Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy
Abstract
:1. Introduction
2. Pancreatic Cancer
2.1. Pancreatic Cancer Biology
- Pancreatic intraepithelial neoplasms (PanIN), which are non-invasive microscopic lesions that occur in small pancreatic ducts (less than 0.5 cm).
- Intraductal papillary mucinous neoplasms (IPMN), precursor lesions of pancreatic cancer.
- Mucinous cystic neoplasms (MCN), which are also considered premalignant lesions of the pancreas and occur more frequently in women [29].
- Adenosquamous carcinoma, which has the worst prognosis.
- Mucinous carcinoma, with a favorable prognosis and is related to the lesion called intraductal papillary mucinous neoplasia.
- Undifferentiated anaplastic carcinoma, which is considered the most aggressive of the subtypes, with an extremely low survival rate due to its atypical cells mixed with osteoclast-like giant cells.
2.2. Clinical Aspects of Pancreatic Cancer
2.3. Current Pancreatic Cancer Treatments
2.4. Surface Protein as Target in Pancreatic Cancer
3. Nanoparticles as a Therapeutic Strategy in Cancer
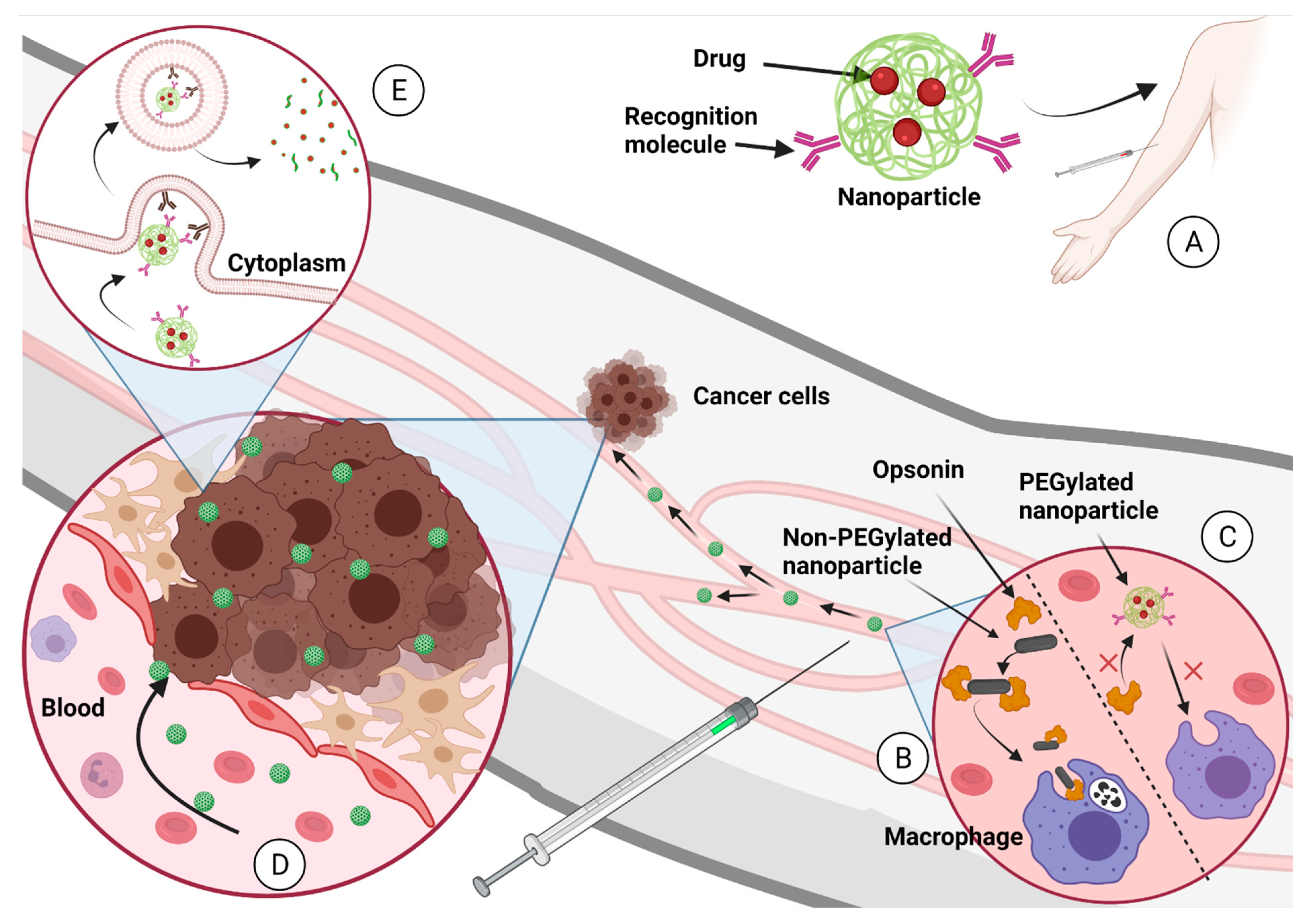
3.1. Nanoparticles for Drug Delivery
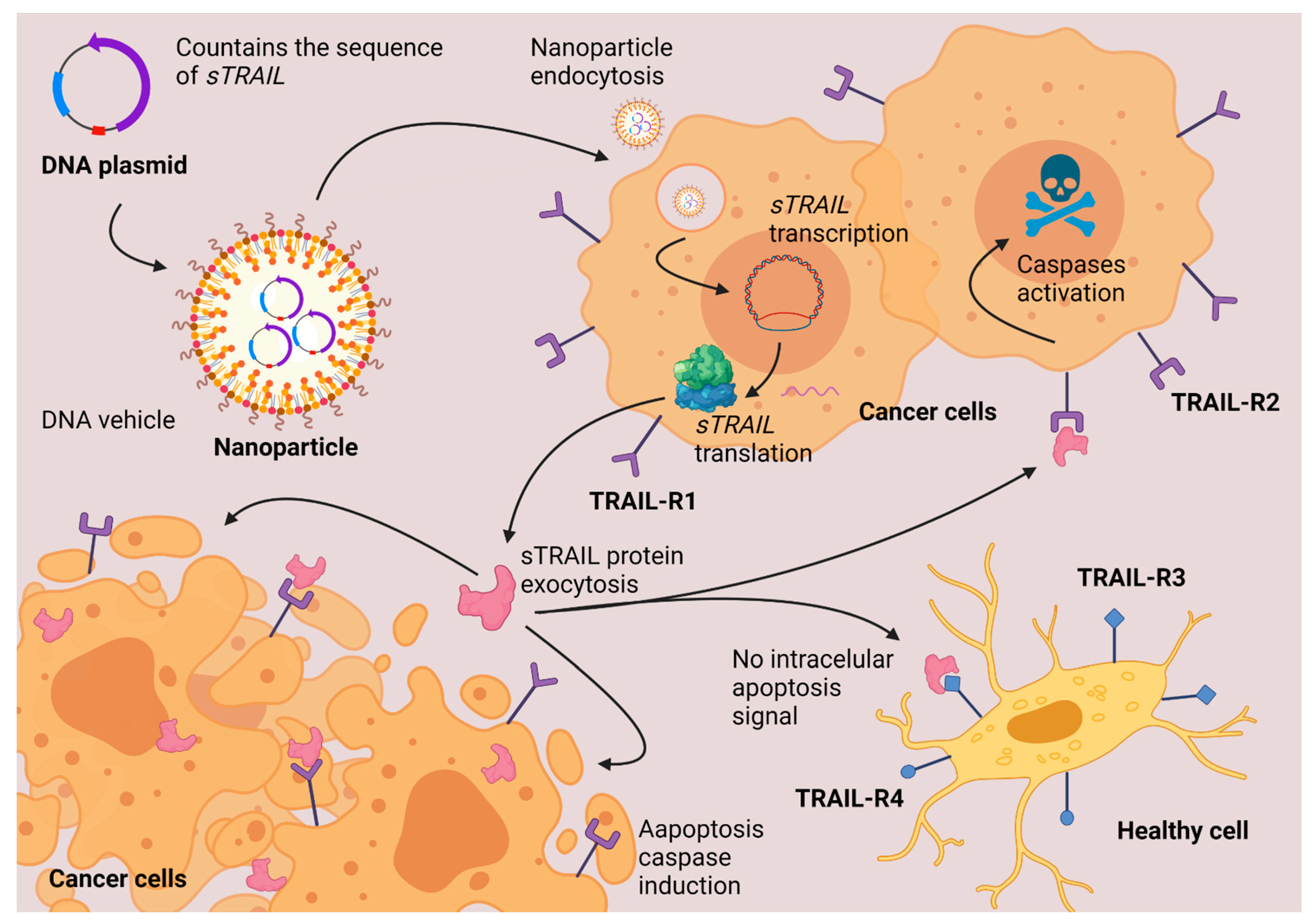
3.2. Nanoparticles as a Vehicle for DNA (Gene Therapy)
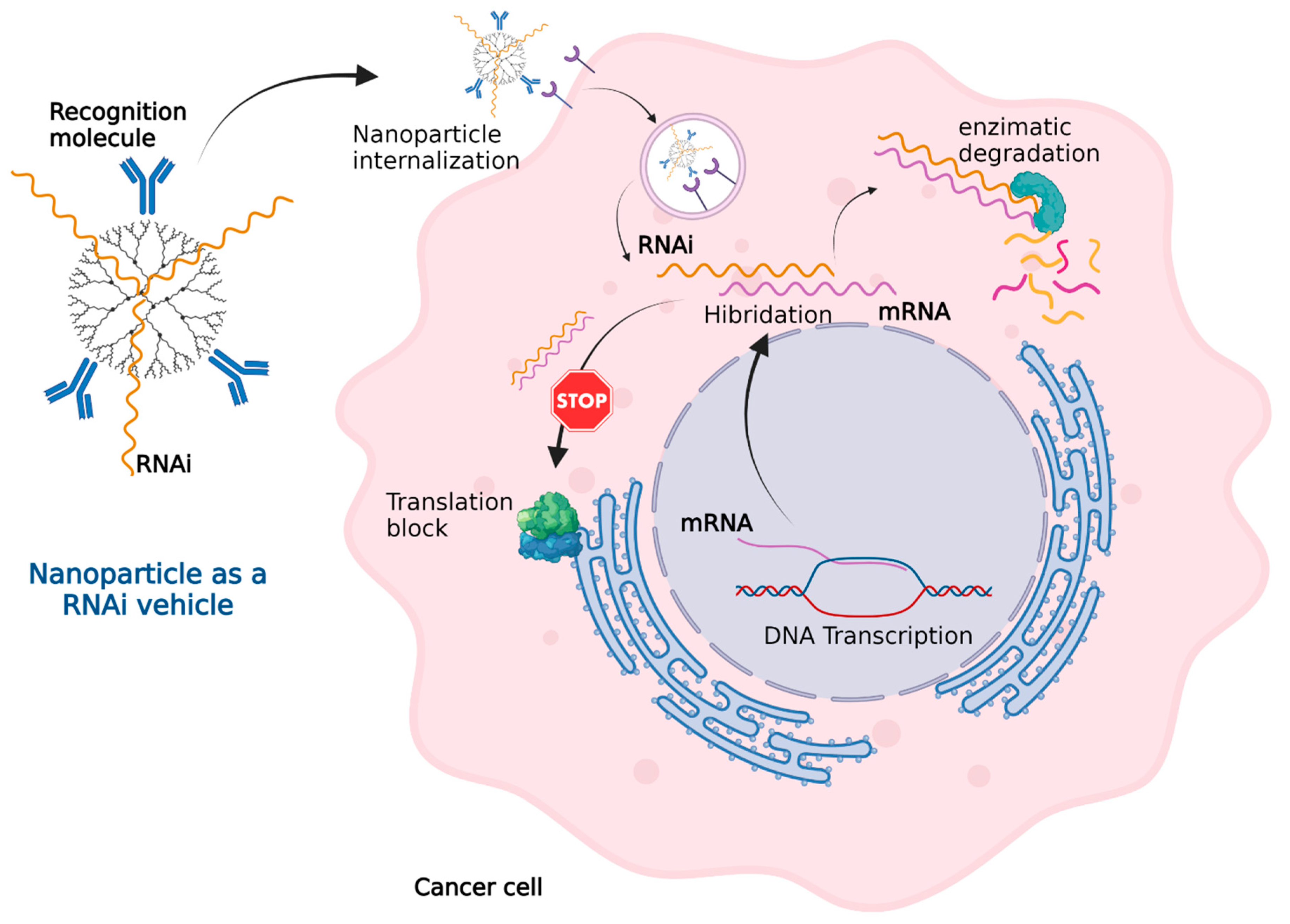
3.3. Nanoparticles as a Vehicle for RNAi (Gene Therapy)
3.4. Nanoparticles for Photothermal Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Roacho-Perez, J.A.; Gallardo-Blanco, H.L.; Sanchez-Dominguez, M.; Garcia-Casillas, P.E.; Chapa-Gonzalez, C.; Sanchez-Dominguez, C.N. Nanoparticles for death-induced gene therapy in cancer (Review). Mol. Med. Rep. 2018, 17, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- He, Q.; Wu, Q.; Feng, X.; Liao, Z.; Peng, W.; Liu, Y.; Peng, D.; Liu, Z.; Mo, M. Interfacing DNA with nanoparticles: Surface science and its applications in biosensing. Int. J. Biol. Macromol. 2020, 151, 757–780. [Google Scholar] [CrossRef]
- Veiga, N.; Diesendruck, Y.; Peer, D. Targeted lipid nanoparticles for RNA therapeutics and immunomodulation in leukocytes. Adv. Drug Deliv. Rev. 2020, 159, 364–376. [Google Scholar] [CrossRef]
- Cao, S.J.; Xu, S.; Wang, H.M.; Ling, Y.; Dong, J.; Xia, R.D.; Sun, X.H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 190. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Fu, Z.; Xiang, J. Aptamer-Functionalized Nanoparticles in Targeted Delivery and Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 9123. [Google Scholar] [CrossRef]
- Zaimy, M.A.; Saffarzadeh, N.; Mohammadi, A.; Pourghadamyari, H.; Izadi, P.; Sarli, A.; Moghaddam, L.K.; Paschepari, S.R.; Azizi, H.; Torkamandi, S.; et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Ther. 2017, 24, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Panneerselvam, J.; Srivastava, A.; Ahmed, R.; Mehta, M.; Munshi, A.; Ramesh, R. Recent Advances in Nanoparticle-Based Cancer Drug and Gene Delivery. Adv. Cancer Res. 2018, 137, 115–170. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, R.; Sanchez-Dominguez, M.; Matutes-Aquino, J.A.; Wennmalm, S.; Kuttuva Rajarao, G. Removal of total organic carbon from sewage wastewater using poly(ethylenimine)-functionalized magnetic nanoparticles. Langmuir 2014, 30, 1036–1044. [Google Scholar] [CrossRef]
- Santana, C.I.; Hoyos, L.M.; Pérez, J.F.; Bustamante, J.; García, A.G. A novel functionalization method for carbon nanotubes to repel ox-LDL in treatments after stent placement. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the Delivery of Chemotherapeutics: Role of Biodegradable Polymeric Nanoparticles. Molecules 2018, 23, 2157. [Google Scholar] [CrossRef] [Green Version]
- Subhan, M.A.; Torchilin, V.P. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl. Res. 2019, 214, 62–91. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, G.; Pan, J.; Torchilin, V.P. Micelle-like nanoparticles as carriers for DNA and siRNA. Mol. Pharm. 2015, 12, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Tingstedt, B.; Andersson, B.; Holmquist, F.; Sturesson, C.; Williamsson, C.; Sasor, A.; Borg, D.; Bauden, M.; Andersson, R. Pancreatic cancer: Yesterday, today and tomorrow. Future Oncol. 2016, 12, 1929–1946. [Google Scholar] [CrossRef] [Green Version]
- Olvera-Granados, C.P.; Leo-Amador, G.E.; Hernández-Montiel, H.L. Páncreas y células beta: Mecanismos de diferenciación, morfogénesis y especificación celular endocrina. ¿Regeneración? Bol. Med. Hosp. Infant. Mex. 2008, 65, 306–324. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1665-11462008000400009&lng=es (accessed on 25 September 2021).
- American Cancer Society. Available online: https://www.cancer.org/es/cancer/cancer-de-pancreas/acerca/que-es-el-cancer-de-pancreas.html (accessed on 25 September 2021).
- Goral, V. Pancreatic Cancer: Pathogenesis and Diagnosis. Asian Pac. J. Cancer Prev. 2015, 16, 5619–5624. [Google Scholar] [CrossRef] [Green Version]
- Esposito, I.; Konukiewitz, B.; Schlitter, A.M.; Klöppel, G. Pathology of pancreatic ductal adenocarcinoma: Facts, challenges and future developments. World J. Gastroenterol. 2014, 20, 13833–13841. [Google Scholar] [CrossRef]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System, 4th ed.; IARC Publications: Lyon, France, 2018; pp. 7–8. [Google Scholar]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Ilic, M.; Ilic, I. Epidemiology of pancreatic cancer. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef]
- Greer, J.B.; Whitcomb, D.C.; Brand, R.E. Genetic predisposition to pancreatic cancer: A brief review. Am. J. Gastroenterol. 2007, 102, 2564–2569. [Google Scholar] [CrossRef]
- Slebos, R.J.; Hoppin, J.A.; Tolbert, P.E.; Holly, E.A.; Brock, J.W.; Zhang, R.H.; Bracci, P.M.; Foley, J.; Stockton, P.; McGregor, L.M.; et al. K-ras and p53 in pancreatic cancer: Association with medical history, histopathology, and environmental exposures in a population-based study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1223–1232. [Google Scholar]
- Loveday, B.P.T.; Lipton, L.; Thomson, B.N. Pancreatic cancer: An update on diagnosis and management. Aust. J. Gen. Pract. 2019, 48, 826–831. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Alberta Health Services. Adenocarcinoma of the Pancreas Clinical Practice Guideline (GI 004). Available online: https://www.albertahealthservices.ca/info/cancerguidelines.aspx (accessed on 1 October 2021).
- Guía de Práctica Clínica GPC Diagnóstico y Tratamiento del Adenocarcinoma de Páncreas en el Adulto. Available online: http://www.imss.gob.mx/sites/all/statics/guiasclinicas/324GRR.pdf (accessed on 1 October 2021).
- Cancer Cleveland Clinic. “5-Fluorouracil” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/fluorouracil.aspx (accessed on 1 October 2021).
- Cancer Cleveland Clinic. “Capecitabine” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/capecitabine.aspx (accessed on 1 October 2021).
- Cancer Cleveland Clinic. “Gemcitabine” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/gemcitabine.aspx (accessed on 1 October 2021).
- Philip, P.A.; Lacy, J.; Portales, F.; Sobrero, A.; Pazo-Cid, R.; Manzano Mozo, J.L.; Kim, E.J.; Dowden, S.; Zakari, A.; Borg, C.; et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol. Hepatol. 2020, 5, 285–294. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Barone, D.; Mahalingam, D.; Bekaii-Saab, T.; Shao, S.H.; Wolf, J.; Rosano, M.; Krause, S.; Richards, D.A.; Yu, K.H.; et al. Randomised phase II trial of gemcitabine and nab-paclitaxel with necuparanib or placebo in untreated metastatic pancreas ductal adenocarcinoma. Eur. J. Cancer 2020, 132, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.; Oh, D.Y.; Tabernero, J.; Reni, M.; Van Cutsem, E.; Hendifar, A.; Waldschmidt, D.T.; Starling, N.; Bachet, J.B.; Chang, H.M.; et al. Ibrutinib in combination with nab-paclitaxel and gemcitabine for first-line treatment of patients with metastatic pancreatic adenocarcinoma: Phase III RESOLVE study. Ann. Oncol. 2021, 32, 600–608. [Google Scholar] [CrossRef]
- Ko, A.H.; Murphy, P.B.; Peyton, J.D.; Shipley, D.L.; Al-Hazzouri, A.; Rodriguez, F.A.; Womack, M.S., IV; Xiong, H.Q.; Waterhouse, D.M.; Tempero, M.A.; et al. A Randomized, Double-Blinded, Phase II Trial of Gemcitabine and Nab-Paclitaxel Plus Apatorsen or Placebo in Patients with Metastatic Pancreatic Cancer: The RAINIER Trial. Oncologist 2017, 22, 1427-e129. [Google Scholar] [CrossRef] [Green Version]
- Cancer Cleveland Clinic. “Paclitaxel Protein-Bound” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/Paclitaxel-Proteinbound.aspx (accessed on 26 October 2021).
- Cancer Cleveland Clinic. “Cisplatine” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/cisplatine.aspx (accessed on 1 October 2021).
- Cancer Cleveland Clinic. “Oxaliplatine” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/oxaliplatine.aspx (accessed on 1 October 2021).
- Cancer Cleveland Clinic. “Irinotecan” Chemocare. Available online: https://chemocare.com/chemotherapy/drug-info/irinotecan.aspx (accessed on 1 October 2021).
- Aslan, M.; Shahbazi, R.; Ulubayram, K.; Ozpolat, B. Targeted Therapies for Pancreatic Cancer and Hurdles Ahead. Anticancer Res. 2018, 38, 6591–6606. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, H.; Han, D.; Peng, B.; Zhang, H.; Zhang, L.; Li, J.; Liu, J.; Cui, C.; Fang, S.; et al. Elucidation and Structural Modeling of CD71 as a Molecular Target for Cell-Specific Aptamer Binding. J. Am. Chem. Soc. 2019, 141, 10760–10769. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Putt, K.S.; Singhal, S.; Han, H.; Visscher, D.W.; Murphy, L.M.; Low, P.S. Assessment of folate receptor alpha and beta expression in selection of lung and pancreatic cancer patients for receptor targeted therapies. Oncotarget 2017, 9, 4485–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.K.; Wang, Z.S.; Hua, Y.F.; Lu, C.D. Membrane expression and significance of TRAIL death receptors DR4 and DR5 in Pancreatic cancer. JPAHS 2020, 7, 54–61. [Google Scholar] [CrossRef]
- Kim, I.K.; Lee, Y.S.; Kim, H.S.; Dong, S.M.; Park, J.S.; Yoon, D.S. Specific protein 1(SP1) regulates the epithelial-mesenchymal transition via lysyl oxidase-like 2(LOXL2) in pancreatic ductal adenocarcinoma. Sci. Rep. 2019, 9, 5933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Pang, T.C.Y.; Liu, A.C.; Pothula, S.P.; Mekapogu, A.R.; Perera, C.J.; Murakami, T.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Targeting the HGF/c-MET pathway in advanced pancreatic cancer: A key element of treatment that limits primary tumour growth and eliminates metastasis. Br. J. Cancer 2020, 122, 1486–1495. [Google Scholar] [CrossRef]
- Soares, K.C.; Rucki, A.A.; Wu, A.A.; Olino, K.; Xiao, Q.; Chai, Y.; Wamwea, A.; Bigelow, E.; Lutz, E.; Liu, L.; et al. PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J. Immunother. 2015, 38, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lutz, E.R.; Wu, A.A.; Bigelow, E.; Sharma, R.; Mo, G.; Soares, K.; Solt, S.; Dorman, A.; Wamwea, A.; Yager, A.; et al. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol. Res. 2014, 2, 616–631. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Wei, X.; Jiang, H.; Lan, C.; Yang, S.; Wang, H.; Yang, Y.; Tian, C.; Xu, Z.; et al. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Sig. Transduct. Target. Ther. 2020, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.; Baba, H.; Fukuda, T.; Takashima, M.; Sugimachi, K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 2000, 88, 2239–2245. [Google Scholar] [CrossRef]
- Liang, Q.L.; Wang, B.R.; Chen, G.Q.; Li, G.H.; Xu, Y.Y. Clinical significance of vascular endothelial growth factor and connexin43 for predicting pancreatic cancer clinicopathologic parameters. Med. Oncol. 2010, 27, 1164–1170. [Google Scholar] [CrossRef]
- Gupta, S.; El-Rayes, B.F. Small molecule tyrosine kinase inhibitors in pancreatic cancer. Biologics 2008, 2, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.H.; Ryu, K.H.; Kwon, A.Y. The Prognostic Impact of HER2 Genetic and Protein Expression in Pancreatic Carcinoma-HER2 Protein and Gene in Pancreatic Cancer. Diagnostics 2021, 11, 653. [Google Scholar] [CrossRef]
- Morse, D.L.; Balagurunathan, Y.; Hostetter, G.; Trissal, M.; Tafreshi, N.K.; Burke, N.; Lloyd, M.; Enkemann, S.; Coppola, D.; Hruby, V.J.; et al. Identification of novel pancreatic adenocarcinoma cell-surface targets by gene expression profiling and tissue microarray. Biochem. Pharmacol. 2010, 80, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karandish, F.; Mallik, S. Biomarkers and Targeted Therapy in Pancreatic Cancer. Biomark. Cancer 2016, 8 (Suppl. 1), 27–35. [Google Scholar] [CrossRef]
- Bloomston, M.; Bhardwaj, A.; Ellison, E.C.; Frankel, W.L. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig. Surg. 2006, 23, 74–79. [Google Scholar] [CrossRef]
- Kwon, J.; Stephan, S.; Mukhopadhyay, A.; Muders, M.H.; Dutta, S.K.; Lau, J.S.; Mukhopadhyay, D. Insulin receptor substrate-2 mediated insulin-like growth factor-I receptor overexpression in pancreatic adenocarcinoma through protein kinase Cdelta. Cancer Res. 2009, 69, 1350–1357. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.; Ballard, B.; Yi, X.; Malankar, A.; Collinson-Pautz, M.R.; Becerra, C.R.; Woodard, J.P.; Foster, A.E. Tumor infiltration and cytokine biomarkers of prostate stem cell antigen (PSCA)-directed GOCAR-T cells in patients with advanced pancreatic tumors. J. Clin. Oncol. 2020, 38, 734. [Google Scholar] [CrossRef]
- Byrne, K.T.; Vonderheide, R.H. CD40 therapy and surgery: A potential immunologic partnership. J. Immunother. 2013, 36, 359–361. [Google Scholar] [CrossRef] [Green Version]
- Beatty, G.L.; Torigian, D.A.; Chiorean, E.G.; Saboury, B.; Brothers, A.; Alavi, A.; Troxel, A.B.; Sun, W.; Teitelbaum, U.R.; Vonderheide, R.H.; et al. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2013, 19, 6286–6295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonderheide, R.H. Prospect of targeting the CD40 pathway for cancer therapy. Clin. Cancer Res. 2007, 13, 1083–1088. [Google Scholar] [CrossRef] [Green Version]
- Danaee, H.; Kalebic, T.; Wyant, T.; Fassan, M.; Mescoli, C.; Gao, F.; Trepicchio, W.L.; Rugge, M. Consistent expression of guanylyl cyclase-C in primary and metastatic gastrointestinal cancers. PLoS ONE 2017, 12, e0189953. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.E.; Lee, K.T.; Lee, J.K.; Paik, S.W.; Rhee, J.C.; Choi, K.W. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J. Gastroenterol. Hepatol. 2004, 19, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, A.R.; Nguyen, A.; Bagby, S.M.; Yacob, B.; Quackenbush, K.; Guy, J.L.; Crowell, T.; Stringer, B.; Danaee, H.; Kalebic, T.; et al. Evaluation of TAK-264, a novel antibody-drug conjugate in pancreatic cancer cell lines and patient-derived xenograft models. In Proceedings of the AACR-NCI-EORTC, International Conference: Molecular Targets and Cancer Therapeutics, Philadelphia, PA, USA, 26–30 October 2017; AACR: Philadelphia, PA, USA, 2018. [Google Scholar]
- Winter, J.M.; Yeo, C.J.; Brody, J.R. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J. Surg. Oncol. 2013, 107, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer, H.; Saurí, T.; Macarulla, T. Predictive and prognostic biomarkers in personalized gastrointestinal cancer treatment. J. Gastrointest. Oncol. 2017, 8, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podhajcer, O.L.; Benedetti, L.; Girotti, M.R.; Prada, F.; Salvatierra, E.; Llera, A.S. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008, 27, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Tomitaka, A.; Takemura, Y. Magnetic Relaxation of Intracellular Magnetic Nanoparticles for Hyperthermia. Crit. Rev. Biomed. Eng. 2019, 47, 489–494. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. Int. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Lollo, G.; Matha, K.; Bocchiardo, M.; Bejaud, J.; Marigo, I.; Virgone-Carlotta, A.; Dehoux, T.; Rivière, C.; Rieu, J.P.; Briançon, S.; et al. Drug delivery to tumours using a novel 5-FU derivative encapsulated into lipid nanocapsules. J. Drug Target. 2019, 27, 634–645. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, T.; Li, L.; Zhu, Z.; Zhang, W.; Cui, G.; Li, W. Co-delivery of Cisplatin(IV) and Capecitabine as an Effective and Non-toxic Cancer Treatment. Front. Pharmacol. 2019, 10, 110. [Google Scholar] [CrossRef]
- Parsian, M.; Mutlu, P.; Yalcin, S.; Gunduz, U. Characterization of Gemcitabine Loaded Polyhydroxybutyrate Coated Magnetic Nanoparticles for Targeted Drug Delivery. Anticancer Agents Med. Chem. 2020, 20, 1233–1240. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; He, D.; Xu, B.; Tu, J.; Shen, Y. Long-Circulating Thermosensitive Liposomes for the Targeted Drug Delivery of Oxaliplatin. Int. J. Nanomed. 2020, 15, 6721–6734. [Google Scholar] [CrossRef] [PubMed]
- Juang, V.; Chang, C.H.; Wang, C.S.; Wang, H.E.; Lo, Y.L. pH-Responsive PEG-Shedding and Targeting Peptide-Modified Nanoparticles for Dual-Delivery of Irinotecan and microRNA to Enhance Tumor-Specific Therapy. Small 2019, 15, e1903296. [Google Scholar] [CrossRef]
- Mozar, F.S.; Chowdhury, E.H. PEGylation of Carbonate Apatite Nanoparticles Prevents Opsonin Binding and Enhances Tumor Accumulation of Gemcitabine. J. Pharm. Sci. 2018, 107, 2497–2508. [Google Scholar] [CrossRef]
- Partikel, K.; Korte, R.; Stein, N.C.; Mulac, D.; Herrmann, F.C.; Humpf, H.U.; Langer, K. Effect of nanoparticle size and PEGylation on the protein corona of PLGA nanoparticles. Eur. J. Pharm. Biopharm. 2019, 141, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Jiang, H.; Xu, B.; Yu, Y.; Chen, X. Effects of Protein Corona on Active and Passive Targeting of Cyclic RGD Peptide-Functionalized PEGylation Nanoparticles. Mol. Pharm. 2018, 15, 5019–5030. [Google Scholar] [CrossRef]
- Wang, K.; Kievit, F.M.; Zhang, M. Nanoparticles for cancer gene therapy: Recent advances, challenges, and strategies. Pharmacol. Res. 2016, 114, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Von Karstedt, S.; Montinaro, A.; Walczak, H. Exploring the TRAILs less travelled: TRAIL in cancer biology and therapy. Nat. Rev. Cancer 2017, 17, 352–366. [Google Scholar] [CrossRef]
- Guimarães, P.P.G.; Gaglione, S.; Sewastianik, T.; Carrasco, R.D.; Langer, R.; Mitchell, M.J. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano 2018, 12, 912–931. [Google Scholar] [CrossRef] [PubMed]
- Belkahla, H.; Herlem, G.; Picaud, F.; Gharbi, T.; Hémadi, M.; Ammar, S.; Micheau, O. TRAIL-NP hybrids for cancer therapy: A review. Nanoscale 2017, 9, 5755–5768. [Google Scholar] [CrossRef]
- Yuan, X.; Gajan, A.; Chu, Q.; Xiong, H.; Wu, K.; Wu, G.S. Developing TRAIL/TRAIL death receptor-based cancer therapies. Cancer Metastasis Rev. 2018, 37, 733–748. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Buscail, L.; Bournet, B.; Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 153–168. [Google Scholar] [CrossRef]
- Zhao, Y.; Lee, R.J.; Liu, L.; Dong, S.; Zhang, J.; Zhang, Y.; Yao, Y.; Lu, J.; Meng, Q.; Xie, J.; et al. Multifunctional drug carrier based on PEI derivatives loaded with small interfering RNA for therapy of liver cancer. Int. J. Pharm. 2019, 564, 214–224. [Google Scholar] [CrossRef]
- Wan, X.; Sun, R.; Bao, Y.; Zhang, C.; Wu, Y.; Gong, Y. In Vivo Delivery of siRNAs Targeting EGFR and BRD4 Expression by Peptide-Modified Redox Responsive PEG-PEI Nanoparticles for the Treatment of Triple-Negative Breast Cancer. Mol. Pharm. 2021, 18, 3990–3998. [Google Scholar] [CrossRef]
- Kodama, Y.; Kuramoto, H.; Mieda, Y.; Muro, T.; Nakagawa, H.; Kurosaki, T.; Sakaguchi, M.; Nakamura, T.; Kitahara, T.; Sasaki, H. Application of biodegradable dendrigraft poly-l-lysine to a small interfering RNA delivery system. J. Drug Target. 2017, 25, 49–57. [Google Scholar] [CrossRef]
- Lallana, E.; Rios de la Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Rostami, N.; Nikkhoo, A.; Khazaei-Poul, Y.; Farhadi, S.; Sadat Haeri, M.; Moghadaszadeh Ardebili, S.; Aghaei Vanda, N.; Atyabi, F.; Namdar, A.; Baghaei, M.; et al. Coinhibition of S1PR1 and GP130 by siRNA-loaded alginate-conjugated trimethyl chitosan nanoparticles robustly blocks development of cancer cells. J. Cell. Physiol. 2020, 235, 9702–9717. [Google Scholar] [CrossRef] [PubMed]
- Oyaghire, S.N.; Quijano, E.; Piotrowski-Daspit, A.S.; Saltzman, W.M.; Glazer, P.M. Poly(Lactic-co-Glycolic Acid) Nanoparticle Delivery of Peptide Nucleic Acids In Vivo. Methods Mol. Biol. 2020, 2105, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Ickenstein, L.M.; Garidel, P. Lipid-based nanoparticle formulations for small molecules and RNA drugs. Expert Opin. Drug Deliv. 2019, 16, 1205–1226. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and Tumor-Penetrating Mesoporous Silica Nanoparticles for siRNA/miRNA Combination Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308–4322. [Google Scholar] [CrossRef]
- Edwards, C.H.; Christie, C.R.; Masotti, A.; Celluzzi, A.; Caporali, A.; Campbell, E.M. Dendrimer-coated carbon nanotubes deliver dsRNA and increase the efficacy of gene knockdown in the red flour beetle Tribolium castaneum. Sci. Rep. 2020, 10, 12422. [Google Scholar] [CrossRef]
- Rahme, K.; Guo, J.; Holmes, J.D. Bioconjugated Gold Nanoparticles Enhance siRNA Delivery in Prostate Cancer Cells. Methods Mol. Biol. 2019, 1974, 291–301. [Google Scholar] [CrossRef]
- Trejo-Santillan, I.; Mendoza-Guevara, C.C.; Ramos-Godinez, M.D.P.; Ramon-Gallegos, E. Biosecurity test of conjugated nanoparticles of chitosan-protoporphyrin IX-vitamin B9 for their use in photodynamic therapy. IEEE Trans. Nanobiosci. 2021, 23. [Google Scholar] [CrossRef]


| Nanoparticle | Unique Properties | Medical Use in Cancer | References |
|---|---|---|---|
| Magnetic nanoparticles (MNPs) | Can be detected and manipulated by remote magnetic fields, can generate heat when exposed to an alternating magnetic field. | Magnetic biosensing, magnetic imaging, and magnetic separation (diagnostics). Drug and gene delivery, and hyperthermia therapy. | [5,16,17] |
| Gold nanoparticles (AuNPs) | Surface plasmon resonance, surface multi-functionalization, facile synthesis, stable nature, non-toxic and non-immunogenic nature, high permeability and retention effect, easy penetration and accumulation at tumor, can absorb near-infrared (NIR) light at 650–900 nm and convert it to heat. | Tumor detection by imaging (diagnostics). Treatment of cancer by drug delivery, photothermal and photodynamic therapy. | [5,17,18] |
| Polymeric nanoparticles | Biodegradable, increase the circulation time of drugs in the body, can target molecules with minimal side-effects, non-activation of the mononuclear phagocyte system. | Polymeric nanocarrier system for drug delivery in chemotherapy. Cationic charged polymers can carry nucleic acids (gene therapy). Controlled drug delivery. | [19,20] |
| Liposomes | Biocompatible, highly flexible, can carry different types of therapeutic molecules, can be tailored to extend blood circulation time, can be targeted. Several liposomes (lipidic nanoparticles) are on the market. | Drug delivery, long-circulating (PEGylated) liposomes, gene therapy, ligand-targeted liposomes, liposomes containing combinations of drugs. Delivery of anti-fungal, antibiotic, anesthetic, and anti-inflammatory drugs. | [21,22] |
| Micelles | Self-assembly, condensation and protection of nucleic acids, cell association, gene transfection, low toxicity. | Gene delivery | [23] |
| Carbon nanotubes (CNTs) | Can penetrate cell membranes, the sp2 hybridization of all carbons enables their functionalization with almost every biomolecule or compound, allowing them to target cells and deliver drugs under the appropriate environmental stimuli, can absorb near-infrared (NIR) light at 650–900 nm and convert it to heat. | Drug delivery and hyperthermia therapy. | [24] |
| Drug | Action Pathway | Common Adverse Side Effects (>30%) | Less Common Adverse Side Effects (<30%) | References |
|---|---|---|---|---|
| 5-FU Capecitabine Gemcitabine | Pirimidin antagonist | Diarrhea, occasional nausea, vomiting, mouth sores, poor appetite, watery eyes, sensitivity to light (photophobia), metallic taste in the mouth during the infusion, anemia. | Skin reactions: dryness, cracking, peeling of the skin, darkening of the skin due to hypersensitization to radiation, skin rash, swelling, redness, pain, peeling of the skin on the palms of the hands and the soles of the feet. Hair thinning, nail discoloration, falling of the nails, hand-and-foot syndrome (palmar-plantar erythrodysesthesia). | [40,41,42] |
| Paclitaxel (Abraxane®) | Mitotic block by stabilizing microtubules. | Low blood counts, hair loss, peripheral neuropathy, abnormal ECG, nausea, weakness, fatigue, diarrhea, poor appetite, arthralgias, myalgias, edema, and fever. | Infections, dehydration, constipation, taste changes, skin rash, headache, eye problems, depression, mouth sores, shortness of breath, cough, nose bleeds. | [43,44,45,46,47] |
| Cisplatin Oxaliplatin | Chelant | Nausea and vomiting. Nausea can last up to 1 week after treatment. Renal toxicity occurs 10 to 20 days after treatment and is usually reversible. Reduction of the concentration of magnesium, calcium, and potassium. Leukopenia and anemia. | Peripheral neuropathy: despite being rare, a serious side effect of decreased sensation and paresthesia can be observed. Sensory loss, numbness and tingling, and difficulty walking can last at least during therapy. These side effects can get progressively worse with treatment. The neurological effects can be irreversible. High frequency deafness. Ringing in the ears. Lack of appetite, alterations in taste, metallic taste. Increased values in blood tests that measure liver function. Hair loss, fever. Cisplatin can also affect fertility. | [48,49] |
| Irinotecan (Onivyde®) | Topoisomerase I inhibitor | Early diarrhea occurs within 24 h of drug administration. It is accompanied by symptoms such as a runny nose, increased salivation, tearing, sweating, erythema, and abdominal cramps. This type of diarrhea can occur during drug administration. Late diarrhea occurs 24 h after drug administration and usually reaches its highest intensity around 11 days after treatment. Dehydration and electrolyte imbalance. Nausea, vomiting, weakness, leukopenia, anemia. | Hair loss, poor appetite, fever, weight loss, constipation, dyspnea, insomnia, cough, headache, dehydration, shaking chills, acne, flatulence, erythema of the face, mouth sores, heartburn, swelling in the feet and ankles. | [50] |
| Surface Protein in Pancreatic Cancer 1 | Relevance | References |
|---|---|---|
| TFRC | Transferrin receptors (TFRC) are over expressed in 93% of the pancreatic cells. In 2019, Wu demonstrated that nanoparticles can be targeted to pancreatic cancer cells using an aptamer that binds with transferrin receptor protein 1 also known as CD71. | [51,52] |
| FC | Folate receptor (FR) is a glycosylphosphatidylinositol expressed in more than the 80% of the pancreatic cancer patients. It has a limited expression in healthy cells. | [51,53] |
| DR5 | DR5 is significantly higher than stage II, III, and IV tumors than in stage I tumors. DR5 is associated with TRAIL resistance. | [54] |
| LOXL2 | Regulates the expression of EMT markers. LOXL2 overexpression correlates with poor prognosis in patients with pancreatic cancer. | [55] |
| HGF | Modulate multiple cell functions, including proliferation, motility, migration, and invasion. | [56] |
| PD-1/PDL1 | PD-L1 is expressed in PDAC, and its overexpression is associated with a poor prognosis. Previous studies reported divergent tumoral PD-L1 levels, ranging from 12 to 90%. | [57,58,59] |
| VEGF | An important factor regulating angiogenesis, VEGF, is over expressed in more than 90% of PDACs and correlates with a worse prognosis. Seo et al. demonstrated that 93% of PDAC were positive for VEGF protein. | [60,61,62] |
| HER2 or ERBB2 | HER2 protein expression is associated with decreased survival rate. HER2 is overexpressed in 45% of PDAC. | [63] |
| EGRF | EGFR is overexpressed in 40–70% of pancreatic cancers. Overexpression is correlated with metastasis to other organs. | [64,65,66] |
| IGF-IR | Overexpression and excessive activation of IGF-IR are associated with malignant transformation, increment of tumor aggressiveness, and protection from apoptosis. IGF-IR targets 70 to 100% of the core metabolic pathways that are often altered in PDAC pathogenesis. | [67,68] |
| PSCA | Overexpressed in approximately 60% of pancreatic cancers. | [69] |
| CD40 | CD40 agonists tumor growth suppression and extended survival. | [70,71,72] |
| GCC | GCC is a transmembrane G protein cell-surface receptor activated by the endogenous hormones guanylin and uroguanylin and bacterial heat-stable enterotoxins that plays a role in regulation of fluid and electrolyte balance. It is highly expressed in colorectal cancer and about 60–70% of pancreatic cancers. It is shown to inhibit the growth-suppressing activity of GCC in pancreatic cancer cell lines and pancreatic-patient-derived xenograft (PDX) models. | [73,74,75] |
| CA19-9 | An attractive therapeutic target for PCAD is carbohydrate antigen 19-9 (CA19-9), known as sialyl Lewis A (sLea). It represents a validated biomarker widely used for diagnostic and prognostic in pancreatic cancer. It is a useful predictor of tumor stage and resectability and response to therapy, and is useful for assessing overall survival. A reduction in CA19-9 is an indicator of treatment benefit. | [76,77] |
| SLC44A4 | Localized in tumor stroma, fibroblasts, and tumor epithelial cells. This protein has been evaluated as a prognostic and predictive biomarker. | [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Delgado-Gonzalez, P.; G-Buentello, Z.; Delgado-Gallegos, J.L.; Chapa-Gonzalez, C.; Sánchez-Domínguez, M.; Sánchez-Domínguez, C.N.; Islas, J.F. Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy. Life 2021, 11, 1187. https://doi.org/10.3390/life11111187
Roacho-Pérez JA, Garza-Treviño EN, Delgado-Gonzalez P, G-Buentello Z, Delgado-Gallegos JL, Chapa-Gonzalez C, Sánchez-Domínguez M, Sánchez-Domínguez CN, Islas JF. Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy. Life. 2021; 11(11):1187. https://doi.org/10.3390/life11111187
Chicago/Turabian StyleRoacho-Pérez, Jorge A., Elsa N. Garza-Treviño, Paulina Delgado-Gonzalez, Zuca G-Buentello, Juan Luis Delgado-Gallegos, Christian Chapa-Gonzalez, Margarita Sánchez-Domínguez, Celia N. Sánchez-Domínguez, and Jose Francisco Islas. 2021. "Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy" Life 11, no. 11: 1187. https://doi.org/10.3390/life11111187
APA StyleRoacho-Pérez, J. A., Garza-Treviño, E. N., Delgado-Gonzalez, P., G-Buentello, Z., Delgado-Gallegos, J. L., Chapa-Gonzalez, C., Sánchez-Domínguez, M., Sánchez-Domínguez, C. N., & Islas, J. F. (2021). Target Nanoparticles against Pancreatic Cancer: Fewer Side Effects in Therapy. Life, 11(11), 1187. https://doi.org/10.3390/life11111187









