Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review
Abstract
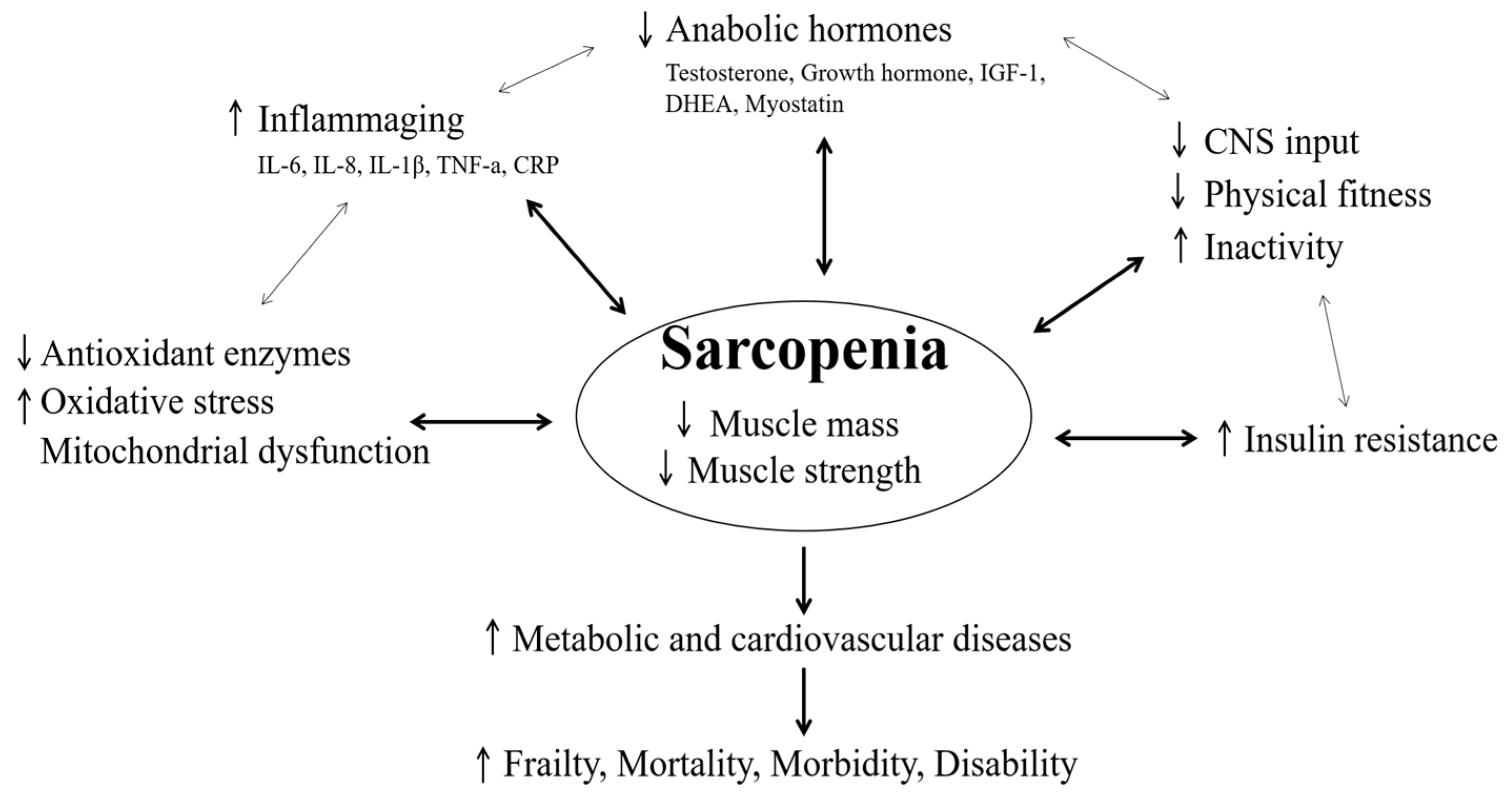
1. Introduction
2. Clinical and Physiological Point in Sarcopenia
2.1. Physical Fitness
2.2. Inflammation Responses
2.3. Antioxidants and Mitochondrial Function
2.4. Hormones
2.5. Biochemical Properties of Muscles
2.6. Metabolic and Cardiovascular Diseases
3. Resistance Training in Hypoxia for Muscular Function and Hypertrophy
3.1. Effect of Resistance Training in Hypoxia on Muscle Morphological and Function Change
3.2. Potential Mechanism of Resistance Training in Hypoxia on Muscle Morphological and Function Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, H.Y.; Hwang, H.; Park, J.; Lee, S.; Lim, K. The effects of altitude/hypoxic training on oxygen delivery capacity of the blood and aerobic exercise capacity in elite athletes—A meta-analysis. J. Exerc. Nutr. Biochem. 2016, 20, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, W.; Lim, K. Living High-Training Low for 21 Days Enhances Exercise Economy, Hemodynamic Function, and Exercise Performance of Competitive Runners. J. Sports Sci. Med. 2019, 18, 427–437. [Google Scholar] [PubMed]
- Kayser, B.; Verges, S. Hypoxia, energy balance and obesity: From pathophysiological mechanisms to new treatment strategies. Obes. Rev. 2013, 14, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Brocherie, F.; Millet, G.P.; Hauser, A.; Steiner, T.; Rysman, J.; Wehrlin, J.P.; Girard, O. “Live High-Train Low and High” Hypoxic Training Improves Team-Sport Performance. Med. Sci. Sports Exerc. 2015, 47, 2140–2149. [Google Scholar] [CrossRef] [PubMed]
- van der Zwaard, S.; Brocherie, F.; Kom, B.L.G.; Millet, G.P.; Deldicque, L.; van der Laarse, W.J.; Girard, O.; Jaspers, R.T. Adaptations in muscle oxidative capacity, fiber size, and oxygen supply capacity after repeated-sprint training in hypoxia combined with chronic hypoxic exposure. J. Appl. Physiol. 2018, 124, 1403–1412. [Google Scholar] [CrossRef]
- Nam, S.S.; Park, H.Y. Effects of endurance exercise under hypoxia on acid-base and ion balance in healthy males. Phys. Act. Nutr. 2020, 24, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, A.; González-Muniesa, P.; Portillo, M.P.; Martínez, J.A. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J. Physiol. Biochem. 2012, 68, 289–304. [Google Scholar] [CrossRef]
- Verges, S.; Chacaroun, S.; Godin-Ribuot, D.; Baillieul, S. Hypoxic Conditioning as a New Therapeutic Modality. Front. Pediatr. 2015, 3, 58. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.; Park, M.Y.; Chung, N.; Hwang, H.; Nam, S.S.; Lim, K. Exposure and Exercise Training in Hypoxic Conditions as a New Obesity Therapeutic Modality: A Mini Review. J. Obes. Metab. Syndr. 2018, 27, 93–101. [Google Scholar] [CrossRef]
- Sinex, J.A.; Chapman, R.F. Hypoxic training methods for improving endurance exercise performance. J. Sport Health Sci. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic Use of Exercising in Hypoxia: Promises and Limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.; Malatesta, D.; Millet, G.P. Walking in Hypoxia: An Efficient Treatment to Lessen Mechanical Constraints and Improve Health in Obese Individuals? Front. Physiol. 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jung, W.S.; Kim, J.; Lim, K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Inness, M.W.; Billaut, F.; Walker, E.J.; Petersen, A.C.; Sweeting, A.J.; Aughey, R.J. Heavy Resistance Training in Hypoxia Enhances 1RM Squat Performance. Front. Physiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Ohiwa, N.; Honda, A.; Matsubayashi, T.; Ikeda, T.; Akimoto, T.; Suzuki, Y.; Hirano, Y.; Russell, A.P. Effects of systemic hypoxia on human muscular adaptations to resistance exercise training. Physiol. Rep. 2014, 2, e12033. [Google Scholar] [CrossRef] [PubMed]
- Kurobe, K.; Huang, Z.; Nishiwaki, M.; Yamamoto, M.; Kanehisa, H.; Ogita, F. Effects of resistance training under hypoxic conditions on muscle hypertrophy and strength. Clin. Physiol. Funct. Imaging 2015, 35, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Lim, K. Effects of Hypoxic Training versus Normoxic Training on Exercise Performance in Competitive Swimmers. J. Sports Sci. Med. 2017, 16, 480–488. [Google Scholar] [PubMed]
- Martínez-Guardado, M.; Sánchez-Ureña, B.; Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Olcina, G.; Timón, R. Effects of strength training under hypoxic conditions on muscle performance, body composition and haematological variables. Biol. Sport 2020, 37, 121–129. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Scott, B.R.; Alcaraz, P.E.; Rubio-Arias, J.A. The efficacy of resistance training in hypoxia to enhance strength and muscle growth: A systematic review and meta-analysis. Eur. J. Sport Sci. 2018, 18, 92–103. [Google Scholar] [CrossRef]
- Manimmanakorn, A.; Hamlin, M.J.; Ross, J.J.; Taylor, R.; Manimmanakorn, N. Effects of low-load resistance training combined with blood flow restriction or hypoxia on muscle function and performance in netball athletes. J. Sci. Med. Sport 2013, 16, 337–342. [Google Scholar] [CrossRef]
- Martínez-Guardado, I.; Ramos-Campo, D.J.; Olcina, G.J.; Rubio-Arias, J.A.; Chung, L.H.; Marín-Cascales, E.; Alcaraz, P.E.; Timón, R. Effects of high-intensity resistance circuit-based training in hypoxia on body composition and strength performance. Eur. J. Sport Sci. 2019, 19, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Chycki, J.; Czuba, M.; Gołaś, A.; Zając, A.; Fidos-Czuba, O.; Młynarz, A.; Smółka, W. Neuroendocrine Responses and Body Composition Changes Following Resistance Training Under Normobaric Hypoxia. J. Hum. Kinet. 2016, 53, 91–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayo, B.; Miles, C.; Sims, S.; Driller, M. The Effect of Resistance Training in a Hypoxic Chamber on Physical Performance in Elite Rugby Athletes. High Alt. Med. Biol. 2018, 19, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Törpel, A.; Peter, B.; Schega, L. Effect of Resistance Training Under Normobaric Hypoxia on Physical Performance, Hematological Parameters, and Body Composition in Young and Older People. Front. Physiol. 2020, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett, W.E., Jr. Muscle changes in aging: Understanding sarcopenia. Sports Health 2014, 6, 36–40. [Google Scholar] [CrossRef]
- Ali, S.; Garcia, J.M. Sarcopenia, cachexia and aging: Diagnosis, mechanisms and therapeutic options—A mini-review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef]
- Baumgartner, R.N.; Koehler, K.M.; Gallagher, D.; Romero, L.; Heymsfield, S.B.; Ross, R.R.; Garry, P.J.; Lindeman, R.D. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998, 147, 755–763. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N.; Roubenoff, R.; Mayer, J.; Nair, K.S. Sarcopenia. J. Lab. Clin. Med. 2001, 137, 231–243. [Google Scholar] [CrossRef]
- Rom, O.; Kaisari, S.; Aizenbud, D.; Reznick, A.Z. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med. J. 2012, 3, e0024. [Google Scholar] [CrossRef] [PubMed]
- Hughes, V.A.; Frontera, W.R.; Roubenoff, R.; Evans, W.J.; Singh, M.A. Longitudinal changes in body composition in older men and women: Role of body weight change and physical activity. Am. J. Clin. Nutr. 2002, 76, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- McCarthy, L.H.; Bigal, M.E.; Katz, M.; Derby, C.; Lipton, R.B. Chronic pain and obesity in elderly people: Results from the Einstein aging study. J. Am. Geriatr. Soc. 2009, 57, 115–119. [Google Scholar] [CrossRef]
- Bainbridge, D.; Seow, H.; Sussman, J.; Pond, G.; Martelli-Reid, L.; Herbert, C.; Evans, W. Multidisciplinary health care professionals’ perceptions of the use and utility of a symptom assessment system for oncology patients. J. Oncol. Pract. 2011, 7, 19–23. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 2012, 71, 109–114. [Google Scholar] [CrossRef]
- Auyeung, T.W.; Lee, J.S.; Leung, J.; Kwok, T.; Woo, J. Adiposity to muscle ratio predicts incident physical limitation in a cohort of 3153 older adults--an alternative measurement of sarcopenia and sarcopenic obesity. Age 2013, 35, 1377–1385. [Google Scholar] [CrossRef]
- Marsh, A.P.; Rejeski, W.J.; Espeland, M.A.; Miller, M.E.; Church, T.S.; Fielding, R.A.; Gill, T.M.; Guralnik, J.M.; Newman, A.B.; Pahor, M. Muscle strength and BMI as predictors of major mobility disability in the Lifestyle Interventions and Independence for Elders pilot (LIFE-P). J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 1376–1383. [Google Scholar] [CrossRef]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Li, R.; Xia, J.; Zhang, X.; Gathirua-Mwangi, W.G.; Guo, J.; Li, Y.; McKenzie, S.; Song, Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med. Sci. Sports Exerc. 2018, 50, 458. [Google Scholar] [CrossRef] [PubMed]
- Wandrag, L.; Siervo, M.; Riley, H.L.; Khosravi, M.; Fernandez, B.O.; Leckstrom, C.A.; Martin, D.S.; Mitchell, K.; Levett, D.Z.H.; Montgomery, H.E.; et al. Does hypoxia play a role in the development of sarcopenia in humans? Mechanistic insights from the Caudwell Xtreme Everest Expedition. Redox Biol. 2017, 13, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395. [Google Scholar] [CrossRef]
- Chien, M.Y.; Kuo, H.K.; Wu, Y.T. Sarcopenia, cardiopulmonary fitness, and physical disability in community-dwelling elderly people. Phys. Ther. 2010, 90, 1277–1287. [Google Scholar] [CrossRef]
- Hida, T.; Ishiguro, N.; Shimokata, H.; Sakai, Y.; Matsui, Y.; Takemura, M.; Terabe, Y.; Harada, A. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr. Gerontol. Int. 2013, 13, 413–420. [Google Scholar] [CrossRef]
- Singh, A.S.; Chin, A.P.M.J.; Bosscher, R.J.; van Mechelen, W. Cross-sectional relationship between physical fitness components and functional performance in older persons living in long-term care facilities. BMC Geriatr. 2006, 6, 4. [Google Scholar] [CrossRef]
- Shephard, R.J. Maximal oxygen intake and independence in old age. Br. J. Sports Med. 2009, 43, 342–346. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Topinková, E.; Michel, J.P. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 1–7. [Google Scholar] [CrossRef]
- Xue, Q.L.; Walston, J.D.; Fried, L.P.; Beamer, B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011, 171, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Nishiguchi, S.; Fukutani, N.; Tanigawa, T.; Yukutake, T.; Kayama, H.; Aoyama, T.; Arai, H. Prevalence of sarcopenia in community-dwelling Japanese older adults. J. Am. Med. Dir. Assoc. 2013, 14, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Moreland, J.D.; Richardson, J.A.; Goldsmith, C.H.; Clase, C.M. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J. Am. Geriatr. Soc. 2004, 52, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Streeper, T.; Cawthon, P.; Baldwin, K.; Taaffe, D.R.; Harris, T.B. Sarcopenia: Etiology, clinical consequences, intervention, and assessment. Osteoporos. Int. 2010, 21, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Wang, Z.; Heymsfield, S.B.; Schautz, B.; Bosy-Westphal, A. Advances in the understanding of specific metabolic rates of major organs and tissues in humans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 501–508. [Google Scholar] [CrossRef]
- Waters, D.L.; Baumgartner, R.N.; Garry, P.J.; Vellas, B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: An update. Clin. Interv. Aging 2010, 5, 259–270. [Google Scholar] [CrossRef]
- Cesari, M.; Pahor, M.; Lauretani, F.; Zamboni, V.; Bandinelli, S.; Bernabei, R.; Guralnik, J.M.; Ferrucci, L. Skeletal muscle and mortality results from the InCHIANTI Study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 377–384. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Stressed out about obesity and insulin resistance. Nat. Med. 2006, 12, 41–42, discussion 42. [Google Scholar] [CrossRef]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Visser, M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006, 119, 526.e9–526.e17. [Google Scholar] [CrossRef]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011, 10, 319–329. [Google Scholar] [CrossRef]
- Jensen, G.L. Inflammation: Roles in aging and sarcopenia. JPEN J. Parenter. Enteral Nutr. 2008, 32, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Schaap, L.A.; Pluijm, S.M.; Deeg, D.J.; Harris, T.B.; Kritchevsky, S.B.; Newman, A.B.; Colbert, L.H.; Pahor, M.; Rubin, S.M.; Tylavsky, F.A.; et al. Higher inflammatory marker levels in older persons: Associations with 5-year change in muscle mass and muscle strength. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Niess, A.M.; Passek, F.; Lorenz, I.; Schneider, E.M.; Dickhuth, H.H.; Northoff, H.; Fehrenbach, E. Expression of the antioxidant stress protein heme oxygenase-1 (HO-1) in human leukocytes. Free Radic. Biol. Med. 1999, 26, 184–192. [Google Scholar] [CrossRef]
- Cerullo, M.A.; Fleck, D.E.; Eliassen, J.C.; Smith, M.S.; DelBello, M.P.; Adler, C.M.; Strakowski, S.M. A longitudinal functional connectivity analysis of the amygdala in bipolar I disorder across mood states. Bipolar Disord. 2012, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Meng, S.J.; Yu, L.J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Calvani, R.; Joseph, A.M.; Adhihetty, P.J.; Miccheli, A.; Bossola, M.; Leeuwenburgh, C.; Bernabei, R.; Marzetti, E. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 2013, 394, 393–414. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Buford, T.W.; Lorenzi, M.; Behnke, B.J.; Leeuwenburgh, C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. Int. J. Biochem. Cell Biol. 2013, 45, 2288–2301. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, X.; Chen, D. Myostatin: A novel insight into its role in metabolism, signal pathways, and expression regulation. Cell. Signal. 2011, 23, 1441–1446. [Google Scholar] [CrossRef]
- Kamel, H.K.; Maas, D.; Duthie, E.H., Jr. Role of hormones in the pathogenesis and management of sarcopenia. Drugs Aging 2002, 19, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.D. Aging and sarcopenia. J. Musculoskelet. Neuronal Interact. 2007, 7, 344–345. [Google Scholar] [PubMed]
- Balagopal, P.; Proctor, D.; Nair, K.S. Sarcopenia and hormonal changes. Endocrine 1997, 7, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Serra Rexach, J.A. Clinical consequences of sarcopenia. Nutr. Hosp. 2006, 21 (Suppl. S3), 46–50. [Google Scholar] [PubMed]
- Bray, G.A. Medical consequences of obesity. J. Clin. Endocrinol. Metab. 2004, 89, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, R.C.; Salomon, F.; Wiles, C.M.; Hesp, R.; Sönksen, P.H. Growth hormone treatment in growth hormone-deficient adults. II. Effects on exercise performance. J. Appl. Physiol. 1991, 70, 695–700. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Ensrud, K.E.; Laughlin, G.A.; Cauley, J.A.; Dam, T.T.; Barrett-Connor, E.; Fink, H.A.; Hoffman, A.R.; Lau, E.; Lane, N.E.; et al. Sex hormones and frailty in older men: The osteoporotic fractures in men (MrOS) study. J. Clin. Endocrinol. Metab. 2009, 94, 3806–3815. [Google Scholar] [CrossRef]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef]
- Bodine, S.C.; Stitt, T.N.; Gonzalez, M.; Kline, W.O.; Stover, G.L.; Bauerlein, R.; Zlotchenko, E.; Scrimgeour, A.; Lawrence, J.C.; Glass, D.J.; et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 2001, 3, 1014–1019. [Google Scholar] [CrossRef]
- Seo, D.Y.; Hwang, B.G. Effects of exercise training on the biochemical pathways associated with sarcopenia. Phys. Act. Nutr. 2020, 24, 32–38. [Google Scholar] [CrossRef]
- Pende, M. mTOR, Akt, S6 kinases and the control of skeletal muscle growth. Bull. Cancer 2006, 93, E39–E43. [Google Scholar] [PubMed]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle: The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Kolch, W. Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat. Rev. Mol. Cell Biol. 2005, 6, 827–837. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Morley, J.E.; Malmstrom, T.K.; Rodriguez-Mañas, L.; Sinclair, A.J. Frailty, sarcopenia and diabetes. J. Am. Med. Dir. Assoc. 2014, 15, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Karakelides, H.; Nair, K.S. Sarcopenia of aging and its metabolic impact. Curr. Top. Dev. Biol. 2005, 68, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Ruts, E.; Kim, J.; Janumala, I.; Heymsfield, S.; Gallagher, D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am. J. Clin. Nutr. 2004, 79, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007, 102, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Hyogo, H.; Sumida, Y.; Eguchi, Y.; Ono, N.; Kuwashiro, T.; Tanaka, K.; Takahashi, H.; Mizuta, T.; Ozaki, I.; et al. Severity of non-alcoholic steatohepatitis is associated with substitution of adipose tissue in skeletal muscle. J. Gastroenterol. Hepatol. 2013, 28, 1507–1514. [Google Scholar] [CrossRef]
- van Loon, L.J.; Goodpaster, B.H. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflug. Arch. 2006, 451, 606–616. [Google Scholar] [CrossRef]
- Meigs, J.B. Epidemiology of the metabolic syndrome, 2002. Am. J. Manag. Care 2002, 8, S283–S292, quiz S293-286. [Google Scholar]
- Roubenoff, R. Sarcopenic obesity: The confluence of two epidemics. Obes. Res. 2004, 12, 887–888. [Google Scholar] [CrossRef]
- Levine, M.E.; Crimmins, E.M. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity 2012, 20, 2101–2106. [Google Scholar] [CrossRef]
- Parr, E.B.; Coffey, V.G.; Hawley, J.A. Sarcobesity: A metabolic conundrum. Maturitas 2013, 74, 109–113. [Google Scholar] [CrossRef]
- Lenaz, G.; Bovina, C.; D’Aurelio, M.; Fato, R.; Formiggini, G.; Genova, M.L.; Giuliano, G.; Merlo Pich, M.; Paolucci, U.; Parenti Castelli, G.; et al. Role of mitochondria in oxidative stress and aging. Ann. N. Y. Acad. Sci. 2002, 959, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.S.; Kim, Y.Y.; Park, H.Y. Circuit Training Improvements in Korean Women with Sarcopenia. Percept. Mot. Skills 2019, 126, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Nourmahnad, A.; Sinha, I. Optimizing Skeletal Muscle Anabolic Response to Resistance Training in Aging. Front. Physiol. 2020, 11, 874. [Google Scholar] [CrossRef]
- Bong, Y.; Song, W. The effects of elastic band exercises and nutritional education on frailty, strength, and nutritional intake in elderly women. Phys. Act. Nutr. 2020, 24, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.S. Exercise in aging: Its important role in mortality, obesity and insulin resistance. Aging Health 2010, 6, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Brooks, N.; Layne, J.E.; Gordon, P.L.; Roubenoff, R.; Nelson, M.E.; Castaneda-Sceppa, C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. Int. J. Med. Sci. 2006, 4, 19–27. [Google Scholar] [CrossRef]
- Bloomer, R.J. The role of nutritional supplements in the prevention and treatment of resistance exercise-induced skeletal muscle injury. Sports Med. 2007, 37, 519–532. [Google Scholar] [CrossRef]
- Flack, K.D.; Davy, K.P.; Hulver, M.W.; Winett, R.A.; Frisard, M.I.; Davy, B.M. Aging, resistance training, and diabetes prevention. J. Aging Res. 2010, 2011, 127315. [Google Scholar] [CrossRef]
- Strasser, B.; Schobersberger, W. Evidence for resistance training as a treatment therapy in obesity. J. Obes. 2011, 2011. [Google Scholar] [CrossRef]
- Hurley, B.F.; Roth, S.M. Strength training in the elderly: Effects on risk factors for age-related diseases. Sports Med. 2000, 30, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Ouellette, M.M.; LeBrasseur, N.K.; Bean, J.F.; Phillips, E.; Stein, J.; Frontera, W.R.; Fielding, R.A. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke 2004, 35, 1404–1409. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.M.; Bezerra, L.M.; Rabelo, H.T.; Silva, M.A.; Silva, A.J.; Bottaro, M.; de Oliveira, R.J. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J. Clin. Densitom. 2009, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Verdijk, L.B.; Gleeson, B.G.; Jonkers, R.A.; Meijer, K.; Savelberg, H.H.; Dendale, P.; van Loon, L.J. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Kryger, A.I.; Andersen, J.L. Resistance training in the oldest old: Consequences for muscle strength, fiber types, fiber size, and MHC isoforms. Scand. J. Med. Sci. Sports 2007, 17, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef]
- de Oliveira Silva, A.; Dutra, M.T.; de Moraes, W.; Funghetto, S.S.; Lopes de Farias, D.; Dos Santos, P.H.F.; Vieira, D.C.L.; Nascimento, D.D.C.; Orsano, V.S.M.; Schoenfeld, B.J.; et al. Resistance training-induced gains in muscle strength, body composition, and functional capacity are attenuated in elderly women with sarcopenic obesity. Clin. Interv. Aging 2018, 13, 411–417. [Google Scholar] [CrossRef]
- Grgic, J.; Garofolini, A.; Orazem, J.; Sabol, F.; Schoenfeld, B.J.; Pedisic, Z. Effects of Resistance Training on Muscle Size and Strength in Very Elderly Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2020, 50, 1983–1999. [Google Scholar] [CrossRef]
- Centner, C.; Wiegel, P.; Gollhofer, A.; König, D. Effects of Blood Flow Restriction Training on Muscular Strength and Hypertrophy in Older Individuals: A Systematic Review and Meta-Analysis. Sports Med. 2019, 49, 95–108. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, M.; Camacho-Cardenosa, A.; Brazo-Sayavera, J.; Olcina, G.; Tomas-Carus, P.; Timón, R. Evaluation of 18-Week Whole-Body Vibration Training in Normobaric Hypoxia on Lower Extremity Muscle Strength in an Elderly Population. High Alt. Med. Biol. 2019, 20, 157–164. [Google Scholar] [CrossRef]
- Nishimura, A.; Sugita, M.; Kato, K.; Fukuda, A.; Sudo, A.; Uchida, A. Hypoxia increases muscle hypertrophy induced by resistance training. Int. J. Sports Physiol. Perform. 2010, 5, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, B.; Kinscherf, R.; Borisch, S.; Richter, G.; Bärtsch, P.; Billeter, R. Effects of low-resistance/high-repetition strength training in hypoxia on muscle structure and gene expression. Pflug. Arch. 2003, 446, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Feriche, B.; García-Ramos, A.; Morales-Artacho, A.J.; Padial, P. Resistance Training Using Different Hypoxic Training Strategies: A Basis for Hypertrophy and Muscle Power Development. Sports Med. Open 2017, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guardado, I.; Sánchez-Ureña, B.; Olcina, G.; Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Timón, R. Bench press performance during an intermittent hypoxic resistance training to muscle failure. J. Sports Med. Phys. Fit. 2019, 59, 1138–1143. [Google Scholar] [CrossRef]
- Scott, B.R.; Slattery, K.M.; Sculley, D.V.; Lockhart, C.; Dascombe, B.J. Acute Physiological Responses to Moderate-Load Resistance Exercise in Hypoxia. J. Strength Cond. Res. 2017, 31, 1973–1981. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Rubio-Arias, J.A.; Dufour, S.; Chung, L.; Ávila-Gandía, V.; Alcaraz, P.E. Biochemical responses and physical performance during high-intensity resistance circuit training in hypoxia and normoxia. Eur. J. Appl. Physiol. 2017, 117, 809–818. [Google Scholar] [CrossRef]
- Suga, T.; Okita, K.; Morita, N.; Yokota, T.; Hirabayashi, K.; Horiuchi, M.; Takada, S.; Takahashi, T.; Omokawa, M.; Kinugawa, S.; et al. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J. Appl. Physiol. (1985) 2009, 106, 1119–1124. [Google Scholar] [CrossRef]
- Tesch, P.A.; Colliander, E.B.; Kaiser, P. Muscle metabolism during intense, heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 362–366. [Google Scholar] [CrossRef]
- Kon, M.; Ikeda, T.; Homma, T.; Akimoto, T.; Suzuki, Y.; Kawahara, T. Effects of acute hypoxia on metabolic and hormonal responses to resistance exercise. Med. Sci. Sports Exerc. 2010, 42, 1279–1285. [Google Scholar] [CrossRef]
- Kon, M.; Ikeda, T.; Homma, T.; Suzuki, Y. Effects of low-intensity resistance exercise under acute systemic hypoxia on hormonal responses. J. Strength Cond. Res. 2012, 26, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.J.; Castracane, V.D.; Hollander, D.B.; Tryniecki, J.L.; Bamman, M.M.; O’Neal, S.; Hebert, E.P.; Kraemer, R.R. Hormonal responses from concentric and eccentric muscle contractions. Med. Sci. Sports Exerc. 2003, 35, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Ishii, N.; Kizuka, T.; Takamatsu, K. The impact of metabolic stress on hormonal responses and muscular adaptations. Med. Sci. Sports Exerc. 2005, 37, 955–963. [Google Scholar]
- Kraemer, W.J.; Ratamess, N.A. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Smilios, I.; Pilianidis, T.; Karamouzis, M.; Tokmakidis, S.P. Hormonal responses after various resistance exercise protocols. Med. Sci. Sports Exerc. 2003, 35, 644–654. [Google Scholar] [CrossRef]
- Favier, F.B.; Costes, F.; Defour, A.; Bonnefoy, R.; Lefai, E.; Baugé, S.; Peinnequin, A.; Benoit, H.; Freyssenet, D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1659–R1666. [Google Scholar] [CrossRef]
- Hayot, M.; Rodriguez, J.; Vernus, B.; Carnac, G.; Jean, E.; Allen, D.; Goret, L.; Obert, P.; Candau, R.; Bonnieu, A. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol. Cell. Endocrinol. 2011, 332, 38–47. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Green, H.J.; Sutton, J.R.; Coates, G.; Cymerman, A.; Young, P.; Houston, C.S. Operation Everest II: Structural adaptations in skeletal muscle in response to extreme simulated altitude. Acta Physiol. Scand. 1991, 142, 421–427. [Google Scholar] [CrossRef]
- Etheridge, T.; Atherton, P.J.; Wilkinson, D.; Selby, A.; Rankin, D.; Webborn, N.; Smith, K.; Watt, P.W. Effects of hypoxia on muscle protein synthesis and anabolic signaling at rest and in response to acute resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E697–E702. [Google Scholar] [CrossRef]
- Slivka, D.R. Skeletal muscle response to hypoxia. Acta Physiol. 2017, 220, 9–10. [Google Scholar] [CrossRef]
- Mason, S.; Johnson, R.S. The role of HIF-1 in hypoxic response in the skeletal muscle. Adv. Exp. Med. Biol. 2007, 618, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Nam, S.S.; Tanaka, H.; Lee, D.J. Hemodynamic, Hematological, and Hormonal Responses to Submaximal Exercise in Normobaric Hypoxia in Pubescent Girls. Pediatr. Exerc. Sci. 2016, 28, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology 2009, 24, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Kawada, S. What phenomena do occur in blood flow-restricted muscle? Int. J. KAATSU Train. Res. 2005, 1, 37–44. [Google Scholar] [CrossRef]
- Loenneke, J.P.; Young, K.C.; Fahs, C.A.; Rossow, L.M.; Bemben, D.A.; Bemben, M.G. Blood flow restriction: Rationale for improving bone. Med. Hypotheses 2012, 78, 523–527. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Sandri, M. Autophagy in skeletal muscle. FEBS Lett. 2010, 584, 1411–1416. [Google Scholar] [CrossRef]
- Britto, F.A.; Gnimassou, O.; De Groote, E.; Balan, E.; Warnier, G.; Everard, A.; Cani, P.D.; Deldicque, L. Acute environmental hypoxia potentiates satellite cell-dependent myogenesis in response to resistance exercise through the inflammation pathway in human. FASEB J. 2020, 34, 1885–1900. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.-S.; Kim, S.-W.; Kim, J.-W.; Park, H.-Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review. Life 2021, 11, 106. https://doi.org/10.3390/life11020106
Jung W-S, Kim S-W, Kim J-W, Park H-Y. Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review. Life. 2021; 11(2):106. https://doi.org/10.3390/life11020106
Chicago/Turabian StyleJung, Won-Sang, Sung-Woo Kim, Jeong-Weon Kim, and Hun-Young Park. 2021. "Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review" Life 11, no. 2: 106. https://doi.org/10.3390/life11020106
APA StyleJung, W.-S., Kim, S.-W., Kim, J.-W., & Park, H.-Y. (2021). Resistance Training in Hypoxia as a New Therapeutic Modality for Sarcopenia—A Narrative Review. Life, 11(2), 106. https://doi.org/10.3390/life11020106







