Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation
Abstract
1. Introduction
2. Materials and Methods
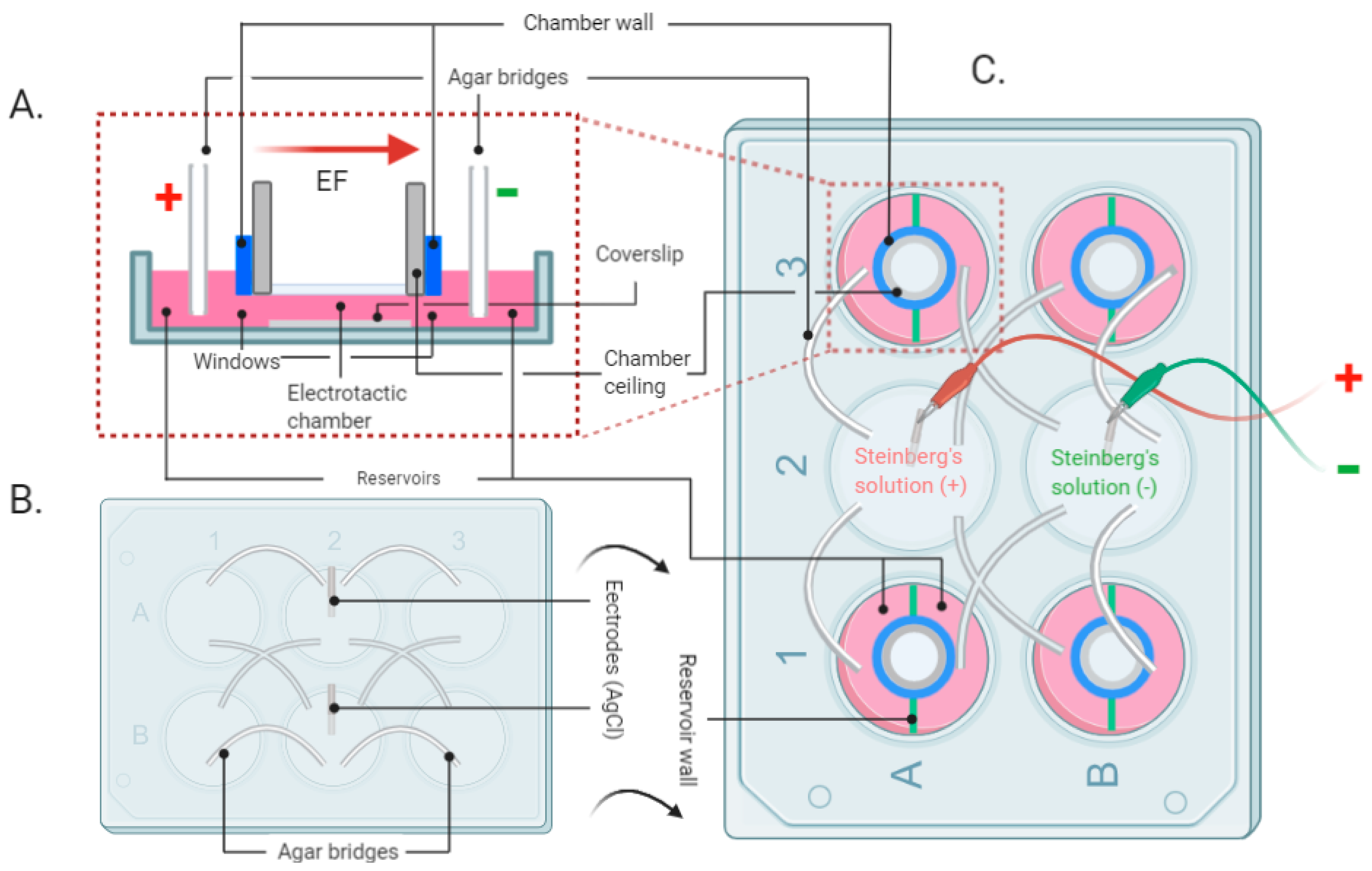
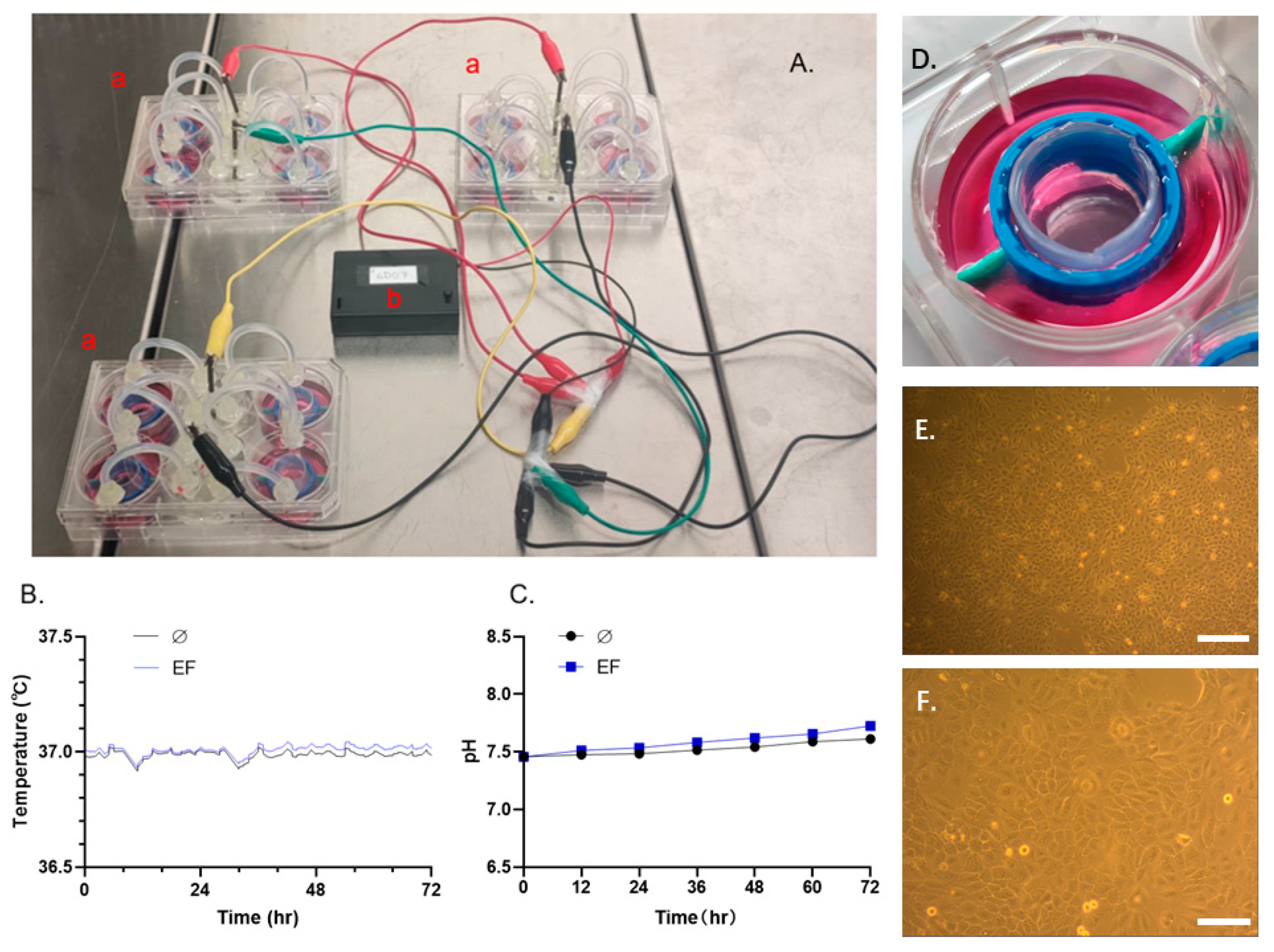
2.1. The EF Stimulation Setup and EF Application
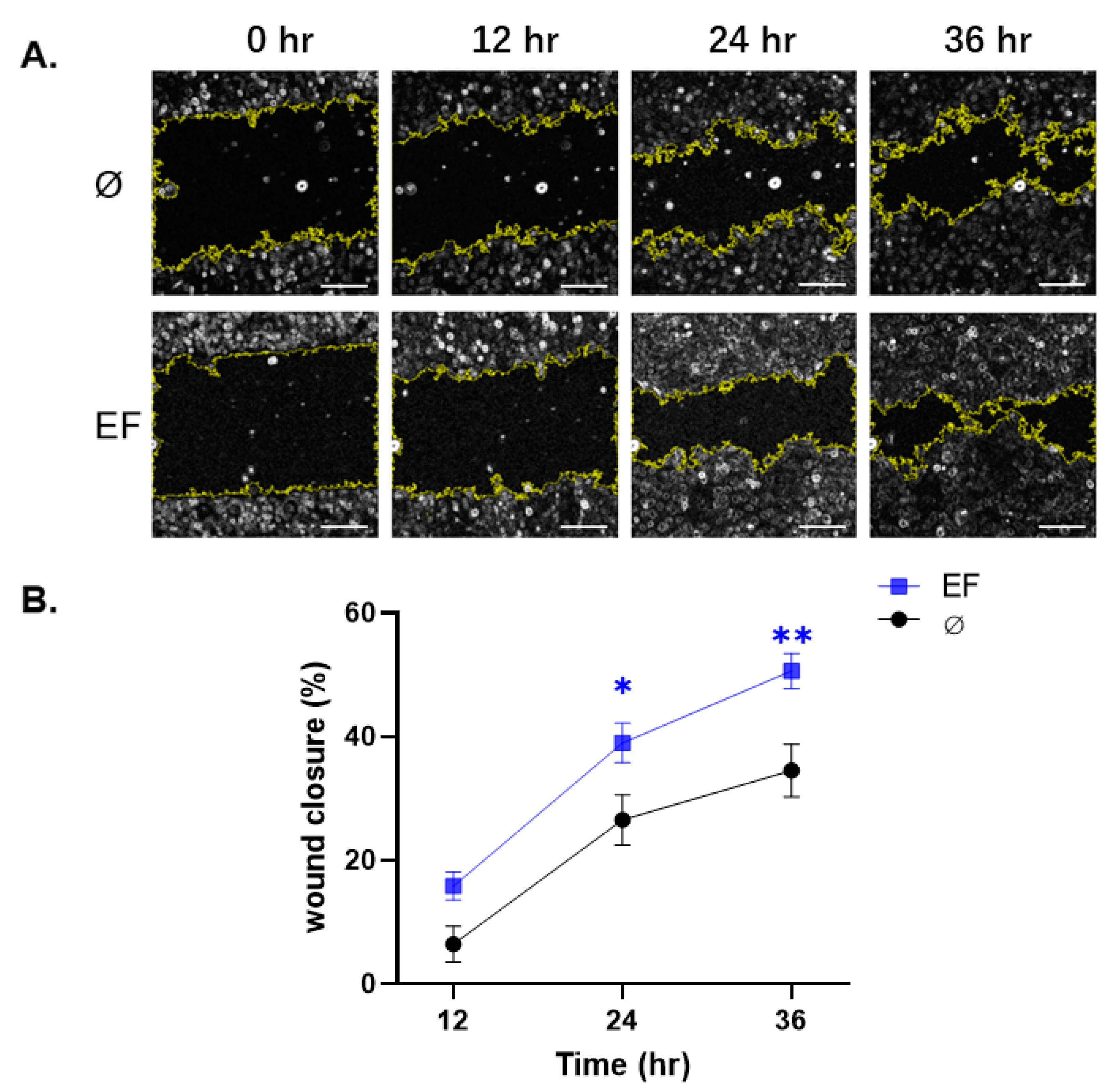
2.2. Scratch Wound Closure Assay
2.3. Resazurin Conversion Assay
2.4. Sulforhodamine B (SRB) Staining for Total Protein Content
2.5. Quantification of Total DNA
2.6. Hoechst and Calcein-AM Staining
2.7. Measurement of Cytokine Expression using the Antibody Array Method
2.8. Western Blot
2.9. Statistics
3. Results
3.1. Finalizing the EF Experimental System
3.2. Effect EF on Scratch Wound Closure in HaCaT cells
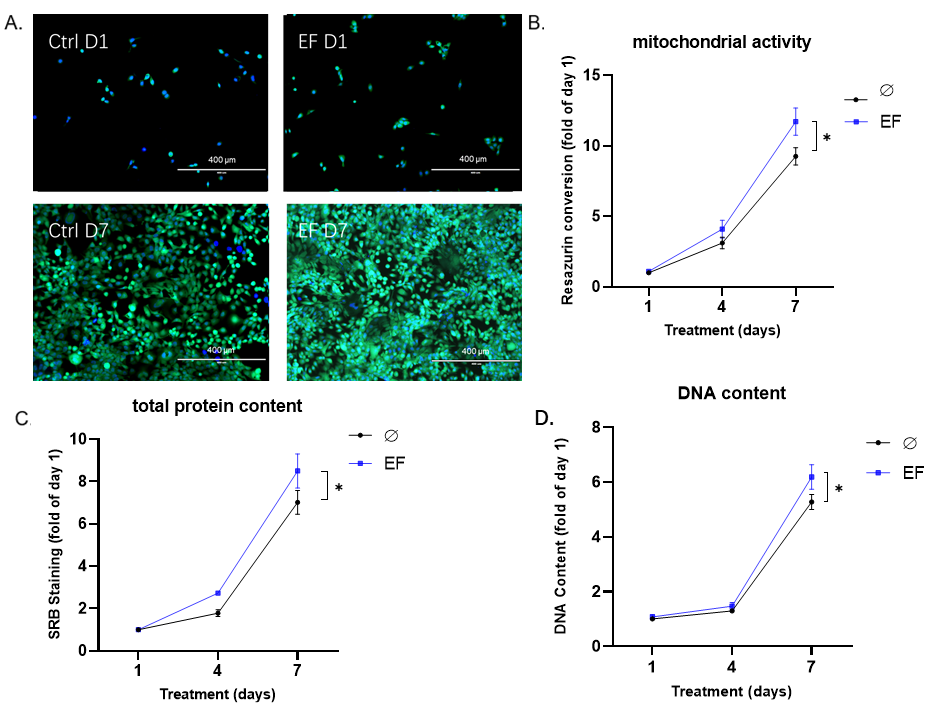
3.3. EF Application Improves the Proliferation of HaCaT Cells
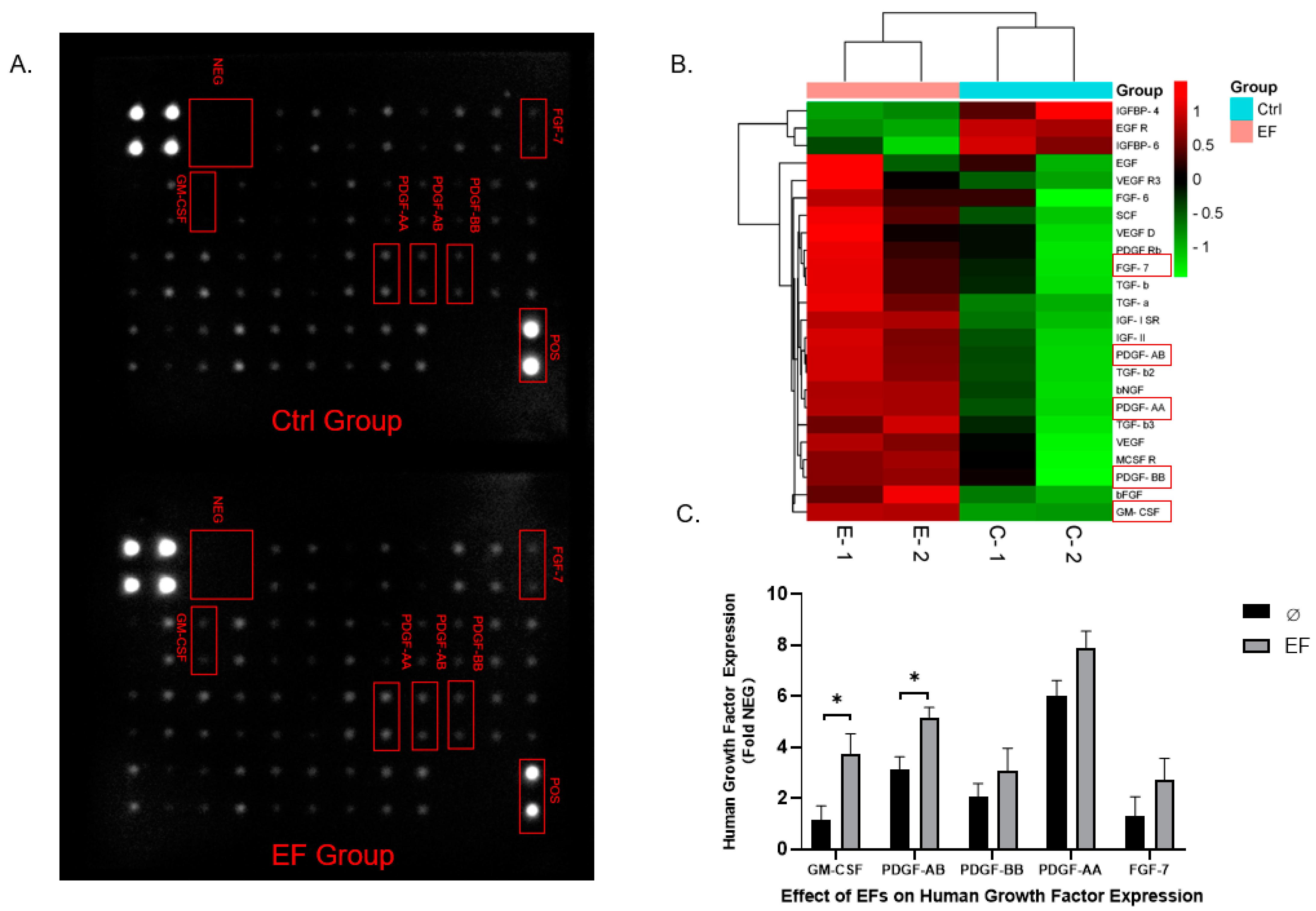
3.4. Differential Expression of Growth Factors in HaCaT cells after EF exposure
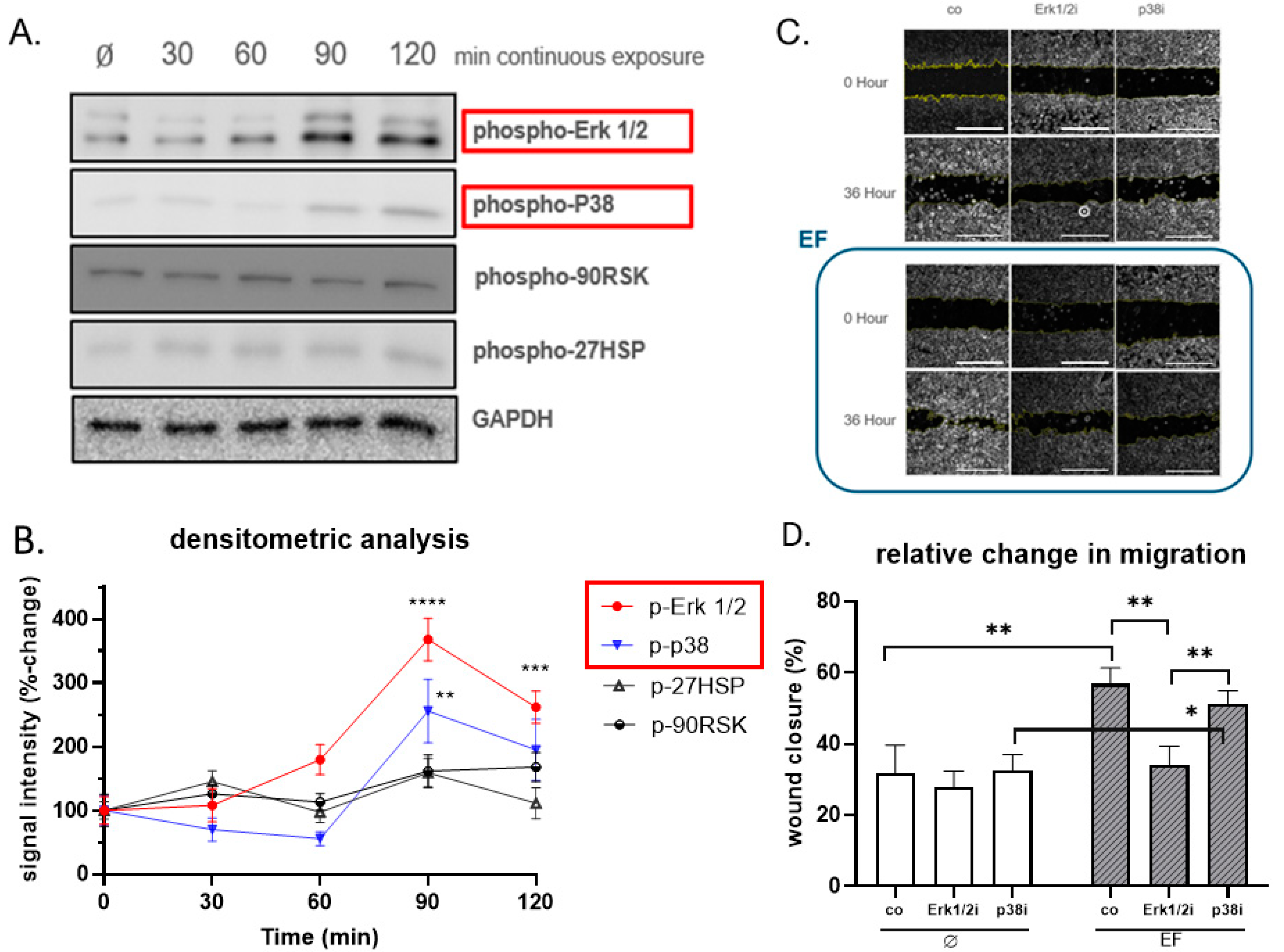
3.5. Erk1/2 Is Crucial for Responding to the Stimulation of EF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMEM | Dulbecco’s modified eagle medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| EFs | Electrical fields |
| EF | Electrical field |
| EMFs | Electromagnetic fields |
| FDA | United States Food and Drug Administration |
| FGF-7 | Fibroblast growth factor-7 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HaCaT | Human keratinocyte cell line |
| MAPK | Mitogen-activated protein kinase |
| PDGF | Platelet-derived growth factor |
| SRB | Sulforhodamine B |
| TEP | Transepithelial potential |
References
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Klyce, S.D.; Crosson, C.E. Transport processes across the rabbit corneal epithelium: A review. Curr. Eye Res. 1985, 4, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R. Endogenous ionic currents and DC electric fields in multicellular animal tissues. Bioelectromagnetics 1992, 13, 147–157. [Google Scholar] [CrossRef]
- Nuccitelli, R. A Role for Endogenous Electric Fields in Wound Healing. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 58, pp. 1–26. [Google Scholar] [CrossRef]
- Gardner, S.; Frantz, R.A.; Schmidt, F.L. Effect of electrical stimulation on chronic wound healing: A meta-analysis. Wound Repair Regen. 1999, 7, 495–503. [Google Scholar] [CrossRef]
- Molsberger, A.; McCaig, C.D. Percutaneous direct current stimulation—a new electroceutical solution for severe neurological pain and soft tissue injuries. Med. Devices (Auckl.) 2018, 11, 205–214. [Google Scholar] [CrossRef]
- Deyo, R.A.; Walsh, N.E.; Martin, D.C.; Schoenfeld, L.S.; Ramamurthy, S. A Controlled Trial of Transcutaneous Electrical Nerve Stimulation (TENS) and Exercise for Chronic Low Back Pain. N. Engl. J. Med. 1990, 322, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Chen, X.M.; Chen, Z.Q. Osteocyte: The impresario in the electrical stimulation for bone fracture healing. Med. Hypotheses 2008, 70, 287–290. [Google Scholar] [CrossRef]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of Cancer Cell Replication by Alternating Electric Fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef]
- Hartig, M.; Joos, U.; Wiesmann, H.-P. Capacitively coupled electric fields accelerate proliferation of osteoblast-like primary cells and increase bone extracellular matrix formation in vitro. Eur. Biophys. J. 2000, 29, 499–506. [Google Scholar] [CrossRef] [PubMed]
- 11. Patruno, A.; Costantini, E.; Ferrone, A.; Pesce, M.; Diomede, F.; Trubiani, O.; Reale, M. Short ELF-EMF exposure targets SIRT1/Nrf2/HO-1 signaling in THP-1 cells. Int. J. Mol. Sci. 2020, 21, 7284. [Google Scholar] [CrossRef]
- McCaig, C.; Allan, D.; Erskine, L.; Rajnicek, A.; Stewart, R. Growing Nerves in an Electric Field. Neuroprotocols 1994, 4, 134–141. [Google Scholar] [CrossRef]
- Pelletier, S.J.; Lagacé, M.; St-Amour, I.; Arsenault, D.; Cisbani, G.; Chabrat, A.; Fecteau, S.; Lévesque, M.; Cicchetti, F. The morphological and molecular changes of brain cells exposed to direct current electric field stimulation. Int. J. Neuropsychopharmacol. 2015, 18, pyu090. [Google Scholar] [CrossRef]
- Cho, Y.; Son, M.; Jeong, H.; Shin, J.H. Electric field–induced migration and intercellular stress alignment in a collective epithelial monolayer. Mol. Biol. Cell 2018, 29, 2292–2302. [Google Scholar] [CrossRef]
- Rajnicek, A.M.; Zhao, Z.; Vico, J.M.; Cruz, A.M.; McCaig, C.D.; Casañ-Pastor, N. Controlling Nerve Growth with an Electric Field Induced Indirectly in Transparent Conductive Substrate Materials. Adv. Health Mater. 2018, 7, e1800473. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechnology 2016, 60, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.T.; Jaffe, L.F.; Vanable, J.W. The glabrous epidermis of cavies contains a powerful battery. Am. J. Physiol. Integr. Comp. Physiol. 1982, 242, R358–R366. [Google Scholar] [CrossRef] [PubMed]
- Schoop, V.M.; Fusenig, N.E.; Mirancea, N. Epidermal Organization and Differentiation of HaCaT Keratinocytes in Organotypic Coculture with Human Dermal Fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Song, B.; Gu, Y.; Pu, J.; Reid, B.; Zhao, Z.; Zhao, M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2007, 2, 1479. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V. A Manual of Experimental Embryology; The University of Chicago Press: Chicago, IL, USA, 1942. [Google Scholar]
- Ehnert, S.; Linnemann, C.; Aspera-Werz, R.H.; Bykova, D.; Biermann, S.; Fecht, L.; De Zwart, P.M.; Nussler, A.K.; Stuby, F. Immune Cell Induced Migration of Osteoprogenitor Cells Is Mediated by TGF-β Dependent Upregulation of NOX4 and Activation of Focal Adhesion Kinase. Int. J. Mol. Sci. 2018, 19, 2239. [Google Scholar] [CrossRef]
- Ehnert, S.; Van Griensven, M.; Unger, M.; Scheffler, H.; Falldorf, K.; Fentz, A.-K.; Seeliger, C.; Schroter, S.; Nussler, A.K.; Balmayor, E.R. Co-Culture with Human Osteoblasts and Exposure to Extremely Low Frequency Pulsed Electromagnetic Fields Improve Osteogenic Differentiation of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 994. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. JNCI J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Linnemann, C.; Braun, B.; Botsch, J.; Leibiger, K.; Hemmann, P.; Nussler, A.K. One-Step ARMS-PCR for the Detection of SNPs—Using the Example of the PADI4 Gene. Methods Protoc. 2019, 2, 63. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Ehnert, S.; Müller, M.; Zhu, S.; Chen, T.; Weng, W.; Jacoby, J.; Nussler, A.K. Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes. World J. Stem Cells 2020, 12, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Ehnert, S.; Aspera-Werz, R.H.; Ihle, C.; Trost, M.; Zirn, B.; Flesch, I.; Schröter, S.; Relja, B.; Nussler, A.K. Smoking Dependent Alterations in Bone Formation and Inflammation Represent Major Risk Factors for Complications Following Total Joint Arthroplasty. J. Clin. Med. 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Wang, Z.; Ehnert, S.; Ihle, C.; Schyschka, L.; Pscherer, S.; Nussler, N.C.; Braun, K.; van Griensven, M.; Wang, G.; Burgkart, R.; et al. Increased Oxidative Stress Response in Granulocytes from Older Patients with a Hip Fracture May Account for Slow Regeneration. Oxidative Med. Cell. Longev. 2014, 2014, 819847. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation: A Novel Tool for Tissue Engineering. Tissue Eng. Part B Rev. 2013, 19, 48–57. [Google Scholar] [CrossRef]
- Piccolino, M. Animal electricity and the birth of electrophysiology: The legacy of Luigi Galvani. Brain Res. Bull. 1998, 46, 381–407. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef]
- Schuetze, S.M. The discovery of the action potential. Trends Neurosci. 1983, 6, 164–168. [Google Scholar] [CrossRef]
- Levin, M. Large-scale biophysics: Ion flows and regeneration. Trends Cell Biol. 2007, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.; Borgens, R.; Pascuzzi, R.; Roos, K.; Groff, M.; Purvines, S.; Ben Rodgers, R.; Hagy, S.; Nelson, P. Oscillating field stimulation for complete spinal cord injury in humans: A Phase 1 trial. J. Neurosurg. Spine 2005, 2, 3–10. [Google Scholar] [CrossRef]
- Rajnicek, A.M.; Stump, R.F.; Robinson, K.R. An endogenous sodium current may mediate wound healing in Xenopus neurulae. Dev. Biol. 1988, 128, 290–299. [Google Scholar] [CrossRef]
- Carroll, D.; Moore, A.R.; McQuay, H.H.; Fairman, F.S.; Tramèr, M.; Leijon, G.G. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst. Rev. 2000, CD003222. [Google Scholar] [CrossRef]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-derived antibacterial peptide, defensin-1, promotes wound re-epithelialisation in vitro and in vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.D.; Oreffo, R.; Healy, E.; Thurner, P.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-Y.; Choi, B.H.; Lee, S.; Jang, Y.H.; Choi, J.-S.; Kim, Y.-M. Regulation of Wound Healing by Granulocyte-Macrophage Colony-Stimulating Factor after Vocal Fold Injury. PLoS ONE 2013, 8, e54256. [Google Scholar] [CrossRef][Green Version]
- Mann, A.; Niekisch, K.; Schirmacher, P.; Blessing, M. Granulocyte–Macrophage Colony-Stimulating Factor Is Essential for Normal Wound Healing. J. Investig. Dermatol. Symp. Proc. 2006, 11, 87–92. [Google Scholar] [CrossRef]
- Bussolino, F.; Ziche, M.; Wang, J.M.; Alessi, D.; Morbidelli, L.; Cremona, O.; Bosia, A.; Marchisio, P.C.; Mantovani, A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J. Clin. Investig. 1991, 87, 986–995. [Google Scholar] [CrossRef]
- Caux, C.; Dezutter-Dambuyant, C.; Schmitt, D.; Banchereau, J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nat. Cell Biol. 1992, 360, 258–261. [Google Scholar] [CrossRef]
- Breuhahn, K.; Mann, A.; Müller, G.; Wilhelmi, A.; Schirmacher, P.; Enk, A.; Blessing, M. Epidermal overexpression of granulocyte-macrophage colony-stimulating factor induces both keratinocyte proliferation and apoptosis. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2000, 11, 111–121. [Google Scholar]
- Braunstein, S.; Kaplan, G.; Gottlieb, A.B.; Schwartz, M.; Walsh, G.; Abalos, R.M.; Fajardo, T.T.; Guido, L.S.; Krueger, J.G. GM-CSF Activates Regenerative Epidermal Growth and Stimulates Keratinocyte Proliferation in Human Skin In Vivo. J. Investig. Dermatol. 1994, 103, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.P.; Griffiths, G.D.; Schor, A.M.; Leese, G.P.; Schor, S.L. Growth factors in the treatment of diabetic foot ulcers. BJS 2003, 90, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Niessen, F.B.; Andriessen, M.P.; Schalkwijk, J.; Visser, L.; Timens, W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J. Pathol. J. Pathol. Soc. Gt. Br. Irel. 2001, 194, 207–216. [Google Scholar] [CrossRef]
- Uutela, M.; Wirzenius, M.; Paavonen, K.; Rajantie, I.; He, Y.; Karpanen, T.; Lohela, M.; Wiig, H.; Salven, P.; Pajusola, K.; et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood 2004, 104, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.C.; Galiano, R.D. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biol. Targets Ther. 2008, 2, 1. [Google Scholar]
- Wu, L.; Pierce, G.F.; Galiano, R.D.; Mustoe, T.A. Keratinocyte growth factor induces granulation tissue in ischemic dermal wounds: Importance of epithelial-mesenchymal cell interactions. Arch. Surg. 1996, 131, 660–666. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Cardinali, G.; Aspite, N.; Picardo, M.; Marchese, C.; Torrisi, M.R.; Mancini, P. Cortactin involvement in the keratinocyte growth factor and fibroblast growth factor 10 promotion of migration and cortical actin assembly in human keratinocytes. Exp. Cell Res. 2007, 313, 1758–1777. [Google Scholar] [CrossRef]
- Raja, S.K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Chang, Z.; Niu, J.; Peng, B.; Xia, Q.; Lu, W.; Huang, P.; Tsao, M.; Chiao, P. Keratinocyte Growth Factor/Fibroblast Growth Factor-7-Regulated Cell Migration and Invasion through Activation of NF-kappaB Transcription Factors; AACR: San Diego, CA, USA, 2008; Volume 68. [Google Scholar]
- Ehnert, S.; Falldorf, K.; Fentz, A.-K.; Ziegler, P.; Schröter, S.; Freude, T.; Ochs, B.G.; Stacke, C.; Ronniger, M.; Sachtleben, J.; et al. Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure—Clinical implication possible. Bone Rep. 2015, 3, 48–56. [Google Scholar] [CrossRef]
- Yumoto, H.; Hirao, K.; Tominaga, T.; Bando, N.; Takahashi, K.; Matsuo, T. Electromagnetic wave irradiation promotes osteoblastic cell proliferation and up-regulates growth factors via activation of the ERK1/2 and p38 MAPK pathways. Cell. Physiol. Biochem. 2015, 35, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Farboud, B.; Nuccitelli, R.; Isseroff, R.R. Power-line frequency electromagnetic fields do not induce changes in phosphorylation, localization, or expression of the 27-kilodalton heat shock protein in human keratinocytes. Environ. Health Perspect. 2003, 111, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Seger, R.; Krebs, E.G. The MAPK signaling cascade. FASEB J. 1995, 9, 726–735. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Kolbenschlag, J.; Nüssler, A.K.; Ehnert, S.; McCaig, C.D.; Čebron, U.; Daigeler, A.; Prahm, C. Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life 2021, 11, 1195. https://doi.org/10.3390/life11111195
Lu C, Kolbenschlag J, Nüssler AK, Ehnert S, McCaig CD, Čebron U, Daigeler A, Prahm C. Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life. 2021; 11(11):1195. https://doi.org/10.3390/life11111195
Chicago/Turabian StyleLu, Chao, Jonas Kolbenschlag, Andreas K. Nüssler, Sabrina Ehnert, Colin D. McCaig, Urška Čebron, Adrien Daigeler, and Cosima Prahm. 2021. "Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation" Life 11, no. 11: 1195. https://doi.org/10.3390/life11111195
APA StyleLu, C., Kolbenschlag, J., Nüssler, A. K., Ehnert, S., McCaig, C. D., Čebron, U., Daigeler, A., & Prahm, C. (2021). Direct Current Electrical Fields Improve Experimental Wound Healing by Activation of Cytokine Secretion and Erk1/2 Pathway Stimulation. Life, 11(11), 1195. https://doi.org/10.3390/life11111195






