Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients
Abstract
1. Introduction
2. Biology and Mechanisms of Oxidative Stress
3. Oncologic Treatment Related Cardiotoxicity: The Role of Oxidative Stress
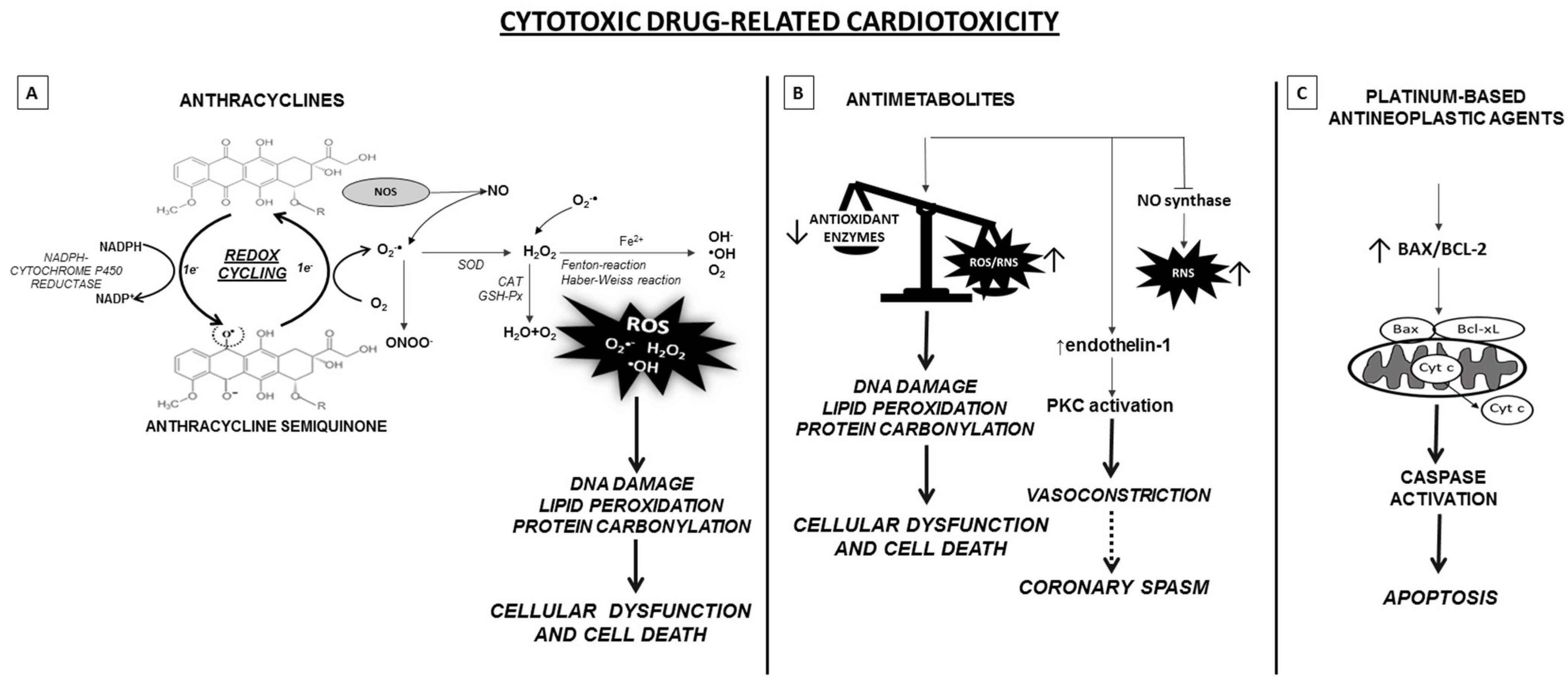
3.1. Anthracyclines
3.2. Taxanes
3.3. Antimetabolites
3.4. Platinum-Based Antineoplastic Agents
3.4.1. Targeted Cancer Therapy
3.4.2. TKIs
3.4.3. mAbs
3.4.4. Immune Checkpoint Inhibitor Based Immunotherapy
4. Molecules to Fight the Onset and Progression of CVD
4.1. Vitamins and Nutraceuticals
4.1.1. Vitamins
4.1.2. Polyphenols
4.1.3. Astaxanthin
4.2. Anti-Oxidative Properties of Common Drugs
4.2.1. NO Donors
4.2.2. Anti-Hypertensives
4.2.3. Anti-Diabetic Agents
4.2.4. Statins
5. Novel Potential Strategy to Revert Antioxidative Stress in CVD
5.1. Mitochondrial-Targeted Antioxidative Based Therapy
5.2. mTOR Signaling Pathway Inhibition
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACE | angiotensin converting enzyme |
| ALL | acute lymphocytic leukemia |
| AMPK | AMP-activated protein kinase |
| AT | angiotensin receptor |
| ATP | adenosine triphosphate |
| BiP | binding immunoglobulin protein |
| CML | chronic myeloid leukemia |
| CTLA-4 | cytotoxic-T-lymphocyte-associated antigen 4 |
| CVD | cardiovascular diseases |
| DDP-4 | Dipeptidyl peptidase-4 |
| DUOX2 | dual oxidase 2 |
| EGFR | epidermal growth factor receptor |
| eIF2α | eukaryotic initiation factor 2α |
| eNOS | endothelial NO synthase |
| ER | endoplasmic reticulum |
| ETC | electron transfer chain |
| FGFR | fibroblast growth factor receptor |
| GIST | gastrointestinal stromal tumor |
| GLP-1 | glucagon-like peptide-1 |
| Grp78 | glucose-regulated protein 78 |
| GSH-Px | glutathione peroxidase |
| HCC | hepatocellular carcinoma |
| hPSC | human pluripotent stem cell |
| H2O2 | hydrogen peroxide |
| ICI | immune checkpoint inhibitors |
| iPS-CMs | induced pluripotent stem cells |
| LV | left ventricular |
| mAbs | monoclonal antibodies |
| MitoQ | mitoquinone |
| mTOR | mammalian target of rapamycin |
| NHL | non-Hodgkin lymphoma |
| NO | nitric oxide |
| NOXs | NADH/NADPH oxidases |
| NSCLC | non-small-cell-lung-cancer |
| ONOO− | peroxynitrite |
| O2− | superoxide |
| PDGFR | platelet-derived growth factor receptor |
| PD-1 | programmed cell death 1 |
| PI3K | phosphatidylinositol 3-kinase |
| RCC | renal cell carcinoma |
| RNS | reactive nitrogen species |
| ROS | radical oxygen species |
| ROS1 | ROS proto-oncogene 1 |
| RTK | receptor tyrosine kinases |
| SGLT2 | sodium-glucose cotransporter 2 |
| SOD | superoxide dismutase |
| STAT1 | signal transducer and activator of transcription 1 |
| TKIs | tyrosine kinase inhibitors |
| VEGFR | vascular endothelial growth factor receptors |
| 5-FU | 5-fluorouracil |
| OH− | hydroxyl radicals |
References
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arnér, E.S. Reactive Oxygen Species, Antioxidants, and the Mammalian Thioredoxin System. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, K. Mitohormesis: Promoting Health and Lifespan by Increased Levels of Reactive Oxygen Species (ROS). Dose Response 2014, 12, 288–341. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic Reticulum Stress and Oxidative Stress in Cell Fate Decision and Human Disease. Antioxid. Redox Signal. 2014, 21, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Simeon, V.; Russomanno, G.; Manzo, V.; Ferrara, N.; Filippelli, A. Aging-Related Changes in Oxidative Stress Response of Human Endothelial Cells. Aging Clin. Exp. Res. 2015, 27, 547–553. [Google Scholar] [CrossRef]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 196–211. [Google Scholar] [CrossRef]
- Ciancarelli, I.; di Massimo, C.; de Amicis, D.; Carolei, A.; Ciancarelli, M.G.T. Evidence of Redox Unbalance in Post-Acute Ischemic Stroke Patients. Curr. Neurovasc. Res. 2012, 9, 85–90. [Google Scholar] [CrossRef]
- Conti, V.; Forte, M.; Corbi, G.; Russomanno, G.; Formisano, L.; Landolfi, A.; Izzo, V.; Filippelli, A.; Vecchione, C.; Carrizzo, A. Sirtuins: Possible Clinical Implications in Cardio and Cerebrovascular Diseases. Curr. Drug Targets 2017, 18, 473–484. [Google Scholar] [CrossRef]
- Brand, M.D. The Sites and Topology of Mitochondrial Superoxide Production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA Mutations, Oxidative Stress, and Apoptosis in Mammalian Aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Otín, C.L.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Komuro, I. Vascular Aging: Insights from Studies on Cellular Senescence, Stem Cell Aging, and Progeroid Syndromes. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Glancy, B.; Balaban, R.S. Role of Mitochondrial Ca2+ in the Regulation of Cellular Energetics. Biochemistry 2012, 51, 2959–2973. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D.; Dorn, G.W. Cytoplasmic Signaling Pathways That Regulate Cardiac Hypertrophy. Annu. Rev. Physiol. 2001, 63, 391–426. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimers Dis. 2014, 42 (Suppl. 3), S125–S152. [Google Scholar] [CrossRef]
- Tublin, J.M.; Adelstein, J.M.; del Monte, F.; Combs, C.K.; Wold, L.E. Getting to the Heart of Alzheimer Disease. Circ. Res. 2019, 124, 142–149. [Google Scholar] [CrossRef]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef]
- Harrison, D.G. Endothelial Function and Oxidant Stress. Clin. Cardiol. 1997, 20, II-17. [Google Scholar] [CrossRef]
- Zhang, P.-Y.; Xu, X.; Li, X.-C. Cardiovascular Diseases: Oxidative Damage and Antioxidant Protection. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3091–3096. [Google Scholar]
- Perez, I.E.; Alam, S.T.; Hernandez, G.A.; Sancassani, R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin. Med. Insights Cardiol. 2019, 13, 1179546819866445. [Google Scholar] [CrossRef]
- Castaldo, S.A.; Freitas, J.R.; Conchinha, N.V.; Madureira, P.A. The Tumorigenic Roles of the Cellular REDOX Regulatory Systems. Oxid. Med. Cell. Longev. 2016, 2016, 8413032. [Google Scholar] [CrossRef] [PubMed]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-Induced Cardiotoxicity: Overview of the Roles of Oxidative Stress. Oxid. Med. Cell. Longev. 2015, 2015, 795602. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Eldridge, S. Spontaneously Occurring Cardiovascular Lesions in Commonly Used Laboratory Animals. Cardiooncology 2019, 5, 6. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhang, X.; Xie, Y.; Chen, R.; Chen, H. C57BL/6 Mice Are More Appropriate than BALB/C Mice in Inducing Dilated Cardiomyopathy with Short-Term Doxorubicin Treatment. Acta Cardiol. Sin. 2012, 28, 236–240. [Google Scholar]
- Qi, W.; Boliang, W.; Xiaoxi, T.; Guoqiang, F.; Jianbo, X.; Gang, W. Cardamonin Protects against Doxorubicin-Induced Cardiotoxicity in Mice by Restraining Oxidative Stress and Inflammation Associated with Nrf2 Signaling. Biomed. Pharmacother. 2020, 122, 109547. [Google Scholar] [CrossRef]
- Chakraborti, S.; Pramanick, A.; Saha, S.; Roy, S.S.; Chaudhuri, A.R.; das, M.; Ghosh, S.; Stewart, A.; Maity, B. Atypical G Protein Β5 Promotes Cardiac Oxidative Stress, Apoptosis, and Fibrotic Remodeling in Response to Multiple Cancer Chemotherapeutics. Cancer Res. 2017, 78, 528–541. [Google Scholar] [CrossRef]
- Cappetta, D.; de Angelis, A.; Sapio, L.; Prezioso, L.; Illiano, M.; Quaini, F.; Rossi, F.; Berrino, L.; Naviglio, S.; Urbanek, K. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid. Med. Cell. Longev. 2017, 2017, 1521020. [Google Scholar] [CrossRef]
- Schwach, V.; Slaats, R.H.; Passier, R. Human Pluripotent Stem Cell-Derived Cardiomyocytes for Assessment of Anticancer Drug-Induced Cardiotoxicity. Front. Cardiovasc. Med. 2020, 7, 50. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Herman, E.H. Anthracycline Cardiotoxicity: The Importance of Horizontally Integrating Pre-Clinical and Clinical Research. Cardiovasc. Res. 2018, 114, 205–209. [Google Scholar] [CrossRef]
- Marinello, J.; Delcuratolo, M.; Capranico, G. Anthracyclines as Topoisomerase II Poisons: From Early Studies to New Perspectives. Int. J. Mol. Sci. 2018, 19, 3480. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, P.A. Anthracycline Cardiotoxicity: An Update on Mechanisms, Monitoring and Prevention. Heart 2018, 104, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Vivar, J.V.; Martasek, P.; Hogg, N.; Masters, B.S.; Pritchard, K.A.; Kalyanaraman, B. Endothelial Nitric Oxide Synthase-Dependent Superoxide Generation from Adriamycin. Biochemistry 1997, 36, 11293–11297. [Google Scholar] [CrossRef] [PubMed]
- Gjyrezi, A.; Xie, F.; Voznesensky, O.; Khanna, P.; Calagua, C.; Bai, Y.; Kung, J.; Wu, J.; Corey, E.; Montgomery, B.; et al. Taxane Resistance in Prostate Cancer Is Mediated by Decreased Drug-Target Engagement. J. Clin. Investig. 2020, 130, 3287–3298. [Google Scholar] [CrossRef]
- Fitzpatrick, J.M.; de Wit, R. Taxane Mechanisms of Action: Potential Implications for Treatment Sequencing in Metastatic Castration-Resistant Prostate Cancer. Eur. Urol. 2014, 65, 1198–1204. [Google Scholar] [CrossRef]
- Ng, R.; Better, N.; Green, M.D. Anticancer Agents and Cardiotoxicity. Semin. Oncol. 2006, 33, 2–14. [Google Scholar] [CrossRef]
- Perotti, A.; Cresta, S.; Grasselli, G.; Capri, G.; Minotti, G.; Gianni, L. Cardiotoxic Effects of Anthracycline-Taxane Combinations. Expert Opin. Drug Saf. 2003, 2, 59–71. [Google Scholar] [CrossRef]
- Putt, M.; Hahn, V.S.; Januzzi, J.L.; Sawaya, H.; Sebag, I.A.; Plana, J.C.; Picard, M.H.; Carver, J.R.; Halpern, E.F.; Kuter, I.; et al. Longitudinal Changes in Multiple Biomarkers Are Associated with Cardiotoxicity in Breast Cancer Patients Treated with Doxorubicin, Taxanes, and Trastuzumab. Clin. Chem. 2015, 61, 1164–1172. [Google Scholar] [CrossRef]
- Bines, J.; Earl, H.; Buzaid, A.C.; Saad, E.D. Anthracyclines and Taxanes in the Neo/Adjuvant Treatment of Breast Cancer: Does the Sequence Matter? Ann. Oncol. 2014, 25, 1079–1085. [Google Scholar] [CrossRef]
- Salvatorelli, E.; Menna, P.; Gianni, L.; Minotti, G. Defective Taxane Stimulation of Epirubicinol Formation in the Human Heart: Insight into the Cardiac Tolerability of Epirubicin-Taxane Chemotherapies. J. Pharmacol. Exp. Ther. 2007, 320, 790–800. [Google Scholar] [CrossRef]
- Deac, A.-L.; Burz, C.C.; Bocşe, H.F.; Bocşan, I.C.; Buzoianu, A.-D. A Review on the Importance of Genotyping and Phenotyping in Fluoropyrimidine Treatment. Med. Pharm. Rep. 2020, 93, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Layoun, M.E.; Wickramasinghe, C.D.; Peralta, M.V.; Yang, E.H. Fluoropyrimidine-Induced Cardiotoxicity: Manifestations, Mechanisms, and Management. Curr. Oncol. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Parekh, H.; Allegra, C.; George, T.J.; Starr, J.S. 5-FU Induced Cardiotoxicity: Case Series and Review of the Literature. Cardiooncology 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Polk, A.; Vistisen, K.; Nilsen, M.V.; Nielsen, D.L. A Systematic Review of the Pathophysiology of 5-Fluorouracil-Induced Cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.; Porto, S.; Marra, M.; Zappavigna, S.; Grimaldi, A.; Feola, D.; Pesce, D.; Naviglio, S.; Spina, A.; Sannolo, N.; et al. 5-Fluorouracil Induces Apoptosis in Rat Cardiocytes through Intracellular Oxidative Stress. J. Exp. Clin. Cancer Res. 2012, 31, 60. [Google Scholar] [CrossRef] [PubMed]
- Millart, H.; Brabant, L.; Lorenzato, M.; Lamiable, D.; Albert, O.; Choisy, H. The Effects of 5-Fluorouracil on Contractility and Oxygen Uptake of the Isolated Perfused Rat Heart. Anticancer Res. 1992, 12, 571–576. [Google Scholar] [PubMed]
- Durak, I.; Karaayvaz, M.; Kavutcu, M.; Cimen, M.Y.; Kaçmaz, M.; Büyükkoçak, S.; Oztürk, H.S. Reduced Antioxidant Defense Capacity in Myocardial Tissue from Guinea Pigs Treated with 5-Fluorouracil. J. Toxicol. Environ. Health A 2000, 59, 585–589. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular Mechanisms of Action and Drug Resistance Development in Cancer Chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, B.; Zhao, B.; Mei, D.; Gu, Q.; Tian, Z. Cisplatin-Induced Cardiotoxicity with Midrange Ejection Fraction: A Case Report and Review of the Literature. Medicine (Baltimore) 2018, 97, e13807. [Google Scholar] [CrossRef]
- Demkow, U.; Emmel, A.S. Cardiotoxicity of Cisplatin-Based Chemotherapy in Advanced Non-Small Cell Lung Cancer Patients. Respir. Physiol. Neurobiol. 2013, 187, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Qian, P.; Yan, L.-J.; Li, Y.-Q.; Yang, H.-T.; Duan, H.-Y.; Wu, J.-T.; Fan, X.-W.; Wang, S.-L. Cyanidin Ameliorates Cisplatin-Induced Cardiotoxicity via Inhibition of ROS-Mediated Apoptosis. Exp. Ther. Med. 2018, 15, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus Carboplatin: Comparative Review of Therapeutic Management in Solid Malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Juan, S.-H.; Chen, J.-J.; Chao, Y.-C.; Chen, H.-H.; Lian, W.-S.; Lu, C.-Y.; Chang, C.-I.; Chiu, T.-H.; Lin, H. Pravastatin Attenuates Carboplatin-Induced Cardiotoxicity via Inhibition of Oxidative Stress Associated Apoptosis. Apoptosis 2008, 13, 883–894. [Google Scholar] [CrossRef] [PubMed]
- He, P.-J.; Ge, R.-F.; Mao, W.-J.; Chung, P.-S.; Ahn, J.-C.; Wu, H.-T. Oxidative Stress Induced by Carboplatin Promotes Apoptosis and Inhibits Migration of HN-3 Cells. Oncol. Lett. 2018, 16, 7131–7138. [Google Scholar] [CrossRef] [PubMed]
- Alcindor, T.; Beauger, N. Oxaliplatin: A Review in the Era of Molecularly Targeted Therapy. Curr. Oncol. 2011, 18, 18–25. [Google Scholar] [CrossRef]
- Polyzos, A.; Tsavaris, N.; Gogas, H.; Souglakos, J.; Vambakas, L.; Vardakas, N.; Polyzos, K.; Tsigris, C.; Mantas, D.; Papachristodoulou, A.; et al. Clinical Features of Hypersensitivity Reactions to Oxaliplatin: A 10-Year Experience. Oncology 2009, 76, 36–41. [Google Scholar] [CrossRef]
- Teppo, H.-R.; Soini, Y.; Karihtala, P. Reactive Oxygen Species-Mediated Mechanisms of Action of Targeted Cancer Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Chen, Y.-X.; Fang, J.-Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Hernández, M.A.R.; de la Cruz-Ojeda, P.; Grueso, M.J.L.; Villarán, E.N.; Aguilar, R.R.; Vega, B.C.; Negrete, M.; Gallego, P.; Ochoa, Á.V.; Victor, V.M. Integrated Molecular Signaling Involving Mitochondrial Dysfunction and Alteration of Cell Metabolism Induced by Tyrosine Kinase Inhibitors in Cancer. Redox Biol. 2020, 36, 101510. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Broekman, F.; Giovannetti, E.; Peters, G.J. Tyrosine Kinase Inhibitors: Multi-Targeted or Single-Targeted? World J. Clin. Oncol. 2011, 2, 80–93. [Google Scholar] [CrossRef]
- Chaar, M.; Kamta, J.; Oudhia, S.A. Mechanisms, Monitoring, and Management of Tyrosine Kinase Inhibitors-Associated Cardiovascular Toxicities. OncoTargets Ther. 2018, 11, 6227–6237. [Google Scholar] [CrossRef]
- Chu, T.F.; Rupnick, M.A.; Kerkela, R.; Dallabrida, S.M.; Zurakowski, D.; Nguyen, L.; Woulfe, K.; Pravda, E.; Cassiola, F.; Desai, J.; et al. Cardiotoxicity Associated with Tyrosine Kinase Inhibitor Sunitinib. Lancet 2007, 370, 2011–2019. [Google Scholar] [CrossRef]
- Narayan, V.; Keefe, S.; Haas, N.; Wang, L.; Puzanov, I.; Putt, M.; Catino, A.; Fang, J.; Agarwal, N.; Hyman, D.; et al. Prospective Evaluation of Sunitinib-Induced Cardiotoxicity in Patients with Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2017, 23, 3601–3609. [Google Scholar] [CrossRef]
- Bouitbir, J.; Alshaikhali, A.; Panajatovic, M.V.; Abegg, V.F.; Paech, F.; Krähenbühl, S. Mitochondrial Oxidative Stress Plays a Critical Role in the Cardiotoxicity of Sunitinib: Running Title: Sunitinib and Oxidative Stress in Hearts. Toxicology 2019, 426, 152281. [Google Scholar] [CrossRef]
- Justice, C.N.; Derbala, M.H.; Baich, T.M.; Kempton, A.N.; Guo, A.S.; Ho, T.H.; Smith, S.A. The Impact of Pazopanib on the Cardiovascular System. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 387–398. [Google Scholar] [CrossRef]
- Singh, A.P.; Umbarkar, P.; Tousif, S.; Lal, H. Cardiotoxicity of the BCR-ABL1 Tyrosine Kinase Inhibitors: Emphasis on Ponatinib. Int. J. Cardiol. 2020, 316, 214–221. [Google Scholar] [CrossRef]
- Paech, F.; Mingard, C.; Grünig, D.; Abegg, V.F.; Bouitbir, J.; Krähenbühl, S. Mechanisms of Mitochondrial Toxicity of the Kinase Inhibitors Ponatinib, Regorafenib and Sorafenib in Human Hepatic HepG2 Cells. Toxicology 2018, 395, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Shao, Y.; Qin, H.-F.; Tai, Y.-H.; Gao, H.-J. ALK-Rearrangement in Non-Small-Cell Lung Cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal Antibodies in Cancer Therapy. Cancer Immun. 2012, 12, 14. [Google Scholar] [PubMed]
- Coulson, A.; Levy, A.; Williams, M.G. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West Indian Med. J. 2014, 63, 650–654. [Google Scholar] [CrossRef]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The Safety and Side Effects of Monoclonal Antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef]
- Genuino, A.J.; Chaikledkaew, U.; The, D.O.; Reungwetwattana, T.; Thakkinstian, A. Adjuvant Trastuzumab Regimen for HER2-Positive Early-Stage Breast Cancer: A Systematic Review and Meta-Analysis. Expert Rev. Clin. Pharmacol. 2019, 12, 815–824. [Google Scholar] [CrossRef]
- Balduzzi, S.; Mantarro, S.; Guarneri, V.; Tagliabue, L.; Pistotti, V.; Moja, L.; D’Amico, R. Trastuzumab-Containing Regimens for Metastatic Breast Cancer. Cochrane Database Syst. Rev. 2014, 2017, CD006242. [Google Scholar] [CrossRef]
- Guarneri, V.; Lenihan, D.J.; Valero, V.; Durand, J.-B.; Broglio, K.; Hess, K.R.; Michaud, L.B.; Angulo, A.M.G.; Hortobagyi, G.N.; Esteva, F.J. Long-Term Cardiac Tolerability of Trastuzumab in Metastatic Breast Cancer: The M.D. Anderson Cancer Center Experience. J. Clin. Oncol. 2006, 24, 4107–4115. [Google Scholar] [CrossRef]
- Mohan, N.; Jiang, J.; Wu, W.J. Implications of Autophagy and Oxidative Stress in Trastuzumab-Mediated Cardiac Toxicities. Austin Pharmacol. Pharm. 2017, 2, 1005. [Google Scholar]
- Kurokawa, Y.K.; Shang, M.R.; Yin, R.T.; George, S.C. Modeling Trastuzumab-Related Cardiotoxicity in Vitro Using Human Stem Cell-Derived Cardiomyocytes. Toxicol. Lett. 2018, 285, 74–80. [Google Scholar] [CrossRef]
- Sendur, M.A.N.; Aksoy, S.; Altundag, K. Pertuzumab-Induced Cardiotoxicity: Safety Compared with Trastuzumab. Future Oncol. 2015, 11, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The History and Advances in Cancer Immunotherapy: Understanding the Characteristics of Tumor-Infiltrating Immune Cells and Their Therapeutic Implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Marone, G.; Criscuolo, G.; Triassi, M.; Bonaduce, D.; Marone, G.; Tocchetti, C.G. Cardiotoxicity of Immune Checkpoint Inhibitors. ESMO Open 2017, 2, e000247. [Google Scholar] [CrossRef] [PubMed]
- Sosnowska, B.; Penson, P.; Banach, M. The Role of Nutraceuticals in the Prevention of Cardiovascular Disease. Cardiovasc. Diagn. Ther. 2017, 7, S21–S31. [Google Scholar] [CrossRef]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The Anti-Ageing Molecule Sirt1 Mediates Beneficial Effects of Cardiac Rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]
- Deruy, E.D.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Grechko, A.V.; Orekhova, V.A.; Chegodaev, Y.S.; Wu, W.-K.; Orekhov, A.N. Oxidative Stress and Antioxidants in Atherosclerosis Development and Treatment. Biology 2020, 9, 60. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative Stress and Cardiovascular Disease: New Insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Tousoulis, D.; Briasoulis, A.; Papageorgiou, N.; Tsioufis, C.; Tsiamis, E.; Toutouzas, K.; Stefanadis, C. Oxidative Stress and Endothelial Function: Therapeutic Interventions. Recent Pat. Cardiovasc. Drug Discov. 2011, 6, 103–114. [Google Scholar] [CrossRef]
- Gori, T.; Münzel, T. Oxidative Stress and Endothelial Dysfunction: Therapeutic Implications. Ann. Med. 2011, 43, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; Clarke, Z.L.; Madhavan, A.K.; Lewis, M.J.; Lang, D. Folic Acid Reverses Endothelial Dysfunction Induced by Inhibition of Tetrahydrobiopterin Biosynthesis. Eur. J. Pharmacol. 2006, 530, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, M.E.; Verma, S.; Rosenfeld, R.J.; Anderson, T.J.; Parsons, H.G. Interaction of 5-Methyltetrahydrofolate and Tetrahydrobiopterin on Endothelial Function. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2167–H2172. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.E.; Kenney, W.L. Role of Folic Acid in Nitric Oxide Bioavailability and Vascular Endothelial Function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hagar, H.H. FOLIC ACID AND VITAMIN B12 SUPPLEMENTATION ATTENUATES ISOPRENALINE-INDUCED MYOCARDIAL INFARCTION IN EXPERIMENTAL HYPERHOMOCYSTEINEMIC RATS. Pharmacol. Res. 2002, 46, 213–219. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Nameni, G.; Hajiluian, G.; Mesgari-Abbasi, M. Cardiac Tissue Oxidative Stress and Inflammation after Vitamin D Administrations in High Fat- Diet Induced Obese Rats. BMC Cardiovasc. Disord. 2017, 17, 161. [Google Scholar] [CrossRef]
- Contreras-Duarte, S.; Chen, P.; Andía, M.; Uribe, S.; Irarrázaval, P.; Kopp, S.; Kern, S.; Marsche, G.; Busso, D.; Wadsack, C.; et al. Attenuation of Atherogenic Apo B-48-Dependent Hyperlipidemia and High Density Lipoprotein Remodeling Induced by Vitamin C and E Combination and Their Beneficial Effect on Lethal Ischemic Heart Disease in Mice. Biol. Res. 2018, 51, 34. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol Preserves Cardiac Function by Reducing Oxidative Stress and Inflammation in Ischemia/Reperfusion Injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Wang, X.; Dong, W.; Yuan, B.; Yang, Y.; Yang, D.; Lin, X.; Chen, C.; Zhang, W. Vitamin E Confers Cytoprotective Effects on Cardiomyocytes under Conditions of Heat Stress by Increasing the Expression of Metallothionein. Int. J. Mol. Med. 2016, 37, 1429–1436. [Google Scholar] [CrossRef]
- Yao, E.-H.; Fukuda, N.; Matsumoto, T.; Kobayashi, N.; Katakawa, M.; Yamamoto, C.; Tsunemi, A.; Suzuki, R.; Ueno, T.; Matsumoto, K. Losartan Improves the Impaired Function of Endothelial Progenitor Cells in Hypertension via an Antioxidant Effect. Hypertens. Res. 2007, 30, 1119–1128. [Google Scholar] [CrossRef]
- Al Khalaf, M.M.; Thalib, L.; Doi, S.A.R. Cardiovascular Outcomes in High-Risk Patients without Heart Failure Treated with ARBs: A Systematic Review and Meta-Analysis. Am. J. Cardiovasc. Drugs 2009, 9, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Saku, K. Recent Progress in the Treatment of Cardiovascular Disease Using Olmesartan. Clin. Exp. Hypertens 2014, 36, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and Anti-inflammatory Mechanisms of Action of Astaxanthin in Cardiovascular Diseases (Review). Int. J. Mol. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, Oxidative Stress, Inflammation and Cardiovascular Disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef]
- Iwamoto, T.; Hosoda, K.; Hirano, R.; Kurata, H.; Matsumoto, A.; Miki, W.; Kamiyama, M.; Itakura, H.; Yamamoto, S.; Kondo, K. Inhibition of Low-Density Lipoprotein Oxidation by Astaxanthin. J. Atheroscler. Thromb. 2000, 7, 216–222. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Mashhadi, N.S.; Zakerkish, M.; Mohammadiasl, J.; Zarei, M.; Mohammadshahi, M.; Haghighizadeh, M.H. Astaxanthin Improves Glucose Metabolism and Reduces Blood Pressure in Patients with Type 2 Diabetes Mellitus. Asia Pac. J. Clin. Nutr. 2018, 27, 341–346. [Google Scholar] [CrossRef]
- Mombouli, J.V.; Vanhoutte, P.M. Kinins and the Vascular Actions of Converting Enzyme Inhibitors. Curr. Opin. Nephrol. Hypertens 1994, 3, 481–484. [Google Scholar]
- Wirth, K.J.; Linz, W.; Wiemer, G.; Schölkens, B.A. Kinins and Cardioprotection. Pharmacol. Res. 1997, 35, 527–530. [Google Scholar] [CrossRef]
- Tschöpe, C.; Gohlke, P.; Zhu, Y.Z.; Linz, W.; Schölkens, B.; Unger, T. Antihypertensive and Cardioprotective Effects after Angiotensin-Converting Enzyme Inhibition: Role of Kinins. J. Card. Fail. 1997, 3, 133–148. [Google Scholar] [CrossRef]
- Honjo, T.; Yamaoka-Tojo, M.; Inoue, N. Pleiotropic Effects of ARB in Vascular Metabolism—Focusing on Atherosclerosis-Based Cardiovascular Disease. Curr. Vasc. Pharmacol. 2011, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Fukuda, N.; Yao, E.-H.; Matsumoto, T.; Kobayashi, N.; Suzuki, R.; Tahira, Y.; Ueno, T.; Matsumoto, K. Effects of an ARB on Endothelial Progenitor Cell Function and Cardiovascular Oxidation in Hypertension. Am. J. Hypertens. 2008, 21, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Fukuda, N.; Katakawa, M.; Tsunemi, A.; Tahira, Y.; Matsumoto, T.; Ueno, T.; Soma, M. Effects of an Angiotensin II Receptor Blocker on the Impaired Function of Endothelial Progenitor Cells in Patients with Essential Hypertension. Am. J. Hypertens. 2014, 27, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Chrysant, S.G. Angiotensin II Receptor Blockers in the Treatment of the Cardiovascular Disease Continuum. Clin. Ther. 2008, 30 Pt 2, 2181–2190. [Google Scholar] [CrossRef]
- Sabbah, Z.A.; Mansoor, A.; Kaul, U. Angiotensin Receptor Blockers—Advantages of the New Sartans. J. Assoc. Physicians India 2013, 61, 464–470. [Google Scholar] [PubMed]
- Akhrass, P.R.; McFarlane, S.I. Telmisartan and Cardioprotection. Vasc. Health Risk Manag. 2011, 7, 677–683. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Zhang, F.; Liu, Y.; Yin, S.; Pang, X.; Li, Z.; Wei, Z. Nebivolol Alleviates Aortic Remodeling through ENOS Upregulation and Inhibition of Oxidative Stress in L-NAME-Induced Hypertensive Rats. Clin. Exp. Hypertens. 2017, 39, 628–639. [Google Scholar] [CrossRef]
- Gums, J.G. Use of ACE Inhibitors in the Treatment of Cardiovascular Disease. Am. Pharm. 1992, NS32, 62–70. [Google Scholar] [CrossRef]
- Borghi, C.; Cosentino, E.; De Sanctis, D. Angiotensin-converting enzyme inhibition and cardiovascular prevention: More than twenty years of clinical success. Ital. Heart J. Suppl. 2005, 6, 769–779. [Google Scholar]
- Song, J.C.; White, C.M. Clinical Pharmacokinetics and Selective Pharmacodynamics of New Angiotensin Converting Enzyme Inhibitors: An Update. Clin. Pharm. 2002, 41, 207–224. [Google Scholar] [CrossRef]
- Hoyer, J.; Schulte, K.L.; Lenz, T. Clinical Pharmacokinetics of Angiotensin Converting Enzyme (ACE) Inhibitors in Renal Failure. Clin. Pharm. 1993, 24, 230–254. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, C.; Munger, M.A. Effect of Angiotensin-Converting Enzyme Inhibitors on Ventricular Remodeling and Survival Following Myocardial Infarction. Ann. Pharm. 1993, 27, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Tummala, P.E.; Chen, X.L.; Sundell, C.L.; Laursen, J.B.; Hammes, C.P.; Alexander, R.W.; Harrison, D.G.; Medford, R.M. Angiotensin II Induces Vascular Cell Adhesion Molecule-1 Expression in Rat Vasculature: A Potential Link between the Renin-Angiotensin System and Atherosclerosis. Circulation 1999, 100, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.F.; Michel, J.B. Angiotensin II Stimulates Endothelial Vascular Cell Adhesion Molecule-1 via Nuclear Factor-KappaB Activation Induced by Intracellular Oxidative Stress. Arter. Thromb. Vasc. Biol. 2000, 20, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Tummala, P.E.; Olbrych, M.T.; Alexander, R.W.; Medford, R.M. Angiotensin II Induces Monocyte Chemoattractant Protein-1 Gene Expression in Rat Vascular Smooth Muscle Cells. Circ. Res. 1998, 83, 952–959. [Google Scholar] [CrossRef]
- Touyz, R.M.; He, G.; El Mabrouk, M.; Diep, Q.; Mardigyan, V.; Schiffrin, E.L. Differential Activation of Extracellular Signal-Regulated Protein Kinase 1/2 and P38 Mitogen Activated-Protein Kinase by AT1 Receptors in Vascular Smooth Muscle Cells from Wistar-Kyoto Rats and Spontaneously Hypertensive Rats. J. Hypertens. 2001, 19, 553–559. [Google Scholar] [CrossRef]
- Mahajan, A.S.; Babbar, R.; Kansal, N.; Agarwal, S.K.; Ray, P.C. Antihypertensive and Antioxidant Action of Amlodipine and Vitamin C in Patients of Essential Hypertension. J. Clin. Biochem. Nutr. 2007, 40, 141–147. [Google Scholar] [CrossRef]
- Umemoto, S.; Tanaka, M.; Kawahara, S.; Kubo, M.; Umeji, K.; Hashimoto, R.; Matsuzaki, M. Calcium Antagonist Reduces Oxidative Stress by Upregulating Cu/Zn Superoxide Dismutase in Stroke-Prone Spontaneously Hypertensive Rats. Hypertens. Res. 2004, 27, 877–885. [Google Scholar] [CrossRef]
- Kouoh, F.; Gressier, B.; Dine, T.; Luyckx, M.; Brunet, C.; Ballester, L.; Cazin, J.C. Antioxidant Effects and Anti-Elastase Activity of the Calcium Antagonist Nicardipine on Activated Human and Rabbit Neutrophils--a Potential Antiatherosclerotic Property of Calcium Antagonists? Cardiovasc. Drugs Ther. 2002, 16, 515–520. [Google Scholar] [CrossRef]
- Fadini, G.P.; Avogaro, A.; Degli Esposti, L.; Russo, P.; Saragoni, S.; Buda, S.; Rosano, G.; Pecorelli, S.; Pani, L. OsMed Health-DB Network Risk of Hospitalization for Heart Failure in Patients with Type 2 Diabetes Newly Treated with DPP-4 Inhibitors or Other Oral Glucose-Lowering Medications: A Retrospective Registry Study on 127,555 Patients from the Nationwide OsMed Health-DB Database. Eur. Heart J. 2015, 36, 2454–2462. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [PubMed]
- Kröller-Schön, S.; Knorr, M.; Hausding, M.; Oelze, M.; Schuff, A.; Schell, R.; Sudowe, S.; Scholz, A.; Daub, S.; Karbach, S.; et al. Glucose-Independent Improvement of Vascular Dysfunction in Experimental Sepsis by Dipeptidyl-Peptidase 4 Inhibition. Cardiovasc. Res. 2012, 96, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Jurk, K.; Kopp, M.; Kröller-Schön, S.; Mikhed, Y.; Schwierczek, K.; Roohani, S.; Kashani, F.; Oelze, M.; Klein, T.; et al. Glucagon-like Peptide-1 Receptor Signalling Reduces Microvascular Thrombosis, Nitro-Oxidative Stress and Platelet Activation in Endotoxaemic Mice. Br. J. Pharmacol. 2017, 174, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 Inhibitor Empagliflozin Improves the Primary Diabetic Complications in ZDF Rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Mak, I.T.; Weglicki, W.B. Protection by Beta-Blocking Agents against Free Radical-Mediated Sarcolemmal Lipid Peroxidation. Circ. Res. 1988, 63, 262–266. [Google Scholar] [CrossRef]
- Kobayashi, N.; Mita, S.; Yoshida, K.; Honda, T.; Kobayashi, T.; Hara, K.; Nakano, S.; Tsubokou, Y.; Matsuoka, H. Celiprolol Activates ENOS through the PI3K-Akt Pathway and Inhibits VCAM-1 Via NF-KappaB Induced by Oxidative Stress. Hypertension 2003, 42, 1004–1013. [Google Scholar] [CrossRef]
- Lu, T.-M.; Ding, Y.-A.; Leu, H.-B.; Yin, W.-H.; Sheu, W.H.-H.; Chu, K.-M. Effect of Rosuvastatin on Plasma Levels of Asymmetric Dimethylarginine in Patients with Hypercholesterolemia. Am. J. Cardiol. 2004, 94, 157–161. [Google Scholar] [CrossRef]
- Wassmann, S.; Laufs, U.; Müller, K.; Konkol, C.; Ahlbory, K.; Bäumer, A.T.; Linz, W.; Böhm, M.; Nickenig, G. Cellular Antioxidant Effects of Atorvastatin in Vitro and in Vivo. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 300–305. [Google Scholar] [CrossRef]
- Stamler, J.S.; Osborne, J.A.; Jaraki, O.; Rabbani, L.E.; Mullins, M.; Singel, D.; Loscalzo, J. Adverse Vascular Effects of Homocysteine Are Modulated by Endothelium-Derived Relaxing Factor and Related Oxides of Nitrogen. J. Clin. Invest. 1993, 91, 308–318. [Google Scholar] [CrossRef]
- McCully, K.S. Homocystinuria, Arteriosclerosis, Methylmalonic Aciduria, and Methyltransferase Deficiency: A Key Case Revisited. Nutr. Rev. 1992, 50, 7–12. [Google Scholar] [CrossRef]
- Libby, P.; Everett, B.M. Novel Antiatherosclerotic Therapies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative Stress in Human Atherothrombosis: Sources, Markers and Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Loffredo, L.; Carnevale, R.; Pignatelli, P.; Pastori, D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxid. Redox Signal. 2017, 27, 1083–1124. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The Antioxidant Therapy: New Insights in the Treatment of Hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database (Oxford) 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Sheen, J.-M.; Hu, W.L.; Hung, Y.-C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell. Longev. 2017, 2017, 8526438. [Google Scholar] [CrossRef]
- Ramos, S. Cancer Chemoprevention and Chemotherapy: Dietary Polyphenols and Signalling Pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Thomas, R.; Kim, M.H. Epigallocatechin Gallate Inhibits HIF-1alpha Degradation in Prostate Cancer Cells. Biochem. Biophys. Res. Commun. 2005, 334, 543–548. [Google Scholar] [CrossRef]
- Moskaug, J.Ø.; Carlsen, H.; Myhrstad, M.C.W.; Blomhoff, R. Polyphenols and Glutathione Synthesis Regulation. Am. J. Clin. Nutr. 2005, 81, 277S–283S. [Google Scholar] [CrossRef]
- Halliwell, B.; Rafter, J.; Jenner, A. Health Promotion by Flavonoids, Tocopherols, Tocotrienols, and Other Phenols: Direct or Indirect Effects? Antioxidant or Not? Am. J. Clin. Nutr. 2005, 81, 268S–276S. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells: Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Chuang, C.-C.; McIntosh, M.K. Potential Mechanisms by Which Polyphenol-Rich Grapes Prevent Obesity-Mediated Inflammation and Metabolic Diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A Systemic Review on the Antioxidant and Anti-Inflammatory Effects of Resveratrol, Curcumin, and Dietary Nitric Oxide Supplementation on Human Cardiovascular Health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus Astaxanthin: Applications for Human Health and Nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, K.; Watanabe, H. Astaxanthin, a Carotenoid with Potential in Human Health and Nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. Multiple Mechanisms of Anti-Cancer Effects Exerted by Astaxanthin. Mar. Drugs 2015, 13, 4310–4330. [Google Scholar] [CrossRef]
- Choi, H.D.; Youn, Y.K.; Shin, W.G. Positive Effects of Astaxanthin on Lipid Profiles and Oxidative Stress in Overweight Subjects. Plant Foods Hum. Nutr. 2011, 66, 363–369. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Carpentero Burdeos, G.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant Effect of Astaxanthin on Phospholipid Peroxidation in Human Erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef]
- Karppi, J.; Rissanen, T.H.; Nyyssönen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of Astaxanthin Supplementation on Lipid Peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of Natural Astaxanthin Increases Serum HDL-Cholesterol and Adiponectin in Subjects with Mild Hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, R.T.; Cysewski, G.R. Commercial Potential for Haematococcus Microalgae as a Natural Source of Astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Münzel, T.; Sinning, C.; Post, F.; Warnholtz, A.; Schulz, E. Pathophysiology, Diagnosis and Prognostic Implications of Endothelial Dysfunction. Ann. Med. 2008, 40, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Kleschyov, A.L.; Oelze, M.; Daiber, A.; Huang, Y.; Mollnau, H.; Schulz, E.; Sydow, K.; Fichtlscherer, B.; Mülsch, A.; Münzel, T. Does Nitric Oxide Mediate the Vasodilator Activity of Nitroglycerin? Circ. Res. 2003, 93, e104–e112. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G.; Stein, D.; Kottenberg, E.; Schnaith, E.M.; Noack, E. In Vivo Effects of Pentaerythrityl-Tetranitrate and Isosorbide-5-Mononitrate on the Development of Atherosclerosis and Endothelial Dysfunction in Cholesterol-Fed Rabbits. J. Cardiovasc. Pharmacol. 1995, 25, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G.; Noack, E. Effects of Pentaerythrityl-Tetranitrate and Isosorbide-5-Mononitrate in Experimental Atherosclerosis. Agents Actions Suppl. 1995, 45, 201–206. [Google Scholar] [CrossRef]
- Wenzel, P.; Mollnau, H.; Oelze, M.; Schulz, E.; Wickramanayake, J.M.D.; Müller, J.; Schuhmacher, S.; Hortmann, M.; Baldus, S.; Gori, T.; et al. First Evidence for a Crosstalk between Mitochondrial and NADPH Oxidase-Derived Reactive Oxygen Species in Nitroglycerin-Triggered Vascular Dysfunction. Antioxid. Redox Signal. 2008, 10, 1435–1447. [Google Scholar] [CrossRef]
- Gori, T.; Burstein, J.M.; Ahmed, S.; Miner, S.E.; Al-Hesayen, A.; Kelly, S.; Parker, J.D. Folic Acid Prevents Nitroglycerin-Induced Nitric Oxide Synthase Dysfunction and Nitrate Tolerance: A Human in Vivo Study. Circulation 2001, 104, 1119–1123. [Google Scholar] [CrossRef]
- Gori, T.; Mak, S.S.; Kelly, S.; Parker, J.D. Evidence Supporting Abnormalities in Nitric Oxide Synthase Function Induced by Nitroglycerin in Humans. J. Am. Coll. Cardiol. 2001, 38, 1096–1101. [Google Scholar] [CrossRef]
- Münzel, T.; Daiber, A.; Mülsch, A. Explaining the Phenomenon of Nitrate Tolerance. Circ. Res. 2005, 97, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Gori, T.; Parker, J.D. Nitrate-Induced Toxicity and Preconditioning: A Rationale for Reconsidering the Use of These Drugs. J. Am. Coll. Cardiol. 2008, 52, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Warnholtz, A.; Nickenig, G.; Schulz, E.; Macharzina, R.; Bräsen, J.H.; Skatchkov, M.; Heitzer, T.; Stasch, J.P.; Griendling, K.K.; Harrison, D.G.; et al. Increased NADH-Oxidase-Mediated Superoxide Production in the Early Stages of Atherosclerosis: Evidence for Involvement of the Renin-Angiotensin System. Circulation 1999, 99, 2027–2033. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; West, N.E.; Black, E.; McDonald, D.; Ratnatunga, C.; Pillai, R.; Channon, K.M. Vascular Superoxide Production by NAD(P)H Oxidase: Association with Endothelial Dysfunction and Clinical Risk Factors. Circ. Res. 2000, 86, E85–E90. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Ghanim, H.; Brooks, D.P. Antioxidant Activity of Carvedilol in Cardiovascular Disease. J. Hypertens 2007, 25, 731–741. [Google Scholar] [CrossRef]
- Feuerstein, G.Z.; Ruffolo, R.R. Carvedilol, a Novel Vasodilating Beta-Blocker with the Potential for Cardiovascular Organ Protection. Eur. Heart J. 1996, 17, 24–29. [Google Scholar] [CrossRef]
- Nakamura, K.; Murakami, M.; Miura, D.; Yunoki, K.; Enko, K.; Tanaka, M.; Saito, Y.; Nishii, N.; Miyoshi, T.; Yoshida, M.; et al. Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals 2011, 4, 1088–1100. [Google Scholar] [CrossRef]
- Wang, R.; Miura, T.; Harada, N.; Kametani, R.; Shibuya, M.; Fukagawa, Y.; Kawamura, S.; Ikeda, Y.; Hara, M.; Matsuzaki, M. Pleiotropic Effects of the Beta-Adrenoceptor Blocker Carvedilol on Calcium Regulation during Oxidative Stress-Induced Apoptosis in Cardiomyocytes. J. Pharmacol. Exp. Ther. 2006, 318, 45–52. [Google Scholar] [CrossRef]
- Ni, L.; Zhou, C.; Duan, Q.; Lv, J.; Fu, X.; Xia, Y.; Wang, D.W. β-AR Blockers Suppresses ER Stress in Cardiac Hypertrophy and Heart Failure. PLoS ONE 2011, 6, e27294. [Google Scholar] [CrossRef]
- Fratta Pasini, A.; Garbin, U.; Nava, M.C.; Stranieri, C.; Davoli, A.; Sawamura, T.; Lo Cascio, V.; Cominacini, L. Nebivolol Decreases Oxidative Stress in Essential Hypertensive Patients and Increases Nitric Oxide by Reducing Its Oxidative Inactivation. J. Hypertens. 2005, 23, 589–596. [Google Scholar] [CrossRef]
- Zepeda, R.J.; Castillo, R.; Rodrigo, R.; Prieto, J.C.; Aramburu, I.; Brugere, S.; Galdames, K.; Noriega, V.; Miranda, H.F. Effect of Carvedilol and Nebivolol on Oxidative Stress-Related Parameters and Endothelial Function in Patients with Essential Hypertension. Basic. Clin. Pharmacol. Toxicol. 2012, 111, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kukin, M.L.; Kalman, J.; Charney, R.H.; Levy, D.K.; Buchholz-Varley, C.; Ocampo, O.N.; Eng, C. Prospective, Randomized Comparison of Effect of Long-Term Treatment with Metoprolol or Carvedilol on Symptoms, Exercise, Ejection Fraction, and Oxidative Stress in Heart Failure. Circulation 1999, 99, 2645–2651. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.-H.; Fukuda, N.; Matsumoto, T.; Katakawa, M.; Yamamoto, C.; Han, Y.; Ueno, T.; Kobayashi, N.; Matsumoto, K. Effects of the Antioxidative Beta-Blocker Celiprolol on Endothelial Progenitor Cells in Hypertensive Rats. Am. J. Hypertens. 2008, 21, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Lopez, L.M.; Chen, L.; Cox, O.E. Alterations in Nitric Oxide Synthase Activity, Superoxide Anion Generation, and Platelet Aggregation in Systemic Hypertension, and Effects of Celiprolol. Am. J. Cardiol. 1994, 74, 901–905. [Google Scholar] [CrossRef]
- Okrucká, A.; Pechán, J.; Balazovjech, I. The Effect of Short-Term Celiprolol Therapy on Platelet Function in Essential Hypertension. Cardiology 1993, 82, 399–404. [Google Scholar] [CrossRef]
- Kobayashi, N.; Mori, Y.; Nakano, S.; Tsubokou, Y.; Shirataki, H.; Matsuoka, H. Celiprolol Stimulates Endothelial Nitric Oxide Synthase Expression and Improves Myocardial Remodeling in Deoxycorticosterone Acetate-Salt Hypertensive Rats. J. Hypertens. 2001, 19, 795–801. [Google Scholar] [CrossRef]
- Cominacini, L.; Fratta Pasini, A.; Garbin, U.; Nava, C.; Davoli, A.; Criscuoli, M.; Crea, A.; Sawamura, T.; Lo Cascio, V. Nebivolol and Its 4-Keto Derivative Increase Nitric Oxide in Endothelial Cells by Reducing Its Oxidative Inactivation. J. Am. Coll. Cardiol. 2003, 42, 1838–1844. [Google Scholar] [CrossRef]
- Filion, K.B.; Azoulay, L.; Platt, R.W.; Dahl, M.; Dormuth, C.R.; Clemens, K.K.; Hu, N.; Paterson, J.M.; Targownik, L.; Turin, T.C.; et al. A Multicenter Observational Study of Incretin-Based Drugs and Heart Failure. NEJM 2016, 374, 1145–1154. [Google Scholar] [CrossRef]
- Kramer, C.K.; Ye, C.; Campbell, S.; Retnakaran, R. Comparison of New Glucose-Lowering Drugs on Risk of Heart Failure in Type 2 Diabetes: A Network Meta-Analysis. JACC Heart Fail. 2018, 6, 823–830. [Google Scholar] [CrossRef]
- Timmers, L.; Henriques, J.P.S.; de Kleijn, D.P.V.; Devries, J.H.; Kemperman, H.; Steendijk, P.; Verlaan, C.W.J.; Kerver, M.; Piek, J.J.; Doevendans, P.A.; et al. Exenatide Reduces Infarct Size and Improves Cardiac Function in a Porcine Model of Ischemia and Reperfusion Injury. J. Am. Coll. Cardiol. 2009, 53, 501–510. [Google Scholar] [CrossRef]
- Hocher, B.; Sharkovska, Y.; Mark, M.; Klein, T.; Pfab, T. The Novel DPP-4 Inhibitors Linagliptin and BI 14361 Reduce Infarct Size after Myocardial Ischemia/Reperfusion in Rats. Int. J. Cardiol. 2013, 167, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, I.; Efentakis, P.; Balafas, E.; Togliatto, G.; Davos, C.H.; Varela, A.; Dimitriou, C.A.; Nikolaou, P.-E.; Maratou, E.; Lambadiari, V.; et al. Empagliflozin Limits Myocardial Infarction in Vivo and Cell Death in Vitro: Role of STAT3, Mitochondria, and Redox Aspects. Front. Physiol. 2017, 8, 1077. [Google Scholar] [CrossRef] [PubMed]
- Oshima, H.; Miki, T.; Kuno, A.; Mizuno, M.; Sato, T.; Tanno, M.; Yano, T.; Nakata, K.; Kimura, Y.; Abe, K.; et al. Empagliflozin, an SGLT2 Inhibitor, Reduced the Mortality Rate after Acute Myocardial Infarction with Modification of Cardiac Metabolomes and Antioxidants in Diabetic Rats. J. Pharmacol. Exp. Ther. 2019, 368, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A.J. Effects of SGLT2 Inhibitors on Systemic and Tissue Low-Grade Inflammation: The Potential Contribution to Diabetes Complications and Cardiovascular Disease. Diabetes Metab. 2018, 44, 457–464. [Google Scholar] [CrossRef]
- Atkin, S.L.; Katsiki, N.; Banach, M.; Mikhailidis, D.P.; Pirro, M.; Sahebkar, A. Effect of Dipeptidyl Peptidase-4 Inhibitors on Circulating Tumor Necrosis Factor-α Concentrations: A Systematic Review and Meta-Analysis of Controlled Trials. J. Diabetes Complicat. 2017, 31, 1458–1464. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of SGLT2 Selective Inhibitor Ipragliflozin on Hyperglycemia, Hyperlipidemia, Hepatic Steatosis, Oxidative Stress, Inflammation, and Obesity in Type 2 Diabetic Mice. Eur. J. Pharmacol. 2013, 715, 246–255. [Google Scholar] [CrossRef]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of Sodium-Glucose Cotransporter 2 Selective Inhibitor Ipragliflozin on Hyperglycaemia, Oxidative Stress, Inflammation and Liver Injury in Streptozotocin-Induced Type 1 Diabetic Rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef]
- Randomised Trial of Cholesterol Lowering in 4444 Patients with Coronary Heart Disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389.
- Sacks, F.M.; Pfeffer, M.A.; Moye, L.A.; Rouleau, J.L.; Rutherford, J.D.; Cole, T.G.; Brown, L.; Warnica, J.W.; Arnold, J.M.; Wun, C.C.; et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. Cholesterol and Recurrent Events Trial Investigators. NEJM 1996, 335, 1001–1009. [Google Scholar] [CrossRef]
- Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of Cardiovascular Events and Death with Pravastatin in Patients with Coronary Heart Disease and a Broad Range of Initial Cholesterol Levels. NEJM 1998, 339, 1349–1357. [CrossRef] [PubMed]
- West of Scotland Coronary Prevention Study: Implications for Clinical Practice. The WOSCOPS Study Group. Eur. Heart J. 1996, 17, 163–164. [Google Scholar] [CrossRef][Green Version]
- Downs, J.R.; Clearfield, M.; Weis, S.; Whitney, E.; Shapiro, D.R.; Beere, P.A.; Langendorfer, A.; Stein, E.A.; Kruyer, W.; Gotto, A.M. Primary Prevention of Acute Coronary Events with Lovastatin in Men and Women with Average Cholesterol Levels: Results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998, 279, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of Cholesterol Lowering with Simvastatin in 20,536 High-Risk Individuals: A Randomised Placebo-Controlled Trial. Lancet 2002, 360, 7–22. [CrossRef]
- Liao, J.K. Clinical Implications for Statin Pleiotropy. Curr. Opin. Lipidol. 2005, 16, 624–629. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. NEJM 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Clearfield, M.; Downs, J.R.; Weis, S.E.; Miles, J.S.; Gotto, A.M. Air Force/Texas Coronary Atherosclerosis Prevention Study Investigators Measurement of C-Reactive Protein for the Targeting of Statin Therapy in the Primary Prevention of Acute Coronary Events. NEJM 2001, 344, 1959–1965. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Pfeffer, M.A.; Sacks, F.; Braunwald, E. Long-Term Effects of Pravastatin on Plasma Concentration of C-Reactive Protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1999, 100, 230–235. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Sabbah, H.N. Targeting Mitochondrial Dysfunction in the Treatment of Heart Failure. Expert Rev. Cardiovasc. Ther. 2016, 14, 1305–1313. [Google Scholar] [CrossRef]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.-C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic Inhibition of Mitochondrial Reactive Oxygen Species with Mito-TEMPO Reduces Diabetic Cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dikalova, A.E.; Bikineyeva, A.T.; Budzyn, K.; Nazarewicz, R.R.; McCann, L.; Lewis, W.; Harrison, D.G.; Dikalov, S.I. Therapeutic Targeting of Mitochondrial Superoxide in Hypertension. Circ. Res. 2010, 107, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.-M.; Chung, J.; Liu, H.; Go, Y.; Gladstein, S.; Farzaneh-Far, A.; Lewandowski, E.D.; Dudley, S.C. Role of Mitochondrial Oxidative Stress in Glucose Tolerance, Insulin Resistance, and Cardiac Diastolic Dysfunction. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-González, S.; Marín-Royo, G.; Jurado-López, R.; Bartolomé, M.V.; Romero-Miranda, A.; Luaces, M.; Islas, F.; Nieto, M.L.; Martínez-Martínez, E.; Cachofeiro, V. The Crosstalk between Cardiac Lipotoxicity and Mitochondrial Oxidative Stress in the Cardiac Alterations in Diet-Induced Obesity in Rats. Cells 2020, 9, 451. [Google Scholar] [CrossRef]
- Kim, S.; Song, J.; Ernst, P.; Latimer, M.N.; Ha, C.-M.; Goh, K.Y.; Ma, W.; Rajasekaran, N.-S.; Zhang, J.; Liu, X.; et al. MitoQ Regulates Redox-Related Noncoding RNAs to Preserve Mitochondrial Network Integrity in Pressure-Overload Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H682–H695. [Google Scholar] [CrossRef] [PubMed]
- Doughan, A.K.; Dikalov, S.I. Mitochondrial Redox Cycling of Mitoquinone Leads to Superoxide Production and Cellular Apoptosis. Antioxid. Redox Signal. 2007, 9, 1825–1836. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Biel, T.G.; Kryndushkin, D.; Rao, V.A. Therapeutic Targeting of the Mitochondria Initiates Excessive Superoxide Production and Mitochondrial Depolarization Causing Decreased MtDNA Integrity. PLoS ONE 2016, 11, e0168283. [Google Scholar] [CrossRef]
- Gibbons, J.J.; Abraham, R.T.; Yu, K. Mammalian Target of Rapamycin: Discovery of Rapamycin Reveals a Signaling Pathway Important for Normal and Cancer Cell Growth. Semin. Oncol. 2009, 36 (Suppl. 3), S3–S17. [Google Scholar] [CrossRef]
- Yang, X.; Yang, C.; Farberman, A.; Rideout, T.C.; de Lange, C.F.M.; France, J.; Fan, M.Z. The Mammalian Target of Rapamycin-Signaling Pathway in Regulating Metabolism and Growth. J. Anim. Sci. 2008, 86, E36–E50. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Testai, L.; Nabavi, S.F.; Battino, M.; Pandima Devi, K.; Tejada, S.; Sureda, A.; Xu, S.; Yousefi, B.; Majidinia, M.; et al. Therapeutic Potential of Polyphenols in Cardiovascular Diseases: Regulation of MTOR Signaling Pathway. Pharmacol. Res. 2020, 152, 104626. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Frati, G.; Sadoshima, J. New Insights Into the Role of MTOR Signaling in the Cardiovascular System. Circ. Res. 2018, 122, 489–505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, J.; Yang, L. Insights for Oxidative Stress and MTOR Signaling in Myocardial Ischemia/Reperfusion Injury under Diabetes. Oxid. Med. Cell. Longev. 2017, 2017, 6437467. [Google Scholar] [CrossRef]
- Elloso, M.M.; Azrolan, N.; Sehgal, S.N.; Hsu, P.-L.; Phiel, K.L.; Kopec, C.A.; Basso, M.D.; Adelman, S.J. Protective Effect of the Immunosuppressant Sirolimus against Aortic Atherosclerosis in Apo E-Deficient Mice. Am. J. Transplant. 2003, 3, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.; Campistol, J.M.; Sancho, D.; Sánchez-Madrid, F.; Casals, E.; Andrés, V. Rapamycin Attenuates Atherosclerosis Induced by Dietary Cholesterol in Apolipoprotein-Deficient Mice through a P27 Kip1 -Independent Pathway. Atherosclerosis 2004, 172, 31–38. [Google Scholar] [CrossRef]
- Chen, W.Q.; Zhong, L.; Zhang, L.; Ji, X.P.; Zhang, M.; Zhao, Y.X.; Zhang, C.; Zhang, Y. Oral Rapamycin Attenuates Inflammation and Enhances Stability of Atherosclerotic Plaques in Rabbits Independent of Serum Lipid Levels. Br. J. Pharmacol. 2009, 156, 941–951. [Google Scholar] [CrossRef]
- Kurdi, A.; Martinet, W.; De Meyer, G.R.Y. MTOR Inhibition and Cardiovascular Diseases: Dyslipidemia and Atherosclerosis. Transplantation 2018, 102, S44–S46. [Google Scholar] [CrossRef]
- Martinet, W.; De Loof, H.; De Meyer, G.R.Y. MTOR Inhibition: A Promising Strategy for Stabilization of Atherosclerotic Plaques. Atherosclerosis 2014, 233, 601–607. [Google Scholar] [CrossRef]
- Xu, X.; Lu, Z.; Fassett, J.; Zhang, P.; Hu, X.; Liu, X.; Kwak, D.; Li, J.; Zhu, G.; Tao, Y.; et al. Metformin Protects against Systolic Overload-Induced Heart Failure Independent of AMP-Activated Protein Kinase A2. Hypertension 2014, 63, 723–728. [Google Scholar] [CrossRef]
- Liu, M.; Wilk, S.A.; Wang, A.; Zhou, L.; Wang, R.-H.; Ogawa, W.; Deng, C.; Dong, L.Q.; Liu, F. Resveratrol Inhibits MTOR Signaling by Promoting the Interaction between MTOR and DEPTOR. J. Biol. Chem. 2010, 285, 36387–36394. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Blagosklonny, M.V. At Concentrations That Inhibit MTOR, Resveratrol Suppresses Cellular Senescence. Cell Cycle 2009, 8, 1901–1904. [Google Scholar] [CrossRef] [PubMed]
- Brito, P.M.; Devillard, R.; Nègre-Salvayre, A.; Almeida, L.M.; Dinis, T.C.P.; Salvayre, R.; Augé, N. Resveratrol Inhibits the MTOR Mitogenic Signaling Evoked by Oxidized LDL in Smooth Muscle Cells. Atherosclerosis 2009, 205, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Huang, Y.; Zheng, W.; Yan, J.; Cheng, M.; Zhao, R.; Chen, L.; Hu, C.; Jia, W. Resveratrol Reduces Intracellular Reactive Oxygen Species Levels by Inducing Autophagy through the AMPK-MTOR Pathway. Front. Med. 2018, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Long, M.; Li, X.; Zhu, S.; Zhang, M.; Yang, Z. Curcumin Activates Autophagy and Attenuates Oxidative Damage in EA.Hy926 Cells via the Akt/MTOR Pathway. Mol. Med. Rep. 2016, 13, 2187–2193. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.-B.; Yang, J.; Wang, J.-R.; Liu, J.-X.; Li, C.-L. Curcumin Alleviates Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis through Inhibition of Autophagy and Activation of MTOR. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7500–7508. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]

| Nutraceutical/Class of Drug | Main Mechanism of Action | Additional Antioxidative Effects | References |
|---|---|---|---|
| Vitamins and nutraceuticals | |||
| B6 | Metabolic Coenzyme in several cellular processes | Reduction of homocysteine levels | [86] |
| Acid folic (B9) | Metabolic Coenzyme in several cellular processes | (i) Prevention of NOS uncoupling and restoration of endothelial dysfunction (ii) Reduction of homocysteine levels (iii) Heart rate and blood pressure restoration | [86,91,92,93,94,95] |
| B12 | Metabolic Coenzyme in several cellular processes | (i) Reduction of homocysteine levels (ii) Heart rate and blood pressure restoration | [86,91,92,93,94,95] |
| C | Antioxidative | Prevention of NOS uncoupling and restoration of endothelial dysfunction | [91,92,93,94] |
| D | Regulation of calcium metabolism | Improvement of cardiac stress and inflammation in obese rats | [96] |
| E | Antioxidative | Restoration of cardiac function and attenuation of atherogenic apo B-48-dependent hyperlipidemia | [97,98,99] |
| Polyphenols | Antioxidative | (i) O2− and peroxynitrite scavenger (ii) Inducers of redox dependent reactions | [100,101,102] |
| Astaxanthin | Antioxidative | (i) Anti-inflammatory effects (ii) Regulation of lipid and glucose metabolisms | [103,104,105,106,107] |
| Anti-hypertensives | |||
| Angiotensin converting enzyme (ACE) inhibitors | ACE inhibition | (i) Reduction of monocyte-macrophage recruitment into vessel wall, smooth muscle cells mitogenesis and extracellular matrix storage (ii) Reduction of ACE mediated ROS production (iii) Increase of bradykinin levels | [91,108,109,110] |
| Angiotensin receptor (AT) blockers | AT blockage | Pleiotropic anti-oxidative effects derived from renin-angiotensin axis blockage without bradykinin-mediated antioxidative effects | [100,101,102,111,112,113,114,115,116] |
| Beta adrenergic receptor blockers | Beta adrenergic receptor blockage | (i) Free radical scavenger function (ii) Decrease of superoxide anions generation (iii) Restoring eNOS expression | [117,118,119,120,121,122,123,124,125,126,127,128,129,130] |
| Anti-diabetic agents | |||
| Dipeptidyl peptidase-4 (DDP-4) inhibitors | DDP-4 inhibition | (i) Decrease of oxidative burst in whole blood (ii) Decrease of the expression of NADPH oxidase (iii) Elevation cAMP and protein kinase A | [131,132,133] |
| Glucagon-like peptide-1 (GLP-1) analogues | GLP-1 functions | (i) Decrease of oxidative burst in whole blood (ii) Decrease of the expression of NADPH oxidase (iii) Elevation cAMP and protein kinase A | [131,132,133] |
| Sodium-glucose cotransporter 2 (SGLT2) inhibitors | SGLT2 blockage | NOS2 and IFNɣ reduction | [131,134] |
| Others | |||
| NO donors | NO and/or NO derived molecule releasing by endothelial cells | NO and/or NO derived molecule releasing by endothelial cells restoring REDOX balance | [92,131,135,136] |
| Statins | 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibition | (i) Increase of eNOS activity (ii) Decrease of both asymmetrical dimethylarginine levels and NADPH oxidase function | [91,137,138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatino, F.; Conti, V.; Liguori, L.; Polcaro, G.; Corbi, G.; Manzo, V.; Tortora, V.; Carlomagno, C.; Vecchione, C.; Filippelli, A.; et al. Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients. Life 2021, 11, 105. https://doi.org/10.3390/life11020105
Sabbatino F, Conti V, Liguori L, Polcaro G, Corbi G, Manzo V, Tortora V, Carlomagno C, Vecchione C, Filippelli A, et al. Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients. Life. 2021; 11(2):105. https://doi.org/10.3390/life11020105
Chicago/Turabian StyleSabbatino, Francesco, Valeria Conti, Luigi Liguori, Giovanna Polcaro, Graziamaria Corbi, Valentina Manzo, Vincenzo Tortora, Chiara Carlomagno, Carmine Vecchione, Amelia Filippelli, and et al. 2021. "Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients" Life 11, no. 2: 105. https://doi.org/10.3390/life11020105
APA StyleSabbatino, F., Conti, V., Liguori, L., Polcaro, G., Corbi, G., Manzo, V., Tortora, V., Carlomagno, C., Vecchione, C., Filippelli, A., & Pepe, S. (2021). Molecules and Mechanisms to Overcome Oxidative Stress Inducing Cardiovascular Disease in Cancer Patients. Life, 11(2), 105. https://doi.org/10.3390/life11020105









