Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedures
2.2.1. Sleep Fragmentation and Collection of Fecal Samples
2.2.2. Sleep Fragmentation and Tissue Collection
2.3. Statistics
3. Results
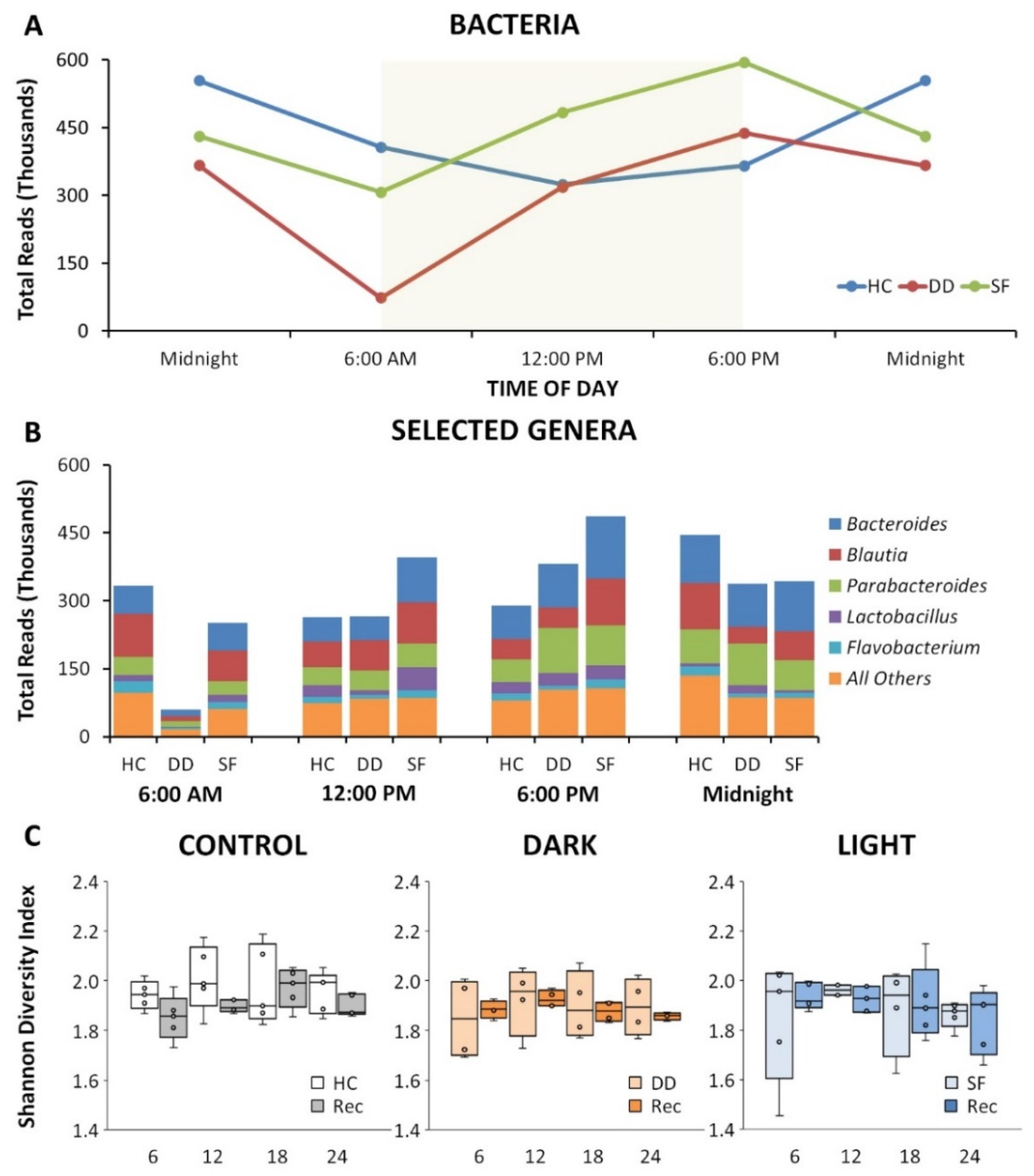
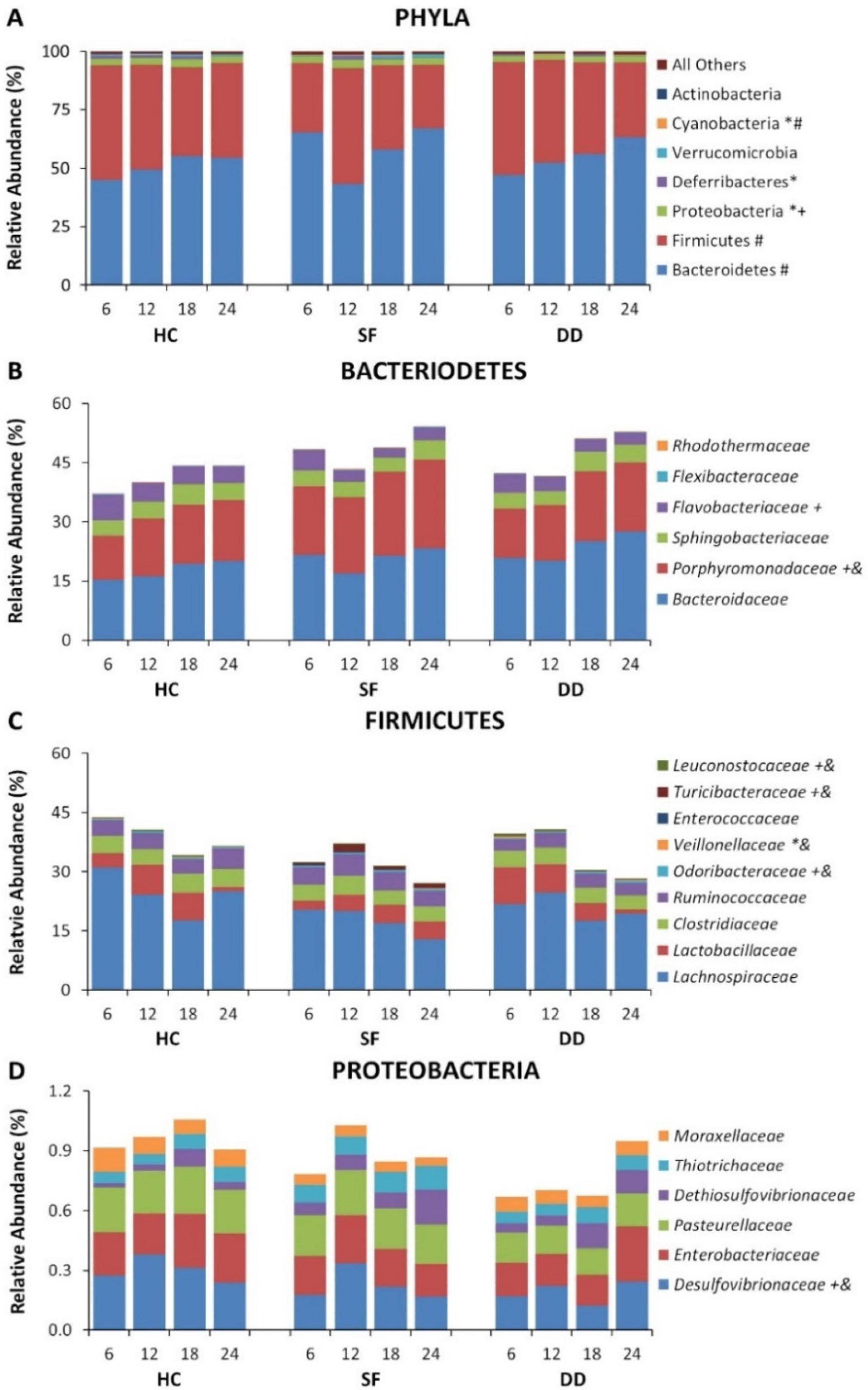
3.1. SF and DD Produce Different Changes in Gut Microbiota
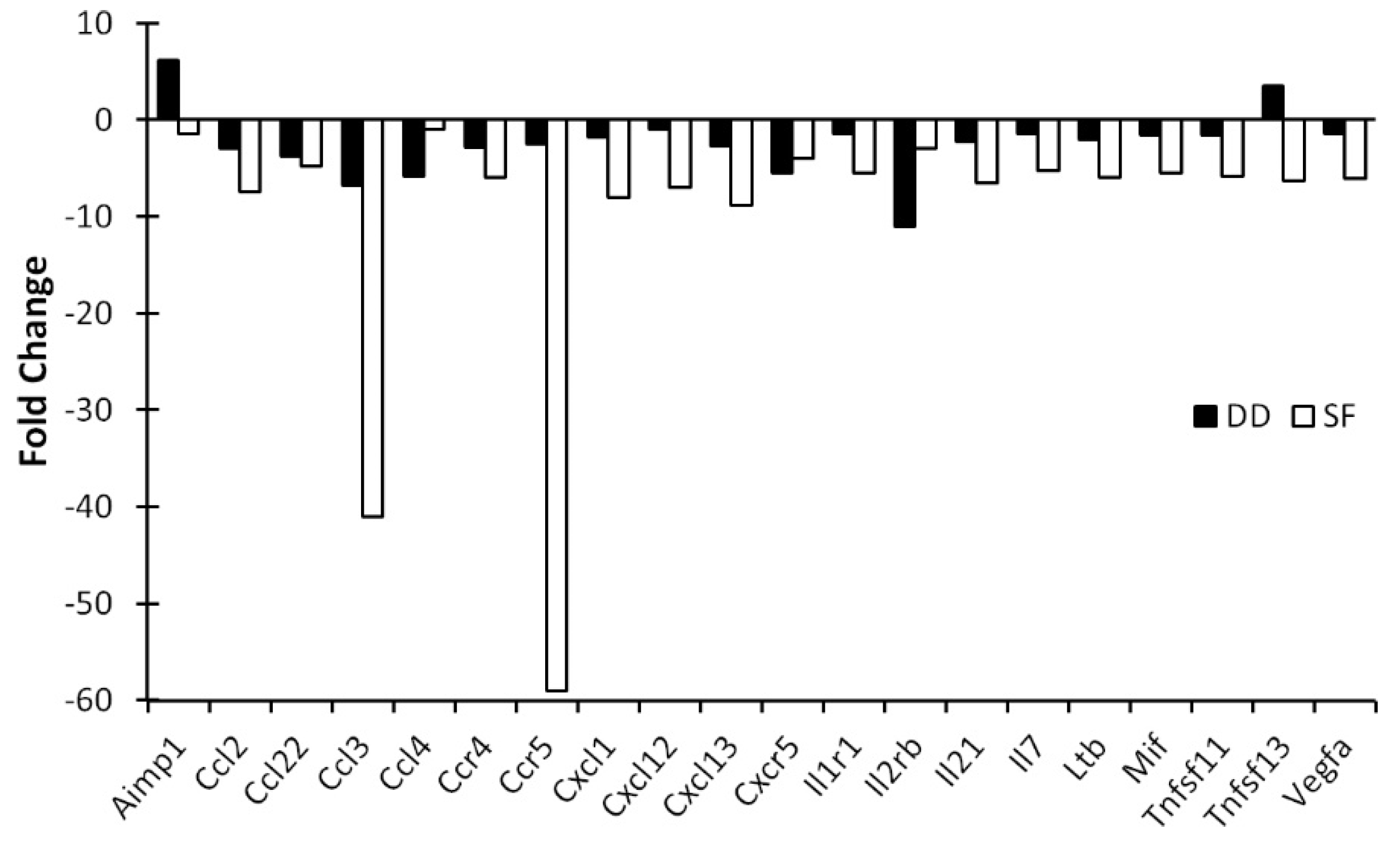
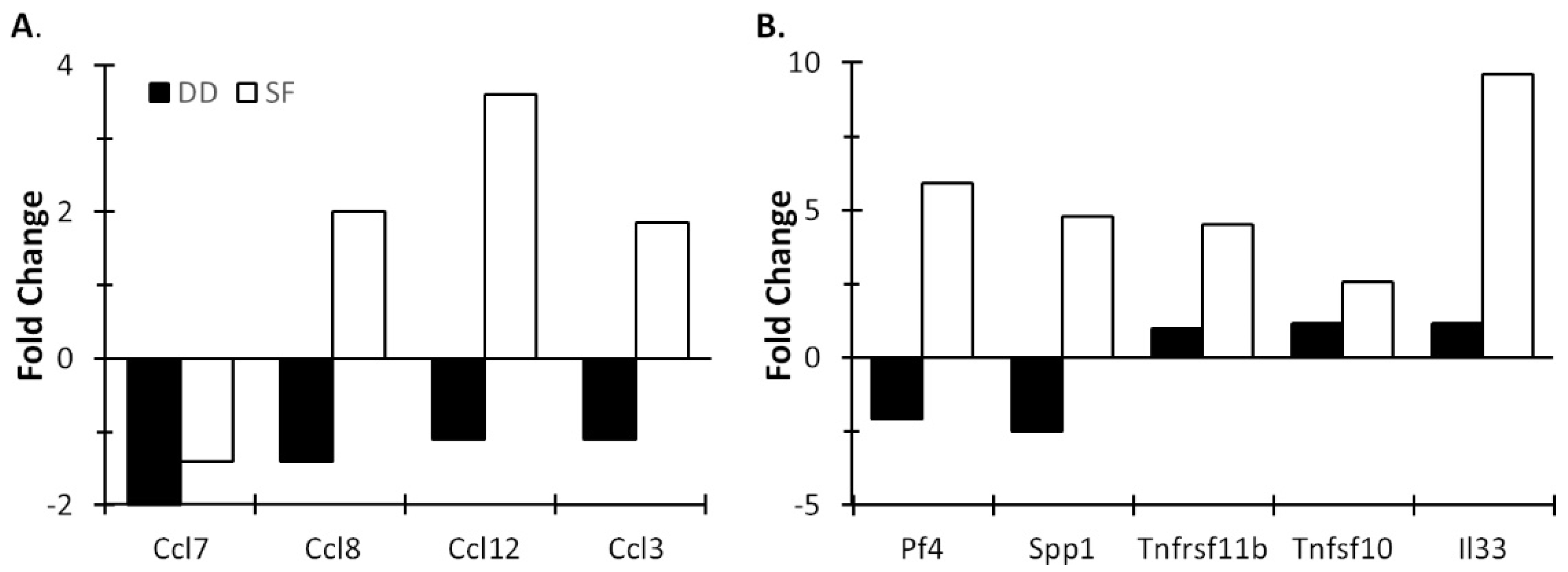
3.2. Light and DD Produce Different Effects on Mucosal and Central Immune System
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayer, E.A.; Knight, R.; Mazmanian, S.K.; Cryan, J.F.; Tillisch, K. Gut microbes and the brain: Paradigm shift in neuroscience. J. Neurosci. 2014, 34, 15490–15496. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. Quart. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Durgan, D.J.; Ganesh, B.P.; Cope, J.L.; Ajami, N.J.; Phillips, S.C.; Petrosino, J.F.; Hollister, E.B.; Bryan, R.M., Jr. Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 2016, 67, 469–474. [Google Scholar] [CrossRef]
- Jackson, M.L.; Butt, H.; Ball, M.; Lewis, D.P.; Bruck, D. Sleep quality and the treatment of intestinal microbiota imbalance in Chronic Fatigue Syndrome: A pilot study. Sleep Sci. 2015, 8, 124–133. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Benedict, C.; Vogel, H.; Jonas, W.; Woting, A.; Blaut, M.; Schurmann, A.; Cedernaes, J. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 2016, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Nair, D.; Zhang, S.X.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-alpha pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef]
- Hakim, F.; Wang, Y.; Zhang, S.X.; Zheng, J.; Yolcu, E.S.; Carreras, A.; Khalyfa, A.; Shirwan, H.; Almendros, I.; Gozal, D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014, 74, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Almendros, I.; Hakim, F. Sleep apnea awakens cancer: A unifying immunological hypothesis. Oncoimmunology 2014, 3, e28326. [Google Scholar] [CrossRef] [PubMed]
- Trammell, R.A.; Verhulst, S.; Toth, L.A. Effects of sleep fragmentation on sleep and markers of inflammation in mice. Comp. Med. 2014, 64, 13–24. [Google Scholar] [PubMed]
- Trammell, R.A.; Toth, L.A. Behavioral perturbation and sleep in healthy and virus-infected inbred mice. Comp. Med. 2014, 64, 283–292. [Google Scholar]
- Everson, C.A.; Toth, L.A. Systemic bacterial invasion induced by sleep deprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R905–R916. [Google Scholar] [CrossRef]
- Everson, C.A. Sustained sleep deprivation impairs host defense. Am. J. Physiol. 1993, 265, R1148–R1154. [Google Scholar] [CrossRef]
- Mezick, E.J.; Matthews, K.A.; Hall, M.; Kamarck, T.W.; Buysse, D.J.; Owens, J.F.; Reis, S.E. Intra-individual variability in sleep duration and fragmentation: Associations with stress. Psychoneuroendocrinology 2009, 34, 1346–1354. [Google Scholar] [CrossRef]
- Miller, M.A. The role of sleep and sleep disorders in the development, diagnosis, and management of neurocognitive disorders. Front. Neurol. 2015, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Zhang, S.X.; Ramesh, V.; Hakim, F.; Kaushal, N.; Wang, Y.; Gozal, D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 2011, 184, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Carreras, A.; Lee, S.; Hakim, F.; Zhang, S.X.; Nair, D.; Ye, H.; Gozal, D. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity 2014, 22, 758–762. [Google Scholar] [CrossRef]
- Balzan, S.; de Almeida Quadros, C.; de Cleva, R.; Zilberstein, B.; Cecconello, I. Bacterial translocation: Overview of mechanisms and clinical impact. J. Gastroenterol. Hepatol. 2007, 22, 464–471. [Google Scholar] [CrossRef]
- Deitch, E.A. Bacterial translocation or lymphatic drainage of toxic products from the gut: What is important in human beings? Surgery 2002, 131, 241–244. [Google Scholar] [CrossRef]
- O’Boyle, C.J.; MacFie, J.; Mitchell, C.J.; Johnstone, D.; Sagar, P.M.; Sedman, P.C. Microbiology of bacterial translocation in humans. Gut 1998, 42, 29–35. [Google Scholar] [CrossRef]
- O’Boyle, C.J.; MacFie, J.; Dave, K.; Sagar, P.S.; Poon, P.; Mitchell, C.J. Alterations in intestinal barrier function do not predispose to translocation of enteric bacteria in gastroenterologic patients. Nutrition 1998, 14, 358–362. [Google Scholar] [CrossRef]
- Magnotti, L.J.; Upperman, J.S.; Xu, D.Z.; Lu, Q.; Deitch, E.A. Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann. Surg. 1998, 228, 518–527. [Google Scholar] [CrossRef]
- Woodcock, N.P.; Robertson, J.; Morgan, D.R.; Gregg, K.L.; Mitchell, C.J.; MacFie, J. Bacterial translocation and immunohistochemical measurement of gut immune function. J. Clin. Pathol. 2001, 54, 619–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Proost, P.; Wuyts, A.; van Damme, J. The role of chemokines in inflammation. Int. J. Clin. Lab. Res. 1996, 26, 211–223. [Google Scholar] [CrossRef]
- Spagnolo, P.; Renzoni, E.A.; Wells, A.U.; Copley, S.J.; Desai, S.R.; Sato, H.; Grutters, J.C.; Abdallah, A.; Taegtmeyer, A.; du Bois, R.M.; et al. C-C chemokine receptor 5 gene variants in relation to lung disease in sarcoidosis. Am. J. Respir. Crit. Care Med. 2005, 172, 721–728. [Google Scholar] [CrossRef]
- Samson, M.; Labbe, O.; Mollereau, C.; Vassart, G.; Parmentier, M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 1996, 35, 3362–3367. [Google Scholar] [CrossRef] [PubMed]
- Raport, C.J.; Gosling, J.; Schweickart, V.L.; Gray, P.W.; Charo, I.F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1beta, and MIP-1alpha. J. Biol. Chem. 1996, 271, 17161–17166. [Google Scholar] [CrossRef] [PubMed]
- Combadiere, C.; Ahuja, S.K.; Tiffany, H.L.; Murphy, P.M. Cloning and functional expression of CC CKR5, a human monocyte CC chemokine receptor selective for MIP-1(alpha), MIP-1(beta), and RANTES. J. Leukoc. Biol. 1996, 60, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Perez-Matute, P.; Perez-Martinez, L.; Aguilera-Lizarraga, J.; Blanco, J.R.; Oteo, J.A. Maraviroc modifies gut microbiota composition in a mouse model of obesity: A plausible therapeutic option to prevent metabolic disorders in HIV-infected patients. Publ. Soc. Esp. Quimioter. 2015, 28, 200–206. [Google Scholar]
- McDonald, K.G.; McDonough, J.S.; Dieckgraefe, B.K.; Newberry, R.D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am. J. Pathol. 2010, 176, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Meek, B.; Doi, Y.; Muramatsu, M.; Chiba, T.; Honjo, T.; Fagarasan, S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc. Natl. Acad. Sci. USA 2004, 101, 1981–1986. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Honjo, T. Regulation of IgA synthesis at mucosal surfaces. Curr. Opin. Immunol. 2004, 16, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Bouskra, D.; Brezillon, C.; Berard, M.; Werts, C.; Varona, R.; Boneca, I.G.; Eberl, G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008, 456, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Sarafi, M.N.; Garcia-Zepeda, E.A.; MacLean, J.A.; Charo, I.F.; Luster, A.D. Murine monocyte chemoattractant protein (MCP)-5: A novel CC chemokine that is a structural and functional homologue of human MCP-1. J. Exp. Med. 1997, 185, 99–109. [Google Scholar] [CrossRef]
- Ohga, E.; Tomita, T.; Wada, H.; Yamamoto, H.; Nagase, T.; Ouchi, Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J. Appl. Physiol. 2003, 94, 179–184. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.H.; Park, C.S.; Kim, B.G.; Kim, S.W.; Cho, J.H. Plasma levels of MCP-1 and adiponectin in obstructive sleep apnea syndrome. Arch. Otolaryngol.-Head Neck Surg. 2010, 136, 896–899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mojsilovic-Petrovic, J.; Callaghan, D.; Cui, H.; Dean, C.; Stanimirovic, D.B.; Zhang, W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J. Neuroinflamm. 2007, 4, 12. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef]
- Liew, F.Y. IL-33: A Janus cytokine. Ann. Rheum. Dis. 2012, 71 (Suppl. 2), i101–i104. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Smirnov, I.; Zheng, J.; Kipnis, J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 2015, 85, 703–709. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Powell, N.D.; Godbout, J.P.; Sheridan, J.F. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 13820–13833. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Opp, M.R. Sleeping to fuel the immune system: Mammalian sleep and resistance to parasites. BMC Evol. Biol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Krueger, J.M.; Majde, J.A. Sleep as a host defense: Its regulation by microbial products and cytokines. Clin. Immunol. Immunopathol. 1990, 57, 188–199. [Google Scholar] [CrossRef]
- Toth, L.A.; Tolley, E.A.; Krueger, J.M. Sleep as a prognostic indicator during infectious disease in rabbits. Proc. Soc. Exp. Biol. Med. 1993, 203, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Karnovsky, M.L. Sleep as a neuroimmune phenomenon: A brief historical perspective. Adv. Neuroimmunol. 1995, 5, 5–12. [Google Scholar] [CrossRef]
- Irwin, M.R.; Opp, M.R. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2017, 42, 129–155. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best practice & research. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef]
- Opp, M.R.; George, A.; Ringgold, K.M.; Hansen, K.M.; Bullock, K.M.; Banks, W.A. Sleep fragmentation and sepsis differentially impact blood-brain barrier integrity and transport of tumor necrosis factor-alpha in aging. Brain Behav. Immun. 2015, 50, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Alvarado, G.; Pavon, L.; Castillo-Garcia, S.A.; Hernandez, M.E.; Dominguez-Salazar, E.; Velazquez-Moctezuma, J.; Gomez-Gonzalez, B. Sleep loss as a factor to induce cellular and molecular inflammatory variations. Clin. Dev. Immunol. 2013, 2013, 801341. [Google Scholar] [CrossRef]
- Patel, S.R.; Zhu, X.; Storfer-Isser, A.; Mehra, R.; Jenny, N.S.; Tracy, R.; Redline, S. Sleep duration and biomarkers of inflammation. Sleep 2009, 32, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Committee on Sleep Medicine and Research. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem; National Academies Press (US): Washington, DC, USA, 2006. [Google Scholar]
- Luyster, F.S.; Strollo, P.J., Jr.; Zee, P.C.; Walsh, J.K. Sleep: A health imperative. Abstract Supplement. Sleep 2012, 35, 727–734. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Young, V.B. The intestinal microbiota in health and disease. Curr. Opin. Gastroenterol. 2012, 28, 63–69. [Google Scholar] [CrossRef]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- Krueger, J.M.; Opp, M.R. Sleep and microbes. Int. Rev. Neurobiol. 2016, 131, 207–225. [Google Scholar] [CrossRef]
- Parekh, P.J.; Oldfield, E.C.T.; Johnson, D.A. The effects of sleep on the commensal microbiota: Eyes wide open? J. Clin. Gastroenterol. 2018, 52, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Conterno, L.; Fava, F.; Viola, R.; Tuohy, K.M. Obesity and the gut microbiota: Does up-regulating colonic fermentation protect against obesity and metabolic disease? Genes Nutr. 2011, 6, 241–260. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Lagier, J.C.; Yahav, D.; Paul, M. Gut bacterial microbiota and obesity. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2013, 19, 305–313. [Google Scholar] [CrossRef]
- Gonzalez, A.; Stombaugh, J.; Lozupone, C.; Turnbaugh, P.J.; Gordon, J.I.; Knight, R. The mind-body-microbial continuum. Dialogues Clin. Neurosci. 2011, 13, 55–62. [Google Scholar] [PubMed]
- Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis in health and disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Regulation of the stress response by the gut microbiota: Implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012, 37, 1369–1378. [Google Scholar] [CrossRef]
- Runtsch, M.C.; Hu, R.; Alexander, M.; Wallace, J.; Kagele, D.; Petersen, C.; Valentine, J.F.; Welker, N.C.; Bronner, M.P.; Chen, X.; et al. MicroRNA-146a constrains multiple parameters of intestinal immunity and increases susceptibility to DSS colitis. Oncotarget 2015, 6, 28556–28572. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Dignass, A.U. Intestinal barrier function. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 685–694. [Google Scholar] [CrossRef]
- Chen, Y.X.; Lai, L.N.; Zhang, H.Y.; Bi, Y.H.; Meng, L.; Li, X.J.; Tian, X.X.; Wang, L.M.; Fan, Y.M.; Zhao, Z.F.; et al. Effect of artesunate supplementation on bacterial translocation and dysbiosis of gut microbiota in rats with liver cirrhosis. World J. Gastroenterol. 2016, 22, 2949–2959. [Google Scholar] [CrossRef]
- Tang, X.; Orchard, S.M.; Liu, X.; Sanford, L.D. Effect of varying recording cable weight and flexibility on activity and sleep in mice. Sleep 2004, 27, 803–810. [Google Scholar] [PubMed]
- Tang, X.; Sanford, L.D. Telemetric recording of sleep and home cage activity in mice. Sleep 2002, 25, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Bai, L.; Goel, N.; Bailey, A.; Jang, C.J.; Bushman, F.D.; Meerlo, P.; Dinges, D.F.; Sehgal, A. Human and rat gut microbiome composition is maintained following sleep restriction. Proc. Natl. Acad. Sci. USA 2017, 114, E1564–E1571. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.J.; Oldfield, E.C.t.; Johnson, D.A. Wake-up call to clinicians: The impact of sleep dysfunction on gastrointestinal health and disease. J. Clin. Gastroenterol. 2018, 52, 194–203. [Google Scholar] [CrossRef]
- Sanford, L.D.; Ciavarra, R.P. Sleep, immune function, and the gut microbiome: Pathophysiologic translational implications. In Sleep Effect on Gastrointestinal Health and Disease: Opportunities for Promoting Health and Optimizing Disease Management; Johnson, D.A., Orr, W.C., Ware, J.C., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018; pp. 17–40. [Google Scholar]
- Decuypere, S.; Meehan, C.J.; Van Puyvelde, S.; De Block, T.; Maltha, J.; Palpouguini, L.; Tahita, M.; Tinto, H.; Jacobs, J.; Deborggraeve, S. Diagnosis of bacterial bloodstream infections: A 16S metagenomics approach. PLoS Negl. Trop. Dis. 2016, 10, e0004470. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanford, L.D.; Wellman, L.L.; Ciavarra, R.P.; Oldfield, E.C.; Shams, R.; Copare, J.L.; Johnson, D.A. Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice. Life 2021, 11, 1283. https://doi.org/10.3390/life11121283
Sanford LD, Wellman LL, Ciavarra RP, Oldfield EC, Shams R, Copare JL, Johnson DA. Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice. Life. 2021; 11(12):1283. https://doi.org/10.3390/life11121283
Chicago/Turabian StyleSanford, Larry D., Laurie L. Wellman, Richard P. Ciavarra, Edward C. Oldfield, Rouzbeh Shams, Jennifer L. Copare, and David A. Johnson. 2021. "Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice" Life 11, no. 12: 1283. https://doi.org/10.3390/life11121283
APA StyleSanford, L. D., Wellman, L. L., Ciavarra, R. P., Oldfield, E. C., Shams, R., Copare, J. L., & Johnson, D. A. (2021). Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice. Life, 11(12), 1283. https://doi.org/10.3390/life11121283






