Potential IFNγ Modulation of Inflammasome Pathway in Chlamydia trachomatis Infected Synovial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Culture Conditions
2.2. Propagation and Titration of C. trachomatis
2.3. Infection of Human Synovial Cells with C. trachomatis and Pretreatment with IFN-γ
2.4. Efficiency of C. trachomatis Infection in Human Synovial and McCoy Cells
2.5. Growth Curve of C. trachomatis in Human Synovial Cells
2.6. TaqMan-Based Real-Time RT-PCR Assay
2.7. ELISA Assay
2.8. Caspase Inhibition in Human Synovial Cells
2.9. Statistical Analysis
3. Results
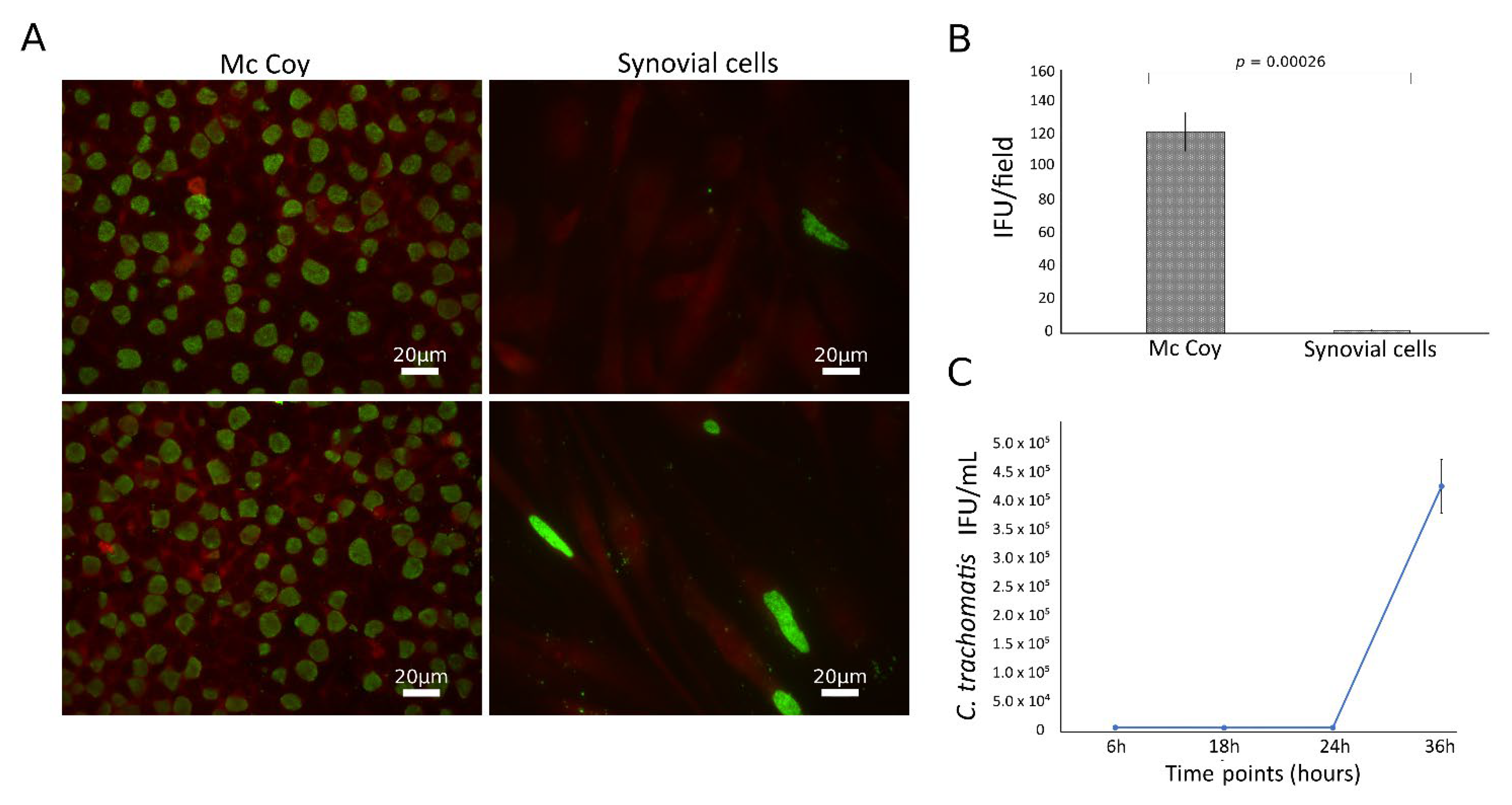
3.1. C. trachomatis Productively Infects Primary Human Synovial Cells
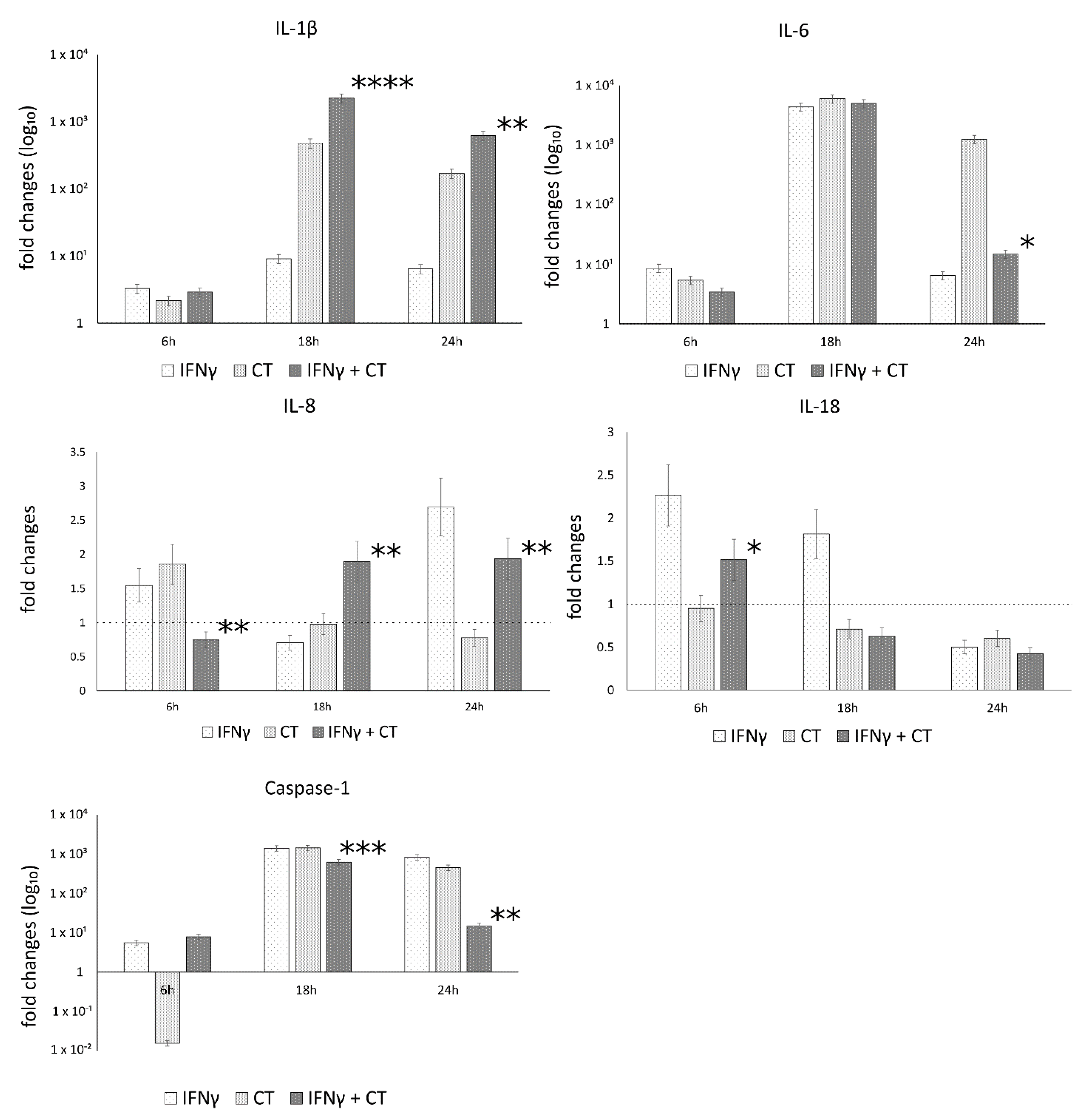
3.2. C. trachomatis Infection of Human Synovial Cells Upregulates the Expression of IL-1β and Caspase-1 Genes Involved in the Host-Cell Inflammasome Pathway
3.3. IFNγ Pretreatment of Human Synovial Cells Modulates the Host Cell Response against C. trachomatis Infection
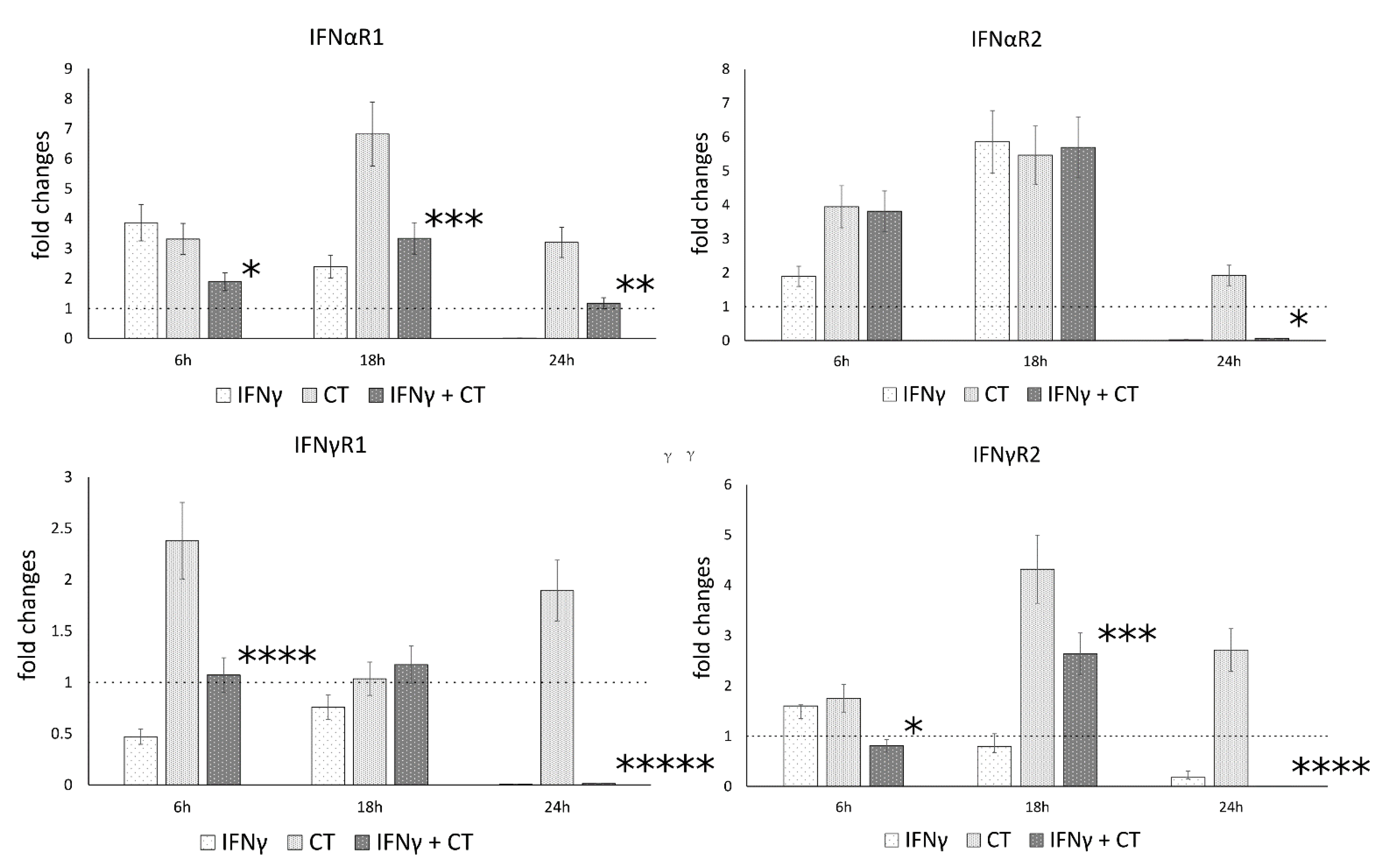
3.4. IFN-γ Pretreatment in C. trachomatis Infected Human Synovial Cells Modulates IFN Receptor Gene Expression
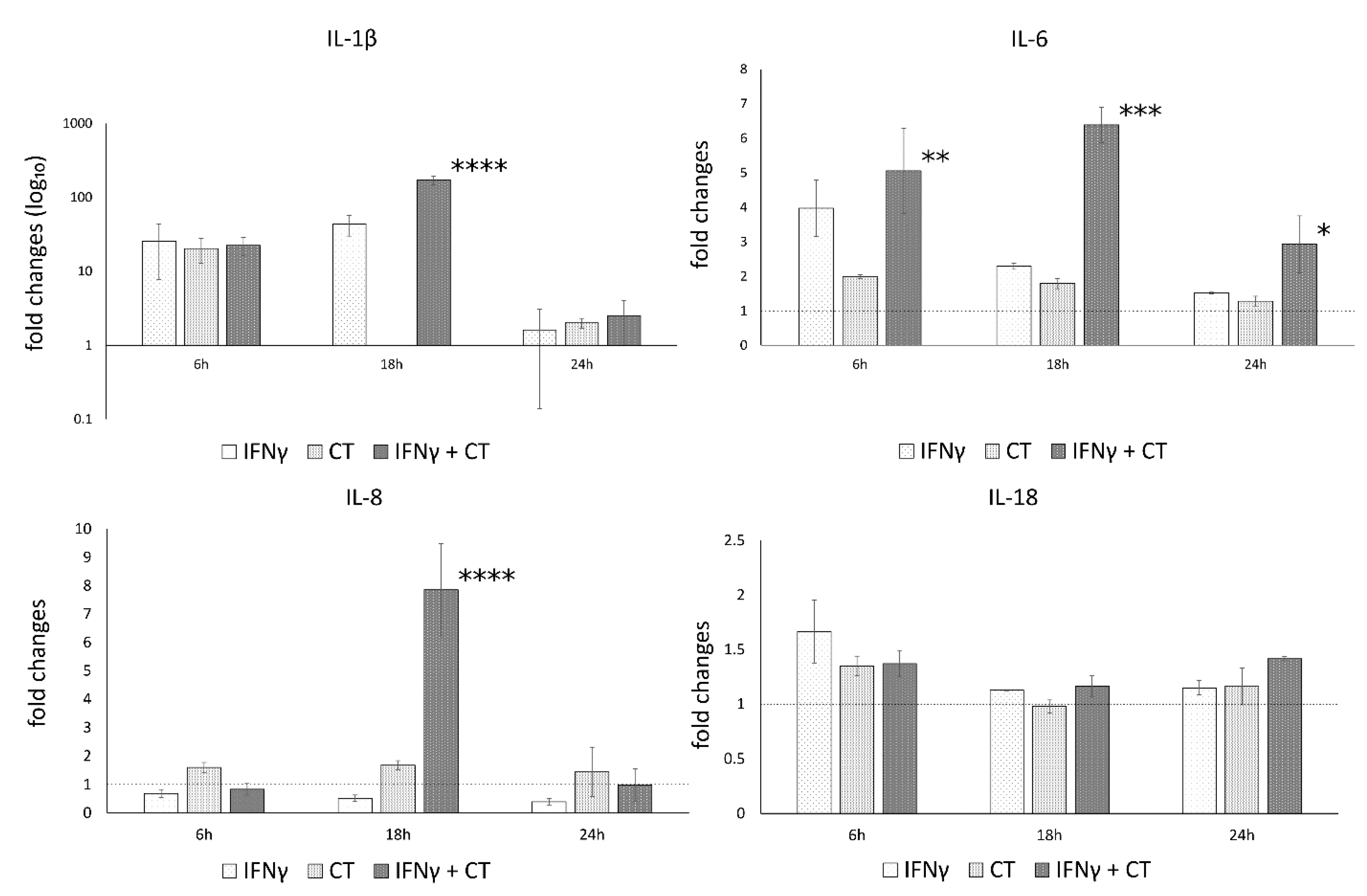
3.5. IFN-γ Pretreatment in C. trachomatis-Infected Human Synovial Cells Increases Inflammatory Cytokine Production
3.6. The Anti-Chlamydial Activity of IFN-γ Is Correlated to the Inhibition of Caspases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowley, J.; Vander Hoorn, S.; Korenromp, E.; Low, N.; Unemo, M.; Abu-Raddad, L.J.; Chico, R.M.; Smolak, A.; Newman, L.; Gottlieb, S.; et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 2019, 97, 548. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Romano, S.; Sessa, R. Chlamydia trachomatis and Chlamydia pneumoniae Interaction with the Host: Latest Advances and Future Prospective. Microorganisms 2019, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.M.; Ferone, M.E. Chlamydia trachomatis Genital Infections. Microb. Cell 2016, 3, 390–403. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; De Santis, F.; Filardo, S.; Ragno, R.; Angiolella, L. Effects ofMentha suaveolensEssential Oil onChlamydia trachomatis. BioMed Res. Int. 2015, 2015, 508071. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; Filardo, S.; Alfano, V.; Pelloni, M.; Spendiani, E.; Po, A.; Paoli, D.; Ferretti, E.; Sessa, R. Chlamydia trachomatis elicits TLR3 expression but disrupts the inflammatory signaling down-modulating NFκB and IRF3 transcription factors in human Sertoli cells. J. Biol. Regul. Homeost. Agents 2020, 34, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Filardo, S.; Skilton, R.J.; O’Neill, C.; Di Pietro, M.; Sessa, R.; Clarke, I.N. Growth kinetics of Chlamydia trachomatis in primary human Sertoli cells. Sci. Rep. 2019, 9, 5847. [Google Scholar] [CrossRef]
- Kumar, R.; A Derbigny, W. TLR3 Deficiency Leads to a Dysregulation in the Global Gene-Expression Profile in Murine Oviduct Epithelial Cells Infected with Chlamydia muridarum. Int. J. Microbiol. Curr. Res. 2018, 1, 1–13. [Google Scholar] [CrossRef][Green Version]
- Yu, P.; Xiao, L.; Lin, L.; Tang, L.; Chen, C.; Wang, F.; Wang, Y. STAT3-mediated TLR2/4 pathway upregulation in an IFN-gamma-inducedChlamydia trachomatispersistent infection model. Pathog. Dis. 2016, 74, ftw076. [Google Scholar] [CrossRef]
- Kavathas, P.B.; Boeras, C.M.; Mulla, M.J.; Abrahams, V.M. Nod1, but not the ASC inflammasome, contributes to induction of IL-1β secretion in human trophoblasts after sensing of Chlamydia trachomatis. Mucosal Immunol. 2012, 6, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, K.R.; Stephens, R.S. The Cytosolic Pattern Recognition Receptor NOD1 Induces Inflammatory Interleukin-8 during Chlamydia trachomatis Infection. Infect. Immun. 2008, 76, 3150–3155. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Frasca, F.; Scagnolari, C.; Manera, M.; Sessa, V.; Antonelli, G.; Sessa, R. Interferon-γ Possesses Anti-Microbial and Immunomodulatory Activity on a Chlamydia trachomatis Infection Model of Primary Human Synovial Fibroblasts. Microorganisms 2020, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Gitsels, A.; Sanders, N.; Vanrompay, D. Chlamydial Infection From Outside to Inside. Front. Microbiol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Panzetta, M.E.; Valdivia, R.H.; Saka, H.A. Chlamydia Persistence: A Survival Strategy to Evade Antimicrobial Effects in-vitro and in-vivo. Front. Microbiol. 2018, 9, 3101. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Di Pietro, M.; Schiavoni, G.; Nardis, C.; Gentile, M.; Sessa, R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 2014, 304, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.M.; Brubaker, S.W.; Monack, D.M. Host inflammasome defense mechanisms and bacterial pathogen evasion strategies. Curr. Opin. Immunol. 2019, 60, 63–70. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- McAllister, M.; Chemaly, M.; Eakin, A.; Gibson, D.; McGilligan, V. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthr. Cartil. 2018, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Puren, A.J.; Fantuzzi, G.; Gu, Y.; Su, M.S.; Dinarello, C.A. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J. Clin. Investig. 1998, 101, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1beta, Interleukin-18, and the Interleukin-1beta Converting Enzymea. Ann. N. Y. Acad. Sci. 1998, 856, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.K.; Gupta, K.; Jordan, S.; Chi, X.; Lensing, S.Y.; Press, C.G.; Geisler, W.M. An Adaptive Chlamydia trachomatis-Specific IFN-γ-Producing CD4+ T Cell Response Is Associated with Protection Against Chlamydia Reinfection in Women. Front. Immunol. 2018, 9, 1981. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Huston, W.M.; Taing, K.; Katouli, M.; Timms, P. In vitro rescue of genital strains of Chlamydia trachomatis from interferon-γ and tryptophan depletion with indole-positive, but not indole-negative Prevotella spp. BMC Microbiol. 2016, 16, 286. [Google Scholar] [CrossRef]
- Sessa, R.; Di Pietro, M.; Filardo, S.; Bressan, A.; Mastromarino, P.; Biasucci, A.V.; Rosa, L.; Cutone, A.; Berlutti, F.; Paesano, R.; et al. Lactobacilli–lactoferrin interplay in Chlamydia trachomatis infection. Pathog. Dis. 2017, 75, ftx054. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, M.; De Santis, F.; Schiavoni, G.; Filardo, S.; Sessa, R. Resveratrol in Chlamydia pneumoniae-induced foam cell formation and interleukin-17A synthesis. J. Boil. Regul. Homeost. Agents 2013, 27, 509–518. [Google Scholar]
- Jorgensen, I.; Bednar, M.M.; Amin, V.; Davis, B.K.; Ting, J.P.; McCafferty, D.G.; Valdivia, R.H. The Chlamydia Protease CPAF Regulates Host and Bacterial Proteins to Maintain Pathogen Vacuole Integrity and Promote Virulence. Cell Host Microbe 2011, 10, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Sater, A.A.; Koo, E.; Häcker, G.; Ojcius, D.M. Inflammasome-dependent Caspase-1 Activation in Cervical Epithelial Cells Stimulates Growth of the Intracellular Pathogen Chlamydia trachomatis. J. Biol. Chem. 2009, 284, 26789–26796. [Google Scholar] [CrossRef]
- Xavier, A.; Al-Zeer, M.A.; Meyer, T.F.; Daumke, O. hGBP1 Coordinates Chlamydia Restriction and Inflammasome Activation through Sequential GTP Hydrolysis. Cell Rep. 2020, 31, 107667. [Google Scholar] [CrossRef]
- Webster, S.J.; Brode, S.; Ellis, L.; Fitzmaurice, T.; Elder, M.J.; Gekara, N.O.; Tourlomousis, P.; Bryant, C.; Clare, S.; Chee, R.; et al. Detection of a microbial metabolite by STING regulates inflammasome activation in response to Chlamydia trachomatis infection. PLoS Pathog. 2017, 13, e1006383. [Google Scholar] [CrossRef] [PubMed]
- Finethy, R.; Jorgensen, I.; Haldar, A.K.; de Zoete, M.R.; Strowig, T.; Flavell, R.A.; Yamamoto, M.; Nagarajan, U.M.; Miao, E.; Coers, J. Guanylate Binding Proteins Enable Rapid Activation of Canonical and Noncanonical Inflammasomes in Chlamydia-Infected Macrophages. Infect. Immun. 2015, 83, 4740–4749. [Google Scholar] [CrossRef]
- Abdul-Sater, A.A.; Saïd-Sadier, N.; Padilla, E.V.; Ojcius, D.M. Chlamydial infection of monocytes stimulates IL-1β secretion through activation of the NLRP3 inflammasome. Microbes Infect. 2010, 12, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.L. The inflammasome: Friend or foe in Chlamydia infection? Biomed. J. 2016, 39, 299–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Detjen, K.M.; Farwig, K.; Welzel, M.; Wiedenmann, B.; Rosewicz, S. Interferon gamma inhibits growth of human pancreatic carcinoma cells via caspase-1 dependent induction of apoptosis. Gut 2001, 49, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Jeong, E.; Benveniste, E.N. Caspase-1 Mediates Fas-Induced Apoptosis and is Up-Regulated by Interferon- in Human Astrocytoma Cells. J. Neuro-Oncol. 2004, 67, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Choi, M.S.K.; Porter, A.G. Expression Analysis of the Human Caspase-1 Subfamily Reveals Specific Regulation of the CASP5 Gene by Lipopolysaccharide and Interferon-γ. J. Biol. Chem. 2000, 275, 39920–39926. [Google Scholar] [CrossRef]
- Khare, S.; Ratsimandresy, R.A.; De Almeida, L.; Cuda, C.; Rellick, S.L.; Misharin, A.; Wallin, M.C.; Gangopadhyay, A.; Forte, E.; Gottwein, E.; et al. The PYRIN domain–only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat. Immunol. 2014, 15, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Yu, N.; Lei, A.; Li, X.; Tan, S.; Huang, L.; Zhou, Z. Insights Into Host Cell Cytokines in Chlamydia Infection. Front. Immunol. 2021, 12, 1915. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fan, Y.; Brunham, R.C.; Yang, X. IFN-Gamma Knockout Mice Show Th2-Associated Delayed-Type Hypersensitivity and the Inflammatory Cells Fail to Localize and Control Chlamydial Infection. Eur. J. Immunol. 1999, 29, 3782–3792. [Google Scholar] [CrossRef]
- Megha, K.; Joseph, X.; Akhil, V.; Mohanan, P. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Eitel, J.; Meixenberger, K.; Van Laak, C.; Orlovski, C.; Hocke, A.; Schmeck, B.; Hippenstiel, S.; N’Guessan, P.D.; Suttorp, N.; Opitz, B. Rac1 Regulates the NLRP3 Inflammasome Which Mediates IL-1beta Production in Chlamydophila pneumoniae Infected Human Mononuclear Cells. PLoS ONE 2012, 7, e30379. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, H.; Dutta, R.; Vats, V.; Gupta, R.; Jha, R.; Jha, H.C.; Srivastava, P.; Bhengraj, A.R.; Mittal, A.S. Persistently Elevated Level of IL-8 inChlamydia trachomatisInfected HeLa 229 Cells is Dependent on Intracellular Available Iron. Mediat. Inflamm. 2009, 2009, 417658. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.; Mielke, L.; Cornish, A.L.; Sutton, C.E.; O’Donnell, J.; Cengia, L.H.; Roberts, A.; Wicks, I.P.; Mills, K.; Croker, B.A. Regulation of interleukin-1β by interferon-γ is species specific, limited by suppressor of cytokine signalling 1 and influences interleukin-17 production. EMBO Rep. 2010, 11, 640–646. [Google Scholar] [CrossRef]
- Bosco, M.C.; Gusella, G.L.; Espinoza-Delgado, I.; Longo, D.L.; Varesio, L. Interferon-Gamma Upregulates In-terleukin-8 Gene Expression in Human Monocytic Cells by a Posttranscriptional Mechanism. Blood 1994, 83, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1 and Its Biologically Related Cytokines. Adv. Immunol. 1989, 44, 153–205. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.J.; Vermeulen, M.W.; Clark, B.D.; Webb, A.C.; Auron, P.E. Human pro-IL-1 beta gene expression in monocytic cells is regulated by two distinct pathways. J. Immunol. 1988, 140, 2267–2273. [Google Scholar] [PubMed]
- Fenton, M.J.; Clark, B.D.; Collins, K.L.; Webb, A.C.; Rich, A.; Auron, P.E. Transcriptional regulation of the human prointerleukin 1 beta gene. J. Immunol. 1987, 138, 3972–3979. [Google Scholar] [PubMed]
- Knudsen, P.J.; Dinarello, C.A.; Strom, T.B. Prostaglandins posttranscriptionally inhibit monocyte expression of interleukin 1 activity by increasing intracellular cyclic adenosine monophosphate. J. Immunol. 1986, 137, 3189–3194. [Google Scholar]
- Huang, T.-J.; Li, Y.-Y.; Weng, Y.-J.; Cheng, C.-C.; Hsu, R.W.-W. Interleukin-6 Protein Expression Is More Important Than Interleukin-6 mRNA Levels in Assessing Surgical Invasiveness. J. Surg. Res. 2007, 142, 53–58. [Google Scholar] [CrossRef]
- Ivanov, P.; Anderson, P. Post-transcriptional regulatory networks in immunity. Immunol. Rev. 2013, 253, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filardo, S.; Di Pietro, M.; Frasca, F.; Diaco, F.; Scordio, M.; Antonelli, G.; Scagnolari, C.; Sessa, R. Potential IFNγ Modulation of Inflammasome Pathway in Chlamydia trachomatis Infected Synovial Cells. Life 2021, 11, 1359. https://doi.org/10.3390/life11121359
Filardo S, Di Pietro M, Frasca F, Diaco F, Scordio M, Antonelli G, Scagnolari C, Sessa R. Potential IFNγ Modulation of Inflammasome Pathway in Chlamydia trachomatis Infected Synovial Cells. Life. 2021; 11(12):1359. https://doi.org/10.3390/life11121359
Chicago/Turabian StyleFilardo, Simone, Marisa Di Pietro, Federica Frasca, Fabiana Diaco, Mirko Scordio, Guido Antonelli, Carolina Scagnolari, and Rosa Sessa. 2021. "Potential IFNγ Modulation of Inflammasome Pathway in Chlamydia trachomatis Infected Synovial Cells" Life 11, no. 12: 1359. https://doi.org/10.3390/life11121359
APA StyleFilardo, S., Di Pietro, M., Frasca, F., Diaco, F., Scordio, M., Antonelli, G., Scagnolari, C., & Sessa, R. (2021). Potential IFNγ Modulation of Inflammasome Pathway in Chlamydia trachomatis Infected Synovial Cells. Life, 11(12), 1359. https://doi.org/10.3390/life11121359







