Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review
Abstract
:1. Introduction
2. Methodology
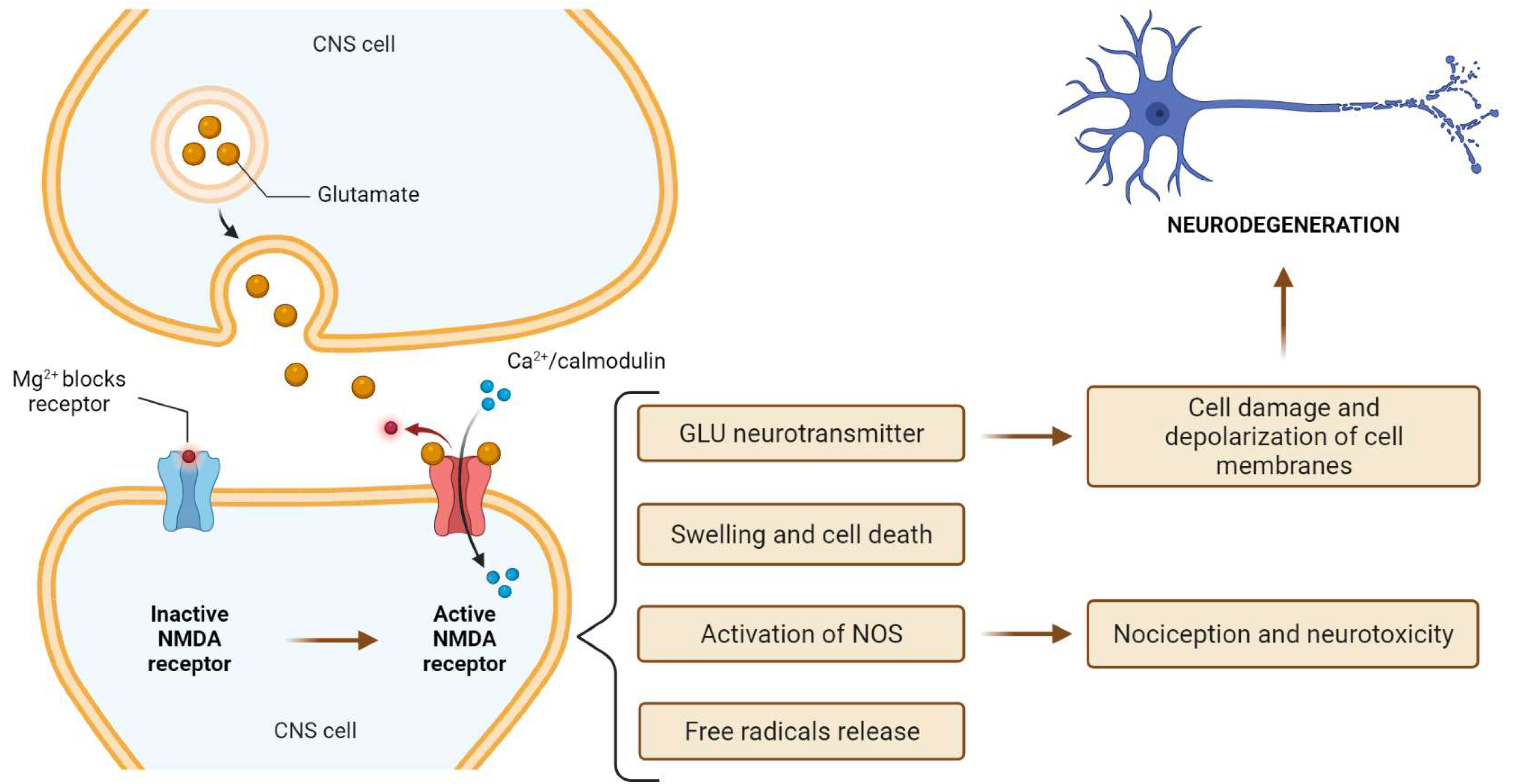
3. N-Methyl-D-Aspartate (NMDA) Receptor
4. Ketamine Characteristics
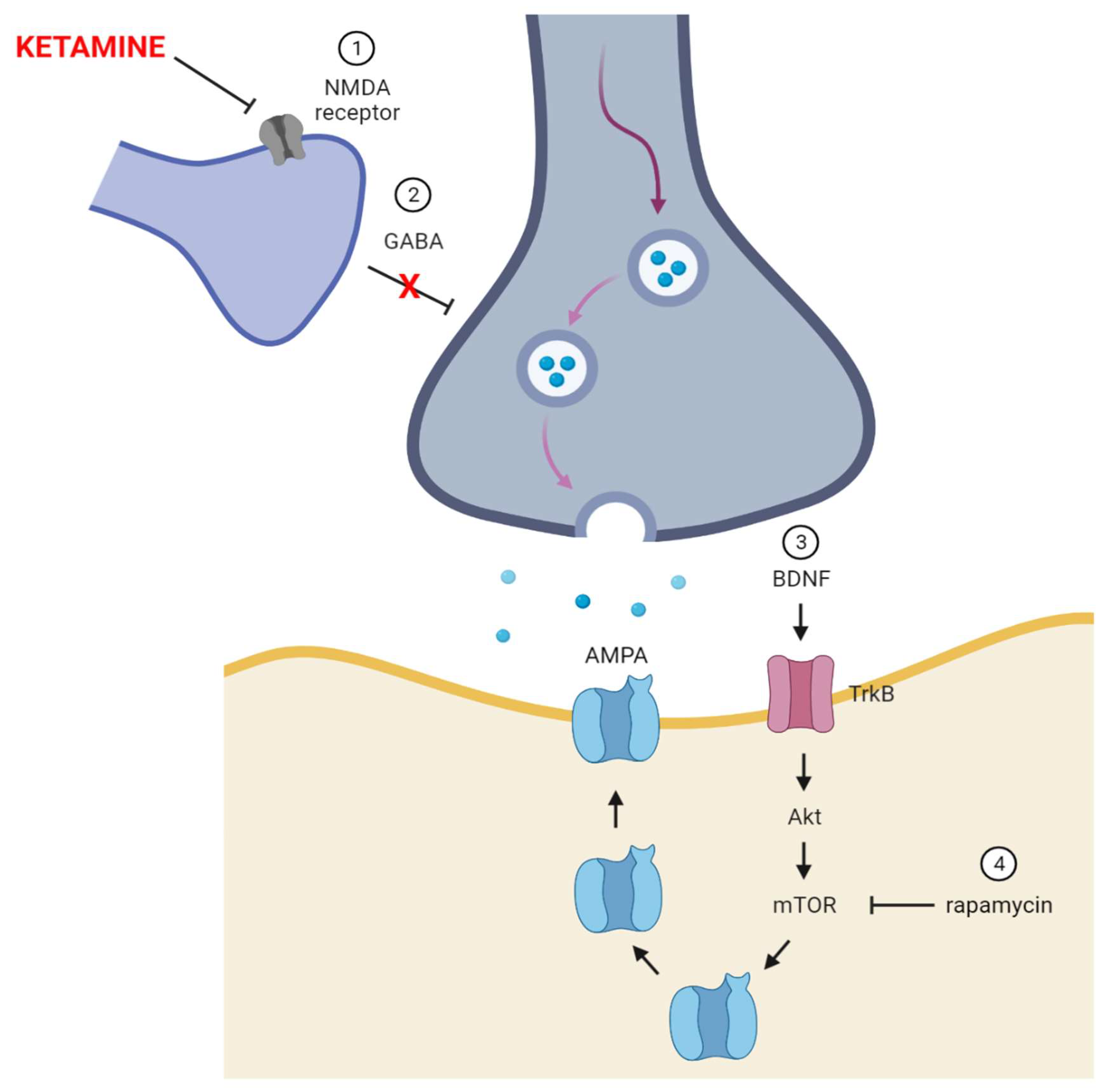
5. Ketamine—The Receptor and Non-Receptor Mechanism of Action
6. Ketamine Toxicity
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Statistics 2021: A Visual Summary. Available online: https://www.who.int/data/stories/world-health-statistics-2021-a-visual-summary (accessed on 29 August 2021).
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Rotenstein, L.S.; Ramos, M.A.; Torre, M.; Segal, J.B.; Peluso, M.J.; Guille, C.; Sen, S.; Mata, D.A. Prevalence of Depression, Depressive Symptoms, and Suicidal Ideation Among Medical Students: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 2214–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Torre, J.A.; Vilagut, G.; Ronaldson, A.; Serrano-Blanco, A.; Martín, V.; Peters, M.; Valderas, J.M.; Dregan, A.; Alonso, J. Prevalence and Variability of Current Depressive Disorder in 27 European Countries: A Population-Based Study. Lancet Public Health 2021. [Google Scholar] [CrossRef]
- Guerrini, C.J.; Schneider, S.C.; Guzick, A.G.; Amos Nwankwo, G.N.; Canfield, I.; Fedson, S.; Gutierrez, A.M.; Sheu, J.C.; Song, A.Y.; Villagran, A.M.; et al. Psychological Distress among the U.S. General Population During the COVID-19 Pandemic. Front. Psychiatry 2021, 12, 810. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, K.; Adolph, C.; Amlag, J.O.; Aravkin, A.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 8. [Google Scholar] [CrossRef]
- Talarowska, M.; Chodkiewicz, J.; Nawrocka, N.; Miniszewska, J.; Biliński, P. Mental Health and the SARS-CoV-2 Epidemic-Polish Research Study. Int. J. Environ. Res. Public Health 2020, 17, 7015. [Google Scholar] [CrossRef]
- Stupin, K.N.; Zenko, M.Y.; Rybnikova, E.A. Comparative Analysis of Pathobiochemical Changes in Major Depression and Post-Traumatic Stress Disorder. Biochemistry 2021, 86, 729–736. [Google Scholar] [CrossRef]
- Ohayon, M.M.; McCue, M.; Krystal, A.; Chrones, L.; Touya, M.; Lawrence, D.; Patel, S.; Cote, M.L. A Longitudinal Study to Assess Antidepressant Treatment Patterns and Outcomes in Individuals with Depression in the General Population. CNS Spectr. 2021, 26, 180. [Google Scholar] [CrossRef]
- Sarno, E.; Moeser, A.J.; Robison, A.J. Neuroimmunology of Depression. Adv. Pharmacol. 2021, 91, 259–292. [Google Scholar] [CrossRef]
- Kennedy, S.H.; Lam, R.W.; McIntyre, R.S.; Tourjman, S.V.; Bhat, V.; Blier, P.; Hasnain, M.; Jollant, F.; Levitt, A.J.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 3. Pharmacological Treatments. Can. J. Psychiatry 2016, 61, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Zonca, V.; Cattane, N.; Pariante, C.M.; Cattaneo, A. MiRNAs in Depression Vulnerability and Resilience: Novel Targets for Preventive Strategies. J. Neural Transm. 2019, 126, 1241–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavi, M.; Henter, I.D.; Park, L.T.; Zarate, C. Key Considerations in the Pharmacological Management of Treatment-Resistant Depression. Expert Opin. Pharmacother. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Popova, V.; Wylie, C.; Fedgchin, M.; Daly, E.; Janik, A.; Ochs-Ross, R.; Lane, R.; Lim, P.; Cooper, K.; et al. Effect of Esketamine Nasal Spray on Olfactory Function and Nasal Tolerability in Patients with Treatment-Resistant Depression: Results from Four Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase III Studies. CNS Drugs 2021, 35, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Lipsitz, O.; Di Vincenzo, J.D.; Rodrigues, N.B.; Kratiuk, K.; Subramaniapillai, M.; Lee, Y.; Arekapudi, A.K.; Abrishami, A.; Chau, E.H.; et al. Real-World Effectiveness of Repeated Ketamine Infusions for Treatment Resistant Depression during the COVID-19 Pandemic. Psychiatry Res. 2021, 303, 114086. [Google Scholar] [CrossRef]
- Ng, J.; Rosenblat, J.D.; Lui, L.M.W.; Teopiz, K.M.; Lee, Y.; Lipsitz, O.; Mansur, R.B.; Rodrigues, N.B.; Nasri, F.; Gill, H.; et al. Efficacy of Ketamine and Esketamine on Functional Outcomes in Treatment-Resistant Depression: A Systematic Review. J. Affect. Disord. 2021, 293, 285–294. [Google Scholar] [CrossRef]
- Sanders, B.; Brula, A.Q. Intranasal Esketamine: From Origins to Future Implications in Treatment-Resistant Depression. J. Psychiatr. Res. 2021, 137, 29–35. [Google Scholar] [CrossRef]
- Popova, V.; Daly, E.J.; Trivedi, M.; Cooper, K.; Lane, R.; Lim, P.; Mazzucco, C.; Hough, D.; Thase, M.E.; Shelton, R.C.; et al. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. AJP 2019, 176, 428–438. [Google Scholar] [CrossRef]
- Bartoli, F.; Riboldi, I.; Crocamo, C.; Di Brita, C.; Clerici, M.; Carrà, G. Ketamine as a Rapid-Acting Agent for Suicidal Ideation: A Meta-Analysis. Neurosci. Biobehav. Rev. 2017, 77, 232–236. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Rodrigues, N.B.; Lipsitz, O.; Nasri, F.; Gill, H.; Lui, L.M.; Subramaniapillai, M.; Kratiuk, K.; Teopiz, K.; Ho, R.; et al. The Effectiveness of Intravenous Ketamine in Adults with Treatment-Resistant Major Depressive Disorder and Bipolar Disorder Presenting with Prominent Anxiety: Results from the Canadian Rapid Treatment Center of Excellence. J. Psychopharmacol. 2021, 35, 128–136. [Google Scholar] [CrossRef]
- Irifune, M.; Shimizu, T.; Nomoto, M.; Fukuda, T. Ketamine-Induced Anesthesia Involves the N-Methyl-D-Aspartate Receptor-Channel Complex in Mice. Brain Res. 1992, 596, 1–9. [Google Scholar] [CrossRef]
- Curtis, D.R.; Phillis, J.W.; Watkins, J.C. Chemical Excitation of Spinal Neurones. Nature 1959, 183, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Porter, R.H.; Greenamyre, J.T. Glutamate and Parkinson’s Disease. Mol. Neurobiol. 1996, 12, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.C.; Krogsgaard-Larsen, P.; Honoré, T. Structure-Activity Relationships in the Development of Excitatory Amino Acid Receptor Agonists and Competitive Antagonists. Trends Pharmacol. Sci. 1990, 11, 25–33. [Google Scholar] [CrossRef]
- Hirota, K.; Lambert, D.G. Ketamine: Its Mechanism(s) of Action and Unusual Clinical Uses. Br. J. Anaesth. 1996, 77, 441–444. [Google Scholar] [CrossRef] [Green Version]
- Doble, A. Excitatory Amino Acid Receptors and Neurodegeneration. Therapie 1995, 50, 319–337. [Google Scholar] [PubMed]
- Lucas, D.R.; Newhouse, J.P. The Toxic Effect of Sodium L-Glutamate on the Inner Layers of the Retina. AMA Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Kainic Acid as a Tool in Neurobiology; McGeer, P.L.; McGeer, E.G.; Olney, J.W. (Eds.) Raven Press: New York, NY, USA, 1978; ISBN 978-0-89004-279-3. [Google Scholar]
- Zarate, C.A.; Du, J.; Quiroz, J.; Gray, N.A.; Denicoff, K.D.; Singh, J.; Charney, D.S.; Manji, H.K. Regulation of Cellular Plasticity Cascades in the Pathophysiology and Treatment of Mood Disorders: Role of the Glutamatergic System. Ann. N. Y. Acad. Sci. 2003, 1003, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Lindsley, C.W.; Shipe, W.D.; Wolkenberg, S.E.; Theberge, C.R.; Williams, D.L.; Sur, C.; Kinney, G.G. Progress towards Validating the NMDA Receptor Hypofunction Hypothesis of Schizophrenia. Curr. Top. Med. Chem. 2006, 6, 771–785. [Google Scholar] [CrossRef]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H.Y. Plasma Concentrations of Excitatory Amino Acids, Serine, Glycine, Taurine and Histidine in Major Depression. Eur. Neuropsychopharmacol. 1995, 5, 71–75. [Google Scholar] [CrossRef]
- Beneyto, M.; Kristiansen, L.V.; Oni-Orisan, A.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal Glutamate Receptor Expression in the Medial Temporal Lobe in Schizophrenia and Mood Disorders. Neuropsychopharmacology 2007, 32, 1888–1902. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Bliss, T.V. Memories of NMDA Receptors and LTP. Trends Neurosci. 1995, 18, 54–56. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Singer, W. Excitatory Amino Acid Receptors and Synaptic Plasticity. Trends Pharmacol. Sci. 1990, 11, 290–296. [Google Scholar] [CrossRef]
- Danysz, W.; Parsons, C.; Bresink, I.; Quack, G. Glutamate in CNS Disorders—A Revived Target for Drug Development. Drug News Perspect. 1995, 8, 261–277. [Google Scholar]
- Ossola, B.; Schendzielorz, N.; Chen, S.-H.; Bird, G.S.; Tuominen, R.K.; Männistö, P.T.; Hong, J.-S. Amantadine Protects Dopamine Neurons by a Dual Action: Reducing Activation of Microglia and Inducing Expression of GDNF in Astroglia [Corrected]. Neuropharmacology 2011, 61, 574–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, N.J.; Barres, B.A. Neuroscience: Glia—More than Just Brain Glue. Nature 2009, 457, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Smiałowska, M.; Szewczyk, B.; Woźniak, M.; Wawrzak-Wleciał, A.; Domin, H. Glial Degeneration as a Model of Depression. Pharmacol. Rep. 2013, 65, 1572–1579. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Wu, B.; Zhou, W. Ketamine Abuse Potential and Use Disorder. Brain Res. Bull. 2016, 126, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Domino, E.F.; Chodoff, P.; Corssen, G. Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clin. Pharmacol. Ther. 1965, 6, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, Y.; Bolon, M.; Boulieu, R. Stability of Ketamine and Its Metabolites Norketamine and Dehydronorketamine in Human Biological Samples. Clin. Chem. 2001, 47, 1713–1715. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.M. Ketamine Hydrochloride in Severe Bronchospasm. Anaesthesia 1977, 32, 771–772. [Google Scholar] [CrossRef]
- Corssen, G.; Domino, E.F. Dissociative Anesthesia: Further Pharmacologic Studies and First Clinical Experience with the Phencyclidine Derivative CI-581. Anesth. Analg. 1966, 45, 29–40. [Google Scholar] [CrossRef]
- Roberts, J.R.; Jerris, R. Hedges. In Roberts and Hedges’ Clinical Procedures in Emergency Medicine, 6th ed.; Saunders: Philadelphia, PA, USA, 2013; ISBN 978-1-4557-0606-8. [Google Scholar]
- White, P.F.; Way, W.L.; Trevor, A.J. Ketamine--Its Pharmacology and Therapeutic Uses. Anesthesiology 1982, 56, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, I.L.; Limoncelli, D.; Gueorguieva, R.; Jatlow, P.; Boutros, N.N.; Trevisan, L.; Gelernter, J.; Krystal, J.H. Altered NMDA Glutamate Receptor Antagonist Response in Individuals with a Family Vulnerability to Alcoholism. Am. J. Psychiatry 2004, 161, 1776–1782. [Google Scholar] [CrossRef]
- Krupitsky, E.M.; Burakov, A.M.; Dunaevsky, I.V.; Romanova, T.N.; Slavina, T.Y.; Grinenko, A.Y. Single versus Repeated Sessions of Ketamine-Assisted Psychotherapy for People with Heroin Dependence. J. Psychoact. Drugs 2007, 39, 13–19. [Google Scholar] [CrossRef]
- Mills, I.H.; Park, G.R.; Manara, A.R.; Merriman, R.J. Treatment of Compulsive Behaviour in Eating Disorders with Intermittent Ketamine Infusions. QJM 1998, 91, 493–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrough, J.W. Ketamine as a Novel Antidepressant: From Synapse to Behavior. Clin. Pharmacol. Ther. 2012, 91, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Aghajanian, G.K. Synaptic Dysfunction in Depression: Potential Therapeutic Targets. Science 2012, 338, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. MTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coyle, C.M.; Laws, K.R. The Use of Ketamine as an Antidepressant: A Systematic Review and Meta-Analysis. Hum. Psychopharmacol. 2015, 30, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A Randomized Trial of an N-Methyl-D-Aspartate Antagonist in Treatment-Resistant Major Depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Barash, P.G.; Cullen, B.F.; Stoelting, R.K.; Cahalan, M.K.; Stock, M.C.; Ortega, R. Clinical Anesthesia: Seventh Edition; Wolters Kluwer Health: Philadelphia, PA, USA, 2013; ISBN 978-1-4511-4419-2. [Google Scholar]
- FRCA, K.; CENG, S.; FRCA, R.; FRCA, M. Introduction to Specialist Therapeutics. In Pharmacology & Pharmacokinetics; Springer: London, UK, 2010; pp. 53–66. ISBN 978-1-84996-145-5. [Google Scholar]
- Niesters, M.; Martini, C.; Dahan, A. Ketamine for Chronic Pain: Risks and Benefits. Br. J. Clin. Pharmacol. 2014, 77, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SPRAVATO® (Esketamine) | SPRAVATO®. Available online: https://www.spravato.com/home-1 (accessed on 30 August 2021).
- Kohrs, R.; Durieux, M.E. Ketamine: Teaching an Old Drug New Tricks. Anesth. Analg. 1998, 87, 1186–1193. [Google Scholar] [CrossRef]
- Miller, K.W. The Nature of Sites of General Anaesthetic Action. Br. J. Anaesth. 2002, 89, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Hustveit, O.; Maurset, A.; Oye, I. Interaction of the Chiral Forms of Ketamine with Opioid, Phencyclidine, Sigma and Muscarinic Receptors. Pharmacol. Toxicol. 1995, 77, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Levänen, J.; Mäkelä, M.L.; Scheinin, H. Dexmedetomidine Premedication Attenuates Ketamine-Induced Cardiostimulatory Effects and Postanesthetic Delirium. Anesthesiology 1995, 82, 1117–1125. [Google Scholar] [CrossRef]
- Martin, D.C.; Introna, R.P.; Aronstam, R.S. Inhibition of Neuronal 5-HT Uptake by Ketamine, but Not Halothane, Involves Disruption of Substrate Recognition by the Transporter. Neurosci. Lett. 1990, 112, 99–103. [Google Scholar] [CrossRef]
- Martin, L.L.; Bouchal, R.L.; Smith, D.J. Ketamine Inhibits Serotonin Uptake in Vivo. Neuropharmacology 1982, 21, 113–118. [Google Scholar] [CrossRef]
- Meng, E.; Chang, H.-Y.; Chang, S.-Y.; Sun, G.-H.; Yu, D.-S.; Cha, T.-L. Involvement of Purinergic Neurotransmission in Ketamine Induced Bladder Dysfunction. J. Urol. 2011, 186, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.E.; Gingrich, K.J.; Kulli, J.C.; Yang, J. Ketamine Blockade of Voltage-Gated Sodium Channels: Evidence for a Shared Receptor Site with Local Anesthetics. Anesthesiology 2001, 95, 1406–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, P.M.; Knudsen, L.; Drummond, P.D. Reduction of Allodynia in Patients with Complex Regional Pain Syndrome: A Double-Blind Placebo-Controlled Trial of Topical Ketamine. Pain 2009, 146, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Friederich, P.; Urban, B.W. Interaction of Intravenous Anesthetics with Human Neuronal Potassium Currents in Relation to Clinical Concentrations. Anesthesiology 1999, 91, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Proescholdt, M.; Heimann, A.; Kempski, O. Neuroprotection of S(+) Ketamine Isomer in Global Forebrain Ischemia. Brain Res. 2001, 904, 245–251. [Google Scholar] [CrossRef]
- Pałucha-Poniewiera, A.; Szewczyk, B.; Pilc, A. Activation of the MTOR Signaling Pathway in the Antidepressant-like Activity of the MGlu5 Antagonist MTEP and the MGlu7 Agonist AMN082 in the FST in Rats. Neuropharmacology 2014, 82, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hay, N.; Sonenberg, N. Upstream and Downstream of MTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Réus, G.Z.; Nacif, M.P.; Abelaira, H.M.; Tomaz, D.B.; dos Santos, M.A.B.; Carlessi, A.S.; Matias, B.I.; da Luz, J.R.; Steckert, A.V.; Jeremias, G.C.; et al. Ketamine Treatment Partly Reverses Alterations in Brain Derived- Neurotrophic Factor, Oxidative Stress and Energy Metabolism Parameters Induced by an Animal Model of Depression. Curr. Neurovasc. Res. 2015, 12, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Heise, C.; Gardoni, F.; Culotta, L.; di Luca, M.; Verpelli, C.; Sala, C. Elongation Factor-2 Phosphorylation in Dendrites and the Regulation of Dendritic MRNA Translation in Neurons. Front. Cell Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Monteggia, L.M.; Gideons, E.; Kavalali, E.T. The Role of Eukaryotic Elongation Factor 2 Kinase in Rapid Antidepressant Action of Ketamine. Biol. Psychiatry 2013, 73, 1199–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Proud, C.G. Eukaryotic Elongation Factor 2 Kinase as a Drug Target in Cancer, and in Cardiovascular and Neurodegenerative Diseases. Acta Pharmacol. Sin. 2016, 37, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Daly, E.; Trivedi, M.; Janik, A. A randomized withdrawal, double-blind, multicenterstudy of esketamine nasal spray plus an oral antidepressant for relapse prevention in treatment-resistant depression. In Proceedings of the American Society of Clinical Psychopharmacology Annual Meeting, Miami, FL, USA, 29 May–1 June 2018. [Google Scholar]
- Daly, E.J.; Singh, J.B.; Fedgchin, M.; Cooper, K.; Lim, P.; Shelton, R.C.; Thase, M.E.; Winokur, A.; Van Nueten, L.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 2018, 75, 139–148. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Husain, M.M.; Trivedi, M.H.; Fava, M.; Warden, D.; Wisniewski, S.R.; Miyahara, S.; Rush, A.J. Residual Symptoms after Remission of Major Depressive Disorder with Citalopram and Risk of Relapse: A STAR*D Report. Psychol. Med. 2010, 40, 41–50. [Google Scholar] [CrossRef]
- Li, N.; Liu, R.-J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. Glutamate N-Methyl-D-Aspartate Receptor Antagonists Rapidly Reverse Behavioral and Synaptic Deficits Caused by Chronic Stress Exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef] [Green Version]
- Gable, R.S. Acute Toxic Effects of Club Drugs. J. Psychoact. Drugs 2004, 36, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shlomo, I.; Rosenbaum, A.; Hadash, O.; Katz, Y. Intravenous Midazolam Significantly Enhances the Lethal Effect of Thiopental but Not That of Ketamine in Mice. Pharmacol. Res. 2001, 44, 509–512. [Google Scholar] [CrossRef]
- Hansen, G.; Jensen, S.B.; Chandresh, L.; Hilden, T. The Psychotropic Effect of Ketamine. J. Psychoact. Drugs 1988, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Orhurhu, V.J.; Vashisht, R.; Claus, L.E.; Cohen, S.P. Ketamine Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bokor, G.; Anderson, P.D. Ketamine: An Update on Its Abuse. J. Pharm. Pract. 2014, 27, 582–586. [Google Scholar] [CrossRef]
- Sollazzi, L.; Modesti, C.; Vitale, F.; Sacco, T.; Ciocchetti, P.; Idra, A.S.; Tacchino, R.M.; Perilli, V. Preinductive Use of Clonidine and Ketamine Improves Recovery and Reduces Postoperative Pain after Bariatric Surgery. Surg. Obes. Relat. Dis. 2009, 5, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Bhatia, A.; Buvanendran, A.; Schwenk, E.S.; Wasan, A.D.; Hurley, R.W.; Viscusi, E.R.; Narouze, S.; Davis, F.N.; Ritchie, E.C.; et al. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 2018, 43, 521–546. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.L.; Fedgchin, M.; Singh, J.; Van Gerven, J.; Zuiker, R.; Lim, K.S.; van der Ark, P.; Wajs, E.; Xi, L.; Zannikos, P.; et al. Effect of Intranasal Esketamine on Cognitive Functioning in Healthy Participants: A Randomized, Double-Blind, Placebo-Controlled Study. Psychopharmacology 2018, 235, 1107–1119. [Google Scholar] [CrossRef] [Green Version]
- Popova, V.; Daly, E.; Trivedi, M. Randomized, Double-Blind Study of Flexiblydosed Intranasal Esketamine plus Oral Antidepressant vs. Active Control in Treatment-Resistant Depression. In Proceedings of the American Psychiatric Association Annual Meeting, New York, NY, USA, 5–9 May 2018. [Google Scholar]
- Ochs-Ross, R.; Daly, E.J.; Yun, Z.; Lane, R.; Lim, P.; Foster, K.; Hough, D.; Manji, H.; Drevets, W.C.; Sanacora, G.; et al. Efficacy and Safety of Intranasal Esketamine plus an Oral Antidepressant in Elderly Patients with Treatment-Resistant Depression. In Proceedings of the American Psychiatric Association Annual Meeting, New York, NY, USA, 5–9 May 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, M.; Kowalczyk, E.; Kwiatkowski, P.; Łopusiewicz, Ł.; Sienkiewicz, M.; Talarowska, M. Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review. Life 2021, 11, 1186. https://doi.org/10.3390/life11111186
Kowalczyk M, Kowalczyk E, Kwiatkowski P, Łopusiewicz Ł, Sienkiewicz M, Talarowska M. Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review. Life. 2021; 11(11):1186. https://doi.org/10.3390/life11111186
Chicago/Turabian StyleKowalczyk, Mateusz, Edward Kowalczyk, Paweł Kwiatkowski, Łukasz Łopusiewicz, Monika Sienkiewicz, and Monika Talarowska. 2021. "Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review" Life 11, no. 11: 1186. https://doi.org/10.3390/life11111186
APA StyleKowalczyk, M., Kowalczyk, E., Kwiatkowski, P., Łopusiewicz, Ł., Sienkiewicz, M., & Talarowska, M. (2021). Ketamine—New Possibilities in the Treatment of Depression: A Narrative Review. Life, 11(11), 1186. https://doi.org/10.3390/life11111186








