Reproduction and the Early Development of Vertebrates in Space: Problems, Results, Opportunities
Abstract
1. Introduction
2. Fishes
3. Amphibians
3.1. Anuran Amphibian Species
3.2. Urodele Amphibian Species
3.2.1. Japanese Red-Bellied Newt
3.2.2. Spanish Ribbed Newt
4. Reptiles
5. Birds
6. Mammals
6.1. Fertilisation and Preimplantation
6.2. Maternal Influences on Development in Mammals
6.2.1. The Cosmos-1514 Biosatellite
6.2.2. NIH.Rodent 1 and NIH.R2 Experiments
6.2.3. Effects of Postnatal Spaceflight
7. Human
8. Conclusions
- Assessing the impact of weightlessness and hypergravity on various stages of pre-and postnatal development;
- Assessing the possibility of existence in space flight conditions during the full cycle of individual development and in a consecutive series of generations;
- Studying the rate of aging, age-related changes in general resistance and reproductive capacity of animals during space flights and after returning to Earth.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, B.; Luderer, U. Reproductive hazards of space travel in women and men. Nat. Rev. Endocrinol. 2019, 15, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Hart, D. Potential impact of space environments on developmental and maturational programs which evolved to meet the boundary conditions of Earth: will maturing humans be able to establish a functional biologic system set point under non-Earth conditions? J. Biomed. Sci. Eng. 2019, 12, 500–513. [Google Scholar] [CrossRef][Green Version]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Neff, A.W.; Yokota, H.; Chung, H.M.; Wakahara, M.; Malacinski, G.M. Early amphibian (anuran) morphogenesis is sensitive to novel gravitational fields. Dev. Biol. 1993, 155, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Neubert, J.; Schatz, A.; Bromeis, B.; Linke-Hommes, A. Effects of gravity on early development. Adv. Space Res. 1998, 22, 265–271. [Google Scholar] [CrossRef]
- Kojima, Y.; Sasaki, S.; Kubota, Y.; Ikeuchi, T.; Hayashi, Y.; Kohri, K. Effects of simulated microgravity on mammalian fertilization and preimplantation embryonic development in vitro. Fertil. Steril. 2000, 74, 1142–1147. [Google Scholar] [CrossRef]
- Tou, J.; Ronca, A.; Grindeland, R.; Wade, C. Models to study gravitational biology of Mammalian reproduction. Biol. Reprod. 2002, 67, 1681–1687. [Google Scholar] [CrossRef]
- Serova, L.V. Ontogenesis of mammals and gravity. J. Gravit. Physiol. 2004, 11, 161–164. [Google Scholar]
- Sajdel-Sulkowska, E.M. Brain development, environment and sex: what can we learn from studying graviperception, gravitransduction and the gravireaction of the developing CNS to altered gravity? Cerebellum 2008, 7, 223–239. [Google Scholar] [CrossRef]
- Serova, L.V. Prisposobitel’nye vozmozhnosti mlekopitaiushchikh v usloviiakh nevesomosti (Adaptive potentials of mammals under conditions of weightlessness). Aviakosm. Ekolog. Med. 1996, 30, 5–11. [Google Scholar]
- Miquel, J.; Souza, K.A. Gravity effects on reproduction, development, and aging. Adv. Space Biol. Med. 1991, 1, 71–97. [Google Scholar] [CrossRef]
- Gualandris-Parisot, L.; Husson, D.; Foulquier, F.; Kan, P.; Davet, J.; Aimar, C.; Dournon, C.; Duprat, A.M. Pleurodeles waltl, amphibian, Urodele, is a suitable biological model for embryological and physiological space experiments on a vertebrate. Adv. Space Res. 2001, 28, 569–578. [Google Scholar] [CrossRef]
- Ijiri, K. A preliminary report on IML-2 medaka experiment: Mating behavior of the fish medaka and development of their eggs in space. Biol. Sci. Space 1994, 8, 231–233. [Google Scholar] [CrossRef]
- Malacinski, G.M.; Neff, A.W. The influence of gravity on the process of development of animal systems. Adv. Space Res. 1984, 4, 315–323. [Google Scholar] [CrossRef]
- Rahmann, H.; Slenzka, K. Influence of gravity on early development of lower aquatic vertebrates. In Life Sciences Research in Space, Proceedings of the Fifth European Symposium, Arcachon, France, 26 September–1 October 1993; Oster, H., Guyenne, T.D., Eds.; European Space Agency: Paris, France, 1994; pp. 147–152. [Google Scholar]
- Orban, J.I.; Piert, S.J.; Guryeva, T.S.; Hester, P.Y. Calcium utilization by quail embryos during activities preceding space flight and during embryogenesis in microgravity aboard the orbital space station MIR. J. Gravit. Physiol. 1999, 6, 33–41. [Google Scholar]
- Ronca, A.E. Mammalian development in space. In Developmental Biology Research in Space; Marthy, H.J., Ed.; Elsevier Science BV: Amsterdam, The Netherlands, 2003; pp. 217–251. [Google Scholar]
- Gazenko, O.G. Ontogenesis of Mammals in Microgravity. In National Aeronautics and Space Administration, NASA Technical Memorandums; Gazenko, O.G., Ed.; NASA: Washington, DC, USA, 1993; p. 184. Available online: https://ntrs.nasa.gov/citations/19940013163 (accessed on 27 December 2020).
- Ijiri, K. Fish mating experiment in space—What it aimed at and how it was prepared. Biol. Sci. Space 1995, 9, 3–16. [Google Scholar] [CrossRef]
- Ijiri, K. Life-cycle experiments of medaka fish aboard the international space station. Adv. Space Biol. Med. 2003, 9, 201–216. [Google Scholar] [CrossRef]
- Kaplansky, A.S.; Savina, E.A.; Portugalov, V.V.; Ilyina-Kakueva, E.I.; Alexeyev, E.I.; Durnova, G.N.; Pankova, A.S.; Plakhuta-Plakutina, G.I.; Shvets, V.N.; Yakovleva, V.I. Results of morphological investigations aboard biosatellites Cosmos. Physiologist 1980, 23, S51–54. [Google Scholar]
- Vinnikov, Y.A.; Gazenko, O.G.; Lychakov, D.V.; Palmbakh, L.R. Formation of the vestibular apparatus in weightlessness. In Development of Auditory and Vestibular Systems; Romand, R., Ed.; Academic Press: New York, NY, USA, 1983; pp. 537–560. [Google Scholar]
- Neubert, J.; Schatz, A.; Bromeis, B.; Briegleb, W. The reaction of Xenopus laevis Daudin (South African toad) to linear accelerations. Adv. Space Res. 1994, 14, 299–303. [Google Scholar] [CrossRef]
- Snetkova, E.; Chelnaya, N.; Serova, L.; Saveliev, S.; Cherdanzova, E.; Pronych, S.; Wassersug, R. Effects of space flight on Xenopus laevis larval development. J. Exp. Zool. 1995, 273, 21–32. [Google Scholar] [CrossRef]
- Ogneva, I.V. Early development under microgravity conditions. Biophysics 2015, 60, 849–858. [Google Scholar] [CrossRef]
- Young, R.S.; Tremor, J.W. The effect of weightlessness on the dividing egg of Rana pipiens. Bioscience 1968, 18, 609–615. [Google Scholar] [CrossRef]
- Souza, K.; Black, S.; Wassersug, R.G.; Ross, M.D. The effects of spaceflight on amphibian fertilization, development and behavior. In Proceedings of the “In Space ’94” Japan Space Utilization Promotion Center, Tokyo, Japan, January 1994; pp. 113–136. [Google Scholar]
- Souza, K.A.; Black, S.D.; Wassersug, R.J. Amphibian development in the virtual absence of gravity. Proc. Natl. Acad. Sci. USA 1995, 92, 1975–1978. [Google Scholar] [CrossRef] [PubMed]
- Savel’ev, S.V.; Barabanov, V.M.; Besova, N.V.; Gulimova, V.I.; Makarov, A.N.; Proshchina, A.E. Vliianie nevesomosti na razvitie nervnoĭ sistemy i perifericheskikh analizatorov u lichinok Xenopus laevis (The effect of weightlessness on development of the nervous system and peripheral analyzers in Xenopus laevis tadpoles). Bull. Eksp. Biol. Med. 1995, 119, 650–653. [Google Scholar]
- Horn, E.; Böser, S.; Membre, H.; Dournon, C.; Husson, D.; Gualandris-Parisot, L. Morphometric investigations of sensory vestibular structures in tadpoles (Xenopus laevis) after a spaceflight: Implications for microgravity-induced alterations of the vestibuloocular reflex. Protoplasma 2006, 229, 193–203. [Google Scholar] [CrossRef]
- Dournon, C.; Durand, D.; Tankosic, C.; Membre, H.; Gualandris-Parisot, L.; Bautz, A. Effects of microgravity on the larval development, metamorphosis and reproduction of the urodele amphibian Pleurodeles Waltl. Dev. Growth Differ. 2001, 43, 315–326. [Google Scholar] [CrossRef]
- Aimar, C.; Bautz, A.; Durand, D.; Membre, H.; Chardard, D.; Gualandris-Parisot, L.; Husson, D.; Dournon, C. Microgravity and hypergravity effects on fertilization of the salamander Pleurodeles waltl (urodele amphibian). Biol. Reprod. 2000, 63, 551–558. [Google Scholar] [CrossRef]
- Mogami, Y.; Imamizo, M.; Yamashita, M.; Izumi-Kurotani, A.; Wiederhold, M.L.; Koike, H.; Asashima, M. AstroNewt: early development of newt in space. Adv. Space Res. 1996, 17, 257–263. [Google Scholar] [CrossRef]
- Yamashita, M.; Izumi-Kurotani, A.; Imamizo, M.; Koike, H.; Okuno, M.; Pfeiffer, C.J.; Komazaki, S.; Sasaki, F.; Ohira, Y.; Kashima, I.; et al. Japanese red-bellied newts in Space—AstroNewt experiment on Space Shuttle IML-2 and Space Flyer Unit. Biol. Sci. Space 2001, 15, S96–103. [Google Scholar] [CrossRef][Green Version]
- Husson, D.; Gualandris-Parisot, L.; Foulquier, F.; Grinfield, S.; Kan, P.; Duprat, A.M. Differentiation in microgravity of neural and muscle cells of a vertebrate (amphibian). Adv. Space Res. 1998, 22, 303–308. [Google Scholar] [CrossRef]
- Duprat, A.M.; Husson, D.; Gualandris-Parisot, L. Does gravity influence the early stages of the development of the nervous system in an amphibian? Brain Res. Brain Res. Rev. 1998, 28, 19–24. [Google Scholar] [CrossRef]
- Gulimova, V.I.; Nikitin, V.B.; Asadchikov, V.E.; Buzmakov, A.V.; Okshtein, I.L.; Almeida, E.A.C.; Ilyin, E.A.; Tairbekov, M.G.; Saveliev, S.V. Effect of 16-day spaceflight on the morphology of thick-toed geckos (Pachydactylus turnery Gray, 1846). J. Gravit. Physiol. 2006, 13, 197–200. [Google Scholar]
- Gulimova, V.I.; Berdiev, R.C.; Barabanov, V.M.; Saveliev, S.V. The first experience of behavior investigation of Ornate day geckos (Phelsuma ornata) in a long orbital experiment. In Proceedings of the ESA/ISGP/CNES Joint Life Sciences Meeting ‘Life in Space for Life on Earth’, Toulouse, France, 5–10 June 2016. [Google Scholar]
- Takaoki, M. Model animals for space experiments—species flown in the past and candidate animals for the future experiments. Biol. Sci. Space 2007, 21, 76–83. [Google Scholar] [CrossRef]
- Guryeva, T.S.; Dadasheva, O.A.; Meleshko, G.I.; Shepelev, Y.Y.; Bod’a, K.; Sabo, V. The quail embryonic development under the conditions of weightlessness. Acta Vet. Brno 1993, 62, S25–30. [Google Scholar] [CrossRef]
- Komissarova, D.V.; Gur’eva, T.S. Rozhdennie v kosmose. K 40-letiju pervogo eksperimenta po inkubirovaniju iaponskogo perepela v kosmose (Born in space. 40 years from the first experiment in incubating Japanese quails in space). In Proceedings of the 54th Scientific Readings in Memory of K.E. Tsiolkovsky; Kaluga, Russia, 17–19 September 2019.
- Gur’eva, T.S.; Dadasheva, O.A.; Grigorian, E.N.; Sychev, V.N.; Mednikova, E.I.; Lebedeva, Z.N. Osobennosti morfogeneza glaza u émbrionov iaponskogo perepela, razvivshikhsia v usloviiakh nevesomosti (Peculiarities of eye morphogenesis in embryonic Japanese quails developed in microgravity). Aviakosm. Ekolog. Med. 2003, 37, 50–55. [Google Scholar]
- Dadasheva, O.A.; Gurieva, T.S.; Sychev, V.N.; Jehns, G. Osobennosti morfogeneza émbrionov iaponskogo perepela v usloviiakh mikrogravitatsii (Characteristics of morphogenesis of the Japanese quail embryos during microgravity). Aviakosm. Ekolog. Med. 1998, 32, 38–41. [Google Scholar]
- Dadasheva, O.A.; Gur’eva, T.S.; Sychev, V.N.; Mednikova, E.I.; Filatova, A.V.; Komissarova, D.V. Osobennosti razvitiia spinnogo mozga u embrionov i ptentsov iaponskogo perepela v usloviiakh kosmicheskogo poleta (Spinal marrow development in Japanese quail embryos and chicklings in the spaceflight environment). Aviakosm. Ekolog. Med. 2013, 47, 3–6. [Google Scholar]
- Kostal, L.; Jurani, M.; Boda, K.; Shepelev, Y.Y.; Guryev, T.S.; Sabo, V.; Dadasheva, O.A. Behaviour of Japanese quail in microgravity on the ‘MIR’ orbital station. Acta Vet. Brna 1993, 61, 65–67. [Google Scholar]
- Bruce, L.L.; Fritzsch, B. The development of vestibular connections in rat embryos in microgravity. J. Gravit. Physiol. 1997, 4, 59–62. [Google Scholar]
- Gurieva, T.S.; Dadasheva, O.A.; Mednikova, E.I.; Grushina, O.A.; Filatova, A.V.; Sychev, V.N. Razvitie myshechnoi tkani oporno-dvigatel’nogo apparata u ptentsov iaponskogo perepela, razvivshikhsia i prozhivshikh do 5 sutok v usloviiakh nevesomosti (Muscular tissue development of the locomotor apparatus of the Japanese quail chicks hatched and stayed alive 5 days in microgravity). Aviakosm. Ekolog. Med. 2017, 51, 26–29. [Google Scholar]
- Dadasheva, O.A.; Gur’eva, T.S.; Iaglov, V.V. Razvitie shchitovidnoĭ zhelezy u émbrionov iaponskogo perepela, inkubirovannykh v usloviiakh mikrogravitatsii (Thyroid gland development in Japanese quail embryos incubating in microgravity). Aviakosm. Ekolog. Med. 2000, 34, 29–31. [Google Scholar] [PubMed]
- Komissarova, D.V.; Gur’eva, T.S.; Dadasheva, O.A.; Sychev, V.N. Gistogenez kostnoi i khriaschevoi tkani embrionov iaponskogo perepela v usloviiakh nevesomosti na rannikh stadiiakh razvitiia (Early bone and cartilage histogenesis in embryonic Japanese quails in the conditions of microgravity). Aviakosm. Ekolog. Med. 2012, 46, 64–67. [Google Scholar] [PubMed]
- Komissarova, D.V.; Dadasheva, O.A.; Gurieva, T.S.; Sychev, V.N. Gistogenez kostnoi i khriaschevoi tkani embrionov iaponskogo perepela v usloviiakh nevesomosti na zavershajuschikh stadiiakh embrional’nogo razvitiia (Histogenesis of Japanese quail bone and cartilage tissues at the final stages of embryonic development in microgravity) Aviakosm. Ekolog. Med. 2013, 47, 24–28. [Google Scholar]
- Gur’eva, T.S.; Dadasheva, O.A.; Mednikova, E.I.; Dadasheva, M.T.; Sychev, V.N. Gistogenez vnutrennikh organov embrionov iaponskogo perepela, razvivshikhsia v usloviiakh nevesomosti (Histogeny of the visceral organs of embryonic Japanese quails developed in the micro-g environment). Aviakosm. Ekolog. Med. 2009, 43, 8–13. [Google Scholar]
- Komissarova, D.V.; Gur’eva, T.S.; Sychev, V.N. Dinamika ispol’zovaniia kal’ciia dliia postroeniia skeleta embrionov iaponskogo perepela v usloviiakh nevesomosti (Dynamics of calcium utilization for skeleton formation in Japanese quail embryos under the microgravity condition). Aviakosm. Ekolog. Med. 2011, 45, 52–53. [Google Scholar]
- Ruden, D.M.; Bolnick, A.; Awonuga, A.; Abdulhasan, M.; Perez, G.; Puscheck, E.E.; Rappolee, D.A. Effects of gravity, microgravity or microgravity simulation on early mammalian development. Stem Cells Dev. 2018, 27, 1230–1236. [Google Scholar] [CrossRef]
- Serova, L.V.; Denisova, L.A. The effect of weightlessness on the reproductive function of mammals. Physiologist 1982, 25, S9–12. [Google Scholar]
- Keefe, J.R. Vertebrate Development in Space. In NASA Developmental Biology Workshop; Souza, K.A., Halstead, T.W., Eds.; NASA: Washington, DC, USA, 1985; 3543p. [Google Scholar]
- Wakayama, S.; Kamada, Y.; Yamanaka, K.; Kohda, T.; Suzuki, H.; Shimazu, T.; Tada, M.N.; Osada, I.; Nagamatsu, A.; Kamimura, S.; et al. Healthy offspring from freeze-dried mouse spermatozoa held on the International Space Station for 9 months. Proc. Natl. Acad. Sci. USA 2017, 114, 5988–5993. [Google Scholar] [CrossRef]
- Alberts, J.R.; Serova, L.V.; Keefe, J.R.; Apanasenko, Z. Early postnatal development of rats gestated during flight of Cosmos 1514. Physiologist 1985, 28, S81–82. [Google Scholar]
- Ronca, A.E.; Fritzsch, B.; Bruce, L.L.; Alberts, J.R. Orbital spaceflight during pregnancy shapes function of mammalian vestibular system. Behav. Neurosci. 2008, 122, 224–232. [Google Scholar] [CrossRef]
- Olenev, S.N.; Danilov, A.R.; Kryuchkova, T.A.; Sorokina, L.M.; Krasnov, I.B. Effect of weightlessness on some indices of brain development (results of residence of pregnant rats aboard the Cosmos-1514 biosatellite and investigation of subsequent development of their offspring on earth). Neurosci. Behav. Physiol. 1989, 19, 191–195. [Google Scholar] [CrossRef] [PubMed]
- The Russian Federation State Research Center—Institute of Biomedical Problem of the RAS Website. Available online: http://www.imbp.ru/webpages/win1251/Science/Serova_actsp.html (accessed on 27 December 2020).
- Proshchina, A.E.; Besova, N.V.; Voronov, K.A.; Gulimova, V.I.; Serova, L.V.; Saveliev, S.V. Morphogenesis of asymmetry of rat brain nuclei under normal conditions and during exposure to microgravitation. Bull. Exp. Biol. Med. 2000, 130, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.V.; Natochkin, I.V.; Nosovskii, A.M.; Shakhmatova, E.I.; Fast, T. Vliianie nevesomosti na sistemu mat’-plod (rezul’taty embriologicheskogo eksperimenta NIH-R1 na korable “Shattl” (Effect of weightlessness on the mother-fetus system (results of embryological experiment NIH-R1 abroad the “Space Shuttle”). Aviakosm. Ekolog. Med. 1996, 30, 4–8. [Google Scholar] [PubMed]
- The NASA Life Sciences Data Archive Website. Available online: https://lsda.jsc.nasa.gov/Experiment/exper/672 (accessed on 27 December 2020).
- Savel’ev, S.V.; Serova, L.V.; Besova, N.V.; Nosovskiĭ, A.M. Vliianie nevesomosti na razvitie neĭroéndokrinnoĭ sistemy u krys (Effect of weightlessness on rats endocrine system development). Aviakosm. Ekolog. Med. 1998, 32, 31–36. [Google Scholar]
- Murakami, D.M.; Hoban-Higgins, T.M.; Tang, I.H.; Fuller, C.A. The effect of spaceflight on retino-hypothalamic tract development. J. Gravit. Physiol. 1997, 4, P67–70. [Google Scholar]
- Mani-Ponset, L.; Masseguin, C.; Davet, J.; Herbuté, S.; Maurel, D.; Ghandour, M.S.; Reiss-Bubenheim, D.; Güell, A.; Gabrion, J. Effects of an 11-day spaceflight on the choroid plexus of developing rats. Brain Res. Dev. Brain Res. 1997, 99, 187–200. [Google Scholar] [CrossRef]
- Maese, A.; Ostrach, L. Neurolab: Final Report for the Ames Research Center Payload (TM-2002-211841); NASA: Moffet Field, CA, USA, 2002; 174p. Available online: http://ntrs.nasa.gov/archive/nasa/casi.ntrs.nasa. gov/20020073543.pdf (accessed on 27 December 2020).
- Harding, S.M.; Singh, N.J.; Walton, K.D. A Sensitive period for the development of motor function in rats: A microgravity study. Gravit. Space Res. 2020, 5, 5–57. [Google Scholar] [CrossRef]
- Raymond, J.; Demêmes, D.; Blanc, E.; Dechesne, C.J. Development of the vestibular system in microgravity. In The Neurolab Spacelab Mission: Neuroscience Research in Space; Buckey, J.C., Homick, J.L., Eds.; NASA: Houston, TX, USA, 2003; pp. 143–149. [Google Scholar]
- DeFelipe, J.; Arellano, J.I.; Merchán-Pérez, A.; González-Albo, M.C.; Walton, K.; Llinás, R. Spaceflight induces changes in the synaptic circuitry of the postnatal developing neocortex. Cereb. Cortex. 2002, 12, 883–891. [Google Scholar] [CrossRef]
- Inglis, F.M.; Zuckerman, K.E.; Kalb, R.G. Experience-dependent development of spinal motor neurons. Neuron 2000, 26, 299–305. [Google Scholar] [CrossRef]
- Walton, K.D.; Benavides, L.; Singh, N.; Hatoum, N. Long-term effects of microgravity on the swimming behaviour of young rats. J. Physiol. 2005, 565, 609–626. [Google Scholar] [CrossRef]
- Walton, K.D.; Harding, S.; Anschel, D.; Harris, Y.T.; Llinás, R. The effects of microgravity on the development of surface righting in rats. J. Physiol. 2005, 565, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, R.S.; Hayes, N.L. The effect of weightlessness on the developing nervous system. In The Neurolab Spacelab Mission: Neuroscience Research in Space; Buckey, J.C., Homick, J.L., Eds.; NASA: Houston, TX, USA, 2003; 169p. [Google Scholar]
- The NASA Life Sciences Data Archive Website. Available online: https://lsda.jsc.nasa.gov/Experiment/exper/742 (accessed on 27 December 2020).
- Ronca, A.E. Altered gravity effects on mothers and offspring: the importance of maternal behavior. J. Gravit. Physiol. 2001, 8, P133–136. [Google Scholar] [PubMed]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of sex and gender on adaptations to space: reproductive health. J. Womens Health 2014, 23, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.T.; Baker, E.S. Gynecological and reproductive issues for women in space: A review. Obstet. Gynecol. Surv. 2000, 55, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Schuster, H.; Peck, S.L. Mars ain’t the kind of place to raise your kid: ethical implications of pregnancy on missions to colonize other planets. Life Sci. Soc. Policy 2016, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Rahmann, H.; Anken, R.H. Gravitational neurobiology of fish. Adv. Space Res. 2000, 25, 1985–1995. [Google Scholar] [CrossRef]
- Gulimova, V.I.; Barabanov, V.M.; Berdiev, R.K.; Proschina, A.E.; Kharlamova, A.S.; Saveliev, S.V. Vidovye razlichiia v sposobnostiakh gekkonov adaptirovatsia k usloviiam dlitel’nogo orbital’nogo eksperimenta na biosputnikakh “BION-M1” i “FOTON-M4” (Species differences in the ability of geckos to adapt to the conditions of long-term orbital experiment onboard “BION-M1” and “FOTON-M4” biosatellites). Aviakosm. Ekolog. Med. 2016, 50, 55–57. [Google Scholar]
- Gulimova, V.I.; Barabanov, V.M.; Berdiev, R.K.; Okshtein, I.L.; Rakov, D.V.; Landau, I.N.; Saveliev, S.V. Zhizneobespecheniye toslstopalykh gekkonov v planiruemom eksperimente na bortu avtomaticheskogo kosmicheskogo apparata: vybor vozmozhnykh variantov korma (Supporting life of Pachydactylus turneri in a projected experiment aboard robotic spacecraft: choosing feed). Aviakosm. Ekolog. Med. 2012, 46, 14–19. [Google Scholar]
- Wassersug, R. Should the space station be an arc? Space Policy 1994, 10, 199–206. [Google Scholar] [CrossRef]
- Correia, M.J. Neuronal plasticity: adaptation and readaptation to the environment of space. Brain Res. Brain Res. Rev. 1998, 28, 61–65. [Google Scholar] [CrossRef]

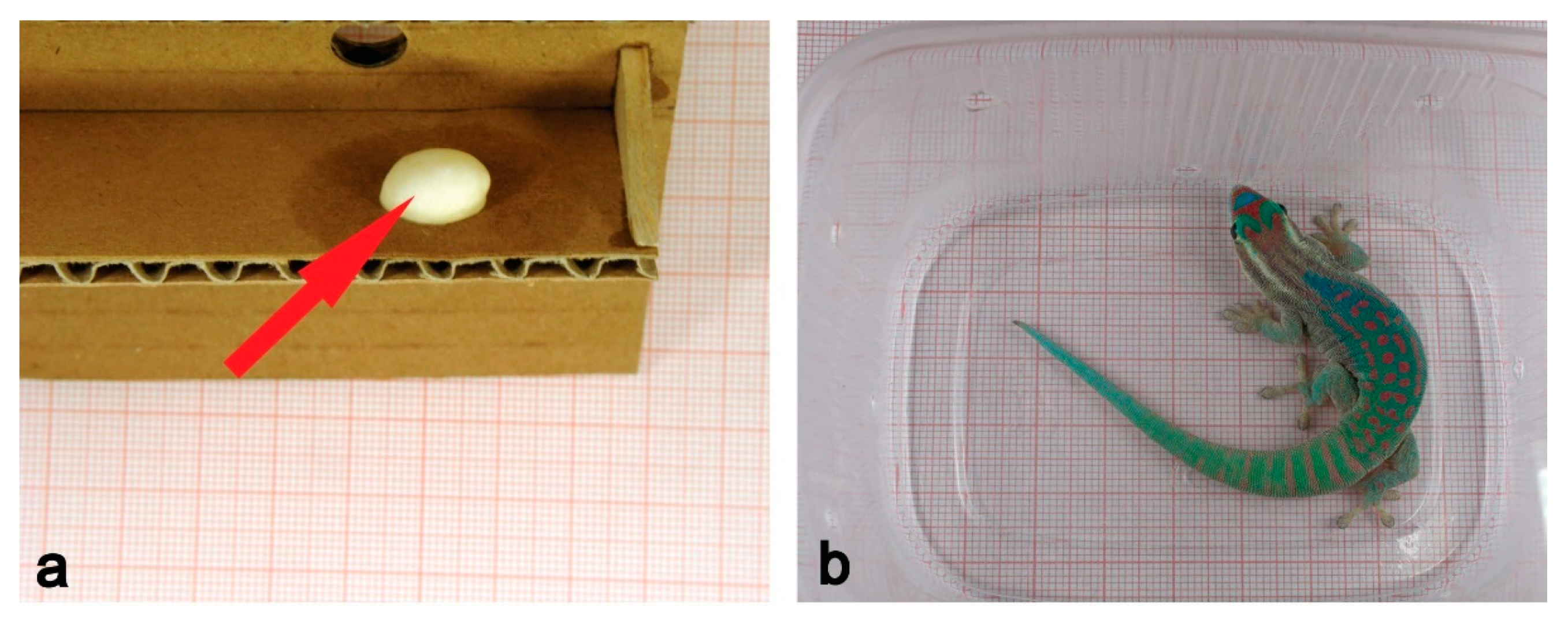
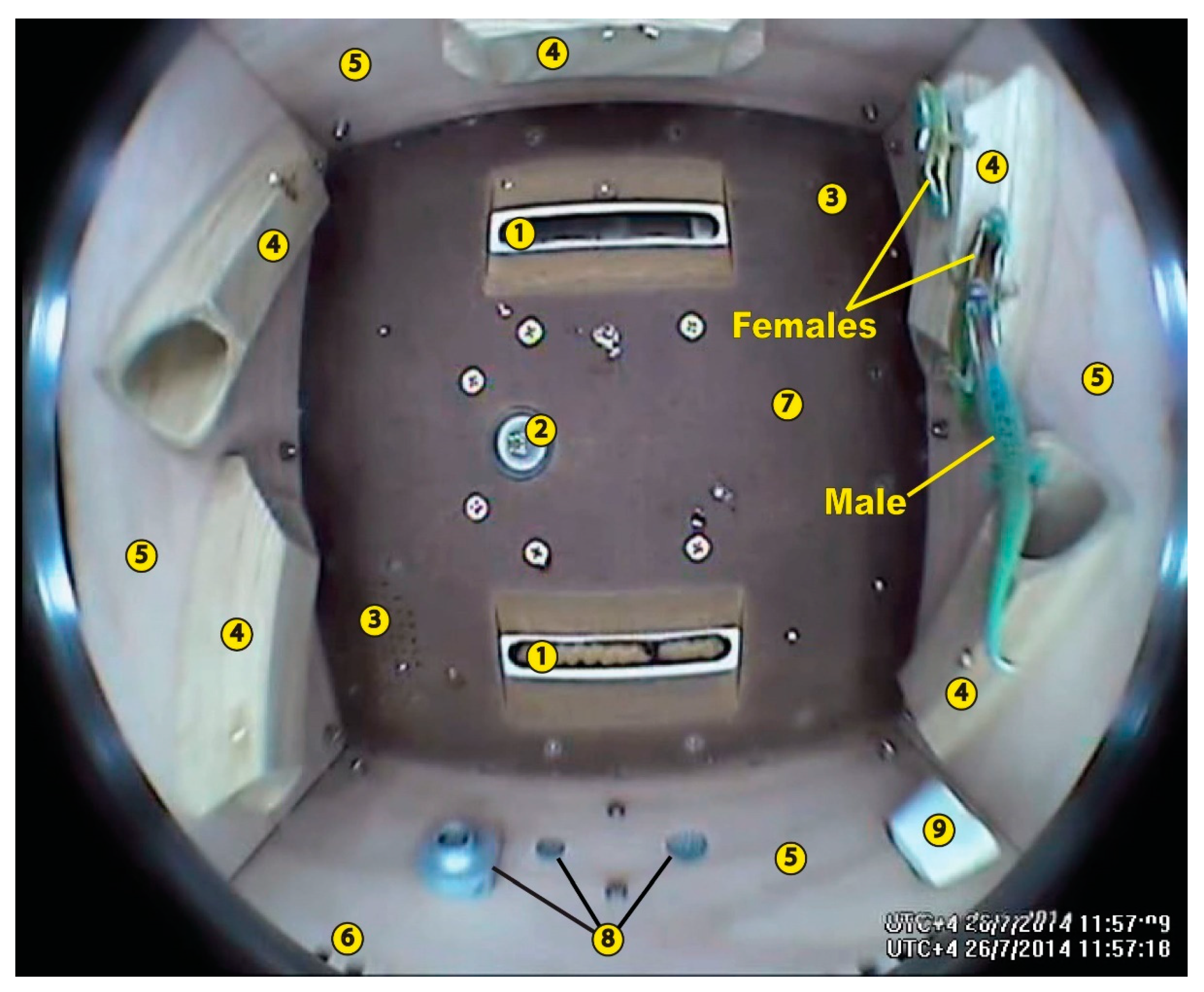
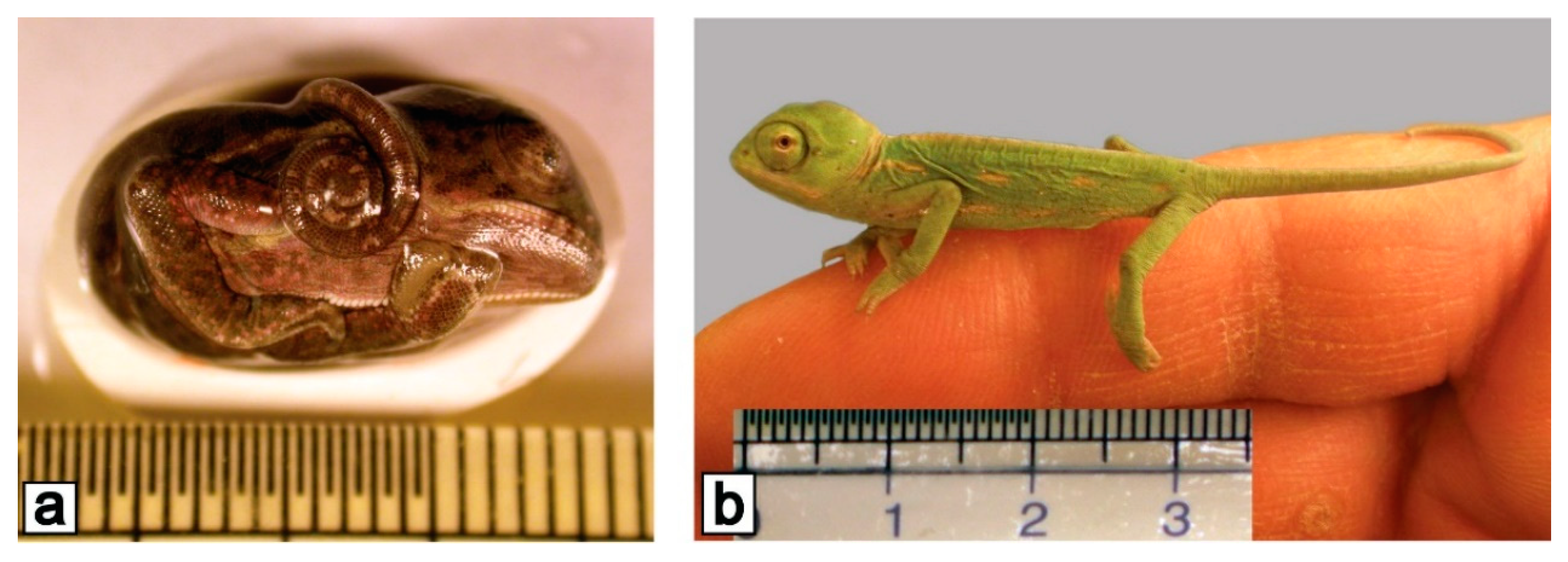

| Year | Species | Mission | Mission Duration | Age of Embryos and Foetuses (Larvae) | |
|---|---|---|---|---|---|
| Launch | Fixation | ||||
| 1966 | American grass (leopard) frog Rana pipiens | Gemini VIII mission | 11 h | two-cell stage | in weightlessness cleavage stages: L + 40 h, L + 130 h, shortly before re-entry |
| 1966 | American grass (leopard) frog Rana pipiens | Gemini XII mission | 3.9 days | two-cell stage | in weightlessness: L + 41 h, L + 85 h, several hours after recovery |
| 1967 | American grass (leopard) frog Rana pipiens | Biosatelllte II | 2.5 days | two-cell stage | in weightlessness: L, L + 1 h, L + 2 h, L + 3 h (early cleavage), L + 32 h, L + 40 h (late gastrula), and L + 68 h (tail bud stage); the last two modules were to return live embryos |
| 1971 | Common frog Rana temporaria | Soyuz 10 | 2 days | Blastula—early gastrula | in weightlessness: early tail bud |
| 1974 | Zebrafish Brachidanio rerio | Soyuz 16 | 6 days | five somites | in weightlessness |
| 1975 | Clawed frog Xenopus laevis | Soyuz 17-Salyut 4 | 16 days | tail bud | in weightlessness |
| 1975 | Killifish Fundulus heteroclitus | Cosmos 782 satellite | 19.5 days | fish eggs | after landing |
| 1977 | Zebrafish Brachidanio rerio | Soyuz 21–Salyut 5 | 9 days | late gastrula | in weightlessness |
| 1976 | Zebrafish Brachidanio rerio | Soyuz 22–Salyut 5 | 8 days | medium gastrula | on second day after landing |
| 1977 | Clawed frog Xenopus laevis | Soyuz 26–Salyut 6 | 20 days | early tail bud | in weightlessness |
| 1979 | Japanese quail Coturnix japonica | Cosmos-1129 satellite | 18.5 days | fertilized eggs | only one of the quail embryos survived |
| 1979 | Brown rat Rattus norvegicus | Cosmos-1129 satellite | 18.5 days | mating in space? | Not applicable |
| 1980 | Clawed frog Xenopus laevis | Soyuz 36–Salyut 6 | 8 days | mid neurula | on second day after landing |
| 1981 | Clawed frog Xenopus laevis | Soyuz 39–Salyut 6 | 9 days | early blastula | on second day after landing SD 45 |
| 1981 | Clawed frog Xenopus laevis | Soyuz 40–Salyut 6 | 8 days | tail bud | on second day after landing SD 46 |
| 1983 | Pregnant brown rat Rattus norvegicus | Cosmos-1514 satellite | 4.5 days | 13 gestational days | after landing: fetuses E18, pups—P0 |
| 1990 | Japanese quail Coturnix japonica | Progress—MIR | 16 days | fertilized eggs | in weightlessness: on days 4,7,10,14,16 of incubation; after landing: 4–5 days after hatching |
| 1992 | Clawed frog Xenopus laevis | STS-47 Spacelab SL-J | 8 days | unfertilized eggs | in weightlessness: two-cell embryos, gastrulae, neurulae and swimming tadpoles |
| 1992 | Japanese quail Coturnix japonica | Soyuz TM-12—MIR | 16 days | fertilized eggs | in weightlessness: on days 3, 7, 10 and 14 of incubation |
| 1992–1993 | Clawed frog Xenopus laevis | BION-10 satellite | 11.5 days | SD 25 | after landing: SD 47 |
| 1993 | Clawed frog Xenopus laevis | STS-55 Spacelab D-2 | 10 days | SD 35 | SD 45 |
| 1994 | Medaka fish Oryzias latipes | STS-65 IML-2 | 15 days | mating and fertilization in space | after landing: hatching stage |
| 1994 | Japanese red-bellied newt Cynops pyrrhogaster | AstroNewt project STS-65–IML-2 | embrios (at SD before the inner ear had formed and at the point just before the otoliths were formed) and fertilization in space | immediately after landing: a) SD-26–36 b) SD 8,9 | |
| 1995 | Japanese red-bellied newt Cynops pyrrhogaster | AstroNewt project unmanned SFU (Space Flyer Unit) | fertilization in space | after nine days from raising temperature | |
| 1994 | Pregnant brown rat Rattus norvegicus | STS-66 NIH.Rodent 1 | 11 days | 9 gestational days | after landing fetuses: E20, pups: P0 |
| 1995 | Pregnant brown rat Rattus norvegicus | STS-70 NIH.Rodent 2 | 9 days | 11 gestational days | after landing fetuses: E20, pups: P0 |
| 1995 | Japanese quail Coturnix japonica | Progress 227–MIR STS-71 INCUBATOR 1 | 16 days | fertilized eggs | in weightlessness on days 7, 10, 14 and 17 of incubation |
| 1995 | Japanese quail Coturnix japonica | STS-71–MIR–STS-74 INCUBATOR 2 | 16 days | fertilized eggs | in weightlessness on days 7, 10, 14 and 16 of incubation |
| 1996 | Rattus norvegicus | STS-72 NIH.Rodent 3 | 9 days | pups: P5, 8, 14 | after landing: P14,17,21 |
| 1996 | Spanish ribbed newt Pleurodeles waltl | Fertile I Neurogenesis Mir Cassiopée expedition | 16 days | fertilization in space | in weightlessness: different stages of the embryonic development. landing: hatching stages SD 31–32 |
| 1996 | Japanese quail Coturnix japonica | STS-76–MIR–STS-79 INCUBATOR 3 | 16 days | fertilized eggs | in weightlessness on days 7, 10, 13 and 16 of incubation |
| 1998 | Rats pups Rattus norvegicus | NEUROLAB (STS-90) | 16 days | pups: P8 and P14 | after landing P24 and P30 |
| 1998 | Pregnant mice Mus musculus | NEUROLAB (STS-90) | 16 days | no data available | |
| 1998 | Spanish ribbed newt Pleurodeles waltl | Fertile II (Mir Pégase expedition) | 21 days | fertilization in space | in weightlessness: different stages of the embryonic development. landing: hatching stages SD 31–32 |
| 1999 | Spanish ribbed newt Pleurodeles waltl | NEUROGENESIS (Mir Perseus expedition) | 21 days | fertilization in space and different stages of the embryonic development (SD—0, 18, 43) | in weightlessness: different stages of the embryonic development; landing: swimming and feeding larvae SD 38, 39, 45 |
| 1999 | Japanese quail Coturnix japonica | “Perepel CK-6” Soyus TM-28 MIR | 13–14 days of incubation | hatching, early postnatal development | |
| 2001 | Clawed frogs Xenopus laevis | French Soyuz taxi flight Andromède mission to the International Space Station | 9.5 days | embryos SD 26–27 tadepoles—45 | after landing: SD—46,47 |
| 2005 | Thick-toed geckos (Chondrodactylus turneri) | Foton-M2 | 16 days | unfertilized eggs, | not applicable |
| 2007 | Thick-toed geckos (Chondrodactylus turneri) | Foton-M3 | 12 days | unfertilized eggs | not applicable |
| 2010 | Thick-toed geckos (Chondrodactylus turneri) | Bion-M1 | 30 days | unfertilized eggs | not applicable |
| 2014 | Ornate day geckos (Phelsuma ornata) | Foton-M4 | 44.5 days instead of the planned 60 | not applicable | not applicable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proshchina, A.; Gulimova, V.; Kharlamova, A.; Krivova, Y.; Besova, N.; Berdiev, R.; Saveliev, S. Reproduction and the Early Development of Vertebrates in Space: Problems, Results, Opportunities. Life 2021, 11, 109. https://doi.org/10.3390/life11020109
Proshchina A, Gulimova V, Kharlamova A, Krivova Y, Besova N, Berdiev R, Saveliev S. Reproduction and the Early Development of Vertebrates in Space: Problems, Results, Opportunities. Life. 2021; 11(2):109. https://doi.org/10.3390/life11020109
Chicago/Turabian StyleProshchina, Alexandra, Victoria Gulimova, Anastasia Kharlamova, Yuliya Krivova, Nadezhda Besova, Rustam Berdiev, and Sergey Saveliev. 2021. "Reproduction and the Early Development of Vertebrates in Space: Problems, Results, Opportunities" Life 11, no. 2: 109. https://doi.org/10.3390/life11020109
APA StyleProshchina, A., Gulimova, V., Kharlamova, A., Krivova, Y., Besova, N., Berdiev, R., & Saveliev, S. (2021). Reproduction and the Early Development of Vertebrates in Space: Problems, Results, Opportunities. Life, 11(2), 109. https://doi.org/10.3390/life11020109






