Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Measurement of Anthropometric and Biochemical Parameters
2.3. Measurement of Markers Related to Obesity and Diabesity
2.4. Statistical Analysis
3. Results
3.1. Demographic, Biochemical and Anthropometric Parameters
3.2. Relationship between Markers Related to Obesity, Diabesity and Metabolic Health Status
4. Discussion
Study Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michałowska, J.; Miller-Kasprzak, E.; Bogdański, P. Incretin hormones in obesity and related cardiometabolic disorders: The clinical perspective. Nutrients 2021, 13, 351. [Google Scholar] [CrossRef]
- World Health Organization Page. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 July 2021).
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, T17–T36. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Peña-Orihuela, P.; Perez-Martinez, P.; Fuentes, F.; Marin, C.; Tunez, I.; Tinahones, F.J.; Perez-Jimenez, F.; Roche, H.M.; et al. Oxidative stress is associated with the number of components of metabolic syndrome: LIPGENE study. Exp. Mol. Med. 2013, 45, e28. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M. Obesity, Metabolic Syndrome, and Cardiovascular Disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically healthy obesity. Endocr. Rev. 2020, 41, 405–420. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Foster, M.C.; Anderson, C.A.M.; Burke, G.L.; Haq, N.; Kalyani, R.R.; Ouyang, P.; Sibley, C.T.; Tracy, R.; Woodward, M.; et al. Metabolically Healthy Obesity, Transition to Metabolic Syndrome, and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 71, 1857–1865. [Google Scholar] [CrossRef]
- Kaess, B.M.; Jóźwiak, J.; Nelson, C.P.; Lukas, W.; Mastej, M.; Windak, A.; Tomasik, T.; Grzeszczak, W.; Tykarski, A.; Gąsowski, J.; et al. The relation of rapid changes in obesity measures to lipid profile—Insights from a nationwide metabolic health survey in 444 Polish cities. PLoS ONE 2014, 9, e86837. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, W.J.; Song, K.-H. Metabolically healthy obesity: A friend or foe? Korean J. Intern. Med. 2017, 32, 611–621. [Google Scholar] [CrossRef]
- Primeau, V.; Coderre, L.; Karelis, A.D.; Brochu, M.; Lavoie, M.E.; Messier, V.; Sladek, R.; Rabasa-Lhoret, R. Characterizing the profile of obese patients who are metabolically healthy. Int. J. Obes. 2011, 35, 971–981. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; Cornejo-Pareja, I.; Tinahones, F. Does Metabolically Healthy Obesity Exist? Nutrients 2016, 8, 320. [Google Scholar] [CrossRef]
- Van Vliet-Ostaptchouk, J.V.; Nuotio, M.-L.; Slagter, S.N.; Doiron, D.; Fischer, K.; Foco, L.; Gaye, A.; Gögele, M.; Heier, M.; Hiekkalinna, T.; et al. The prevalence of metabolic syndrome and metabolically healthy obesity in Europe: A collaborative analysis of ten large cohort studies. BMC Endocr. Disord. 2014, 14, 9. [Google Scholar] [CrossRef]
- Blüher, M. Mechanisms in endocrinology: Are metabolically healthy obese individuals really healthy? Eur. J. Endocrinol. 2014, 171, R209–R219. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.-U.; Hu, F.B.; Schulze, M.B. Metabolically healthy obesity: Epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013, 1, 152–162. [Google Scholar] [CrossRef]
- Osadnik, T.; Osadnik, K.; Pawlas, N.; Strzelczyk, J.; Kasperczyk, J.; Poloński, L.; Gąsior, M. Metabolic and genetic profiling of young adults with and without a family history of premature coronary heart disease (MAGNETIC). Study design and methodology. Arch. Med. Sci. 2019, 15, 590–597. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Cleeman, J.I. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Lejawa, M.; Osadnik, K.; Osadnik, T.; Pawlas, N. Association of metabolically healthy and unhealthy obesity phenotypes with oxidative stress parameters and telomere length in healthy young adult men. Analysis of the magnetic study. Antioxidants 2021, 10, 93. [Google Scholar] [CrossRef]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral adiposity index: A reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing 2019. Available online: https://www.r-project.org/ (accessed on 1 December 2021).
- Stekhoven, D.J.; Bühlmann, P. Missforest-Non-parametric missing value imputation for mixed-type data. Bioinformatics 2012, 28, 112–118. [Google Scholar] [CrossRef]
- Jung, U.; Choi, M.-S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Samocha-Bonet, D.; Dixit, V.D.; Kahn, C.R.; Leibel, R.L.; Lin, X.; Nieuwdorp, M.; Pietiläinen, K.H.; Rabasa-Lhoret, R.; Roden, M.; Scherer, P.E.; et al. Metabolically healthy and unhealthy obese—The 2013 Stock Conference report. Obes. Rev. 2014, 15, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Hamer, M. Healthy obesity as an intermediate state of risk: A critical review. Expert Rev. Endocrinol. Metab. 2016, 11, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Osadnik, K.; Osadnik, T.; Lonnie, M.; Lejawa, M.; Reguła, R.; Fronczek, M.; Gawlita, M.; Wądołowska, L.; Gąsior, M.; Pawlas, N. Metabolically healthy obese and metabolic syndrome of the lean: The importance of diet quality. Analysis of MAGNETIC cohort. Nutr. J. 2020, 19, 19. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; García, E.; Robles, L.; Riaño, D.; Ruiz-Gomez, D.G.; García-Ulloa, A.C.; Melgarejo, M.A.; Zamora, M.; Guillen-Pineda, L.E.; Mehta, R.; et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J. Clin. Endocrinol. Metab. 2008, 93, 4075–4079. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, K.; Wang, L.; Yang, H.; Yan, K.; Pan, H.; Zhu, H.; Gong, F. Serum zag and adiponectin levels were closely related to obesity and the metabolically abnormal phenotype in Chinese population. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3099–3112. [Google Scholar] [CrossRef] [PubMed]
- Halperin, F.; Beckman, J.A.; Patti, M.E.; Trujillo, M.E.; Garvin, M.; Creager, M.A.; Scherer, P.E.; Goldfine, A.B. The role of total and high-molecular-weight complex of adiponectin in vascular function in offspring whose parents both had type 2 diabetes. Diabetologia 2005, 48, 2147–2154. [Google Scholar] [CrossRef]
- De Abreu, V.G.; Martins, C.J.D.M.; De Oliveira, P.A.C.; Francischetti, E.A. High-molecular weight adiponectin/HOMA-IR ratio as a biomarker of metabolic syndrome in urban multiethnic Brazilian subjects. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Baragetti, A.; Catapano, A.L.; Norata, G.D. Translating the biology of adipokines in atherosclerosis and cardiovascular diseases: Gaps and open questions. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Sargsyan, K.; Mangge, H. Hypoadiponectinemia as a Risk Factor for Atherosclerosis? Stroke 2006, 37, 1642. [Google Scholar] [CrossRef][Green Version]
- Chow, W.-S.; Cheung, B.M.Y.; Tso, A.W.K.; Xu, A.; Wat, N.M.S.; Fong, C.H.Y.; Ong, L.H.Y.; Tam, S.; Tan, K.C.B.; Janus, E.D.; et al. Hypoadiponectinemia as a Predictor for the Development of Hypertension. Hypertension 2007, 49, 1455–1461. [Google Scholar] [CrossRef]
- Phillips, C.M.; Perry, I.J. Does Inflammation Determine Metabolic Health Status in Obese and Nonobese Adults? J. Clin. Endocrinol. Metab. 2013, 98, E1610–E1619. [Google Scholar] [CrossRef]
- Fu, J.; Li, Y.; Esangbedo, I.C.; Li, G.; Feng, D.; Li, L.; Xu, L.; Han, L.; Li, M.; Li, C.; et al. Circulating osteonectin and adipokine profiles in relation to metabolically healthy obesity in Chinese children: Findings from BCAMS. J. Am. Heart Assoc. 2018, 7, e009169. [Google Scholar] [CrossRef]
- Pereira, S.; Alvarez-Leite, J. Adipokines: Biological functions and metabolically healthy obese profile. J. Receptor. Ligand Channel Res. 2014, 7, 15. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.; Reilly, M.P. Leptin Resistance. A Possible Interface of Inflammation and Metabolism in Obesity-Related Cardiovascular Disease. J. Am. Coll. Cardiol. 2008, 52, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Cheng, H.; Chen, F.; Yan, Y.; Zhang, M.; Zhao, X.; Hou, D.; Mi, J. Adipokines are Associated with Hypertension in Metabolically Healthy Obese (MHO) Children and Adolescents: A Prospective Population-Based Cohort Study. J. Epidemiol. 2018, 28, 19–26. [Google Scholar] [CrossRef]
- Jamar, G.; Caranti, D.A.; de Cassia Cesar, H.; Masquio, D.C.L.; Bandoni, D.H.; Pisani, L.P. Leptin as a cardiovascular risk marker in metabolically healthy obese. Appetite 2017, 108, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Molli, A.E.; Penas Steinhardt, A.; López, A.P.; González, C.D.; Vilariño, J.; Frechtel, G.D.; Cerrone, G.E. Metabolically healthy obese individuals present similar chronic inflammation level but less insulin-resistance than obese individuals with metabolic syndrome. PLoS ONE 2017, 12, e0190528. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Velho, S.; Waterworth, D.; Waeber, G.; Von Känel, R.; Vollenweider, P. The association between inflammatory biomarkers and metabolically healthy obesity depends of the definition used. Eur. J. Clin. Nutr. 2012, 66, 426–435. [Google Scholar] [CrossRef]
- Song, N.-J.; Kim, S.; Jang, B.-H.; Chang, S.-H.; Yun, U.J.; Park, K.-M.; Waki, H.; Li, D.Y.; Tontonoz, P.; Park, K.W. Small Molecule-Induced Complement Factor D (Adipsin) Promotes Lipid Accumulation and Adipocyte Differentiation. PLoS ONE 2016, 11, e0162228. [Google Scholar] [CrossRef]
- Tafere, G.G.; Wondafrash, D.Z.; Zewdie, K.A.; Assefa, B.T.; Ayza, M.A. Plasma Adipsin as a Biomarker and Its Implication in Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Chatterjee Bhowmick, D.; Murano, I.; Cohen, P.; et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Lee, W.J.; Lee, I.T.; Lin, S.Y.; Lee, W.L.; Liang, K.W.; Sheu, W.H.H. Association between serum adipsin levels and insulin resistance in subjects with various degrees of glucose intolerance. J. Endocr. Soc. 2019, 3, 403–410. [Google Scholar] [CrossRef]
- Vasilenko, M.A.; Kirienkova, E.V.; Skuratovskaia, D.A.; Zatolokin, P.A.; Mironyuk, N.I.; Litvinova, L.S. The Role of Production of Adipsin and Leptin in the Development of Insulin Resistance in Patients with Abdominal Obesity. Dokl. Biochem. Biophys. 2017, 475, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ambrosi, J.; Catalán, V.; Rodríguez, A.; Andrada, P.; Ramírez, B.; Ibáñez, P.; Vila, N.; Romero, S.; Margall, M.A.; Gil, M.J.; et al. Increased cardiometabolic risk factors and inflammation in adipose tissue in obese subjects classified as metabolically healthy. Diabetes Care 2014, 37, 2813–2821. [Google Scholar] [CrossRef]
- Christou, K.A.; Christou, G.A.; Karamoutsios, A.; Vartholomatos, G. The regulation of serum resistin levels in metabolically healthy and unhealthy obese individuals. Hormones 2020, 19, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Barnes, K.; Miner, J. Role of Resistin in Insulin Sensitivity in Rodents and Humans. Curr. Protein Pept. Sci. 2009, 10, 96–107. [Google Scholar] [CrossRef]
- Nogueira, J.P.; Maraninchi, M.; Béliard, S.; Lorec, A.M.; Berthet, B.; Bégu-Le Corroller, A.; Dubois, N.; Grangeot, R.; Mattei, C.; Gaudart, J.; et al. Unacylated Ghrelin is associated with the isolated low HDL-cholesterol obese phenotype independently of insulin resistance and CRP level. Nutr. Metab. 2012, 9, 17. [Google Scholar] [CrossRef]
- Ferrer, R.; Pardina, E.; Rossell, J.; Oller, L.; Viñas, A.; Baena-Fustegueras, J.A.; Lecube, A.; Vargas, V.; Balibrea, J.M.; Caubet, E.; et al. Morbidly “Healthy” Obese Are Not Metabolically Healthy but Less Metabolically Imbalanced Than Those with Type 2 Diabetes or Dyslipidemia. Obes. Surg. 2015, 25, 1380–1391. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Jankiewicz-Wika, J.; Kołomecki, K.; Cywiński, J.; Piestrzeniewicz, K.; Świȩtosławski, J.; Stȩpień, H.; Komorowski, J. Impact of vertical banded gastroplasty on body weight, insulin resistance, adipocytokine, inflammation and metabolic syndrome markers in morbidly obese patients. Endokrynol. Pol. 2011, 62, 109–119. [Google Scholar]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef] [PubMed]
- Basurto, L.; Sánchez, L.; Díaz, A.; Valle, M.; Robledo, A.; Martínez-Murillo, C. Differences between metabolically healthy and unhealthy obesity in PAI-1 level. Thromb. Res. 2019, 180, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.; Guagnano, M.T.; Pacini, G.; Vigneri, S.; Falco, A.; Marinopiccoli, M.; Manigrasso, M.R.; Basili, S.; Davì, G. Association of Inflammation Markers with Impaired Insulin Sensitivity and Coagulative Activation in Obese Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 5321–5326. [Google Scholar] [CrossRef]
- Ito, Y.; Nagaike, H. Increased Angiopoietin Like Protein 4 (Angptl4) Is Associated With Higher Concentration Of Ldl- Triglycerides In Type 2 Diabetes. Atherosclerosis 2019, 287, e80–e81. [Google Scholar] [CrossRef]
- Hassan, M. ANGPLT3: A novel modulator of lipid metabolism. Glob. Cardiol. Sci. Pract. 2015, 2015, 65. [Google Scholar] [CrossRef]
- Schinzari, F.; Vizioli, G.; Campia, U.; Tesauro, M.; Cardillo, C. Variable Changes of Circulating ANGPTL3 and ANGPTL4 in Different Obese Phenotypes: Relationship with Vasodilator Dysfunction. Biomedicines 2021, 9, 1037. [Google Scholar] [CrossRef]
- Garcés, M.F.; Buell-Acosta, J.D.; Rodríguez-Navarro, H.A.; Pulido-Sánchez, E.; Rincon-Ramírez, J.J.; Moreno-Ordóñez, D.C.; Franco-Vega, R.; Roncancio-Muñoz, J.S.; Burgos-Cardenas, A.J.; Lacunza, E.; et al. Serum angiopoietin-like 3 levels are elevated in obese non diabetic men but are unaffected during an oral glucose tolerance test. Sci. Rep. 2020, 10, 21118. [Google Scholar] [CrossRef]
- Cinkajzlová, A.; Mráz, M.; Lacinová, Z.; Kloučková, J.; Kaválková, P.; Kratochvílová, H.; Trachta, P.; Křížová, J.; Haluzíková, D.; Škrha, J.; et al. Angiopoietin-like protein 3 and 4 in obesity, type 2 diabetes mellitus, and malnutrition: The effect of weight reduction and realimentation. Nutr. Diabetes 2018, 8, 21. [Google Scholar] [CrossRef]
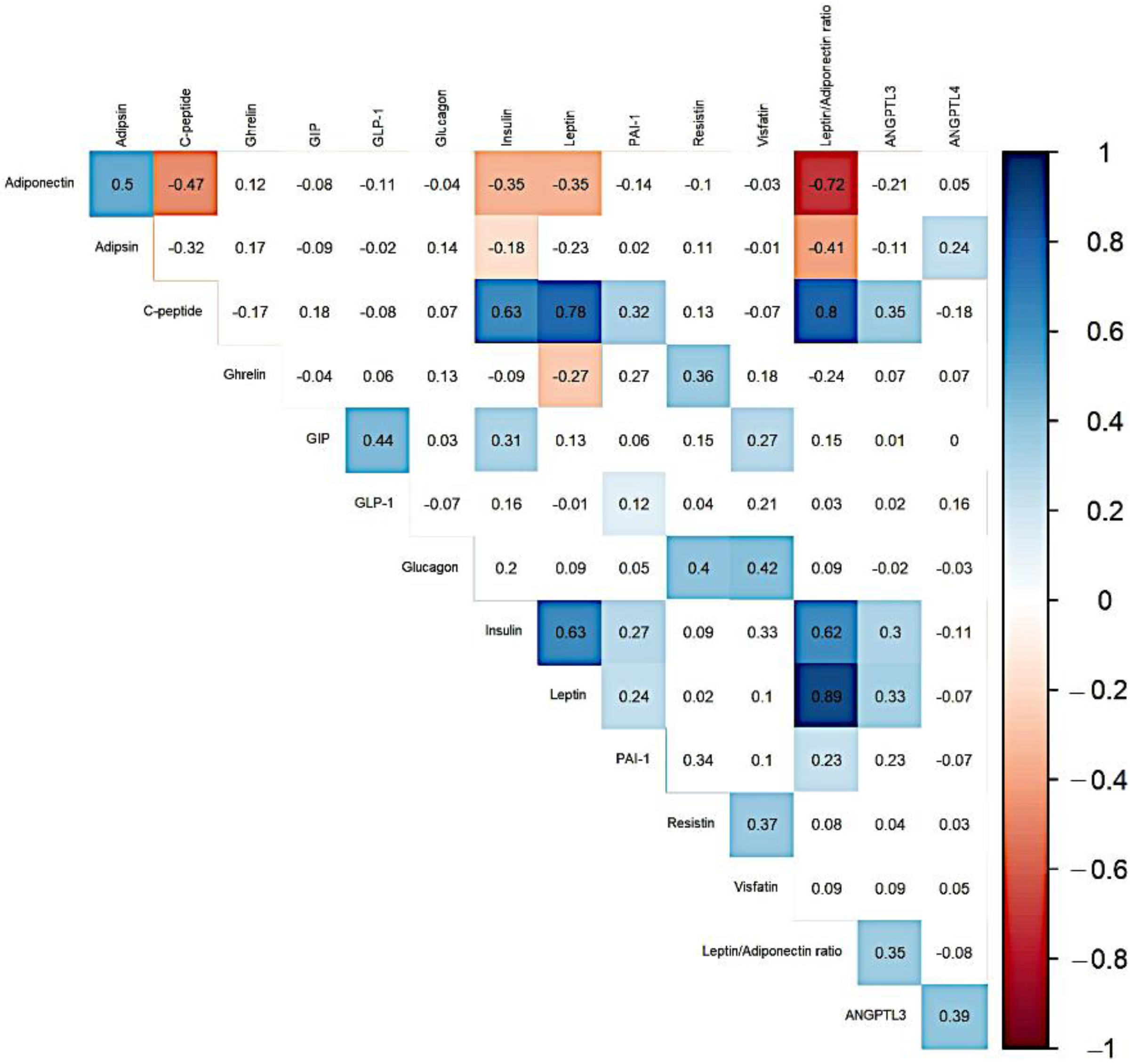
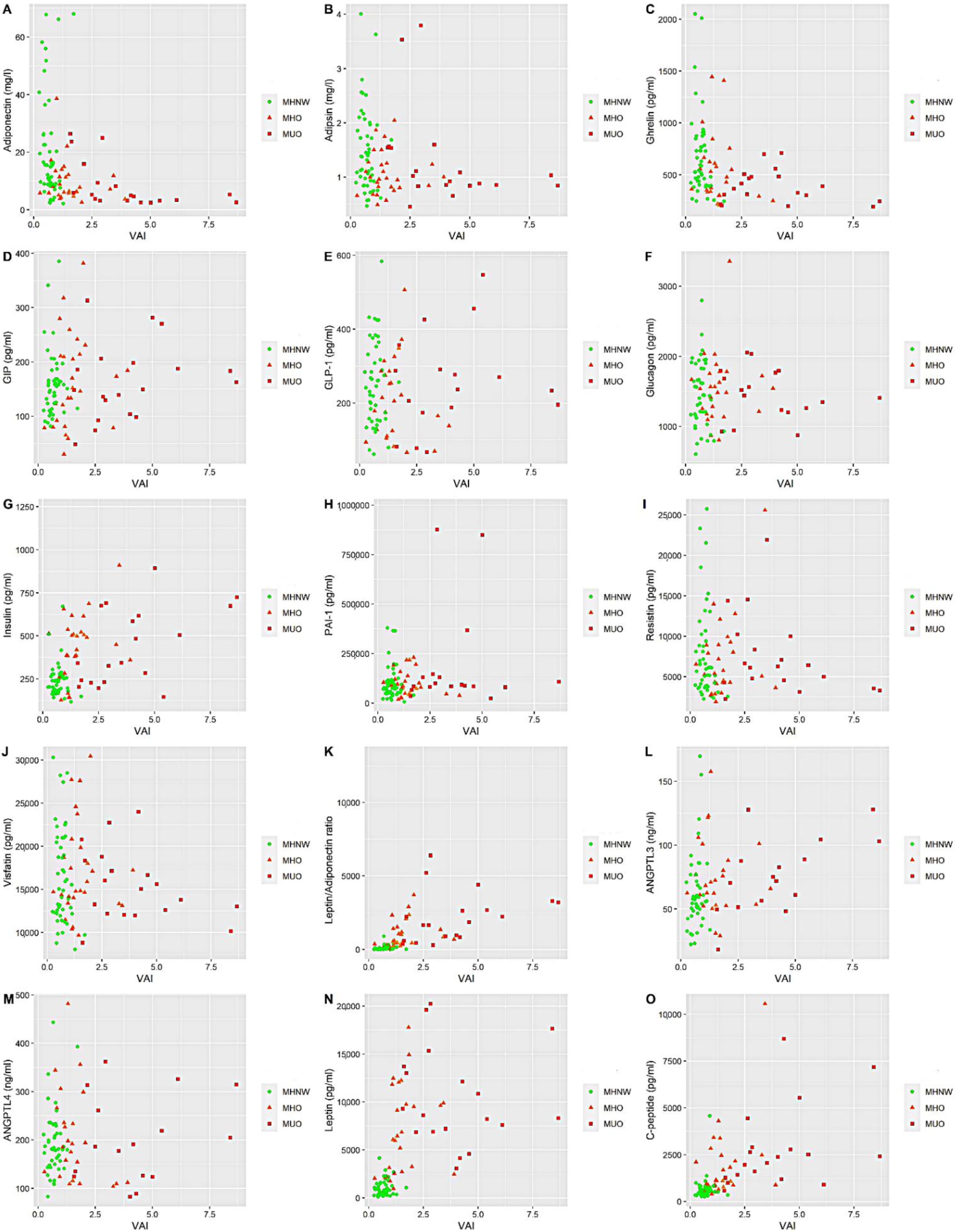
| Variables | MHNW (n = 49) | MHO (n = 27) | MUO (n = 22) | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| Age (years) | 30.96 [27.30–32.42] | 29.88 [26.61–32.67] | 31.82 [28.12–33.10] | >0.05 | >0.05 |
| Currently smoking (vs. past smoker or non-smoker) (%) | 10 (20.41) | 4 (14.81) | 6 (27.27) | >0.05 | >0.05 |
| Alcohol consumption (%) | 42 (85.71) | 21 (77.77) | 18 (81.81) | >0.05 | >0.05 |
| Family history of P-CAD (%) | 21 (42.86) | 18 (66.67) | 16 (72.72) | <0.05 | >0.05 |
| Family history of DM (%) | 6 (12.24) | 4 (14.81) | 5 (22.73) | >0.05 | >0.05 |
| Less than six hours of sleep per night during weekdays (%) | 23 (46.94) | 12 (44.44) | 8 (36.36) | >0.05 | >0.05 |
| Less than six hours of sleep per night during the weekends (%) | 4 (8.16) | 3 (11.11) | 1 (4.54) | >0.05 | >0.05 |
| Low physical activity level (%) | 18 (36.74) | 10 (37.04) | 15 (68.18) | >0.05 | >0.05 |
| Variables | MHNW (n = 49) | MHO (n = 27) | MUO (n = 22) | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| BMI [kg/m2] | 23.36 [21.78–24.10] | 31.38 [30.63–33.05] | 34.02 [33.03–37.02] | <0.001 | <0.001 |
| WHR [cm] | 0.84 [0.81–0.88] | 0.93 [0.88–0.96] | 1.00 [0.97–1.02] | <0.001 | <0.001 |
| VAI | 0.69 [0.49–0.82] | 1.30 [1.09–1.77] | 3.77 [2.53–5.31] | <0.001 | <0.001 |
| SBP [mmHg] | 128.00 [120.00–134.50] | 135.00 [128.00–144.00] | 136.00 [132.00–157.00] | <0.001 | <0.001 |
| DBP [mmHg] | 80.00 [73.75–84.00] | 85.00 [77.50–89.00] | 90.00 [82.00–95.00] | <0.01 | <0.001 |
| TC [mmol/L] | 4.80 [4.27–5.30] | 5.41 [4.74–6.17] | 5.77 [5.04–6.87] | <0.001 | <0.001 |
| HDL-C [mmol/L] | 1.58 [1.40–1.74] | 1.19 [1.15–1.44] | 1.01 [0.89–1.15] | <0.001 | <0.001 |
| HDL% | 33.00 [29.00–38.00] | 24.00 [20.00–27.50] | 17.50 [13.25–21.75] | <0.001 | <0.001 |
| LDL-C [mmol/L] | 2.84 [2.49–3.43] | 3.71 [2.94–4.25] | 3.76 [3.13–4.27] | <0.001 | <0.001 |
| TG [mmol/L] | 0.80 [0.64–1.03] | 1.30 [1.13–1.56] | 2.76 [1.94–3.76] | <0.001 | <0.001 |
| Lp(a) [nmol/L] | 17.00 [5.00–48.00] | 9.00 [4.50–23.50] | 9.50 [3.25–102.75] | >0.05 | >0.05 |
| apoA1 [g/L] | 1.57 [1.49–1.71] | 1.44 [1.41–1.69] | 1.46 [1.32–1.61] | <0.05 | <0.01 |
| apoB [g/L] | 0.85 [0.76–1.05] | 1.12 [0.93–1.25] | 1.23 [1.05–1.42] | <0.001 | <0.001 |
| HbA1c (%) | 4.90 [4.80–5.20] | 5.10 [4.85–5.35] | 5.10 [5.00–5.20] | <0.05 | <0.01 |
| Glucose [mmol/L] | 5.00 [4.70–5.30] | 5.20 [4.85–5.60] | 5.35 [5.10–5.90] | <0.01 | <0.001 |
| HOMA-IR | 1.46 [1.20–1.70] | 2.76 [2.17–3.34] | 3.51 [1.63–4.49] | <0.001 | <0.001 |
| hsCRP [mg/dL] | 0.66 [0.46–1.04] | 1.21 [0.62–1.67] | 1.73 [1.20–2.25] | <0.001 | <0.001 |
| Bilirubin [µmol/L] | 13.00 [9.90–17.60] | 11.00 [7.90–16.35] | 9.05 [6.93–14.03] | <0.05 | <0.01 |
| Uric acid [µmol/L] | 314.00 [296.00–367.00] | 387.00 [353.00–409.50] | 403.50 [364.75–439.00] | <0.001 | <0.001 |
| Fibrinogen [mg/dL] | 248.00 [220.00–297.00] | 256.00 [234.00–278.50] | 303.00 [260.25–334.00] | <0.01 | <0.01 |
| Ceruloplasmin [g/L] | 0.20 [0.18–0.22] | 0.22 [0.21–0.23] | 0.23 [0.21–0.25] | <0.001 | <0.001 |
| Variables | MHNW (n = 49) | MHO (n = 27) | MUO (n = 22) | p-Value * | p-Value ** |
|---|---|---|---|---|---|
| Adiponectin [mg/L] | 15.45 [8.85–26.03] | 7.18 [5.80–12.00] | 4.81 [3.10–8.89] | <0.001 | <0.001 |
| Adipsin [mg/L] | 1.37 [0.92–1.96] | 0.98 [0.81–1.24] | 1.03 [0.85–1.51] | <0.05 | <0.05 |
| Leptin [pg/mL] | 783.15 [409.82–1357.25] | 6086.88 [2505.89–9811.66] | 8464.22 [7045.08–13,405.24] | <0.001 | <0.001 |
| Leptin/Adiponectin ratio | 43.92 [27.47–108.77] | 577.85 [315.54–1374.92] | 2039.48 [843.05–3053.01] | <0.001 | <0.001 |
| Visfatin [pg/mL] | 14,961.90 [12,397.98–20,576.90] | 14,809.15 [13,715.20–19,280.44] | 15,319.60 [12,696.9–17,003.08] | >0.05 | >0.05 |
| Resistin [pg/mL] | 5933.41 [3821.07–8860.00] | 5400.22 [4198.69–8922.45] | 6329.89 [4354.88–9574.26] | >0.05 | >0.05 |
| Ghrelin [pg/mL] | 541.19 [401.98–847.85] | 476.62 [333.62–623.31] | 401.83 [304.74–481.09] | <0.01 | <0.001 |
| GIP [pg/mL] | 157.28 [121.10–168.27] | 151.70 [86.57–212.35] | 162.82 [129.16–198.10] | >0.05 | >0.05 |
| C-peptide [pg/mL] | 525.64 [416.93–742.86] | 1103.01 [872.32–2310.21] | 2453.77 [1471.87–3504.04] | <0.001 | <0.001 |
| GLP-1 [pg/mL] | 245.56 [155.74–324.58] | 205.62 [124.78–285.86] | 235.37 [185.01–307.65] | >0.05 | >0.05 |
| Glucagon [pg/mL] | 1445.99 [1159.42–1828.44] | 1540.74 [1224.63–1744.17] | 1480.60 [1226.07–1768.03] | >0.05 | >0.05 |
| Insulin [pg/mL] | 237.50 [183.22–274.48] | 449.05 [321.08–517.38] | 494.55 [251.64–674.10] | <0.001 | <0.001 |
| PAI-1 [pg/mL] | 77,353.49 [50,801.99–111,687.54] | 86,828.42 [48,879.62–133,191.15] | 92,513.11 [82,278.29–147,186.72] | >0.05 | >0.05 |
| ANGPTL3 [ng/mL] | 56.03 [46.44–71.64] | 68.30 [53.59–88.06] | 75.14 [58.74–95.57] | <0.05 | <0.01 |
| ANGPTL4 [ng/mL] | 181.17 [151.17–225.22] | 188.68 [121.99–243.08] | 185.71 [125.13–259.42] | >0.05 | >0.05 |
| Dependent Variable | Independent Variable | OR | 95% CI | p-Value |
|---|---|---|---|---|
| Adiponectin | MHO | 0.56 | 0.38–0.82 | <0.01 |
| MUO | 0.37 | 0.25–0.56 | <0.001 | |
| Adipsin | MHO | 0.76 | 0.61–0.94 | <0.05 |
| MUO | 0.85 | 0.68–1.07 | >0.05 | |
| Leptin | MHO | 6.75 | 4.69–9.73 | <0.001 |
| MUO | 12.52 | 8.47–18.51 | <0.001 | |
| Leptin/Adiponectin ratio | MHO | 0.08 | 0.05–0.14 | <0.001 |
| MUO | 0.03 | 0.02–0.05 | <0.001 | |
| Visfatin | MHO | 1.05 | 0.91–1.21 | >0.05 |
| MUO | 0.96 | 0.82–1.12 | >0.05 | |
| Resistin | MHO | 0.96 | 0.72–1.27 | >0.05 |
| MUO | 1.02 | 0.75–1.38 | >0.05 | |
| Ghrelin | MHO | 0.82 | 0.65–1.03 | >0.05 |
| MUO | 0.63 | 0.49–0.80 | <0.001 | |
| GIP | MHO | 0.94 | 0.77–1.15 | >0.05 |
| MUO | 1.01 | 0.81–1.26 | >0.05 | |
| C-peptide | MHO | 2.40 | 1.79–3.22 | <0.001 |
| MUO | 4.31 | 3.15–5.90 | <0.001 | |
| GLP-1 | MHO | 0.82 | 0.64–1.05 | >0.05 |
| MUO | 1.02 | 0.78–1.32 | >0.05 | |
| Glucagon | MHO | 1.07 | 0.92–1.23 | >0.05 |
| MUO | 1.01 | 0.86–1.17 | >0.05 | |
| Insulin | MHO | 1.67 | 1.35–2.07 | <0.001 |
| MUO | 1.88 | 1.49–2.36 | <0.001 | |
| PAI-1 | MHO | 1.10 | 0.75–1.62 | >0.05 |
| MUO | 1.72 | 1.14–2.60 | <0.05 | |
| ANGPTL3 | MHO | 1.23 | 1.01–1.49 | <0.05 |
| MUO | 1.28 | 1.05–1.58 | <0.05 | |
| ANGPTL4 | MHO | 1.02 | 0.85–1.22 | >0.05 |
| MUO | 0.99 | 0.82–1.19 | >0.05 |
| Dependent Variable | Covariates | Independent Variables | OR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Adiponectin | Age, smoking, alcohol consumption | MHO | 0.56 | 0.38–0.83 | <0.01 |
| MUO | 0.38 | 0.25–0.57 | <0.001 | ||
| Adipsin | Age, smoking, alcohol consumption | MHO | 0.75 | 0.61–0.93 | <0.05 |
| MUO | 0.88 | 0.70–1.11 | >0.05 | ||
| Leptin | Age, smoking, alcohol consuption | MHO | 6.69 | 4.62–9.70 | <0.001 |
| MUO | 12.52 | 8.40–18.65 | <0.001 | ||
| Leptin/Adiponectin ratio | Age, smoking, alcohol consumption | MHO | 0.08 | 0.05–0.14 | <0.001 |
| MUO | 0.03 | 0.02–0.05 | <0.001 | ||
| Ghrelin | Age, smoking, alcohol consumption | MHO | 0.81 | 0.65–1.02 | >0.05 |
| MUO | 0.65 | 0.51–0.83 | <0.001 | ||
| C-peptide | Age, smoking, alcohol consumption | MHO | 2.40 | 1.79–3.24 | <0.001 |
| MUO | 4.21 | 3.06–5.80 | <0.001 | ||
| Insulin | Age, smoking, alcohol consumption | MHO | 1.67 | 1.34–2.07 | <0.001 |
| MUO | 1.88 | 1.49–2.38 | <0.001 | ||
| PAI-1 | Age, smoking, alcohol consumption | MHO | 1.07 | 0.73–1.58 | >0.05 |
| MUO | 1.76 | 1.16–2.67 | <0.01 | ||
| ANGPTL3 | Age, smoking, alcohol consumption | MHO | 1.23 | 1.01–1.50 | <0.05 |
| MUO | 1.27 | 1.03–1.57 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lejawa, M.; Osadnik, K.; Czuba, Z.; Osadnik, T.; Pawlas, N. Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study. Life 2021, 11, 1350. https://doi.org/10.3390/life11121350
Lejawa M, Osadnik K, Czuba Z, Osadnik T, Pawlas N. Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study. Life. 2021; 11(12):1350. https://doi.org/10.3390/life11121350
Chicago/Turabian StyleLejawa, Mateusz, Kamila Osadnik, Zenon Czuba, Tadeusz Osadnik, and Natalia Pawlas. 2021. "Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study" Life 11, no. 12: 1350. https://doi.org/10.3390/life11121350
APA StyleLejawa, M., Osadnik, K., Czuba, Z., Osadnik, T., & Pawlas, N. (2021). Association of Metabolically Healthy and Unhealthy Obesity Phenotype with Markers Related to Obesity, Diabetes among Young, Healthy Adult Men. Analysis of MAGNETIC Study. Life, 11(12), 1350. https://doi.org/10.3390/life11121350







