Diagnostic and Prognostic Value of a TDI-Derived Systolic Wall Motion Analysis as a Screening Modality for Allograft Rejection after Heart Transplantation
Abstract
:1. Introduction
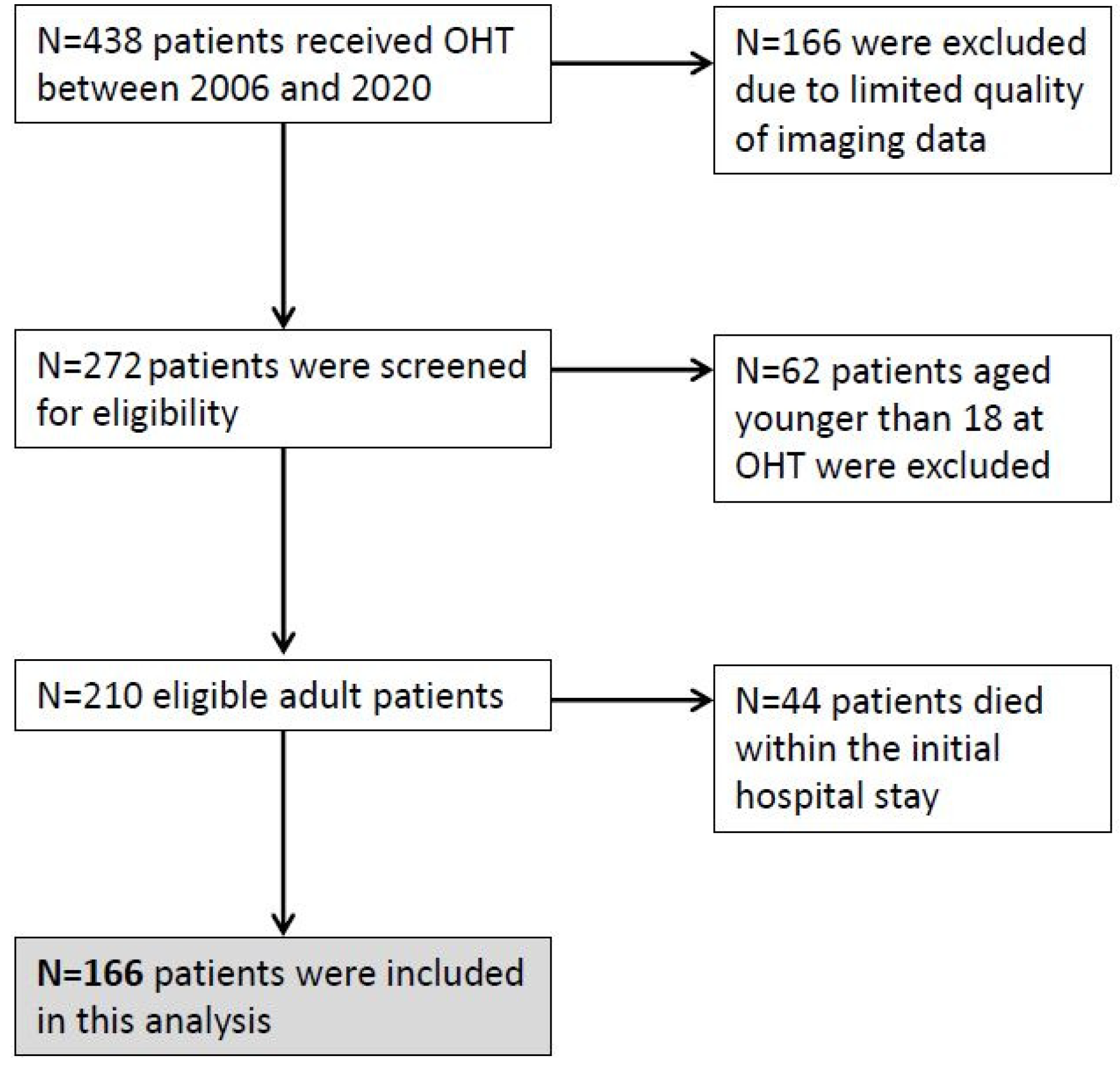
2. Materials and Methods
3. Results
3.1. Patient Characteristics
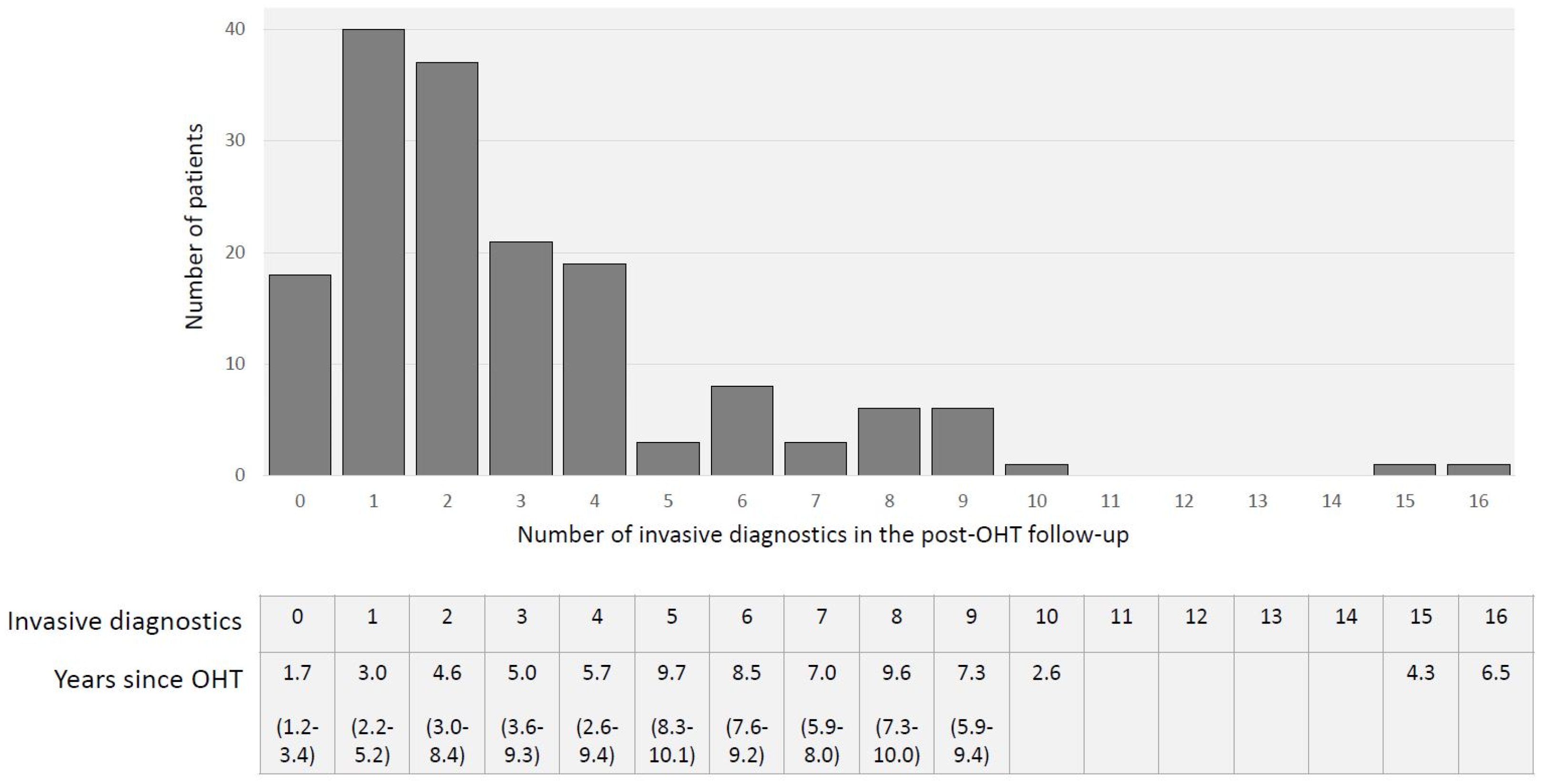
3.2. Invasive Diagnostics in OHT Follow-Up
3.3. Mortality, AR and CAV
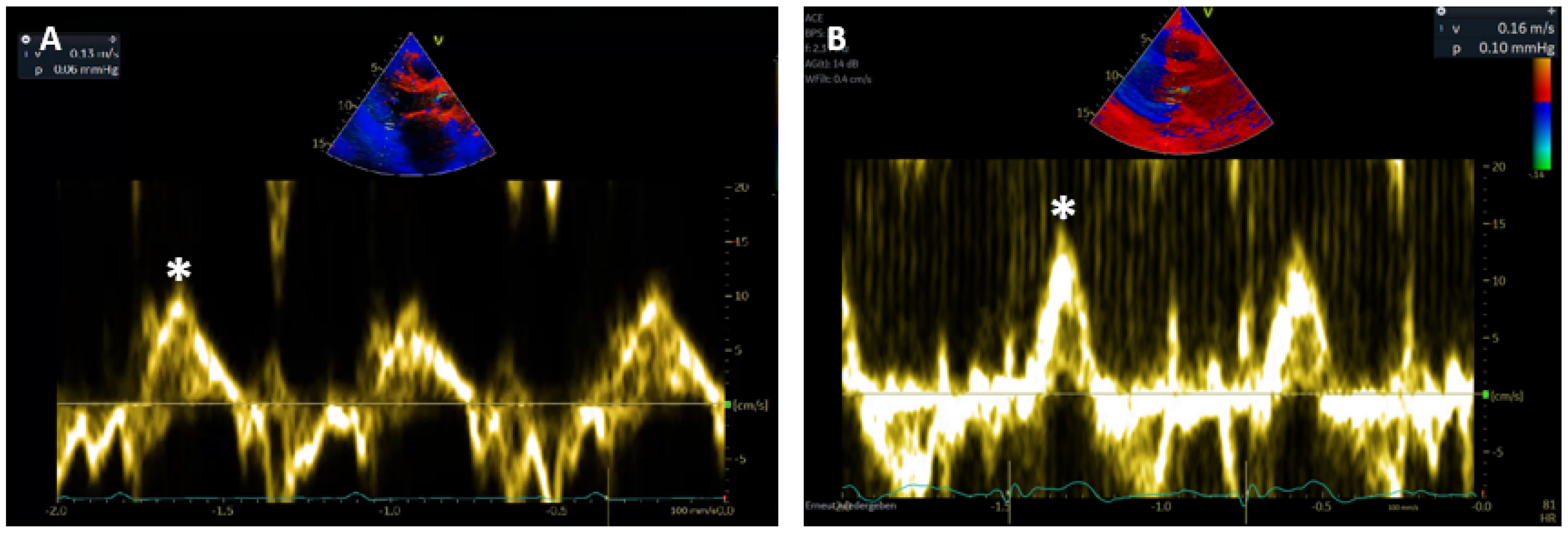
3.4. Sm Course after OHT
3.5. Predictive Value of Sm
3.6. Prognostic Value of Sm
4. Discussion
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chambers, D.C.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hyes, D.J.; Hsish, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic-Organ-Transplant-Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult heart transplantation report—2019; focus theme: Donor and recipient size match. J. Heart Lung Transplant. 2019, 38, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report—2017; Focus theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1037–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S. Allograft vasculopathy: The Achilles’ heel of heart transplantation. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Constanzo, M.R.; Dipchand, A.; Starling, R.; Anderson, A.; Chan, M.; Desai, S.; Fedson, S.; Fisher, P.; Gonzales-Stawinski, G.; Martinelli, L.; et al. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2010, 29, 914–956. [Google Scholar] [CrossRef] [PubMed]
- From, A.M.; Maleszewski, J.J.; Rihal, C.S. Current status of endomyocardial biopsy. Mayo Clin. Proc. 2011, 86, 1095–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, C.K.; Koerner, M.; THohan, V.; Torre-Amione, G. Frequent surveillance biopsies do not improve survival following heart transplantation. J. Heart Lung Transplant. 2004, 23, S74. [Google Scholar] [CrossRef]
- Stewart, S.; Winters, G.L.; Fishbein, M.C.; Tazelaar, H.D.; Kobashigawa, J.; Abrams, J.; Andersen, C.B.; Angelini, A.; Berry, G.J.; Burke, M.M.; et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J. Heart Lung Transplant. 2005, 24, 1710. [Google Scholar] [CrossRef]
- Berry, G.J.; Burke, M.M.; Andersen, C.; Bruneval, P.; Fedrigo, M.; Fishbein, M.C.; Goddard, M.; Hammond, E.H.; Leone, O.; Marboe, C.; et al. The 2013 International Society for Heart and Lung Transplantation working formulation for the standardization of nomenclature in the pathologic diagnosis of antibody-mediated rejection in heart transplantation. J. Heart Lung Transplant. 2013, 32, 1147–1162. [Google Scholar] [CrossRef]
- Tsutsui, H.; Ziada, K.M.; Schoenhagen, P.; Iyisoy, A.; Magyar, W.A.; Crowe, T.D.; Klingensmith, J.D.; Vince, D.G.; Rincon, G.; Hobbs, R.E.; et al. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: Results from a 5-year serial intravascular ultrasound study. Circulation 2001, 104, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Hirohata, A.; Nakamura, M.; Waseda, K.; Honda, Y.; Lee, D.P.; Vagelos, R.H.; Hunt, S.A.; Valantine, H.A.; Yock, P.G.; Fitzgerald, P.J.; et al. Changes in coronary anatomy and physiology after heart transplantation. Am. J. Cardiol. 2007, 99, 1603–1607. [Google Scholar] [CrossRef] [Green Version]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantationworking formulation of a standardized nomenclature forcardiac allograft vasculopathy—2010. J. Heart Lung Transplant. 2010, 29, 717–727. [Google Scholar] [CrossRef]
- Dandel, M.; Hummel, M.; Mueller, J.; Wellnhofer, E.; Meyer, R.; Solowjowa, N.; Ewert, R.; Hetzer, R. Reliability of tissue Doppler wall motion monitoring after heart transplantation for replacement of invasve routine screenings by optimally timed cardiac biopsies and catheterizations. Circulation 2001, 104, I184–I191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dandel, M.; Hummel, M.; Mueller, R.; Ewert, R.; Hetzer, R. Wall motion assessment by tissue Doppler imaging after heart transplantation: Timing of endomyocardial biopsies and facilitation of therapeutic decisions during acute cardiac rejection. J. Heart Lung Transplant. 2001, 20, 213. [Google Scholar] [CrossRef]
- Dandel, M.; Hummel, M.; Wellnhofer, E.; Mueller, J.; Ewert, R.; Hetzer, R. Usefulness of post-transplant wall motion analysis by tissue Doppler imaging in patients with cardiac allograft vasculopathy: Evaluation of functional severity and timing of follow-up cardiac catheterizations. J. Heart Lung Transplant. 2001, 20, 151. [Google Scholar] [CrossRef]
- Sinphurmsukskul, S.; Ariyachaipanich, A.; Siwamogsatham, S.; Thammanatsakul, K.; Puwanant, S.; Benjacholamas, V.; Ongcharit, P. Endomyocardial biopsy and prevalence of acute cellular rejection in heart transplantation. Transplant. Proc. 2021, 53, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Weckbach, L.; Maurer, U.; Schramm, R.; Huber, B.C.; Lackermair, K.; Weiss, M.; Meiser, B.; Hagl, C.; Massberg, S.; Eifert, S.; et al. Lower frequency routine surveillance endomyocardial biopsies after heart transplantation. PLoS ONE 2017, 12, e0182880. [Google Scholar] [CrossRef]
- Stehlik, J.; Starling, R.C.; Movsesian, M.A.; Fang, J.C.; Brown, R.N.; Hess, M.L.; Lewis, N.P.; Kirklin, J.K.; Cardiac Transplant Database Group. Utility of long-term surveillance endomyocardial biopsy: A multi-institutional analysis. J. Heart Lung Transplant. 2006, 25, 1402–1409. [Google Scholar] [CrossRef]
- Orrego, C.M.; Cordero-Reyes, A.M.; Estep, J.D.; Loebe, M.; Torre-Amione, G. Usefulness of routine surveillance endomyocardial biopsy 6 months after heart transplantation. J. Heart Lung Transplant. 2012, 31, 845–849. [Google Scholar] [CrossRef]
- Suarez-Pierre, A.; Lui, C.; Zhou, X.; Fraser, C.D.; Ferrigno, A.S.; Etchill, E.; Giuliano, K.; Higgins, R.S.; Choi, C.W.; Kilic, A. Conditional survival in heart transplantation: An organ procurement and transplantation network database analysis. Ann. Thorac. Surg. 2020, 110, 1339–1347. [Google Scholar] [CrossRef]
- Suarez-Pierre, A.; Zhou, X.; Fraser, C.D.; Grimm, J.C.; Crawford, T.C.; Lui, C.; Valero, V.; Choi, C.W.; Higgins, R.S.; Kilic, A. Survival and functional status after bridge-to-transplant with left ventricular assist device. ASAIO J. 2019, 65, 661–667. [Google Scholar] [CrossRef]
- Sammani, A.; Wind, A.M.; Kirkels, J.H.; Kloepping, C.; Buijsrogge, M.P.; Ramjakhan, F.Z.; Asselbergs, F.W.; de Jonge, N. Thirty years of heart transplantation at the Univercity Medial Centre Utrecht. Neth. Heart J. 2017, 25, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellgren, G.; Geiran, O.; Lemstroem, K.; Gustafsson, F.; Eiskjaer, H.; Koul, B.; Hagermann, I.; Selimovic, N. Nordic Thoracic Transplant Study Group. Three decades of heart transplantation in Scandinavia:long-term follow-up. Eur. J. Heart Fail. 2013, 15, 308–315. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Marcondes Braga, F.G.; Binder, T.; et al. European association of cardiovascular imaging/cardiovascular imaging department of the Brazilian society of cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef] [PubMed]
- Ghali, M.G.Z.; Stewart, R.; Ghali, G.Z.; Blitzer, W. Two dimensional speckle tracking echocardiography detects cardiac allograft stage III vasculopathy in recipients of heart transplants with preserved systolic function. Acta Cardiol. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Logstrup, B.B.; Eiskjaer, H.; Poulsen, S.H. Evaluation of longitudinal myocardial deformation by 2-dimentional speckle tracking echocardiography in heart transplant recipients: Relation to coronary artery vasculopathy. J. Heart Lung Transplant. 2015, 34, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Pichler, P.; Binder, T.; Höfer, P.; Bergler-Klein, J. Two-dimensional speckle tracking echocardiography in heart transplant patients: Three-year Follow-up of deformation parameters and ejection fraction derived from transthoracic echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 181–186. [Google Scholar] [CrossRef] [Green Version]


| Recipient | |
| Age at OHT | 46.2 (±11.4) |
| Sex (male) | 127 (76.5) |
| BMI in kg/m² | 25.2 (±4.0) |
| Waiting list status | |
| Highly urgent | 146 (88.0) |
| Urgent (until February 2011) | 4 (2.4) |
| Transplantable | 16 (9.6) |
| Diagnosis leading to OHT | |
| DCM | 114 (68.7) |
| HCM | 4 (2.4) |
| RCM | 0 |
| ARVC | 3 (1.8) |
| IHD | 35 (21.1) |
| Other * | 10 (6.0) |
| Previous VAD | 84 (50.6) |
| LVAD | 78 (47.0) |
| RVAD | 1 (0.6) |
| BVAD | 6 (3.6) |
| Concomitant disease | |
| CKD | 115 (69.3) |
| DM | 26 (15.7) |
| Type 1 | 4 (2.4) |
| Type 2 | 22 (13.3) |
| HLP | 64 (38.6) |
| Former smoker | 38 (22.9) |
| Donor | |
| Age at donation | 40.8 (±14.1) |
| Sex (male) | 107 (64.5) |
| BMI in kg/m² | 25.2 (±3.7) |
| Echocardiography | |
| LV hypertrophy | 12 (7.2) |
| Concomitant disease | |
| Hypertension | 22 (13.3) |
| CAD | 2 (1.2) |
| DM | 6 (3.6) |
| Former smoker | 26 (15.7) |
| Alcohol abuse | 16 (9.6) |
| Drug abuse † | 7 (4.2) |
| Cardiopulmonary resuscitation | 20 (12.0) |
| Transplantation | |
| Combined OHT | |
| Heart–kidney | 3 (1.8) |
| Ischemic time in min | 259.9 (±63.3) |
| ICU stay in days | 26.7 (±36.3) |
| Hospital stay in days | 62.3 (±50.7) |
| Inotropic support in days | 8 (5–12) |
| Mechanical ventilation in days | 5 (2–18) |
| GFR at discharge in ml/min | |
| >90 | 67 (40.2) |
| 60–89 | 35 (21.1) |
| 40–59 | 22 (13.3) |
| 30–44 | 19 (11.4) |
| 15–29 | 8 (4.8) |
| RRT | 16 (9.6) |
| Immunosuppression induction | |
| Cyclosporine | 162 (97.2) |
| Tacrolimus | 4 (2.4) |
| Mycophenolate mofetil | 9 (5.4) |
| Methylprednisolone | 166 (100.0) |
| Antithymocyte globulin | 100 (60.2) |
| Other § | 4 (2.4) |
| Immunosuppression at discharge | |
| Cyclosporine | 92 (55.4) |
| Tacrolimus | 71 (42.8) |
| Everolimus | 19 (11.4) |
| Mycophenolate mofetil | 132 (79.5) |
| Methylprednisolone | 164 (98.8) |
| Unknown | 2 (1.2) |
| CAV Grade | ISHLT CAV1 n = 5 | ISHLT CAV2 n = 4 | ISHLT CAV3 n = 20 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | CAV1 | Change | Pre | CAV2 | Change | Pre | CAV3 | Change | (CI) p value | |
| Sm rad cm/s | 10.7 | 9.9 | −0.8 | 11.0 | 10.3 | 0.8 | 10.5 | 9.3 | 1.3 | (0.23–2.43) |
| (±1.2) | (±0.7) | (±1.69) | (±2.2) | (±1.5) | (±2.2) | (±1.9) | (±2.9) | (±2.5) | 0.017 | |
| Sm long cm/s | 11.0 | 9.2 | −1.8 | 10.8 | 10.8 | 0 | 10.6 | 9.5 | 1.4 | (0.21–2.66) |
| (±1.4) | (±0.7) | (±1.3) | (±2.2) | (±2.2) | (±2.5) | (±2.2) | (±2.6) | (±2.8) | 0.021 | |
| LV-EF % | 60.0 | 56.0 | −4.0 | 58.8 | 55.5 | −3.75 | 56.9 | 52.1 | 4.8 | (0.42–9.18) |
| (±3.2) | (±11.6) | (±8.6) | (±4.1) | (±3.5) | (±1.1) | (±6.7) | (±12.0) | (±10.0) | 0.031 | |
| ACR grade | ISHLT 1R n = 84 | ISHLT 2R n = 13 | ISHLT 3R n = 8 | |||||||
| pre | 1R | change | (CI) p value | pre | 2R | change | pre | 3R | change | |
| Sm rad cm/s | 11.3 | 9.6 | −1.7 | (1.32–2.08) | 11.3 | 9.1 | −1.9 | 11.4 | 9.4 | −2.6 |
| (±2.0) | (±2.0) | (±1.8) | <0.001 | (±1.7) | (±2.4) | (±2.3) | (±2.1) | (±2.1) | (±3.0) | |
| Sm long cm/s | 11.8 | 10.0 | −2.0 | (1.66–2.34) | 11.7 | 9.1 | −2.3 | 11.9 | 9.1 | −3.4 |
| (±1.7) | (±2.4) | (±1.6) | <0.001 | (±1.8) | (±2.9) | (±2.4) | (±1.8) | (±1.8) | (±1.9) | |
| LV-EF % | 62.6 | 57.9 | −5.3 | (3.85–6.75) | 60.4 | 53.8 | −7.1 | 65.0 | 54.4 | −10.6 |
| (±5.8) | (±8.8) | (±6.8) | <0.001 | (±4.0) | (±12.7) | (±16.2) | (±3.5) | (±14.5) | (±13.3) | |
| ISHLT AMR n = 65 | ||||
|---|---|---|---|---|
| Pre | AMR | Change (%) | (CI) p Value | |
| Sm rad cm/s | 11.7 (±1.9) | 10.1 (±2.1) | −1.6 (±1.9) | (0.77–2.43) <0.001 |
| Sm long cm/s | 11.9 (±1.5) | 10.3 (±2.0) | −1.8 (±2.0) | (0.92–2.67) <0.001 |
| LV-EF % | 60.8 (±6.4) | 56.5 (±9.8) | −4.5 (±7.7) | (1.13–7.87) 0.009 |
| Hazard Ratio | 95% CI | p Value | |
|---|---|---|---|
| Sm long, cm/s | 0.74 | 0.58–0.95 | 0.016 |
| Sm rad, cm/s | 0.77 | 0.63–0.93 | 0.008 |
| IVSd per mm | 0.94 | 0.72–1.21 | 0.607 |
| LV-EF, % | 1.0 | 0.95–1.05 | 0.968 |
| Sm long ≥ 10 cm/s | 0.34 | 0.14–0.84 | 0.019 |
| Sm long < 10 cm/s | 2.92 | 1.19–7.18 | 0.019 |
| Sm rad ≥ 9 cm/s | 0.31 | 0.11–0.83 | 0.020 |
| Sm rad < 9 cm/s | 3.24 | 1.2–8.76 | 0.020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Just, I.A.; Guelfirat, M.; Leser, L.; Uecertas, A.; Kopp Fernandes, L.; Godde, M.; Merke, N.; Stawowy, P.; Hennig, F.; Knosalla, C.; et al. Diagnostic and Prognostic Value of a TDI-Derived Systolic Wall Motion Analysis as a Screening Modality for Allograft Rejection after Heart Transplantation. Life 2021, 11, 1206. https://doi.org/10.3390/life11111206
Just IA, Guelfirat M, Leser L, Uecertas A, Kopp Fernandes L, Godde M, Merke N, Stawowy P, Hennig F, Knosalla C, et al. Diagnostic and Prognostic Value of a TDI-Derived Systolic Wall Motion Analysis as a Screening Modality for Allograft Rejection after Heart Transplantation. Life. 2021; 11(11):1206. https://doi.org/10.3390/life11111206
Chicago/Turabian StyleJust, Isabell A., Meryem Guelfirat, Laura Leser, Ata Uecertas, Laurenz Kopp Fernandes, Maren Godde, Nicolas Merke, Philipp Stawowy, Felix Hennig, Christoph Knosalla, and et al. 2021. "Diagnostic and Prognostic Value of a TDI-Derived Systolic Wall Motion Analysis as a Screening Modality for Allograft Rejection after Heart Transplantation" Life 11, no. 11: 1206. https://doi.org/10.3390/life11111206
APA StyleJust, I. A., Guelfirat, M., Leser, L., Uecertas, A., Kopp Fernandes, L., Godde, M., Merke, N., Stawowy, P., Hennig, F., Knosalla, C., Falk, V., Knierim, J., & Schoenrath, F. (2021). Diagnostic and Prognostic Value of a TDI-Derived Systolic Wall Motion Analysis as a Screening Modality for Allograft Rejection after Heart Transplantation. Life, 11(11), 1206. https://doi.org/10.3390/life11111206






