrossRef]
- Bellaby, T.; Robinson, K.; Wakelin, D. Induction of differential T-helper-cell responses in mice infected with variants of the parasitic nematode Trichuris muris. Infect. Immun. 1996, 64, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Stadnyk, A.W.; Kearsey, J.A. Pattern of proinflammatory cytokine mRNA expression during Trichinella spiralis infection of the rat. Infect. Immun. 1996, 64, 5138–5143. [Google Scholar] [CrossRef]
- Ludwig-Portugall, I.; Layland, L.E. TLRs, Treg, and B celss, an interplay of regulation during helminth infection. Front. Immunol. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sahputra, R.; Else, K.J.; Rückerl, D.; Couper, K.; Müller, W. The essential role played by B cells in supporting protective immunity against Trichuris muris infection is by controlling the Th1/Th2 balance in the mesenteric lymph nodes and depends on host genetic background. Front. Immunol. 2019, 10, 2842. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.J.; McCarthy, N.E.; Giles, E.M.; Davidson, K.L.; Haltalli, M.L.; Hazell, S.; Lindsay, J.O.; Stagg, A.J. Increased production of retinoic acid by intestinal macrophages contributes to their inflammatory phenotype in patients with Crohn’s disease. Gastroenterology 2014, 146, 1278–1288.e1272. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.M.; Cheung, J.; Yen, D.; Li, J.; Zurawski, S.M.; Lo, S.; Menon, S.; Clifford, T.; Hunte, B.; Lesley, R. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001, 15, 985–995. [Google Scholar] [CrossRef]
- Jang, S.; Morris, S.; Lukacs, N.W. TSLP promotes induction of Th2 differentiation but is not necessary during established allergen-induced pulmonary disease. PLoS ONE 2013, 8, e56433. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Lee, H.C.; Nakayama, T.; Ziegler, S.F. TSLP enhances the function of helper type 2 cells. Eur. J. Immunol. 2011, 41, 1862–1871. [Google Scholar] [CrossRef]
- Murakami-Satsutani, N.; Ito, T.; Nakanishi, T.; Inagaki, N.; Tanaka, A.; Vien, P.T.X.; Kibata, K.; Inaba, M.; Nomura, S. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol. Int. 2014, 63, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Marillier, R.G.; Michels, C.; Smith, E.M.; Fick, L.C.; Leeto, M.; Dewals, B.; Horsnell, W.G.; Brombacher, F. IL-4/IL-13 independent goblet cell hyperplasia in experimental helminth infections. BMC Immunol. 2008, 9, 1–9. [Google Scholar] [CrossRef]
- Sharba, S.; Navabi, N.; Padra, M.; Persson, J.; Quintana-Hayashi, M.; Gustafsson, J.; Szeponik, L.; Venkatakrishnan, V.; Sjöling, Å.; Nilsson, S. Interleukin 4 induces rapid mucin transport, increases mucus thickness and quality and decreases colitis and Citrobacter rodentium in contact with epithelial cells. Virulence 2019, 10, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Capron, A.; Dessaint, J.-P.; Haque, A.; Capron, M. Antibody-Dependent Cell-Mediated Cytotoxicity against Parasites. Immun. Concomitant Immun. Infect. Dis. 1982, 31, 234–267. [Google Scholar] [CrossRef]
- Tang, H.; Ming, Z.; Liu, R.; Xiong, T.; Grevelding, C.G.; Dong, H.; Jiang, M. Development of adult worms and granulomatous pathology are collectively regulated by T-and B-cells in mice infected with Schistosoma japonicum. PLoS ONE 2013, 8, e54432. [Google Scholar]
- Matsuda, S.; Tani, Y.; Yamada, M.; Yoshimura, K.; Arizono, N. Type 2-biased expression of cytokine genes in lung granulomatous lesions induced by Nippostrongylus brasiliensis infection. Parasite Immunol. 2001, 23, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.A.; Ovington, K.S. The Role of Eosinophils in Parasitic Helminth Infections: Insights from Genetically Modified Mice. Parasitol. Today 2000, 16, 202–209. [Google Scholar] [CrossRef]
- Klion, A.D.; Nutman, T.B. The role of eosinophils in host defense against helminth parasites. J. Allergy Clin. Immunol. 2004, 113, 30–37. [Google Scholar] [CrossRef]
- BETTS, C.J.; ELSE, K.J. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999, 21, 45–52. [Google Scholar] [CrossRef]
- Yasuda, K.; Kuroda, E. Role of eosinophils in protective immunity against secondary nematode infections. Immunol. Med. 2019, 42, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.J.; Else, K.J. Trichuris muris research revisited: A journey through time. Parasitology 2013, 140, 1325–1339. [Google Scholar] [CrossRef]
- Wakelin, D. Acquired immunity to Trichuris muris in the albino laboratory mouse. Parasitology 1967, 57, 515–524. [Google Scholar] [CrossRef]
- Mirchandani, A.S.; Besnard, A.G.; Yip, E.; Scott, C.; Bain, C.C.; Cerovic, V.; Salmond, R.J.; Liew, F.Y. Type 2 innate lymphoid cells drive CD4+ Th2 cell responses. J. Immunol. 2014, 192, 2442–2448. [Google Scholar] [CrossRef] [PubMed]
- Curry, A.; Else, K.; Jones, F.; Bancroft, A.; Grencis, R.; Dunne, D. Evidence that cytokine-mediated immune interactions induced by Schistosoma mansoni alter disease outcome in mice concurrently infected with Trichuris muris. J. Exp. Med. 1995, 181, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Kim, H.Y.; Albacker, L.A.; Baumgarth, N.; McKenzie, A.N.; Smith, D.E.; Dekruyff, R.H.; Umetsu, D.T. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat. Immunol. 2011, 12, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Chang, Y.J.; Subramanian, S.; Lee, H.H.; Albacker, L.A.; Matangkasombut, P.; Savage, P.B.; McKenzie, A.N.; Smith, D.E.; Rottman, J.B.; et al. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. J. Allergy Clin. Immunol. 2012, 129, 216–227.e6. [Google Scholar] [CrossRef] [PubMed]
- Duerr, C.U.; Fritz, J.H. Isolation of Group 2 Innate Lymphoid Cells from Mouse Lungs. In Innate Antiviral Immunity; Humana Press: Totowa, NJ, USA, 2017; Volume 1656, pp. 253–261. [Google Scholar] [CrossRef]
- Mjosberg, J.; Bernink, J.; Golebski, K.; Karrich, J.J.; Peters, C.P.; Blom, B.; te Velde, A.A.; Fokkens, W.J.; van Drunen, C.M.; Spits, H. The transcription factor GATA3 is essential for the function of human type 2 innate lymphoid cells. Immunity 2012, 37, 649–659. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, L.; Qiu, J.; Chen, X.; Hu-Li, J.; Siebenlist, U.; Williamson, P.R.; Urban, J.F.; Paul, W.E. IL-25-responsive, lineage-negative KLRG1 hi cells are multipotential ‘inflammatory’type 2 innate lymphoid cells. Nat. Immunol. 2015, 16, 161–169. [Google Scholar] [CrossRef]
- Nakamura, S.; Kuroki, K.; Ohki, I.; Sasaki, K.; Kajikawa, M.; Maruyama, T.; Ito, M.; Kameda, Y.; Ikura, M.; Yamamoto, K. Molecular basis for E-cadherin recognition by killer cell lectin-like receptor G1 (KLRG1). J. Biol. Chem. 2009, 284, 27327–27335. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, C.J.; Hwang, Y.Y.; Walker, J.A.; Salimi, M.; Wong, S.H.; Brewer, J.M.; Englezakis, A.; Barlow, J.L.; Hams, E.; Scanlon, S.T.; et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity 2014, 41, 283–295. [Google Scholar] [CrossRef]
- Han, M.; Rajput, C.; Hong, J.Y.; Lei, J.; Hinde, J.L.; Wu, Q.; Bentley, J.K.; Hershenson, M.B. The Innate Cytokines IL-25, IL-33, and TSLP Cooperate in the Induction of Type 2 Innate Lymphoid Cell Expansion and Mucous Metaplasia in Rhinovirus-Infected Immature Mice. J. Immunol. 2017, 199, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Bentley, J.K.; Chung, Y.; Lei, J.; Steenrod, J.M.; Chen, Q.; Sajjan, U.S.; Hershenson, M.B. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2014, 134, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Goleniewska, K.; Zhang, J.; Zhou, W.; Newcomb, D.C.; Zhou, B.; Kita, H.; Boyd, K.L.; Peebles, R.S., Jr. TSLP and IL-33 reciprocally promote each other’s lung protein expression and ILC2 receptor expression to enhance innate type-2 airway inflammation. Allergy 2020, 75, 1606–1617. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ji, M.; Liang, H.E.; Locksley, R.M. Tuft-cell-derived IL-25 regulates an intestinal ILC2-epithelial response circuit. Nature 2016, 529, 221–225. [Google Scholar] [CrossRef]
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Creyns, B.; Cremer, J.; De Hertogh, G.; Boon, L.; Ferrante, M.; Vermeire, S.; Van Assche, G.; Ceuppens, J.L.; Breynaert, C. Fibrogenesis in chronic murine colitis is independent of innate lymphoid cells. Immun. Inflamm. Dis. 2020, 8, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, M.W.; Rickard, M.D. Excretory-secretory products helminth parasite: Effects on host immune responses. Parasitology 1988, 96, S123–S166. [Google Scholar] [CrossRef]
- Hewitson, J.P.; Grainger, J.R.; Maizels, R.M. Helminth immunoregulation: The role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 2009, 167, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Maizels, R.M.; Smits, H.H.; McSorley, H.J. Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules. Immunity 2018, 49, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Briggs, N.; Wei, J.; Versteeg, L.; Zhan, B.; Keegan, B.; Damania, A.; Pollet, J.; Hayes, K.S.; Beaumier, C.; Seid, C.A. Trichuris muris whey acidic protein induces type 2 protective immunity against whipworm. PLoS Pathog. 2018, 14, e1007273. [Google Scholar] [CrossRef] [PubMed]
- Summan, A.; Nejsum, P.; Williams, A.R. Modulation of human dendritic cell activity by Giardia and helminth antigens. Parasite Immunol. 2018, 40, e12525. [Google Scholar] [CrossRef]
- Ottow, M.; Klaver, E.; van der Pouw Kraan, T.; Heijnen, P.; Laan, L.; Kringel, H.; Vogel, D.; Dijkstra, C.; Kooij, G.; Van Die, I. The helminth Trichuris suis suppresses TLR4-induced inflammatory responses in human macrophages. Genes Immun. 2014, 15, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Klaver, E.; van der Pouw Kraan, T.; Laan, L.; Kringel, H.; Cummings, R.; Bouma, G.; Kraal, G.; Van Die, I. Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun. 2015, 16, 378–387. [Google Scholar] [CrossRef]
- Laan, L.C.; Williams, A.R.; Stavenhagen, K.; Giera, M.; Kooij, G.; Vlasakov, I.; Kalay, H.; Kringel, H.; Nejsum, P.; Thamsborg, S.M. The whipworm (Trichuris suis) secretes prostaglandin E2 to suppress proinflammatory properties in human dendritic cells. FASEB J. 2017, 31, 719–731. [Google Scholar] [CrossRef]
- Harnett, W. Secretory products of helminth parasites as immunomodulators. Mol. Biochem. Parasitol. 2014, 195, 130–136. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Duque-Correa, M.A.; Schreiber, F.; Rodgers, F.H.; Goulding, D.; Forrest, S.; White, R.; Buck, A.; Grencis, R.K.; Berriman, M. Development of caecaloids to study host-pathogen interactions: New insights into immunoregulatory functions of Trichuris muris extracellular vesicles in the caecum. Int. J. Parasitol. 2020, 50, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Krivan, S.; Kapelouzou, A.; Vagio, S.; Tsilimigras, D.I.; Katsimpoulas, M.; Moris, D.; Aravanis, C.V.; Demesticha, T.D.; Schizas, D.; Mavroidis, M.; et al. Increased expression of Toll-like receptors 2, 3, 4 and 7 mRNA in the kidney and intestine of a septic mouse model. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wiria, A.E.; Hamid, F.; Wammes, L.J.; Prasetyani, M.A.; Dekkers, O.M.; May, L.; Kaisar, M.M.; Verweij, J.J.; Guigas, B.; Partono, F. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PLoS ONE 2015, 10, e0127746. [Google Scholar]
- Rocha, D.; Caldas, A.; Oliveira, L.; Bressan, J.; Hermsdorff, H. Saturated fatty acids trigger TLR4-mediated inflammatory response. Atherosclerosis 2016, 244, 211–215. [Google Scholar] [CrossRef]
- Qi, X.; Qin, L.; Du, R.; Chen, Y.; Lei, M.; Deng, M.; Wang, J. Lipopolysaccharide upregulated intestinal epithelial cell expression of Fn14 and activation of Fn14 signaling amplify intestinal TLR4-mediated inflammation. Front. Cell. Infect. Microbiol. 2017, 7, 315. [Google Scholar] [CrossRef]
- Kooij, G.; Braster, R.; Koning, J.J.; Laan, L.C.; van Vliet, S.J.; Los, T.; Eveleens, A.M.; van der Pol, S.M.; Förster-Waldl, E.; Boztug, K. Trichuris suis induces human non-classical patrolling monocytes via the mannose receptor and PKC: Implications for multiple sclerosis. Acta Neuropathol. Commun. 2015, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Klaver, E.J.; Kuijk, L.M.; Laan, L.C.; Kringel, H.; van Vliet, S.J.; Bouma, G.; Cummings, R.D.; Kraal, G.; van Die, I. Trichuris suis-induced modulation of human dendritic cell function is glycan-mediated. Int. J. Parasitol. 2013, 43, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bono, M.a.R.; Elgueta, R.l.; Sauma, D.; Pino, K.; Osorio, F.; Michea, P.; Fierro, A.; Rosemblatt, M. The essential role of chemokines in the selective regulation of lymphocyte homing. Cytokine Growth Factor Rev. 2007, 18, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Laufer, J.M.; Legler, D.F. Beyond migration—Chemokines in lymphocyte priming, differentiation, and modulating effector functions. J. Leucoc. Biol. 2018, 104, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, Y. Protein kinases are potential targets to treat inflammatory bowel disease. World J. Gastrointest. Pharmacol. Ther. 2014, 5, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.F.; Chang, Q.; Soper, B.D.; Tepperman, B.L. Protein kinase C mediates experimental colitis in the rat. Am. J. Physiol. 1999, 276, G583–G590. [Google Scholar] [CrossRef]
- Alhallaf, R.; Agha, Z.; Miller, C.M.; Robertson, A.A.; Sotillo, J.; Croese, J.; Cooper, M.A.; Masters, S.L.; Kupz, A.; Smith, N.C. The NLRP3 inflammasome suppresses protective immunity to gastrointestinal helminth infection. Cell Rep. 2018, 23, 1085–1098. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Maslowski, K.M.; Mosconi, I.; Guenat, N.; Marsland, B.J.; Harris, N.L. IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog 2013, 9, e1003531. [Google Scholar] [CrossRef]
- Chenery, A.L.; Alhallaf, R.; Agha, Z.; Ajendra, J.; Parkinson, J.E.; Cooper, M.M.; Chan, B.H.; Eichenberger, R.M.; Dent, L.A.; Robertson, A.A. Inflammasome-independent role for NLRP3 in controlling innate antihelminth immunity and tissue repair in the lung. J. Immunol. 2019, 203, 2724–2734. [Google Scholar] [CrossRef]
- Duque-Correa, M.A.; Karp, N.A.; McCarthy, C.; Forman, S.; Goulding, D.; Sankaranarayanan, G.; Jenkins, T.P.; Reid, A.J.; Cambridge, E.L.; Reviriego, C.B. Exclusive dependence of IL-10Rα signalling on intestinal microbiota homeostasis and control of whipworm infection. PLoS Pathog. 2019, 15, e1007265. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, L.; Urban Jr, J. The pathogenesis of necrotic proliferative colitis in swine is linked to whipworm induced suppression of mucosal immunity to resident bacteria. Vet. Immunol. Immunopathol. 1996, 50, 1–17. [Google Scholar] [CrossRef]
- Montacute, R.; Foley, K.; Forman, R.; Else, K.J.; Cruickshank, S.M.; Allan, S.M. Enhanced susceptibility of triple transgenic Alzheimer’s disease (3xTg-AD) mice to acute infection. J. Neuroinflammation 2017, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Kühn, R.; Löhler, J.; Rennick, D.; Rajewsky, K.; Müller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 1993, 75, 263–274. [Google Scholar] [CrossRef]
- Ebner, F.; Hepworth, M.; Rausch, S.; Janek, K.; Niewienda, A.; Kühl, A.; Henklein, P.; Lucius, R.; Hamelmann, E.; Hartmann, S. Therapeutic potential of larval excretory/secretory proteins of the pig whipworm Trichuris suis in allergic disease. Allergy 2014, 69, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, A.J.; McKenzie, A.N.; Grencis, R.K. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 1998, 160, 3453–3461. [Google Scholar] [PubMed]
- Bancroft, A.J.; Levy, C.W.; Jowitt, T.A.; Hayes, K.S.; Thompson, S.; Mckenzie, E.A.; Ball, M.D.; Dubaissi, E.; France, A.P.; Bellina, B. The major secreted protein of the whipworm parasite tethers to matrix and inhibits interleukin-13 function. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darlan, D.M.; Rozi, M.F.; Yulfi, H. Overview of Immunological Responses and Immunomodulation Properties of Trichuris sp.: Prospects for Better Understanding Human Trichuriasis. Life 2021, 11, 188. https://doi.org/10.3390/life11030188
Darlan DM, Rozi MF, Yulfi H. Overview of Immunological Responses and Immunomodulation Properties of Trichuris sp.: Prospects for Better Understanding Human Trichuriasis. Life. 2021; 11(3):188. https://doi.org/10.3390/life11030188
Chicago/Turabian StyleDarlan, Dewi Masyithah, Muhammad Fakhrur Rozi, and Hemma Yulfi. 2021. "Overview of Immunological Responses and Immunomodulation Properties of Trichuris sp.: Prospects for Better Understanding Human Trichuriasis" Life 11, no. 3: 188. https://doi.org/10.3390/life11030188
APA StyleDarlan, D. M., Rozi, M. F., & Yulfi, H. (2021). Overview of Immunological Responses and Immunomodulation Properties of Trichuris sp.: Prospects for Better Understanding Human Trichuriasis. Life, 11(3), 188. https://doi.org/10.3390/life11030188




