Multiscale Entropy of Resting-State Functional Magnetic Resonance Imaging Differentiates Progressive Supranuclear Palsy and Multiple System Atrophy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Neuropsychological Assessment
2.3. Image Acquisition
2.4. Processing for Functional Imaging
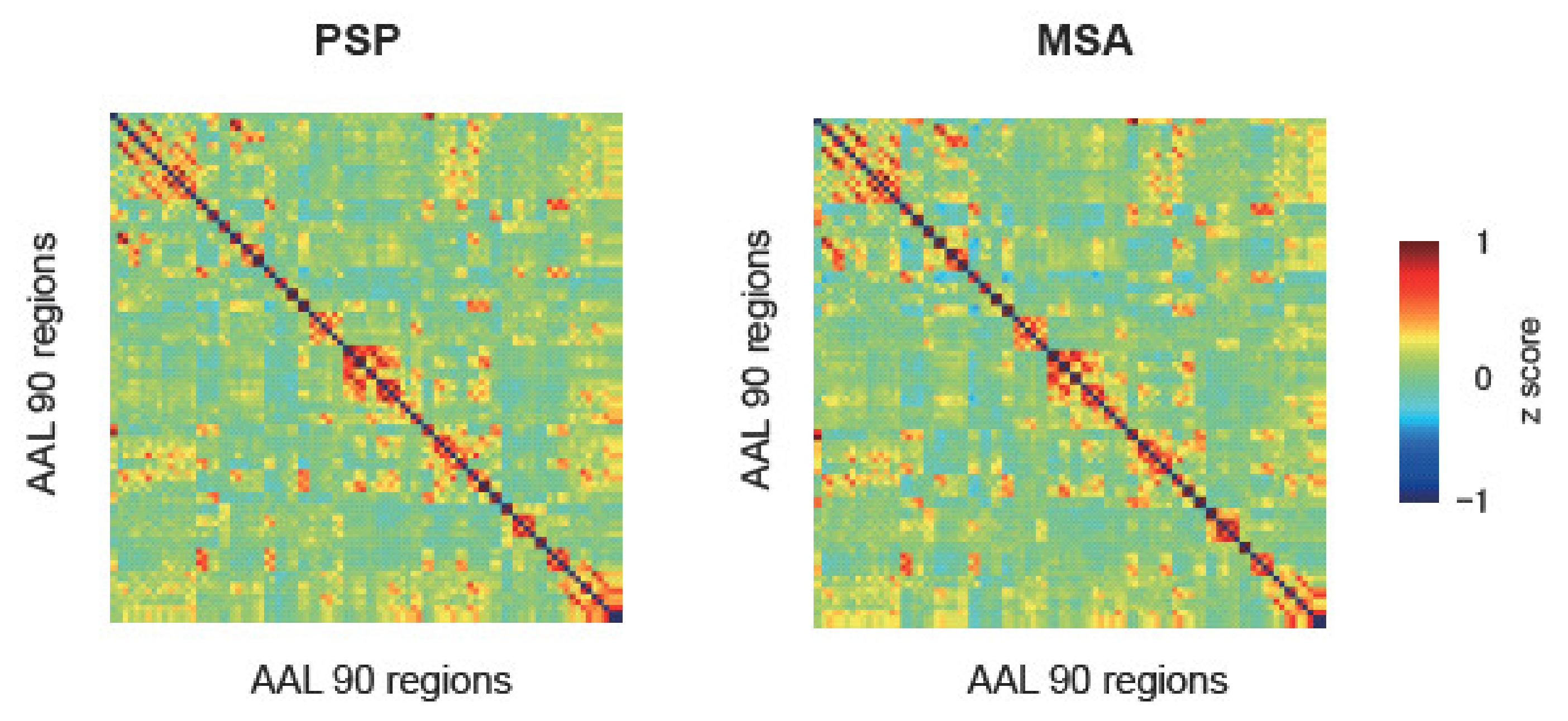
2.5. ROI-to-ROI Analysis
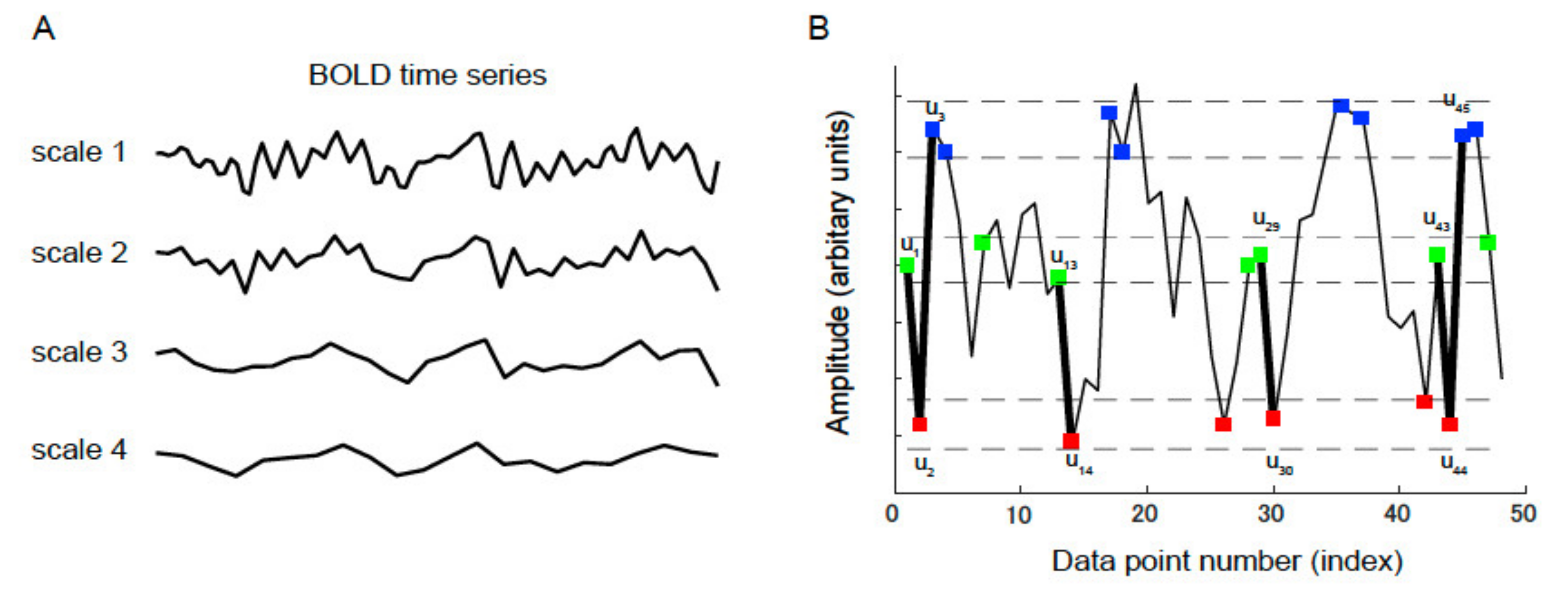
2.6. MSE
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tong, J.; Wong, H.; Guttman, M.; Ang, L.C.; Forno, L.S.; Shimadzu, M.; Rajput, A.H.; Muenter, M.D.; Kish, S.J.; Hornykiewicz, O.; et al. Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: A comparative investigation. Brain 2010, 133, 172–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, D.W. Parkinson’s disease and parkinsonism: Neuropathology. Cold Spring Harb. Perspect. Med. 2012, 2, a2009528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, G.; Niccolini, F.; Politis, M. Imaging in Parkinson’s disease. Clin. Med. 2016, 16, 371–375. [Google Scholar] [CrossRef]
- Koga, S.; Aoki, N.; Uitti, R.J.; van Gerpen, J.A.; Cheshire, W.P.; Josephs, K.A.; Wszolek, Z.K.; Langston, J.W.; Dickson, D.W. When DLB, PD, and PSP masquerade as MSA: An autopsy study of 134 patients. Neurology 2015, 85, 404–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Litvan, I.; Bhatia, K.P.; Burn, D.J.; Goetz, C.G.; Lang, A.E.; McKeith, I.; Quinn, N.; Sethi, K.D.; Shults, C.; Wenning, G.K.; et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov. Disord. 2003, 18, 467–486. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Brier, M.R.; Thomas, J.B.; Fagan, A.M.; Hassenstab, J.; Holtzman, D.M.; Benzinger, T.L.; Morris, J.C.; Ances, B.M. Functional connectivity and graph theory in preclinical Alzheimer’s disease. Neurobiol. Aging 2014, 35, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Bharti, K.; Bologna, M.; Upadhyay, N.; Piattella, M.C.; Suppa, A.; Petsas, N.; Gianni, C.; Tona, F.; Berardelli, A.; Pantano, P. Abnormal resting-state functional connectivity in progressive supranuclear palsy and corticobasal syndrome. Front. Neurol. 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitwell, J.L.; Avula, R.; Master, A.; Vemuri, P.; Senjem, M.L.; Jones, D.T.; Jack, C.R., Jr.; Josephs, K.A. Disrupted thalamocortical connectivity in PSP: A resting-state fMRI, DTI, and VBM study. Parkinsonism Relat. Disord. 2011, 17, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, R.C.; Boxer, A.L.; Trujillo, A.; Mirsky, J.B.; Guo, C.C.; Gennatas, E.D.; Heuer, H.W.; Fine, E.; Zhou, J.; Kramer, J.H.; et al. Intrinsic connectivity network disruption in progressive supranuclear palsy. Ann. Neurol. 2013, 73, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piattella, M.C.; Tona, F.; Bologna, M.; Sbardella, E.; Formica, A.; Petsas, N.; Filippini, N.; Berardelli, A.; Pantano, P. Disrupted resting-state functional connectivity in progressive supranuclear palsy. AJNR Am. J. Neuroradiol. 2015, 36, 915–921. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, N.; Suppa, A.; Piattella, M.C.; Gianni, C.; Bologna, M.; Di Stasio, F.; Petsas, N.; Tona, F.; Fabbrini, G.; Berardelli, A.; et al. Functional disconnection of thalamic and cerebellar dentate nucleus networks in progressive supranuclear palsy and corticobasal syndrome. Parkinsonism Relat. Disord. 2017, 39, 52–57. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wang, J.; Wang, H.; Zang, Y.F.; Zheng, F.L.; Meng, C.L.; Feng, F. Altered regional homogeneity in motor cortices in patients with multiple system atrophy. Neurosci. Lett. 2011, 502, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Zhu, D.; Li, F.; Xiao, C.; Lin, X.; Huang, Q.; Shi, J. Altered functional and causal connectivity of cerebello-cortical circuits between multiple system atrophy (Parkinsonian type) and Parkinson’s disease. Front. Aging Neurosci. 2017, 9, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, K.; Hara, K.; Watanabe, H.; Bagarinao, E.; Ogura, A.; Masuda, M.; Yokoi, T.; Kato, T.; Ohdake, R.; Ito, M.; et al. Alterations in cognition-related cerebello-cerebral networks in multiple system atrophy. Cerebellum 2019, 18, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Goldberger, A.L.; Peng, C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 068102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.C.; Huang, C.C.; Yeh, H.L.; Liu, M.E.; Hong, C.J.; Tu, P.C.; Chen, J.F.; Huang, N.E.; Peng, C.K.; Lin, C.P.; et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: A multiscale entropy analysis. Neurobiol. Aging 2013, 34, 428–438. [Google Scholar] [CrossRef]
- Smith, R.X.; Yan, L.; Wang, D.J. Multiple time scale complexity analysis of resting state FMRI. Brain Imaging Behav. 2014, 8, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Grieder, M.; Wang, D.J.J.; Dierks, T.; Wahlund, L.O.; Jann, K. Default mode network complexity and cognitive decline in mild Alzheimer’s disease. Front. Neurosci. 2018, 12, 770. [Google Scholar] [CrossRef] [Green Version]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dubois, B.; Slachevsky, A.; Litvan, I.; Pillon, B. The FAB: A Frontal Assessment Battery at bedside. Neurology 2000, 55, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Zung, W.W. A self-rating depression scale. Arch. Gen. Psychiatry 1965, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Starkstein, S.E.; Mayberg, H.S.; Preziosi, T.J.; Andrezejewski, P.; Leiguarda, R.; Robinson, R.G. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 1992, 4, 134–139. [Google Scholar]
- Okada, K.; Kobayashi, S.; Aoki, K.; Suyama, N.; Yamaguchi, S. Assessment of motivational loss in poststroke patients using the Japanese version of Starkstein’s apathy scale. Jpn. J. Stroke 1998, 20, 318–323. [Google Scholar] [CrossRef] [Green Version]
- Anthony, J.C.; LeResche, L.; Niaz, U.; von Korff, M.R.; Folstein, M.F. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol. Med. 1982, 12, 397–408. [Google Scholar] [CrossRef]
- Nakaaki, S.; Murata, Y.; Sato, J.; Shinagawa, Y.; Matsui, T.; Tatsumi, H.; Furukawa, T. Reliability and validity of the Japanese version of the Frontal Assessment Battery in patients with the frontal variant of frontotemporal dementia. Psychiatry Clin. Neurosci. 2007, 61, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.C.; Hong, C.J.; Liou, Y.J.; Huang, K.L.; Huang, C.C.; Liu, M.E.; Lo, M.T.; Huang, N.E.; Peng, C.K.; Lin, C.P.; et al. Decreased resting-state brain activity complexity in schizophrenia characterized by both increased regularity and randomness. Hum. Brain Mapp. 2015, 36, 2174–2186. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.J.; Lin, C.P.; Peng, C.K. A strategy to reduce bias of entropy estimates in resting-state fMRI signals. Front. Neurosci. 2018, 12, 398. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wu, H.; Xu, M.; Yang, Y.; Chang, C.; Zeng, W.; Yan, H. Occupational functional plasticity revealed by brain entropy: A resting-state fMRI study of seafarers. Hum. Brain Mapp. 2018, 39, 2997–3004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokunbi, M.O.; Staff, R.T.; Waiter, G.D.; Ahearn, T.S.; Fox, H.C.; Deary, I.J.; Starr, J.M.; Whalley, L.J.; Murray, A.D. Inter-individual differences in fMRI entropy measurements in old age. IEEE Trans. Biomed. Eng. 2011, 58, 3206–3214. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Pandya, D.N. Disconnection syndromes of basal ganglia, thalamus, and cerebrocerebellar systems. Cortex 2008, 44, 1037–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PSP | MSA | p-Value | |
|---|---|---|---|
| N | 14 | 18 | |
| Age (years) | 73.5 ± 6.2 | 69.3 ± 8.7 | 0.132 |
| Sex (female/male) | 9/5 | 10/8 | 0.618 |
| Disease duration (years) | 3.3 ± 1.7 | 5.1 ± 3.2 | 0.073 |
| mRS | 3.2 ± 0.8 | 3.5 ± 0.8 | 0.750 |
| MMSE | 22.8 ± 4.6 | 25.8 ± 3.7 | 0.051 |
| FAB | 9.1 ± 3.4 | 13.2 ± 3.6 | 0.004 |
| SDS | 45.1 ± 10.4 | 39.5 ± 11.4 | 0.161 |
| AS | 17.6 ± 6.7 | 14.8 ± 8.2 | 0.317 |
| SBI (%) | 14.3 | 11.1 | 0.425 |
| CMB (%) | 7.1 | 11.1 | 0.702 |
| PVH (0/1/2, %) | 12.5/42.9/28.6 | 33.3/38.9/27.8 | 0.956 |
| DSWMH (0/1/2/3, %) | 21.4/35.7/28.6/14.3 | 27.8/22.2/33.6/16.7 | 0.868 |
| Head movement (mm) | 0.77 ± 0.57 | 0.69 ± 0.58 | 0.715 |
| Scale 2 | Scale 3 | Scale 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| [x, y, z] | Size | pFDR | [x, y, z] | Size | pFDR | [x, y, z] | size | pFDR | |
| Left PFC | - | - | - | [−26, 36, 9] | 223 | 0.041 | - | - | - |
| Right PFC | [33, 27, 9] | 486 | 0.005 | [39, 36, 3] | 828 | <0.001 | [39, 36, 6] | 364 | <0.001 |
| MMSE | FAB | SDS | AS | ||
|---|---|---|---|---|---|
| Scale 2 | PFC (R) | 0.142 | 0.416 | 0.026 | −0.095 |
| Scale 3 | PFC (L) | 0.492 * | 0.553 * | 0.004 | −0.084 |
| PFC (R) | 0.181 | 0.393 | −0.112 | −0.066 | |
| Scale 4 | PFC (R) | 0.353 | 0.572 * | −0.078 | −0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadota, K.; Onoda, K.; Abe, S.; Hamada, C.; Mitaki, S.; Oguro, H.; Nagai, A.; Kitagaki, H.; Yamaguchi, S. Multiscale Entropy of Resting-State Functional Magnetic Resonance Imaging Differentiates Progressive Supranuclear Palsy and Multiple System Atrophy. Life 2021, 11, 1411. https://doi.org/10.3390/life11121411
Kadota K, Onoda K, Abe S, Hamada C, Mitaki S, Oguro H, Nagai A, Kitagaki H, Yamaguchi S. Multiscale Entropy of Resting-State Functional Magnetic Resonance Imaging Differentiates Progressive Supranuclear Palsy and Multiple System Atrophy. Life. 2021; 11(12):1411. https://doi.org/10.3390/life11121411
Chicago/Turabian StyleKadota, Katsuhiko, Keiichi Onoda, Satoshi Abe, Chizuko Hamada, Shingo Mitaki, Hiroaki Oguro, Atsushi Nagai, Hajime Kitagaki, and Shuhei Yamaguchi. 2021. "Multiscale Entropy of Resting-State Functional Magnetic Resonance Imaging Differentiates Progressive Supranuclear Palsy and Multiple System Atrophy" Life 11, no. 12: 1411. https://doi.org/10.3390/life11121411
APA StyleKadota, K., Onoda, K., Abe, S., Hamada, C., Mitaki, S., Oguro, H., Nagai, A., Kitagaki, H., & Yamaguchi, S. (2021). Multiscale Entropy of Resting-State Functional Magnetic Resonance Imaging Differentiates Progressive Supranuclear Palsy and Multiple System Atrophy. Life, 11(12), 1411. https://doi.org/10.3390/life11121411






