Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
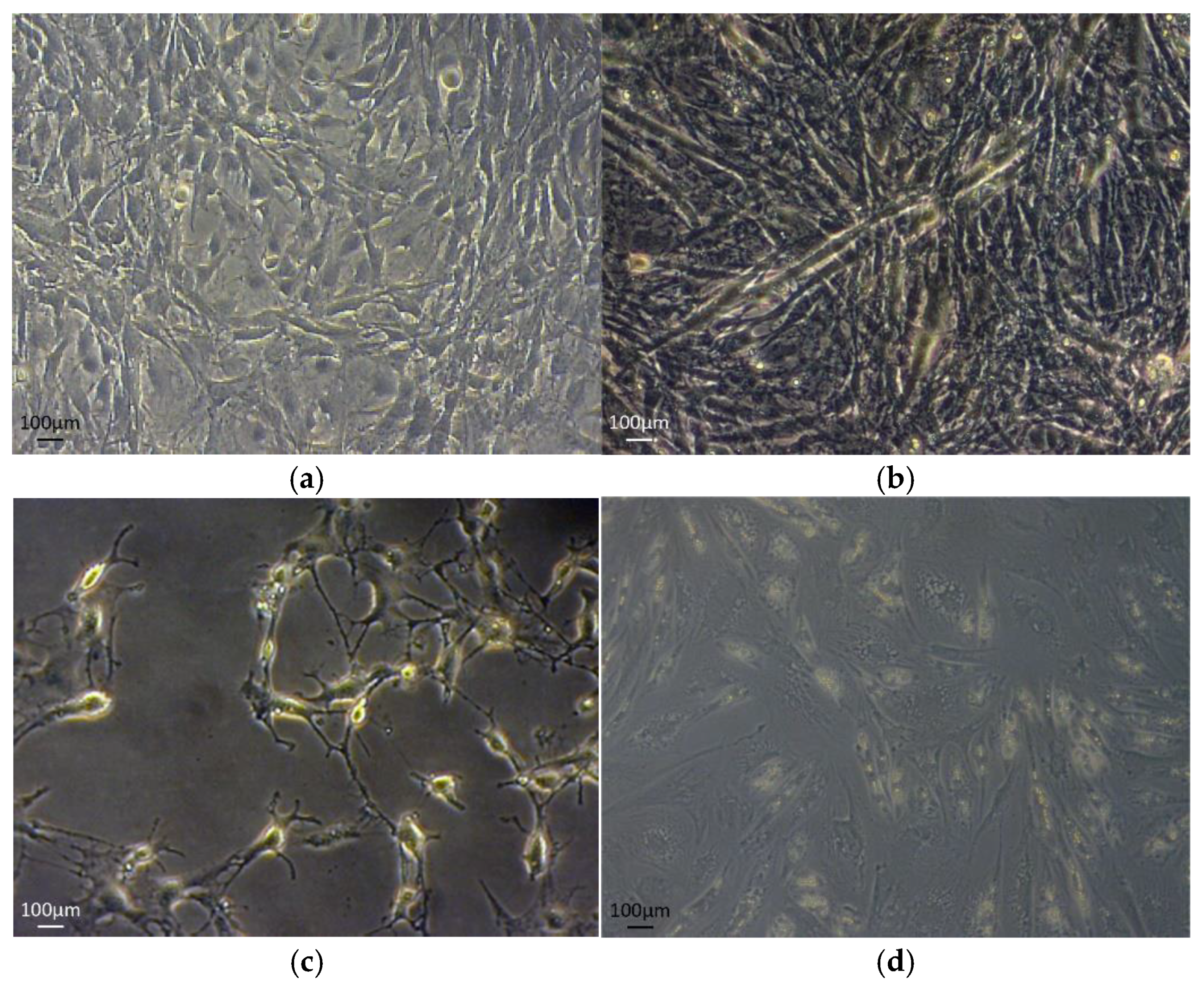
2.1. Cell Lines and Cell Cultures
2.2. C2C12 and 3T3-L1 Differentiation Protocols
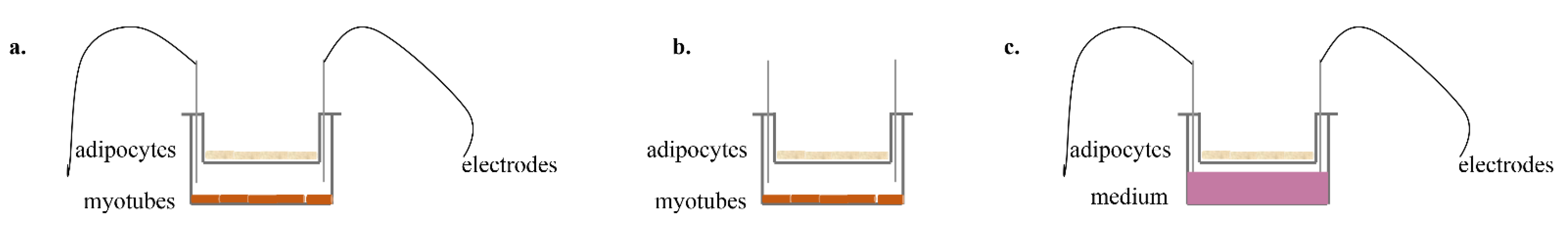
2.3. Co–Culture Protocol
2.4. EPS Protocol
2.5. Lactate Dehydrogenase (LDH) Assay
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
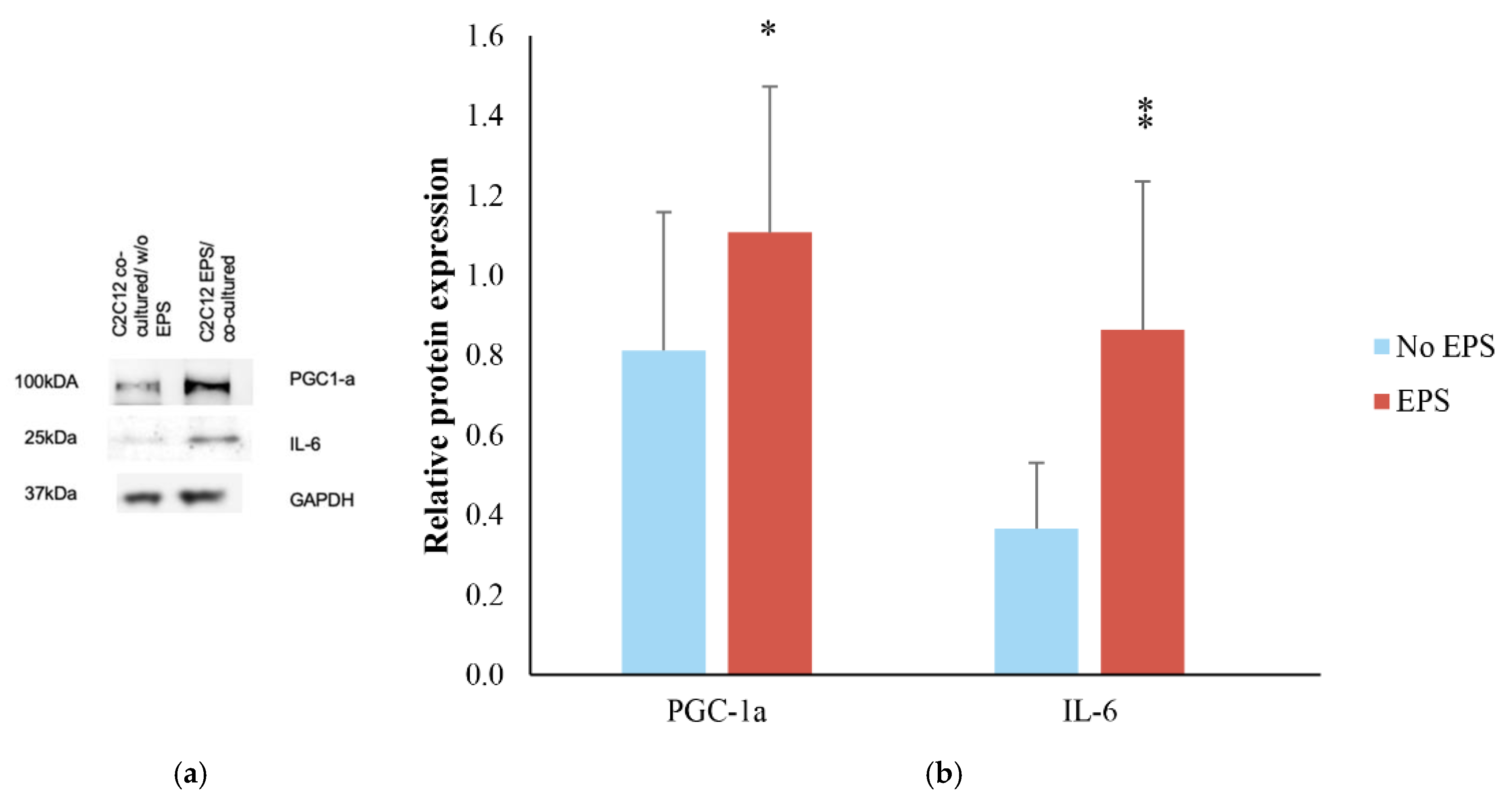
3.1. Effects of EPS Protocol on C2C12 Myotubes
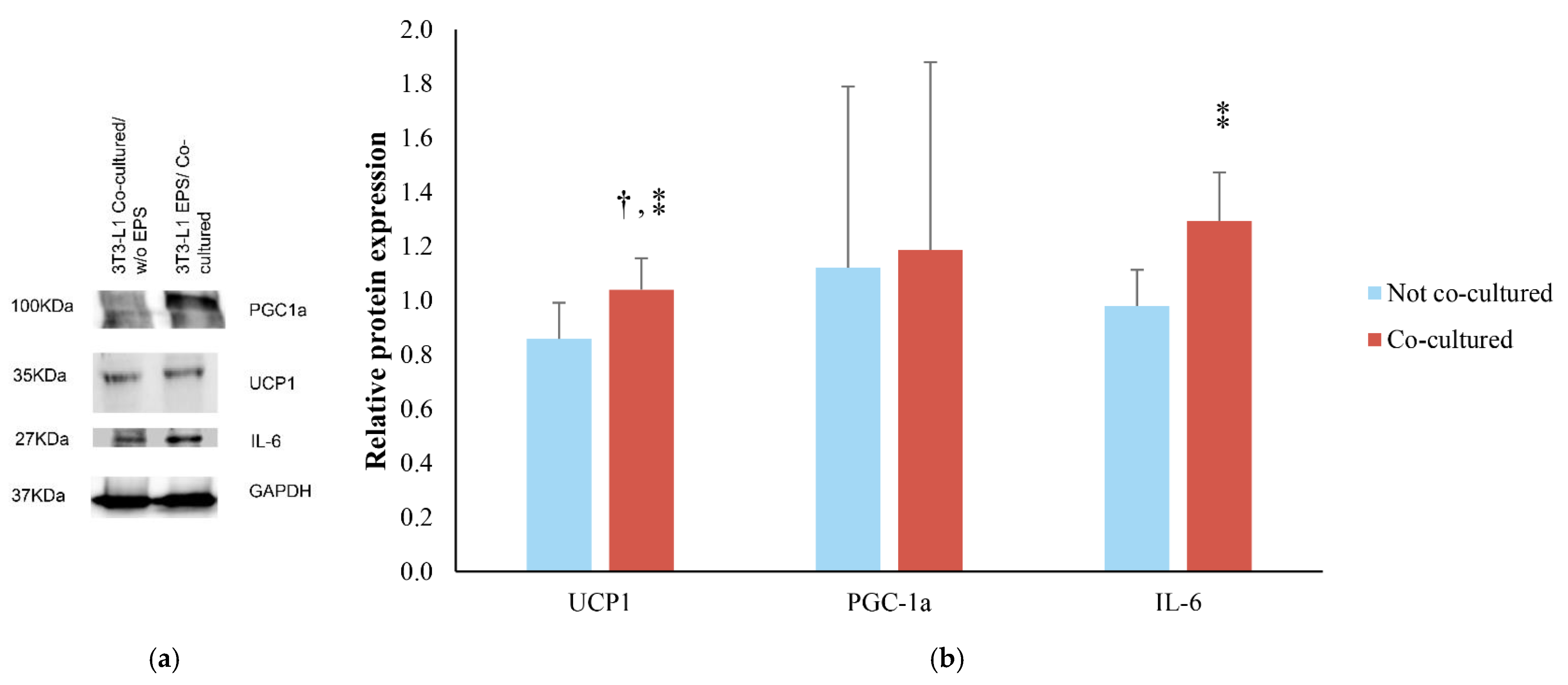
3.2. Effects of EPS Protocol on 3T3-L1/C2C12 Co-Cultured Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vegiopoulos, A.; Rohm, M.; Herzig, S. Adipose tissue: Between the extremes. EMBO J. 2017, 36, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2014, 10, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Mani, M.V.; Mani, A. New targets to treat obesity and the metabolic syndrome. Eur. J. Pharmacol. 2015, 763, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinti, S. Adipose tissues and obesity. Ital. J. Anat. Embryol. 1999, 104, 37–51. [Google Scholar]
- Enerbäck, S. Human brown adipose tissue. Cell Metab. 2010, 11, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Valente, A.; Jamurtas, A.Z.; Koutedakis, Y.; Flouris, A.D. Molecular pathways linking non-shivering thermogenesis and obesity: Focusing on brown adipose tissue development. Biol. Rev. Camb. Philos. Soc. 2015, 90, 77–88. [Google Scholar] [CrossRef]
- Qiu, Y.; Sun, L.; Hu, X.; Zhao, X.; Shi, H.; Liu, Z.; Yin, X. Compromised browning plasticity of primary subcutaneous adipocytes derived from overweight Chinese adults. Diabetol. Metab. Syndr. 2020, 12, 91. [Google Scholar] [CrossRef]
- Wang, W.; Kissig, M.; Rajakumari, S.; Huang, L.; Lim, H.W.; Won, K.J.; Seale, P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl. Acad. Sci. USA 2014, 111, 14466–14471. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef] [Green Version]
- Heinonen, I.; Kalliokoski, K.K.; Hannukainen, J.C.; Duncker, D.J.; Nuutila, P.; Knuuti, J. Organ-specific physiological responses to acute physical exercise and long-term training in humans. Physiology 2014, 29, 421–436. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; Lee, M.Y.; Takahashi, H.; So, K.; Hitchcox, K.M.; Markan, K.R.; Hellbach, K.; Hirshman, M.F.; et al. A novel role for subcutaneous adipose tissue in exercise-induced improvements in glucose homeostasis. Diabetes 2015, 64, 2002–2014. [Google Scholar] [CrossRef] [Green Version]
- Golbidi, S.; Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J. Diabetes Res. 2014, 2014, 726861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Choi, E.Y.; Liu, X.; Martin, A.; Wang, C.; Xu, X.; During, M.J. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011, 14, 324–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- Otero-Díaz, B.; Rodríguez-Flores, M.; Sánchez-Muñoz, V.; Monraz-Preciado, F.; Ordoñez-Ortega, S.; Becerril-Elias, V.; Baay-Guzmán, G.; Obando-Monge, R.; García-García, E.; Palacios-González, B.; et al. Exercise Induces White Adipose Tissue Browning Across the Weight Spectrum in Humans. Front. Physiol. 2018, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-induced ‘browning’ of adipose tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Dinas, P.C.; Lahart, I.M.; Timmons, J.A.; Svensson, P.A.; Koutedakis, Y.; Flouris, A.D.; Metsios, G.S. Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Ιrisin and UCP1 of white adipocytes in humans: A systematic review. F1000Res 2017, 6, 286. [Google Scholar] [CrossRef]
- Dinas, P.C.; Valente, A.; Granzotto, M.; Rossato, M.; Vettor, R.; Zacharopoulou, A.; Carrillo, A.E.; Davies, N.A.; Gkiata, P.; Jamurtas, A.Z.; et al. Browning formation markers of subcutaneous adipose tissue in relation to resting energy expenditure, physical activity and diet in humans. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170008. [Google Scholar]
- Knudsen, J.G.; Murholm, M.; Carey, A.L.; Biensø, R.S.; Basse, A.L.; Allen, T.L.; Hidalgo, J.; Kingwell, B.A.; Febbraio, M.A.; Hansen, J.B.; et al. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS ONE 2014, 9, e84910. [Google Scholar] [CrossRef] [Green Version]
- Daskalopoulou, S.S.; Cooke, A.B.; Gomez, Y.H.; Mutter, A.F.; Filippaios, A.; Mesfum, E.T.; Mantzoros, C.S. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur. J. Endocrinol. 2014, 171, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef] [Green Version]
- Dinas, P.C.; Krase, A.; Nintou, E.; Georgakopoulos, A.; Granzotto, M.; Metaxas, M.; Karachaliou, E.; Rossato, M.; Vettor, R.; Georgoulias, P.; et al. Human white-fat thermogenesis: Experimental and meta-analytic findings. Temperature 2021, 8, 39–52. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Raschke, S.; Eckardt, K.; Bjørklund Holven, K.; Jensen, J.; Eckel, J. Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS ONE 2013, 8, e62008. [Google Scholar] [CrossRef] [Green Version]
- Scheler, M.; Irmler, M.; Lehr, S.; Hartwig, S.; Staiger, H.; Al-Hasani, H.; Beckers, J.; de Angelis, M.H.; Häring, H.U.; Weigert, C. Cytokine response of primary human myotubes in an in vitro exercise model. Am. J. Physiol. Cell Physiol. 2013, 305, C877–C886. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Górniak, B.; Puszczewicz, M. A review on irisin, a new protagonist that mediates muscle-adipose-bone-neuron connectivity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4687–4693. [Google Scholar] [PubMed]
- Nikolić, N.; Bakke, S.S.; Kase, E.T.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Nyasha, M.R.; Koide, M.; Tsuchiya, M.; Suzuki, N.; Hagiwara, Y.; Aoki, M.; Kanzaki, M. In vitro exercise model using contractile human and mouse hybrid myotubes. Sci. Rep. 2019, 9, 11914. [Google Scholar] [CrossRef] [PubMed]
- Burch, N.; Arnold, A.S.; Item, F.; Summermatter, S.; Brochmann Santana Santos, G.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric Pulse Stimulation of Cultured Murine Muscle Cells Reproduces Gene Expression Changes of Trained Mouse Muscle. PLoS ONE 2010, 5, e10970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.E.; Jones, D.E.; Walker, M.; Newton, J.L. Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS ONE 2015, 10, e0122982. [Google Scholar]
- Christensen, C.S.; Christensen, D.P.; Lundh, M.; Dahllof, M.S.; Haase, T.N.; Velasquez, J.M.; Laye, M.J.; Mandrup-Poulsen, T.; Solomon, T.P.J. Skeletal muscle to pancreatic beta-cell cross-talk: The effect of humoral mediators liberated by muscle contraction and acute exercise on beta-cell apoptosis. J. Clin. Endocrinol. Metab. 2015, 100, E1289–E1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattamaprapanont, P.; Garde, C.; Fabre, O.; Barrès, R. Muscle Contraction Induces Acute Hydroxymethylation of the Exercise-Responsive Gene Nr4a3. Front. Endocrinol. 2016, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Ishiuchi, Y.; Sato, H.; Tsujimura, K.; Kawaguchi, H.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M.; Nedachi, T. Skeletal muscle cell contraction reduces a novel myokine, chemokine (C-X-C motif) ligand 10 (CXCL10): Potential roles in exercise-regulated angiogenesis. Biosci. Biotechnol. Biochem. 2018, 82, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.-J.; Ma, J.; Li, S.-B.; Chen, X.-F.; Zhang, J. Electric pulse stimulation inhibited lipid accumulation on C2C12 myotubes incubated with oleic acid and palmitic acid. Arch. Physiol. Biochem. 2021, 127, 344–350. [Google Scholar] [CrossRef]
- Nieuwoudt, S.; Mulya, A.; Fealy, C.E.; Martelli, E.; Dasarathy, S.; Naga Prasad, S.V.; Kirwan, J.P. In vitro Contraction Protects Against Palmitate-Induced Insulin Resistance in C2C12 Myotubes. Am. J. Physiol. Cell Physiol. 2017, 313, C575–C583. [Google Scholar] [CrossRef]
- Tamura, K.; Goto-Inoue, N.; Miyata, K.; Furuichi, Y.; Fujii, N.L.; Manabe, Y. Effect of treatment with conditioned media derived from C2C12 myotube on adipogenesis and lipolysis in 3T3-L1 adipocytes. PLoS ONE 2020, 15, e0237095. [Google Scholar] [CrossRef]
- UCSC. Encyclopedia of DNA Elements at UCSC; UCSC: Santa Cruz, CA, USA, 2003. [Google Scholar]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Pandurangan, M.; Jeong, D.; Amna, T.; van Ba, H.; Hwang, I. Co-culture of C2C12 and 3T3-L1 preadipocyte cells alters the gene expression of calpains, caspases and heat shock proteins. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 577–582. [Google Scholar] [CrossRef]
- Marotta, M.; Bragós, R.; Gómez-Foix, A.M. Design and performance of an electrical stimulator for long-term contraction of cultured muscle cells. Biotechniques 2004, 36, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Nomura, M.; Yamana, I.; Uchiyama, A.; Furuichi, Y.; Manabe, Y.; Fujii, N.L. A new in vitro muscle contraction model and its application for analysis of mTORC1 signaling in combination with contraction and beta-hydroxy-beta-methylbutyrate administration. Biosci. Biotechnol. Biochem. 2019, 83, 1851–1857. [Google Scholar] [CrossRef]
- Chan, F.K.; Moriwaki, K.; de Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar]
- Wood, E.J. Molecular Cloning. A Laboratory Manual; Maniatis, T., Fritsch, E.F., Sambrook, J., Eds.; Cold Spring Harbor Laboratory: New York, NY, USA, 1982; Volume 11, p. 82. [Google Scholar]
- Karpen, S.C. P value problems. Am. J. Pharm. Educ. 2017, 81, 6570. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1988. [Google Scholar]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Xu, X.; Ying, Z.; Cai, M.; Xu, Z.; Li, Y.; Jiang, S.Y.; Tzan, K.; Wang, A.; Parthasarathy, S.; He, G.; et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1115–R1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullahi, A.; Chen, P.; Stanojcic, M.; Sadri, A.R.; Coburn, N.; Jeschke, M.G. IL-6 Signal from the Bone Marrow is Required for the Browning of White Adipose Tissue Post Burn Injury. Shock 2017, 47, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Lira, V.A.; Benton, C.R.; Yan, Z.; Bonen, A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E145–E161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedachi, T.; Fujita, H.; Kanzaki, M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1191–E1204. [Google Scholar] [CrossRef] [Green Version]
- Pettersson-Klein, A.T.; Izadi, M.; Ferreira, D.M.S.; Cervenka, I.; Correia, J.C.; Martinez-Redondo, V.; Southern, M.; Cameron, M.; Kamenecka, T.; Agudelo, L.Z.; et al. Small molecule PGC-1alpha1 protein stabilizers induce adipocyte Ucp1 expression and uncoupled mitochondrial respiration. Mol. Metab. 2018, 9, 28–42. [Google Scholar] [CrossRef]
- Shabalina, I.G.; Petrovic, N.; de Jong, J.M.; Kalinovich, A.V.; Cannon, B.; Nedergaard, J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013, 5, 1196–1203. [Google Scholar] [CrossRef] [Green Version]
- Rohleder, N.; Aringer, M.; Boentert, M. Role of interleukin-6 in stress, sleep, and fatigue. Ann. NY Acad. Sci. 2012, 1261, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Aboouf, M.A.; Armbruster, J.; Thiersch, M.; Gassmann, M.; Gödecke, A.; Gnaiger, E.; Kristiansen, G.; Bicker, A.; Hankeln, T.; Zhu, H.; et al. Myoglobin, expressed in brown adipose tissue of mice, regulates the content and activity of mitochondria and lipid droplets. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2021, 1866, 159026. [Google Scholar] [CrossRef] [PubMed]
- Kristóf, E.; Klusóczki, Á.; Veress, R.; Shaw, A.; Combi, Z.S.; Varga, K.; Győry, F.; Balajthy, Z.; Bai, P.; Bacso, Z.; et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp. Cell Res. 2019, 377, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, A.-C.; Paz, H.A.; Wankhade, U.D. Beige Adipose Tissue Identification and Marker Specificity—Overview. Front. Endocrinol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef] [Green Version]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glassman, A.R. Are P Values Enough? JAMA Ophthalmol. 2020, 138, 567–568. [Google Scholar] [CrossRef]
- Dufau, J.; Shen, J.X.; Couchet, M.; De Castro Barbosa, T.; Mejhert, N.; Massier, L.; Griseti, E.; Mouisel, E.; Amri, E.Z.; Lauschke, V.M.; et al. In vitro and ex vivo models of adipocytes. Am. J. Physiol. Cell Physiol. 2021, 320, C822–C841. [Google Scholar] [CrossRef]
- Samuelson, I.; Vidal-Puig, A. Studying Brown Adipose Tissue in a Human in vitro Context. Front. Endocrinol. (Lausanne) 2020, 11, 629. [Google Scholar] [CrossRef]
- Carter, S.; Solomon, T.P.J. In vitro experimental models for examining the skeletal muscle cell biology of exercise: The possibilities, challenges and future developments. Pflugers Arch 2019, 471, 413–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nintou, E.; Karligiotou, E.; Vliora, M.; Fatouros, I.G.; Jamurtas, A.Z.; Sakellaridis, N.; Dimas, K.; Flouris, A.D. Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes. Life 2021, 11, 1227. https://doi.org/10.3390/life11111227
Nintou E, Karligiotou E, Vliora M, Fatouros IG, Jamurtas AZ, Sakellaridis N, Dimas K, Flouris AD. Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes. Life. 2021; 11(11):1227. https://doi.org/10.3390/life11111227
Chicago/Turabian StyleNintou, Eleni, Eleni Karligiotou, Maria Vliora, Ioannis G. Fatouros, Athanasios Z. Jamurtas, Nikos Sakellaridis, Konstantinos Dimas, and Andreas D. Flouris. 2021. "Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes" Life 11, no. 11: 1227. https://doi.org/10.3390/life11111227
APA StyleNintou, E., Karligiotou, E., Vliora, M., Fatouros, I. G., Jamurtas, A. Z., Sakellaridis, N., Dimas, K., & Flouris, A. D. (2021). Effects of In Vitro Muscle Contraction on Thermogenic Protein Levels in Co-Cultured Adipocytes. Life, 11(11), 1227. https://doi.org/10.3390/life11111227







