Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations
Abstract
1. Introduction
1.1. Age Related Macular Degeneration
1.2. Phototoxicity Models
1.3. LED Phototoxicity Models
1.3.1. Diffuse LED Phototoxicity Models
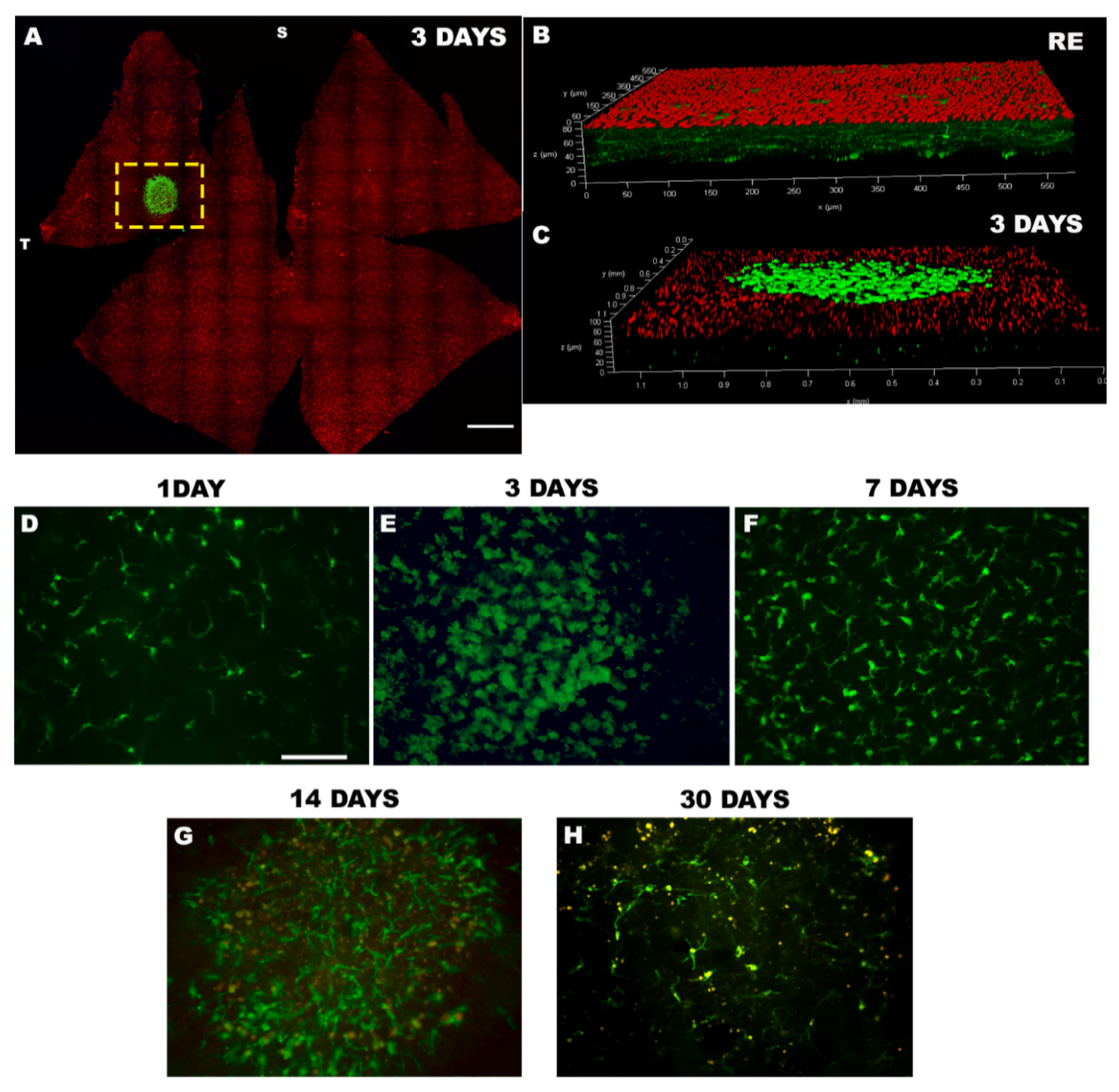
1.3.2. Focal LED Phototoxicity Models
1.4. Distribution of Cone Population in Rats and Mice
2. Toxic Effects of Focal LIP on the Retina
2.1. Retinal Thickness Reduction and Cone Degeneration in Models of LED-Induced Focal Photoreceptor Phototoxicity
2.1.1. In Vivo SD-OCT Observations in the Focal LIP
2.1.2. Ex Vivo Histological Observations in Focal LIP
2.2. Microglial Reaction in Focal Phototoxicity Models
2.3. RPE Degeneration in a Focal Phototoxicity Model
3. Neuroprotection in Focal Phototoxicity Models
3.1. Neurotrophic Factors
3.1.1. Basic Fibroblastic Growth Factor (bFGF)
3.1.2. Brain Derived Neurotrophic Factor (BDNF)
3.1.3. Ciliary Neurotrophic Factor (CNTF)
3.1.4. Pigment Epithelium Derived Factor (PEDF)
3.2. Brimonidine (BMD)
3.3. Minocycline
4. Limitations of the Model
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J.; Eye Diseases Prevalence Research, G. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar]
- Klein, R.; Cruickshanks, K.J.; Nash, S.D.; Krantz, E.M.; Nieto, F.J.; Huang, G.H.; Pankow, J.S.; Klein, B.E. The prevalence of age-related macular degeneration and associated risk factors. Arch. Ophthalmol. 2010, 128, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.; Stevens, G.A.; White, R.A.; Smith, J.L.; Flaxman, S.R.; Price, H.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; et al. Causes of vision loss worldwide, 1990–2010: A systematic analysis. Lancet Glob. Health 2013, 1, e339–e349. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Nowak, J.Z. AMD—The retinal disease with an unprecised etiopathogenesis: In search of effective therapeutics. Acta Pol. Pharm. 2014, 71, 900–916. [Google Scholar] [PubMed]
- Thakkinstian, A.; Han, P.; McEvoy, M.; Smith, W.; Hoh, J.; Magnusson, K.; Zhang, K.; Attia, J. Systematic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum. Mol. Genet. 2006, 15, 2784–2790. [Google Scholar] [CrossRef]
- Cooke Bailey, J.N.; Hoffman, J.D.; Sardell, R.J.; Scott, W.K.; Pericak-Vance, M.A.; Haines, J.L. The Application of Genetic Risk Scores in Age-Related Macular Degeneration: A Review. J. Clin. Med. 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.J.; Milton, R.C.; Klein, R.; Gensler, G.; Taylor, A. Dietary carbohydrate and the progression of age-related macular degeneration: A prospective study from the Age-Related Eye Disease Study. Am. J. Clin. Nutr. 2007, 86, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Chua, B.; Flood, V.; Rochtchina, E.; Wang, J.J.; Smith, W.; Mitchell, P. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch. Ophthalmol. 2006, 124, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, U.; Wong, T.Y.; Fletcher, A.; Piault, E.; Evans, C.; Zlateva, G.; Buggage, R.; Pleil, A.; Mitchell, P. Clinical risk factors for age-related macular degeneration: A systematic review and meta-analysis. BMC Ophthalmol. 2010, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Wormald, R.; Evans, J.; Virgili, G.; Keller, P.R.; Lawrenson, J.G.; Li, T. Analysis of a Systematic Review About Blue Light-Filtering Intraocular Lenses for Retinal Protection: Understanding the Limitations of the Evidence. JAMA Ophthalmol. 2019, 137, 694–697. [Google Scholar] [CrossRef]
- Margrain, T.H.; Boulton, M.; Marshall, J.; Sliney, D.H. Do blue light filters confer protection against age-related macular degeneration? Prog. Retin. Eye Res. 2004, 23, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Alaimo, A.; Linares, G.G.; Bujjamer, J.M.; Gorojod, R.M.; Alcon, S.P.; Martinez, J.H.; Baldessari, A.; Grecco, H.E.; Kotler, M.L. Toxicity of blue led light and A2E is associated to mitochondrial dynamics impairment in ARPE-19 cells: Implications for age-related macular degeneration. Arch. Toxicol. 2019, 93, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.Y.; Liu, G.C.; Liu, G.Y.; Gao, Y.Y.; Deng, Y.; Wang, W.Y.; Tong, S.H.; Wang, L. Is sunlight exposure a risk factor for age-related macular degeneration? A systematic review and meta-analysis. Br. J. Ophthalmol. 2013, 97, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Ragauskaite, L.; Heckathorn, R.C.; Gaillard, E.R. Environmental effects on the photochemistry of A2-E, a component of human retinal lipofuscin. Photochem. Photobiol. 2001, 74, 483–488. [Google Scholar] [CrossRef]
- Youssef, P.N.; Sheibani, N.; Albert, D.M. Retinal light toxicity. Eye 2011, 25, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye 2016, 30, 169–172. [Google Scholar] [CrossRef]
- Di Carlo, E.; Augustin, A.J. Prevention of the Onset of Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 3297. [Google Scholar] [CrossRef] [PubMed]
- Marquioni-Ramella, M.D.; Suburo, A.M. Photo-damage, photo-protection and age-related macular degeneration. Photochem. Photobiol. Sci. 2015, 14, 1560–1577. [Google Scholar] [CrossRef] [PubMed]
- Noell, W.K.; Walker, V.S.; Kang, B.S.; Berman, S. Retinal damage by light in rats. Investig. Ophthalmol. 1966, 5, 450–473. [Google Scholar]
- Behar-Cohen, F.; Martinsons, C.; Vienot, F.; Zissis, G.; Barlier-Salsi, A.; Cesarini, J.P.; Enouf, O.; Garcia, M.; Picaud, S.; Attia, D. Light-emitting diodes (LED) for domestic lighting: Any risks for the eye? Prog. Retin. Eye Res. 2011, 30, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Retinal damage induced by commercial light emitting diodes (LEDs). Free Radic. Biol. Med. 2015, 84, 373–384. [Google Scholar] [CrossRef]
- Krigel, A.; Berdugo, M.; Picard, E.; Levy-Boukris, R.; Jaadane, I.; Jonet, L.; Dernigoghossian, M.; Andrieu-Soler, C.; Torriglia, A.; Behar-Cohen, F. Light-induced retinal damage using different light sources, protocols and rat strains reveals LED phototoxicity. Neuroscience 2016, 339, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wu, M.R.; Huang, W.J.; Chow, D.S.; Hsiao, G.; Cheng, Y.W. Low-Luminance Blue Light-Enhanced Phototoxicity in A2E-Laden RPE Cell Cultures and Rats. Int. J. Mol. Sci. 2019, 20, 1799. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Kukielczak, B.M.; Hu, D.N.; Miller, D.S.; Bilski, P.; Sik, R.H.; Motten, A.G.; Chignell, C.F. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem. Photobiol. 2002, 75, 184–190. [Google Scholar] [CrossRef]
- Shang, Y.M.; Wang, G.S.; Sliney, D.; Yang, C.H.; Lee, L.L. White light-emitting diodes (LEDs) at domestic lighting levels and retinal injury in a rat model. Environ. Health Perspect. 2014, 122, 269–276. [Google Scholar] [CrossRef]
- Wielgus, A.R.; Collier, R.J.; Martin, E.; Lih, F.B.; Tomer, K.B.; Chignell, C.F.; Roberts, J.E. Blue light induced A2E oxidation in rat eyes--experimental animal model of dry AMD. Photochem. Photobiol. Sci. 2010, 9, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.; Wenzel, A.; Hafezi, F.; Yu, S.; Redmond, T.M.; Reme, C.E. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat. Genet. 2000, 25, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Hu, Q.; Li, L.; Tang, X.; Zou, J.; Huang, L.; Li, X. Protective effects of autophagy against blue light-induced retinal degeneration in aged mice. Sci. China Life Sci. 2019, 62, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Choi, C.Y. Effects of blue light spectra on retinal stress and damage in goldfish (Carassius auratus). Fish Physiol. Biochem. 2019, 45, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Song, J.A.; Kim, N.N.; Choi, Y.J.; Choi, C.Y. Effect of green light spectra on the reduction of retinal damage and stress in goldfish, Carassius auratus. Biochem. Biophys. Res. Commun. 2016, 476, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Villalpando Rodriguez, G.E.; Boulenguez, P.; Chahory, S.; Carre, S.; Savoldelli, M.; Jonet, L.; Behar-Cohen, F.; Martinsons, C.; Torriglia, A. Effects of white light-emitting diode (LED) exposure on retinal pigment epithelium in vivo. J. Cell Mol. Med. 2017, 21, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Galindo-Romero, C.; Di Pierdomenico, J.; Vidal-Sanz, M.; Agudo-Barriuso, M.; Villegas Perez, M.P. Light-induced retinal degeneration causes a transient downregulation of melanopsin in the rat retina. Exp. Eye Res. 2017, 161, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ayuso, D.; Salinas-Navarro, M.; Agudo-Barriuso, M.; Alarcon-Martinez, L.; Vidal-Sanz, M.; Villegas-Perez, M.P. Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration. Mol. Vis. 2011, 17, 1716–1733. [Google Scholar] [PubMed]
- Montalban-Soler, L.; Alarcon-Martinez, L.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Galindo-Romero, C.; Bezerra de Sa, F.; Garcia-Ayuso, D.; Aviles-Trigueros, M.; Vidal-Sanz, M.; Agudo-Barriuso, M.; et al. Retinal compensatory changes after light damage in albino mice. Mol. Vis. 2012, 18, 675–693. [Google Scholar] [PubMed]
- Kim, G.H.; Kim, H.I.; Paik, S.S.; Jung, S.W.; Kang, S.; Kim, I.B. Functional and morphological evaluation of blue light-emitting diode-induced retinal degeneration in mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Ortin-Martinez, A.; Valiente-Soriano, F.J.; Garcia-Ayuso, D.; Alarcon-Martinez, L.; Jimenez-Lopez, M.; Bernal-Garro, J.M.; Nieto-Lopez, L.; Nadal-Nicolas, F.M.; Villegas-Perez, M.P.; Wheeler, L.A.; et al. A novel in vivo model of focal light emitting diode-induced cone-photoreceptor phototoxicity: Neuroprotection afforded by brimonidine, BDNF, PEDF or bFGF. PLoS ONE 2014, 9, e113798. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Ortin-Martinez, A.; Miralles de Imperial-Ollero, J.A.; Gallego-Ortega, A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Becerra, S.P.; Vidal-Sanz, M. Pigment Epithelium-Derived Factor (PEDF) Fragments Prevent Mouse Cone Photoreceptor Cell Loss Induced by Focal Phototoxicity In Vivo. Int. J. Mol. Sci. 2020, 21, 7242. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Soriano, F.J.; Ortin-Martinez, A.; Di Pierdomenico, J.; Garcia-Ayuso, D.; Gallego-Ortega, A.; Miralles de Imperial-Ollero, J.A.; Jimenez-Lopez, M.; Villegas-Perez, M.P.; Wheeler, L.A.; Vidal-Sanz, M. Topical Brimonidine or Intravitreal BDNF, CNTF, or bFGF Protect Cones Against Phototoxicity. Transl. Vis. Sci. Technol. 2019, 8, 36. [Google Scholar] [CrossRef]
- Miralles de Imperial-Ollero, J.A.; Gallego-Ortega, A.; Norte-Munoz, M.; Di Pierdomenico, J.; Valiente-Soriano, F.J.; Vidal-Sanz, M. An in vivo model of focal light emitting diode-induced cone photoreceptor phototoxicity in adult pigmented mice: Protection with bFGF. Exp. Eye Res. 2021, 211, 108746. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Tejedor, J.; Marchena, M.; Ramirez, L.; Garcia-Ayuso, D.; Gomez-Vicente, V.; Sanchez-Ramos, C.; de la Villa, P.; Germain, F. Removal of the blue component of light significantly decreases retinal damage after high intensity exposure. PLoS ONE 2018, 13, e0194218. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kuse, Y.; Tsuruma, K.; Shimazawa, M.; Hara, H. The Involvement of the Oxidative Stress in Murine Blue LED Light-Induced Retinal Damage Model. Biol. Pharm. Bull. 2017, 40, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yako, T.; Kuse, Y.; Inoue, Y.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Exposure to excessive blue LED light damages retinal pigment epithelium and photoreceptors of pigmented mice. Exp. Eye Res. 2018, 177, 1–11. [Google Scholar] [CrossRef]
- Meer, A.V.; Berger, T.; Muller, F.; Foldenauer, A.C.; Johnen, S.; Walter, P. Establishment and Characterization of a Unilateral UV-Induced Photoreceptor Degeneration Model in the C57Bl/6J Mouse. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [PubMed]
- Choi, J.Y.; Kim, T.H.; Choi, Y.J.; Kim, N.N.; Oh, S.Y.; Choi, C.Y. Effects of various LED light spectra on antioxidant and immune response in juvenile rock bream, Oplegnathus fasciatus exposed to bisphenol A. Environ. Toxicol. Pharm. 2016, 45, 140–149. [Google Scholar] [CrossRef]
- Garcia-Ayuso, D.; Di Pierdomenico, J.; Hadj-Said, W.; Marie, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Picaud, S.; Villegas-Perez, M.P. Taurine Depletion Causes ipRGC Loss and Increases Light-Induced Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Marco-Gomariz, M.A.; Hurtado-Montalban, N.; Vidal-Sanz, M.; Lund, R.D.; Villegas-Perez, M.P. Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J. Comp. Neurol. 2006, 498, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Wielgus, A.R.; Chignell, C.F.; Ceger, P.; Roberts, J.E. Comparison of A2E cytotoxicity and phototoxicity with all-trans-retinal in human retinal pigment epithelial cells. Photochem. Photobiol. 2010, 86, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Kuse, Y.; Ogawa, K.; Tsuruma, K.; Shimazawa, M.; Hara, H. Damage of photoreceptor-derived cells in culture induced by light emitting diode-derived blue light. Sci. Rep. 2014, 4, 5223. [Google Scholar] [CrossRef] [PubMed]
- Miralles de Imperial-Ollero, J.A.; Gallego-Ortega, A.; Norte-Muñoz, M.; Di Pierdomenico, J.; Bernal-Garro, J.M.; Valiente-Soriano, F.J.; Vidal-Sanz, M. Short- and Long-Term Study of the Impact of Focal Blue Light-Emitting Diode-Induced Phototoxicity in Adult Albino Rats. Int. J. Mol. Sci. 2021, 22, 9742. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Wu, M.R.; Li, C.H.; Cheng, H.W.; Huang, S.H.; Tsai, C.H.; Lin, F.L.; Ho, J.D.; Kang, J.J.; Hsiao, G.; et al. Editor’s Highlight: Periodic Exposure to Smartphone-Mimic Low-Luminance Blue Light Induces Retina Damage Through Bcl-2/BAX-Dependent Apoptosis. Toxicol. Sci. 2017, 157, 196–210. [Google Scholar] [CrossRef]
- Moon, J.; Yun, J.; Yoon, Y.D.; Park, S.I.; Seo, Y.J.; Park, W.S.; Chu, H.Y.; Park, K.H.; Lee, M.Y.; Lee, C.W.; et al. Blue light effect on retinal pigment epithelial cells by display devices. Integr. Biol. 2017, 9, 436–443. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, J.B.; Khazova, M.; Price, L.L. Low-energy light bulbs, computers, tablets and the blue light hazard. Eye 2016, 30, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Jaadane, I.; Chahory, S.; Lepretre, C.; Omri, B.; Jonet, L.; Behar-Cohen, F.; Crisanti, P.; Torriglia, A. The activation of the atypical PKC zeta in light-induced retinal degeneration and its involvement in L-DNase II control. J. Cell Mol. Med. 2015, 19, 1646–1655. [Google Scholar] [CrossRef]
- Ortin-Martinez, A.; Jimenez-Lopez, M.; Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Alarcon-Martinez, L.; Sauve, Y.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Automated quantification and topographical distribution of the whole population of S- and L-cones in adult albino and pigmented rats. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Ortin-Martinez, A.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Alburquerque-Bejar, J.J.; Nieto-Lopez, L.; Garcia-Ayuso, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Number and distribution of mouse retinal cone photoreceptors: Differences between an albino (Swiss) and a pigmented (C57/BL6) strain. PLoS ONE 2014, 9, e102392. [Google Scholar] [CrossRef]
- Tsukahara, N.; Tani, Y.; Kikuchi, H.; Sugita, S. Light transmission of the ocular media in birds and mammals. J. Vet. Med. Sci. 2014, 76, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Navarro, M.; Mayor-Torroglosa, S.; Jimenez-Lopez, M.; Aviles-Trigueros, M.; Holmes, T.M.; Lund, R.D.; Villegas-Perez, M.P.; Vidal-Sanz, M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vis. Res. 2009, 49, 115–126. [Google Scholar] [CrossRef]
- Wu, J.; Seregard, S.; Algvere, P.V. Photochemical damage of the retina. Surv. Ophthalmol. 2006, 51, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Reme, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef] [PubMed]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Ouyang, X.; Yang, J.; Hong, Z.; Wu, Y.; Xie, Y.; Wang, G. Mechanisms of blue light-induced eye hazard and protective measures: A review. Biomed. Pharm. 2020, 130, 110577. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Kaidzu, S.; Anderson, R.E. Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats. Exp. Eye Res. 2006, 83, 1493–1504. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Hua, T.; Han, L.K.; Zhang, Y.; Li, G.Y.; Zhang, Q.L.; Su, G.F. Antiapoptotic role of the cellular repressor of E1A-stimulated genes (CREG) in retinal photoreceptor cells in a rat model of light-induced retinal injury. Biomed. Pharm. 2018, 103, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Denman, D.J.; Siegle, J.H.; Koch, C.; Reid, R.C.; Blanche, T.J. Spatial Organization of Chromatic Pathways in the Mouse Dorsal Lateral Geniculate Nucleus. J. Neurosci. 2017, 37, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Kunze, V.P.; Ball, J.M.; Peng, B.T.; Krishnan, A.; Zhou, G.; Dong, L.; Li, W. True S-cones are concentrated in the ventral mouse retina and wired for color detection in the upper visual field. eLife 2020, 9, e56840. [Google Scholar] [CrossRef] [PubMed]
- Rio-Hortega, P.D. The microglia. Lancet 1939, 233, 4. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.M.; Wong, W.T. Microglia in the Retina: Roles in Development, Maturity, and Disease. Annu. Rev. Vis. Sci. 2018, 4, 45–77. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Scholz, R.; Rutar, M.; Wong, W.T.; Provis, J.M.; Langmann, T. Retinal microglia: Just bystander or target for therapy? Prog. Retin. Eye Res. 2015, 45, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.; Sanchez-Mejias, E.; Jimenez, S.; Munoz-Castro, C.; Sanchez-Varo, R.; Davila, J.C.; Vizuete, M.; Gutierrez, A.; Vitorica, J. Microglia in Alzheimer’s Disease: Activated, Dysfunctional or Degenerative. Front. Aging Neurosci. 2018, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.I.; de Hoz, R.; Salobrar-Garcia, E.; Salazar, J.J.; Rojas, B.; Ajoy, D.; Lopez-Cuenca, I.; Rojas, P.; Trivino, A.; Ramirez, J.M. The Role of Microglia in Retinal Neurodegeneration: Alzheimer’s Disease, Parkinson, and Glaucoma. Front. Aging Neurosci. 2017, 9, 214. [Google Scholar]
- Streit, W.J.; Xue, Q.S. Life and death of microglia. J. Neuroimmune Pharm. 2009, 4, 371–379. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Role of microglial cells in photoreceptor degeneration. Neural Regen. Res. 2019, 14, 1186–1190. [Google Scholar] [PubMed]
- De Hoz, R.; Ramirez, A.I.; Gonzalez-Martin, R.; Ajoy, D.; Rojas, B.; Salobrar-Garcia, E.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; et al. Bilateral early activation of retinal microglial cells in a mouse model of unilateral laser-induced experimental ocular hypertension. Exp. Eye Res. 2018, 171, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.; Gallego, B.I.; Ramirez, A.I.; Salazar, J.J.; de Hoz, R.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Trivino, A.; et al. Microglia in mouse retina contralateral to experimental glaucoma exhibit multiple signs of activation in all retinal layers. J. Neuroinflamm. 2014, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Silva, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Microglial cells in the retina of Carassius auratus: Effects of optic nerve crush. J. Comp. Neurol. 2000, 417, 431–447. [Google Scholar] [CrossRef]
- Wohl, S.G.; Schmeer, C.W.; Witte, O.W.; Isenmann, S. Proliferative response of microglia and macrophages in the adult mouse eye after optic nerve lesion. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Sobrado-Calvo, P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Microglial dynamics after axotomy-induced retinal ganglion cell death. J. Neuroinflamm. 2017, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The aging rat retina: From function to anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef]
- Sobrado-Calvo, P.; Vidal-Sanz, M.; Villegas-Perez, M.P. Rat retinal microglial cells under normal conditions, after optic nerve section, and after optic nerve section and intravitreal injection of trophic factors or macrophage inhibitory factor. J. Comp. Neurol. 2007, 501, 866–878. [Google Scholar] [CrossRef] [PubMed]
- Jonas, R.A.; Yuan, T.F.; Liang, Y.X.; Jonas, J.B.; Tay, D.K.; Ellis-Behnke, R.G. The spider effect: Morphological and orienting classification of microglia in response to stimuli in vivo. PLoS ONE 2012, 7, e30763. [Google Scholar] [CrossRef] [PubMed]
- Joly, S.; Francke, M.; Ulbricht, E.; Beck, S.; Seeliger, M.; Hirrlinger, P.; Hirrlinger, J.; Lang, K.S.; Zinkernagel, M.; Odermatt, B.; et al. Cooperative phagocytes: Resident microglia and bone marrow immigrants remove dead photoreceptors in retinal lesions. Am. J. Pathol. 2009, 174, 2310–2323. [Google Scholar] [CrossRef] [PubMed]
- Raoul, W.; Keller, N.; Rodero, M.; Behar-Cohen, F.; Sennlaub, F.; Combadiere, C. Role of the chemokine receptor CX3CR1 in the mobilization of phagocytic retinal microglial cells. J. Neuroimmunol. 2008, 198, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Torres-Platas, S.G.; Comeau, S.; Rachalski, A.; Bo, G.D.; Cruceanu, C.; Turecki, G.; Giros, B.; Mechawar, N. Morphometric characterization of microglial phenotypes in human cerebral cortex. J. Neuroinflamm. 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, S.; Zou, C.; Levine, E.M. Retinal pigment epithelium development, plasticity, and tissue homeostasis. Exp. Eye Res. 2014, 123, 141–150. [Google Scholar] [CrossRef]
- Hunter, J.J.; Morgan, J.I.; Merigan, W.H.; Sliney, D.H.; Sparrow, J.R.; Williams, D.R. The susceptibility of the retina to photochemical damage from visible light. Prog. Retin. Eye Res. 2012, 31, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Ng, T.K.; Lee, C.; Nakamura, S.; Speck, J.S.; DenBaars, S.P.; Alyamani, A.Y.; El-Desouki, M.M.; Ooi, B.S. Semipolar InGaN quantum-well laser diode with integrated amplifier for visible light communications. Opt. Express 2018, 26, A219–A226. [Google Scholar] [CrossRef]
- Narimatsu, T.; Negishi, K.; Miyake, S.; Hirasawa, M.; Osada, H.; Kurihara, T.; Tsubota, K.; Ozawa, Y. Blue light-induced inflammatory marker expression in the retinal pigment epithelium-choroid of mice and the protective effect of a yellow intraocular lens material in vivo. Exp. Eye Res. 2015, 132, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Sepah, Y.J.; Akhtar, A.; Sadiq, M.A.; Hafeez, Y.; Nasir, H.; Perez, B.; Mawji, N.; Dean, D.J.; Ferraz, D.; Nguyen, Q.D. Fundus autofluorescence imaging: Fundamentals and clinical relevance. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2014, 28, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gliem, M.; Muller, P.L.; Birtel, J.; Herrmann, P.; McGuinness, M.B.; Holz, F.G.; Charbel Issa, P. Quantitative Fundus Autofluorescence and Genetic Associations in Macular, Cone, and Cone-Rod Dystrophies. Ophthalmol. Retin. 2020, 4, 737–749. [Google Scholar] [CrossRef]
- Murdaugh, L.S.; Avalle, L.B.; Mandal, S.; Dill, A.E.; Dillon, J.; Simon, J.D.; Gaillard, E.R. Compositional studies of human RPE lipofuscin. J. Mass Spectrom. JMS 2010, 45, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Paavo, M.; Lee, W.; Allikmets, R.; Tsang, S.; Sparrow, J.R. Photoreceptor cells as a source of fundus autofluorescence in recessive Stargardt disease. J. Neurosci. Res. 2019, 97, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pichi, F.; Abboud, E.B.; Ghazi, N.G.; Khan, A.O. Fundus autofluorescence imaging in hereditary retinal diseases. Acta Ophthalmol. 2018, 96, e549–e561. [Google Scholar] [CrossRef]
- Schmitz-Valckenberg, S.; Holz, F.G.; Bird, A.C.; Spaide, R.F. Fundus autofluorescence imaging: Review and perspectives. Retina 2008, 28, 385–409. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Scholz, R.; Valiente-Soriano, F.J.; Sanchez-Migallon, M.C.; Vidal-Sanz, M.; Langmann, T.; Agudo-Barriuso, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P. Neuroprotective Effects of FGF2 and Minocycline in Two Animal Models of Inherited Retinal Degeneration. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4392–4403. [Google Scholar] [CrossRef]
- Unsicker, K. Neurotrophic molecules in the treatment of neurodegenerative disease with focus on the retina: Status and perspectives. Cell Tissue Res. 2013, 353, 205–218. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, C.; O’Connor, J.; O’Brien, C.J.; Cotter, T.G. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J. Neurochem. 2008, 105, 524–536. [Google Scholar] [CrossRef]
- Nir, I.; Harrison, J.M.; Liu, C.; Wen, R. Extended photoreceptor viability by light stress in the RCS rats but not in the opsin P23H mutant rats. Investig. Ophthalmol. Vis. Sci. 2001, 42, 842–849. [Google Scholar]
- Gao, H.; Hollyfield, J.G. Basic fibroblast growth factor: Increased gene expression in inherited and light-induced photoreceptor degeneration. Exp. Eye Res. 1996, 62, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bjorkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Galindo-Romero, C.; Valiente-Soriano, F.J.; Jimenez-Lopez, M.; Garcia-Ayuso, D.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Effect of brain-derived neurotrophic factor on mouse axotomized retinal ganglion cells and phagocytic microglia. Investig. Ophthalmol. Vis. Sci. 2013, 54, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Parrilla-Reverter, G.; Agudo, M.; Sobrado-Calvo, P.; Salinas-Navarro, M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: A quantitative in vivo study. Exp. Eye Res. 2009, 89, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Migallon, M.C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. Apoptotic Retinal Ganglion Cell Death After Optic Nerve Transection or Crush in Mice: Delayed RGC Loss with BDNF or a Caspase 3 Inhibitor. Investig. Ophthalmol. Vis. Sci. 2016, 57, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Galindo-Romero, C.; Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Ortin-Martinez, A.; Rovere, G.; Salinas-Navarro, M.; Lucas-Ruiz, F.; Sanchez-Migallon, M.C.; Sobrado-Calvo, P.; et al. Shared and Differential Retinal Responses against Optic Nerve Injury and Ocular Hypertension. Front. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; Valiente-Soriano, F.J.; Ortin-Martinez, A.; Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Salinas-Navarro, M.; Alarcon-Martinez, L.; Garcia-Ayuso, D.; Aviles-Trigueros, M.; Agudo-Barriuso, M.; et al. Retinal neurodegeneration in experimental glaucoma. Prog. Brain Res. 2015, 220, 1–35. [Google Scholar] [PubMed]
- Rovere, G.; Nadal-Nicolas, F.M.; Wang, J.; Bernal-Garro, J.M.; Garcia-Carrillo, N.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. Melanopsin-Containing or Non-Melanopsin-Containing Retinal Ganglion Cells Response to Acute Ocular Hypertension with or without Brain-Derived Neurotrophic Factor Neuroprotection. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6652–6661. [Google Scholar] [CrossRef]
- Valiente-Soriano, F.J.; Nadal-Nicolas, F.M.; Salinas-Navarro, M.; Jimenez-Lopez, M.; Bernal-Garro, J.M.; Villegas-Perez, M.P.; Agudo-Barriuso, M.; Vidal-Sanz, M. BDNF Rescues RGCs But Not Intrinsically Photosensitive RGCs in Ocular Hypertensive Albino Rat Retinas. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1924–1936. [Google Scholar] [CrossRef] [PubMed]
- Cerri, E.; Origlia, N.; Falsini, B.; Barloscio, D.; Fabiani, C.; Sanso, M.; Ottino, S.; Giovannini, L.; Domenici, L. Conjunctivally Applied BDNF Protects Photoreceptors from Light-Induced Damage. Transl. Vis. Sci. Technol. 2015, 4, 1. [Google Scholar] [CrossRef]
- Gauthier, R.; Joly, S.; Pernet, V.; Lachapelle, P.; Di Polo, A. Brain-derived neurotrophic factor gene delivery to muller glia preserves structure and function of light-damaged photoreceptors. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3383–3392. [Google Scholar] [CrossRef] [PubMed]
- Kano, T.; Abe, T.; Tomita, H.; Sakata, T.; Ishiguro, S.; Tamai, M. Protective effect against ischemia and light damage of iris pigment epithelial cells transfected with the BDNF gene. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3744–3753. [Google Scholar]
- Ju, W.K.; Lee, M.Y.; Hofmann, H.D.; Kirsch, M.; Chun, M.H. Expression of CNTF in Muller cells of the rat retina after pressure-induced ischemia. Neuroreport 1999, 10, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sato, K.; Gordon, W.C.; Sendtner, M.; Bazan, N.G.; Jin, M. Ciliary neurotrophic factor (CNTF) protects retinal cone and rod photoreceptors by suppressing excessive formation of the visual pigments. J. Biol. Chem. 2018, 293, 15256–15268. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.; Valter, K.; Stone, J. Cellular and subcellular patterns of expression of bFGF and CNTF in the normal and light stressed adult rat retina. Exp. Eye Res. 2001, 72, 495–501. [Google Scholar] [CrossRef]
- Dulz, S.; Bassal, M.; Flachsbarth, K.; Riecken, K.; Fehse, B.; Schlichting, S.; Bartsch, S.; Bartsch, U. Intravitreal Co-Administration of GDNF and CNTF Confers Synergistic and Long-Lasting Protection against Injury-Induced Cell Death of Retinal Ganglion Cells in Mice. Cells 2020, 9, 2082. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.K.; Guo, Y.; Langenberg, P.; Bernstein, S.L. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br. J. Ophthalmol. 2015, 99, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Cheng, T.; Song, Y.; Matthes, M.T.; Yasumura, D.; LaVail, M.M.; Steinberg, R.H. Continuous exposure to bright light upregulates bFGF and CNTF expression in the rat retina. Curr. Eye Res. 1998, 17, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Becerra, S.P. Focus on Molecules: Pigment epithelium-derived factor (PEDF). Exp. Eye Res. 2006, 82, 739–740. [Google Scholar] [CrossRef] [PubMed]
- Becerra, S.P. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv. Exp. Med. Biol. 1997, 425, 223–237. [Google Scholar]
- Broadhead, M.L.; Becerra, S.P.; Choong, P.F.; Dass, C.R. The applied biochemistry of PEDF and implications for tissue homeostasis. Growth Factors 2010, 28, 280–285. [Google Scholar] [CrossRef]
- Michelis, G.; German, O.L.; Villasmil, R.; Soto, T.; Rotstein, N.P.; Politi, L.; Becerra, S.P. Pigment epithelium-derived factor (PEDF) and derived peptides promote survival and differentiation of photoreceptors and induce neurite-outgrowth in amacrine neurons. J. Neurochem. 2021. [Google Scholar] [CrossRef]
- Subramanian, P.; Locatelli-Hoops, S.; Kenealey, J.; DesJardin, J.; Notari, L.; Becerra, S.P. Pigment epithelium-derived factor (PEDF) prevents retinal cell death via PEDF Receptor (PEDF-R): Identification of a functional ligand binding site. J. Biol. Chem. 2013, 288, 23928–23942. [Google Scholar] [CrossRef] [PubMed]
- Tombran-Tink, J.; Barnstable, C.J. PEDF: A multifaceted neurotrophic factor. Nat. Rev. Neurosci. 2003, 4, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Polato, F.; Samardzija, M.; Abu-Asab, M.; Grimm, C.; Crawford, S.E.; Becerra, S.P. PEDF deficiency increases the susceptibility of rd10 mice to retinal degeneration. Exp. Eye Res. 2020, 198, 108121. [Google Scholar] [CrossRef]
- Miyazaki, M.; Ikeda, Y.; Yonemitsu, Y.; Goto, Y.; Sakamoto, T.; Tabata, T.; Ueda, Y.; Hasegawa, M.; Tobimatsu, S.; Ishibashi, T.; et al. Simian lentiviral vector-mediated retinal gene transfer of pigment epithelium-derived factor protects retinal degeneration and electrical defect in Royal College of Surgeons rats. Gene Ther. 2003, 10, 1503–1511. [Google Scholar] [CrossRef]
- Rapp, M.; Woo, G.; Al-Ubaidi, M.R.; Becerra, S.P.; Subramanian, P. Pigment epithelium-derived factor protects cone photoreceptor-derived 661W cells from light damage through Akt activation. Adv. Exp. Med. Biol. 2014, 801, 813–820. [Google Scholar] [PubMed]
- Kenealey, J.; Subramanian, P.; Comitato, A.; Bullock, J.; Keehan, L.; Polato, F.; Hoover, D.; Marigo, V.; Becerra, S.P. Small Retinoprotective Peptides Reveal a Receptor-binding Region on Pigment Epithelium-derived Factor. J. Biol. Chem. 2015, 290, 25241–25253. [Google Scholar] [CrossRef] [PubMed]
- Lusthaus, J.A.; Goldberg, I. Brimonidine and brinzolamide for treating glaucoma and ocular hypertension; a safety evaluation. Expert Opin. Drug Saf. 2017, 16, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Kawasaki, R.; Takahashi, H.; Maekawa, S.; Tsuda, S.; Omodaka, K.; Nakazawa, T. Effects of Brimonidine and Timolol on the Progression of Visual Field Defects in Open-angle Glaucoma: A Single-center Randomized Trial. J. Glaucoma 2019, 28, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Trigueros, M.; Mayor-Torroglosa, S.; Garcia-Aviles, A.; Lafuente, M.P.; Rodriguez, M.E.; Miralles de Imperial, J.; Villegas-Perez, M.P.; Vidal-Sanz, M. Transient ischemia of the retina results in massive degeneration of the retinotectal projection: Long-term neuroprotection with brimonidine. Exp. Neurol. 2003, 184, 767–777. [Google Scholar] [CrossRef]
- Lafuente, M.P.; Villegas-Perez, M.P.; Mayor, S.; Aguilera, M.E.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective effects of brimonidine against transient ischemia-induced retinal ganglion cell death: A dose response in vivo study. Exp. Eye Res. 2002, 74, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.P.; Villegas-Perez, M.P.; Sobrado-Calvo, P.; Garcia-Aviles, A.; Miralles de Imperial, J.; Vidal-Sanz, M. Neuroprotective effects of alpha(2)-selective adrenergic agonists against ischemia-induced retinal ganglion cell death. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2074–2084. [Google Scholar]
- Mayor-Torroglosa, S.; De la Villa, P.; Rodriguez, M.E.; Lopez-Herrera, M.P.; Aviles-Trigueros, M.; Garcia-Aviles, A.; de Imperial, J.M.; Villegas-Perez, M.P.; Vidal-Sanz, M. Ischemia results 3 months later in altered ERG, degeneration of inner layers, and deafferented tectum: Neuroprotection with brimonidine. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3825–3835. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Sanz, M.; de la Villa, P.; Avilés-Trigueros, M.; Mayor-Torroglosa, S.; Salinas-Navarro, M.; Alarcón-Martínez, L.; Villegas-Pérez, M.P. Neuroprotection of retinal ganglion cell function and their central nervous system targets. Eye 2007, 21, S42–S45. [Google Scholar] [CrossRef]
- Lambert, W.S.; Ruiz, L.; Crish, S.D.; Wheeler, L.A.; Calkins, D.J. Brimonidine prevents axonal and somatic degeneration of retinal ganglion cell neurons. Mol. Neurodegener. 2011, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Gil, D.W.; WoldeMussie, E. Role of alpha-2 adrenergic receptors in neuroprotection and glaucoma. Surv Ophthalmol. 2001, 45, S290–S294. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Woldemussie, E. Alpha-2 adrenergic receptor agonists are neuroprotective in experimental models of glaucoma. Eur. J. Ophthalmol. 2001, 11, S30–S35. [Google Scholar] [CrossRef]
- Conti, F.; Romano, G.L.; Eandi, C.M.; Toro, M.D.; Rejdak, R.; Di Benedetto, G.; Lazzara, F.; Bernardini, R.; Drago, F.; Cantarella, G.; et al. Brimonidine is Neuroprotective in Animal Paradigm of Retinal Ganglion Cell Damage. Front. Pharm. 2021, 12, 705405. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, E.; Lopatina, T.; Mazzeo, A.; Arroba, A.I.; Valverde, A.M.; Hernandez, C.; Simo, R.; Porta, M. Effects of the neuroprotective drugs somatostatin and brimonidine on retinal cell models of diabetic retinopathy. Acta Diabetol. 2016, 53, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, L.; Ghosn, C.; Tamhane, M.; Almazan, A.; Andrews-Jones, L.; Kulkarni, A.; Christie, L.A.; Burke, J.; Lopez, F.J.; Engles, M. A nonhuman primate model of blue light-induced progressive outer retina degeneration showing brimonidine drug delivery system-mediated cyto- and neuroprotection. Exp. Eye Res. 2021, 209, 108678. [Google Scholar] [CrossRef] [PubMed]
- Castanares, M.; Vera, Y.; Erkkila, K.; Kyttanen, S.; Lue, Y.; Dunkel, L.; Wang, C.; Swerdloff, R.S.; Hikim, A.P. Minocycline up-regulates BCL-2 levels in mitochondria and attenuates male germ cell apoptosis. Biochem. Biophys. Res. Commun. 2005, 337, 663–669. [Google Scholar] [CrossRef]
- Chang, C.J.; Cherng, C.H.; Liou, W.S.; Liao, C.L. Minocycline partially inhibits caspase-3 activation and photoreceptor degeneration after photic injury. Ophthalmic Res. 2005, 37, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Grotegut, P.; Perumal, N.; Kuehn, S.; Smit, A.; Dick, H.B.; Grus, F.H.; Joachim, S.C. Minocycline reduces inflammatory response and cell death in a S100B retina degeneration model. J. Neuroinflamm. 2020, 17, 375. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhary, T.; Mishra, J. Minocycline modulates neuroprotective effect of hesperidin against quinolinic acid induced Huntington’s disease like symptoms in rats: Behavioral, biochemical, cellular and histological evidences. Eur. J. Pharm. 2013, 720, 16–28. [Google Scholar]
- Scholz, R.; Sobotka, M.; Caramoy, A.; Stempfl, T.; Moehle, C.; Langmann, T. Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J. Neuroinflamm. 2015, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Yu, A.C.; Lau, L.T.; Lee, C.; Wu le, M.; Zhu, X.; Tso, M.O. Minocycline inhibits LPS-induced retinal microglia activation. Neurochem. Int. 2005, 47, 152–158. [Google Scholar] [CrossRef]
- Baptiste, D.C.; Powell, K.J.; Jollimore, C.A.; Hamilton, C.; LeVatte, T.L.; Archibald, M.L.; Chauhan, B.C.; Robertson, G.S.; Kelly, M.E. Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience 2005, 134, 575–582. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Kalev-Landoy, M.; Habot-Wilner, Z.; Melamed, S. Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch. Ophthalmol. 2006, 124, 520–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Ilia, P. Minocycline mechanism of neuroprotection involves the Bcl-2 gene family in optic nerve transection. Int. J. Neurosci. 2014, 124, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Levkovitch-Verbin, H.; Waserzoog, Y.; Vander, S.; Makarovsky, D.; Piven, I. Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, Z.; Pei, S.; Zheng, D.; Zhu, L. Neuroprotective effect of minocycline on rat retinal ischemia-reperfusion injury. Mol. Vis. 2021, 27, 438–456. [Google Scholar] [PubMed]
- Shimazawa, M.; Yamashima, T.; Agarwal, N.; Hara, H. Neuroprotective effects of minocycline against in vitro and in vivo retinal ganglion cell damage. Brain Res. 2005, 1053, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, R.; Kohno, H.; Watanabe, S.; Saito, S.; Watanabe, A.; Nakano, T. Minocycline decreases CCR2-positive monocytes in the retina and ameliorates photoreceptor degeneration in a mouse model of retinitis pigmentosa. PLoS ONE 2021, 16, e0239108. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, B.; Lam, T.T.; Yang, F.; Sinha, D.; Tso, M.O. Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2753–2759. [Google Scholar] [CrossRef]
- Di Pierdomenico, J.; Garcia-Ayuso, D.; Jimenez-Lopez, M.; Agudo-Barriuso, M.; Vidal-Sanz, M.; Villegas-Perez, M.P. Different Ipsi- and Contralateral Glial Responses to Anti-VEGF and Triamcinolone Intravitreal Injections in Rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3533–3544. [Google Scholar] [CrossRef] [PubMed]

| Author | Rodent Strain | Wavelength | Time Exposure | Intensity | Time of Study |
|---|---|---|---|---|---|
| Shang et al., 2014 [26] | Sprague-Dawley rats | White and blue (460 nm) | On/Off cycles of 12 h for 3, 9 or 28 days | 750 lux | After LED exposure |
| Jaadane et al., 2015 [54] | Wistar rats | White, blue (449, 467, 473 nm) and blue-green (507 nm) | Constant for 6, 12, 18, 24, 48 or 72 h | White: 2680 cd/m2; Blue: 102, 234, 268 cd/m2; Blue-Green 643 cd/m2 | After LED exposure |
| Jaadane et al., 2017 [32] | Wistar rats | White | Constant for 4, 5, 6 12, 18 or 24 h | White: 2680 cd/m2 | After LED exposure |
| Krigel et al., 2016 [23] | Wistar and Long Evans rats | White, blue (460 ± 5 nm) and green (530 ± 10 nm) | Constant for acute exposure: 24 h | Acute exposure: White: 500, 1000, 1500, 6000 lux Blue and green: 500 lux | 7 days |
| On/Off cycles of 12 h for long-term exposure: 8 or 28 days | 500 lux | After LED exposure | |||
| Lin et al., 2017 [51] | Brown-Norway rats | Blue (460 nm) | Periodic cycles from 30 min to 3 h per day for 28 days | 150 lux | After LED exposure |
| Kim et al., 2016 [36] | BALB/c mice | Blue (460 ± 10 nm) | Constant for 2 h | Illuminance-dependent (ID) groups: 1000, 2000, 3000, 6000 lux; Time-dependent (TD) group: 2000 lux | ID groups: 5 days. TD groups: 24, 48, 72 h |
| Nakamura et al., 2017 [42] | ddY mice | Blue (456 nm) | Constant for 2 h | 400 and 800 lux | 5 days |
| Nakamura et al., 2018 [43] | C57BL/6 mice | Blue (456 nm) | Constant for 3 h for 3 consecutive days | 1100 lux | 7 days |
| Wielgus et al., 2010 [27] | Sprague-Dawley rats | Blue (450 nm) | Constant for 6 h | 750 lux | After LED exposure |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miralles de Imperial-Ollero, J.A.; Gallego-Ortega, A.; Ortín-Martínez, A.; Villegas-Pérez, M.P.; Valiente-Soriano, F.J.; Vidal-Sanz, M. Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations. Life 2021, 11, 1137. https://doi.org/10.3390/life11111137
Miralles de Imperial-Ollero JA, Gallego-Ortega A, Ortín-Martínez A, Villegas-Pérez MP, Valiente-Soriano FJ, Vidal-Sanz M. Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations. Life. 2021; 11(11):1137. https://doi.org/10.3390/life11111137
Chicago/Turabian StyleMiralles de Imperial-Ollero, Juan A., Alejandro Gallego-Ortega, Arturo Ortín-Martínez, María Paz Villegas-Pérez, Francisco J. Valiente-Soriano, and Manuel Vidal-Sanz. 2021. "Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations" Life 11, no. 11: 1137. https://doi.org/10.3390/life11111137
APA StyleMiralles de Imperial-Ollero, J. A., Gallego-Ortega, A., Ortín-Martínez, A., Villegas-Pérez, M. P., Valiente-Soriano, F. J., & Vidal-Sanz, M. (2021). Animal Models of LED-Induced Phototoxicity. Short- and Long-Term In Vivo and Ex Vivo Retinal Alterations. Life, 11(11), 1137. https://doi.org/10.3390/life11111137








