Neuromuscular Activity during Cycling Performance in Hot/Dry and Hot/Humid Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Preliminary Testing
2.3. Trial for Familiarization
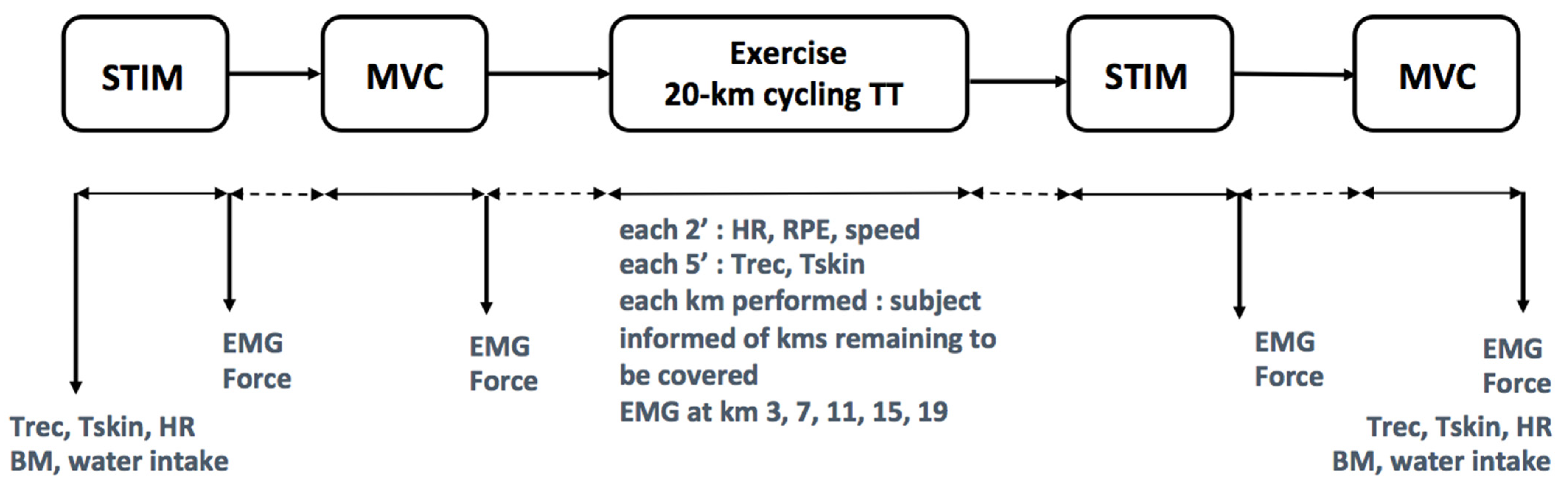
2.4. Design
2.5. Twitch Force of the Vastus Lateralis Muscle
2.6. Testing for Maximal Voluntary Contraction Testing
2.7. EMG Testing
2.8. Temperatures
2.9. Statistical Analysis
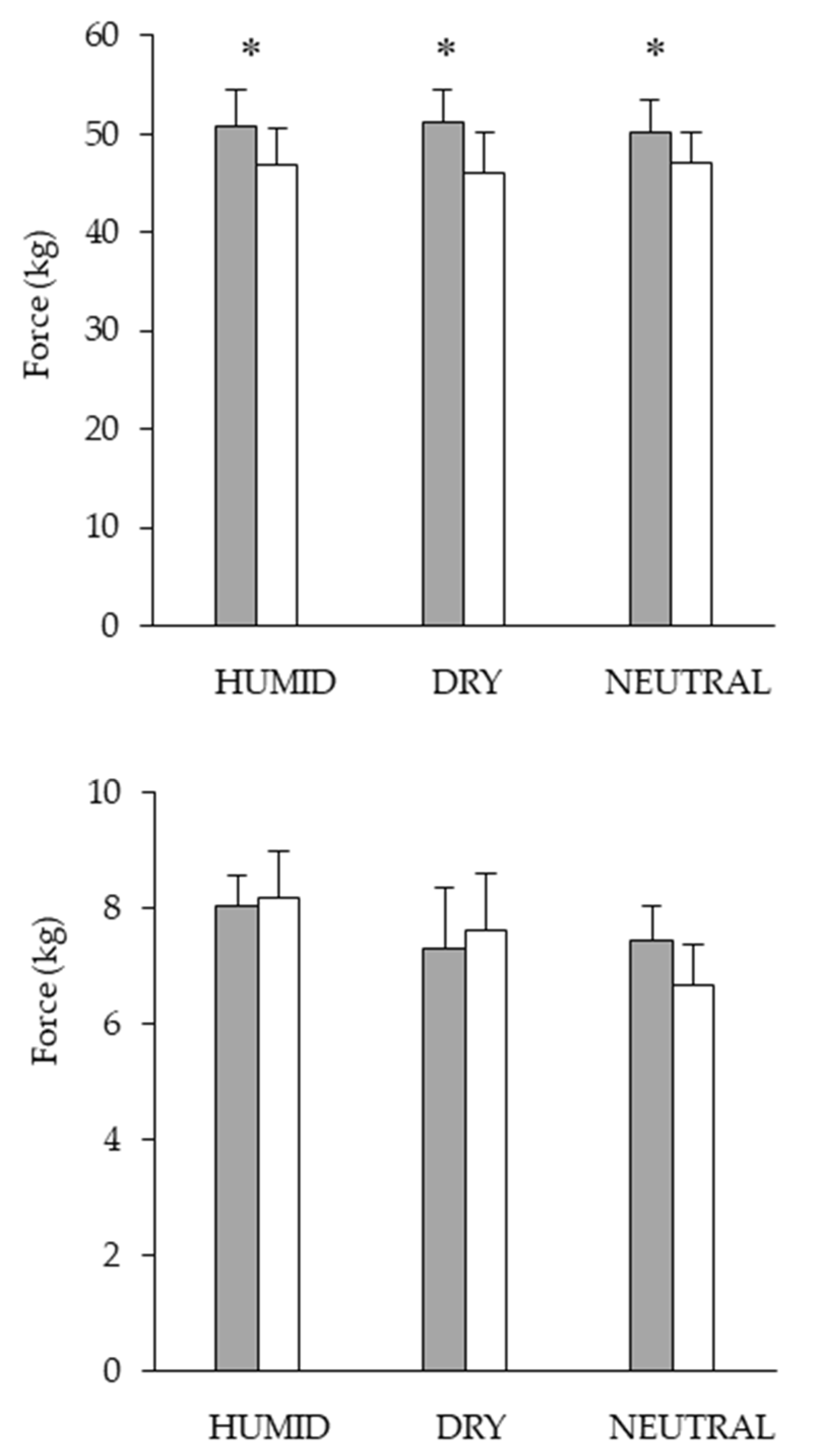
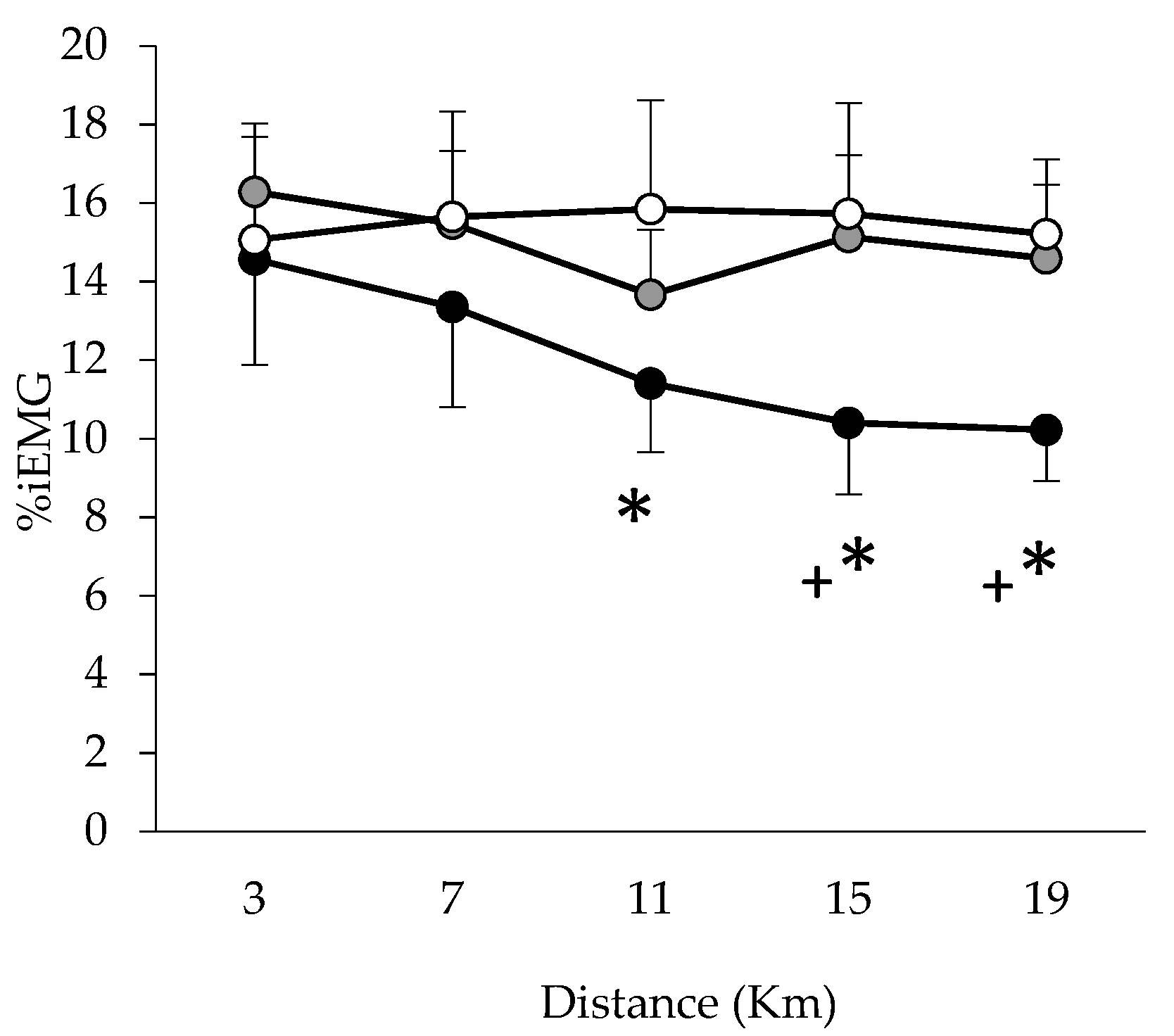
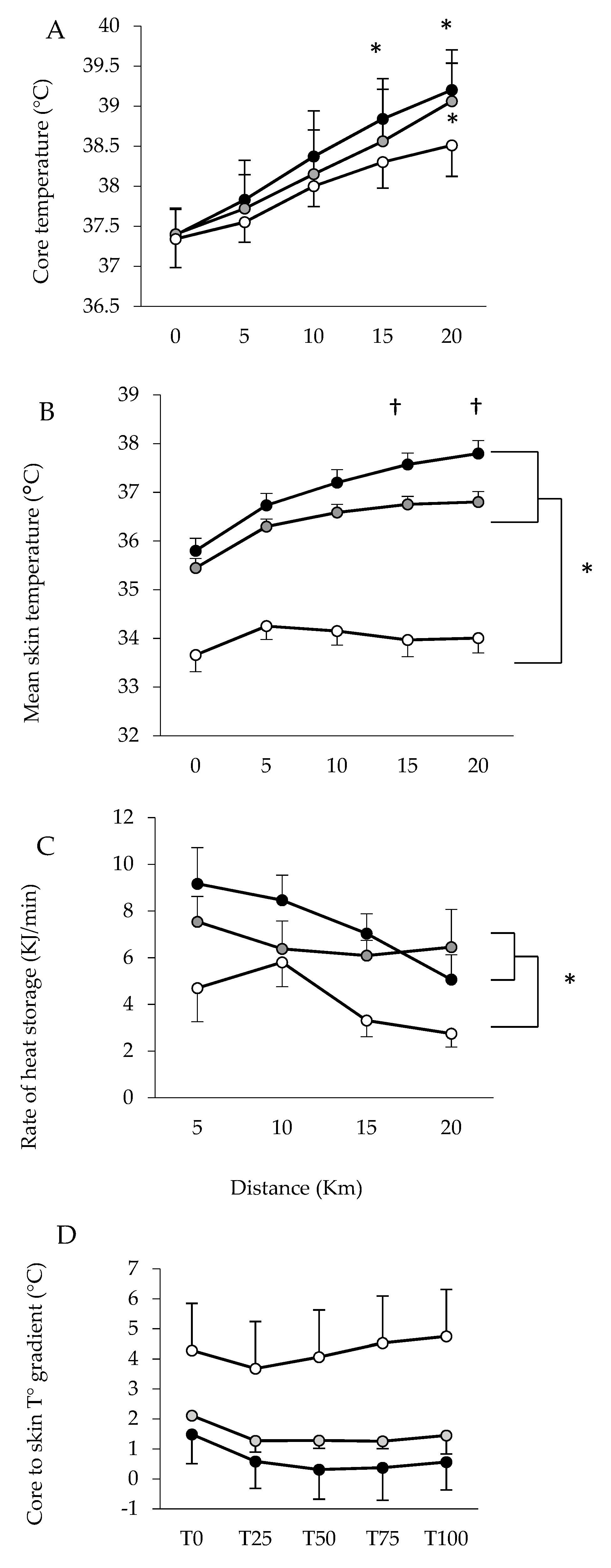
3. Results
Performance
4. Discussion
5. Practical Applications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nybo, L.; Rasmussen, P.; Sawka, M.N. Performance in the Heat-Physiological Factors of Importance for Hyperthermia-Induced Fatigue. Compr. Physiol. 2014, 4, 657–689. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J.; Teller, C.; Andersen, S.L.; Jensen, F.B.; Hyldig, T.; Nielsen, B. Influence of Body Temperature on the Development of Fatigue during Prolonged Exercise in the Heat. J. Appl. Physiol. 1999, 86, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatterson, A.J.; Hahn, A.G.; Martin, D.T.; Febbraio, M.A. Effects of Heat Stress on Physiological Responses and Exercise Performance in Elite Cyclists. J. Sci. Med. Sport 2000, 3, 186–193. [Google Scholar] [CrossRef]
- Nybo, L.; Nielsen, B. Hyperthermia and Central Fatigue during Prolonged Exercise in Humans. J. Appl. Physiol. 2001, 91, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- González-Alonso, J.; Crandall, C.G.; Johnson, J.M. The Cardiovascular Challenge of Exercising in the Heat. J. Physiol. 2008, 586, 45–53. [Google Scholar] [CrossRef]
- Hue, O. The Challenge of Performing Aerobic Exercise in Tropical Environments: Applied Knowledge and Perspectives. Int. J. Sports Physiol. Perform 2011, 6, 443–454. [Google Scholar] [CrossRef] [Green Version]
- Huan, L.; Schlader, Z.; Che Muhamed, A.; Zheng, H.; Stannard, S.; Kondo, N.; Cotter, J.; Mundel, T. Differences in dry-bulb temperature do not influence moderate-duration exercise performance in warm environments when vapor pressure is equivalent. Eur. J. Appl. Physiol. 2020, 120, 841–852. [Google Scholar] [CrossRef]
- Tucker, R.; Rauch, L.; Harley, Y.X.; Noakes, T.D. Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch. 2004, 448, 422–430. [Google Scholar] [CrossRef]
- Baillot, M.; Hue, O. Hydration and thermoregulation during a half-ironman performed in tropical climate. J. Sports Sci. Med. 2015, 14, 263–268. [Google Scholar]
- Baillot, M.; Le Bris, S.; Hue, O. Fluid replacement strategy during a 27-Km trail run in hot and humid conditions. Int. J. Sports Med. 2014, 35, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Polkey, M.I.; Kyroussis, D.; Hamnegard, C.H.; Mills, G.H.; Green, M.; Moxham, J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve 1996, 19, 549–555. [Google Scholar] [CrossRef]
- Hamnegård, C.H.; Sedler, M.; Polkey, M.I.; Bake, B. Quadriceps strength assessed by magnetic stimulation of the femoral nerve in normal subjects. Clin. Physiol. Funct. Imaging 2004, 24, 276–280. [Google Scholar] [CrossRef]
- Kay, D.; Marino, F.E.; Cannon, J.; St Clair Gibson, A.; Lambert, M.I.; Noakes, T.D. Evidence for Neuromuscular Fatigue during High-Intensity Cycling in Warm, Humid Conditions. Eur. J. Appl. Physiol. 2001, 84, 115–121. [Google Scholar] [CrossRef]
- Hunter, A.M.; St Clair Gibson, A.; Lambert, M.; Noakes, T.D. Electromyographic (EMG) Normalization Method for Cycle Fatigue Protocols. Med. Sci. Sports Exerc. 2002, 34, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Schabort, E.J.; Hawley, J.A.; Hopkins, W.G.; Mujika, I.; Noakes, T.D. A New Reliable Laboratory Test of Endurance Performance for Road Cyclists. Med. Sci. Sports Exerc. 1998, 30, 1744–1750. [Google Scholar] [CrossRef]
- Saltin, B.; Hermansen, L. Esophageal, Rectal, and Muscle Temperature during Exercise. J. Appl. Physiol. 1966, 21, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Tucker, R.; Marle, T.; Lambert, E.V.; Noakes, T.D. The rate of heat storage mediates an anticipatory reduction in exercise intensity during cycling at a fixed rating of perceived exertion. J. Physiol. 2006, 574 Pt 3, 905–915. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Cuddy, J.S.; Hailes, W.S.; Ruby, B.C. A reduced core to skin temperature gradient, not a critical core temperature, affects aerobic capacity in the heat. J. Therm. Biol. 2014, 43, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Walters, T.J.; Ryan, K.L.; Tate, L.M.; Mason, P.A. Exercise in the Heat Is Limited by a Critical Internal Temperature. J. Appl. Physiol. (1985) 2000, 89, 799–806. [Google Scholar] [CrossRef] [Green Version]
- González-Alonso, J.; Calbet, J.A.; Nielsen, B. Muscle Blood Flow Is Reduced with Dehydration during Prolonged Exercise in Humans. J. Physiol. 1998, 513 Pt 3, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Otani, H.; Watson, P. Influence of Relative Humidity on Prolonged Exercise Capacity in a Warm Environment. Eur. J. Appl. Physiol. 2012, 112, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Kenefick, R.W.; Montain, S.J.; Sawka, M.N. Mechanisms of Aerobic Performance Impairment with Heat Stress and Dehydration. J. Appl. Physiol. 2010, 109, 1989–1995. [Google Scholar] [CrossRef] [Green Version]
- Dugas, J.P.; Oosthuizen, U.; Tucker, R.; Noakes, T.D. Rates of Fluid Ingestion Alter Pacing but Not Thermoregulatory Responses during Prolonged Exercise in Hot and Humid Conditions with Appropriate Convective Cooling. Eur. J. Appl. Physiol. 2009, 105, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.W.; Nio, A.Q.X.; Lim, C.L.; Teo, E.Y.N.; Byrne, C. Thermoregulation, Pacing and Fluid Balance during Mass Participation Distance Running in a Warm and Humid Environment. Eur. J. Appl. Physiol. 2010, 109, 887–898. [Google Scholar] [CrossRef]
- Beis, L.Y.; Wright-Whyte, M.; Fudge, B.; Noakes, T.; Pitsiladis, Y.P. Drinking Behaviors of Elite Male Runners during Marathon Competition. Clin. J. Sport Med. 2012, 22, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Maughan, R.J. Urine Osmolality and Conductivity as Indices of Hydration Status in Athletes in the Heat. Med. Sci. Sports Exerc. 1998, 30, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Che Muhamed, A.M.; Yusof, H.A.; Stannard, S.R.; Mündel, T.; Thompson, M.W. The Efficacy of Ingesting Water on Thermoregulatory Responses and Running Performance in a Warm-Humid Condition. Front. Physiol. 2019, 10, 507. [Google Scholar] [CrossRef] [Green Version]
- Candas, V.; Libert, J.P.; Vogt, J.J. Effect of Hidromeiosis on Sweat Drippage during Acclimation to Humid Heat. Eur. J. Appl. Physiol. Occup. Physiol. 1980, 44, 123–133. [Google Scholar] [CrossRef]
- Galloway, S.D.; Maughan, R.J. Effects of Ambient Temperature on the Capacity to Perform Prolonged Cycle Exercise in Man. Med. Sci. Sports Exerc. 1997, 29, 1240–1249. [Google Scholar] [CrossRef]
- Sawka, M.N.; Leon, L.R.; Montain, S.J.; Sonna, L.A. Integrated Physiological Mechanisms of Exercise Performance, Adaptation, and Maladaptation to Heat Stress. Compr. Physiol. 2011, 1, 1883–1928. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F.; Wenger, C.B.; Stolwijk, J.A.; Nadel, E.R. Skin Blood Flow and Sweating Changes Following Exercise Training and Heat Acclimation. J. Appl. Physiol. Respir Env. Exerc Physiol. 1977, 43, 133–137. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Simmons, S.E.; Stannard, S.R.; Mündel, T. The Independent Roles of Temperature and Thermal Perception in the Control of Human Thermoregulatory Behavior. Physiol. Behav. 2011, 103, 217–224. [Google Scholar] [CrossRef]
- Kayser, B. Exercise Starts and Ends in the Brain. Eur. J. Appl. Physiol. 2003, 90, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran Trong, T.; Riera, F.; Rinaldi, K.; Briki, W.; Hue, O. Ingestion of a cold temperature/menthol beverage increases outdoor exercise performance in a hot, humid environment. PLoS ONE 2015, 10, e0123815. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, K.; Tran Trong, T.; Riera, F.; Appel, K.; Hue, O. Immersion with menthol improves recovery between 2 cycling exercises in hot and humid environment. Appl. Physiol. Nutr. Metab. 2017, 43, 902–908. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baillot, M.; Hue, O.; Tran, T.T.; Antoine-Jonville, S. Neuromuscular Activity during Cycling Performance in Hot/Dry and Hot/Humid Conditions. Life 2021, 11, 1149. https://doi.org/10.3390/life11111149
Baillot M, Hue O, Tran TT, Antoine-Jonville S. Neuromuscular Activity during Cycling Performance in Hot/Dry and Hot/Humid Conditions. Life. 2021; 11(11):1149. https://doi.org/10.3390/life11111149
Chicago/Turabian StyleBaillot, Michelle, Olivier Hue, Trong Than Tran, and Sophie Antoine-Jonville. 2021. "Neuromuscular Activity during Cycling Performance in Hot/Dry and Hot/Humid Conditions" Life 11, no. 11: 1149. https://doi.org/10.3390/life11111149





