PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pharmacokinetic Analysis Software
2.3. PBPK Modeling and Simulation
3. Results
3.1. Influence of Dose Regimens and Biological Sex
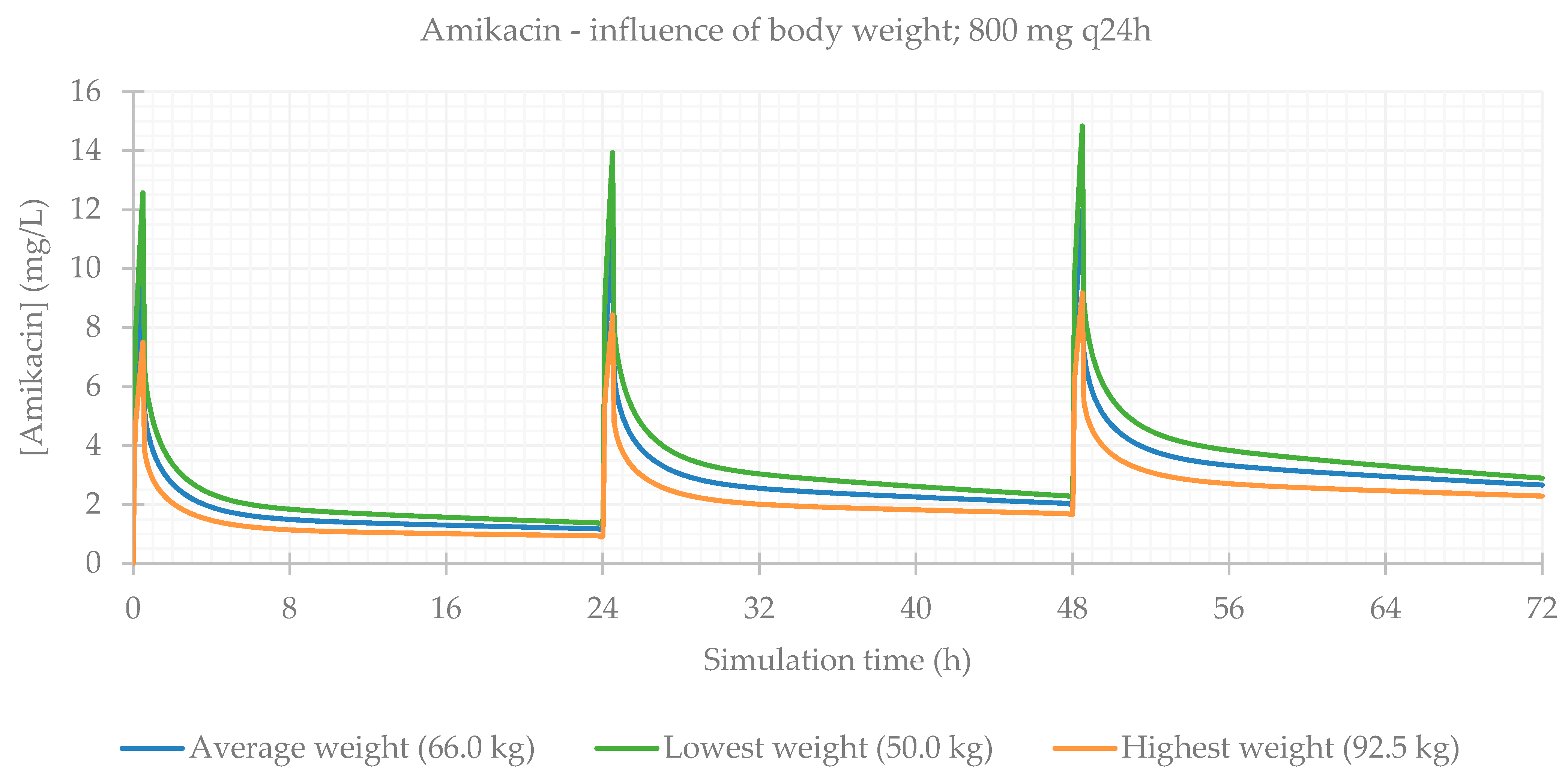
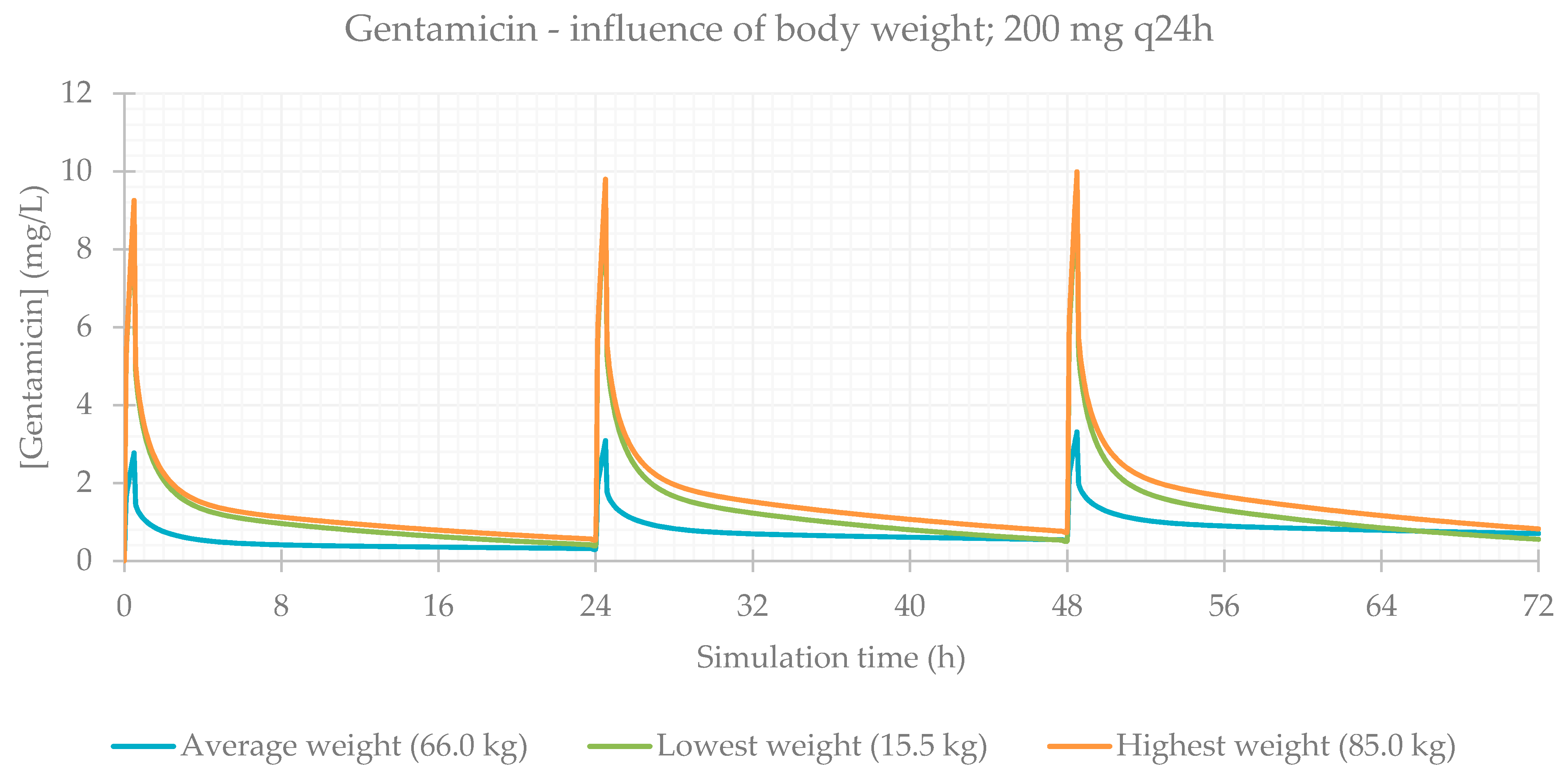
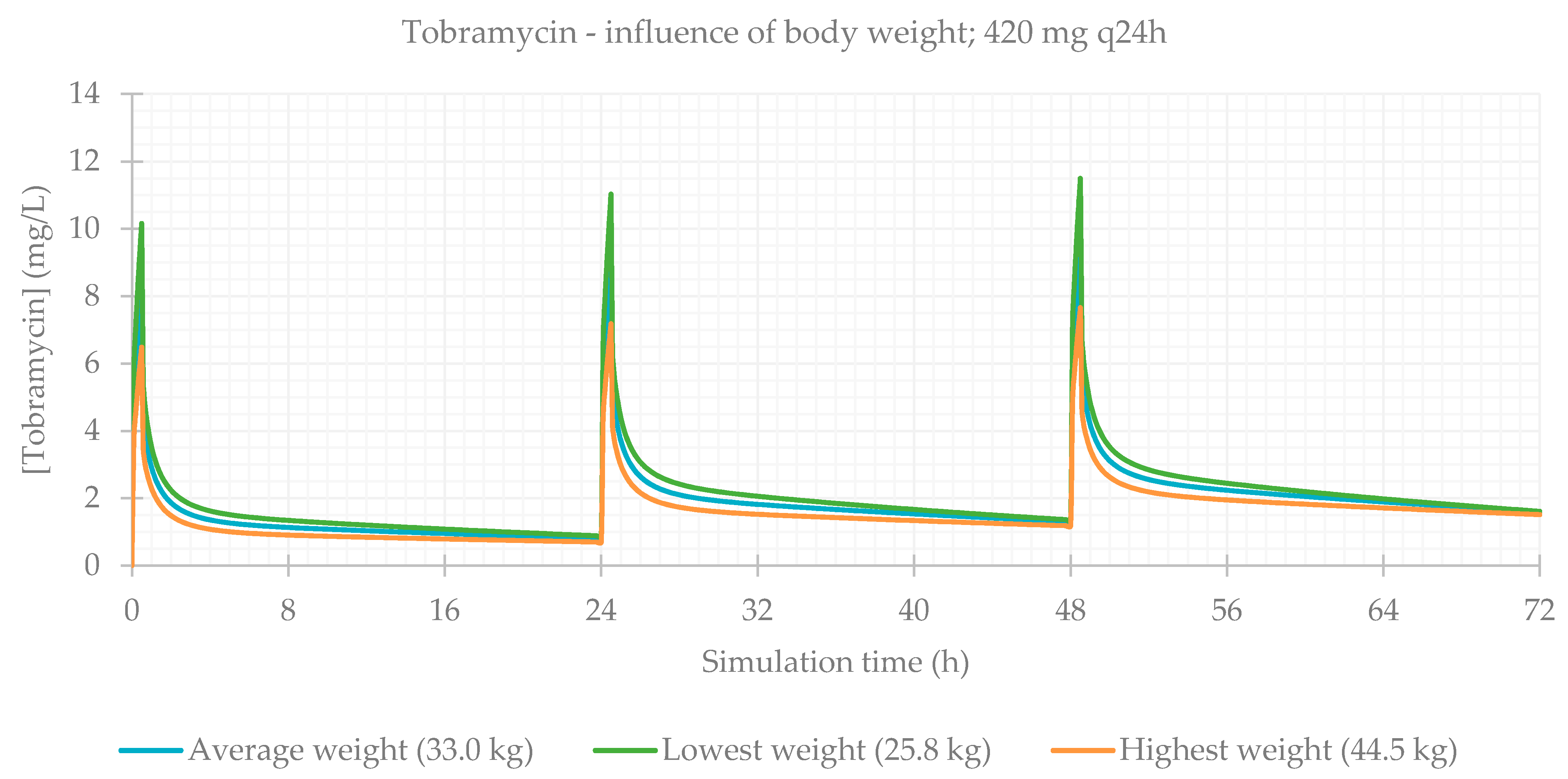
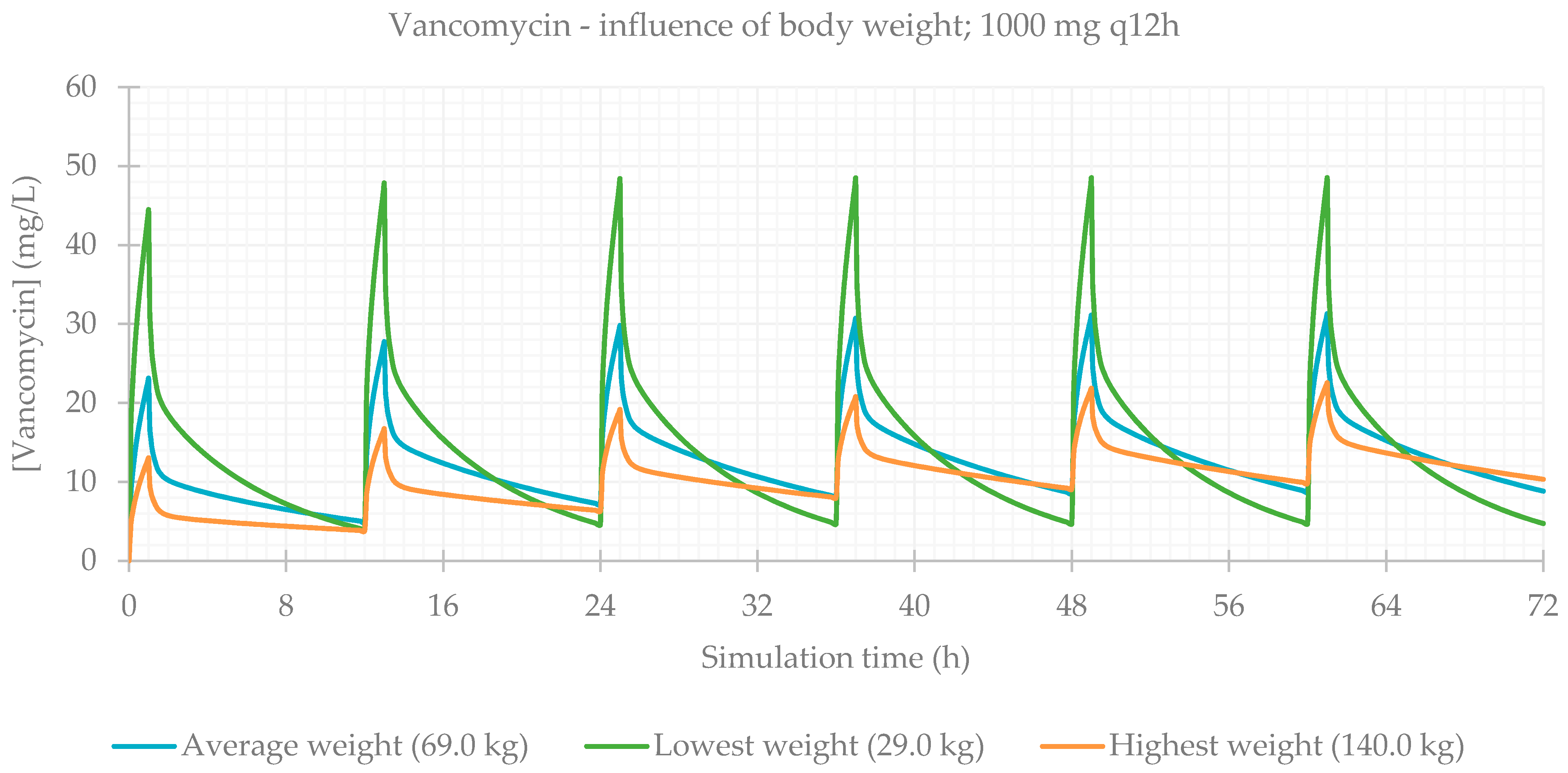
3.2. Influence of Total Body Weight
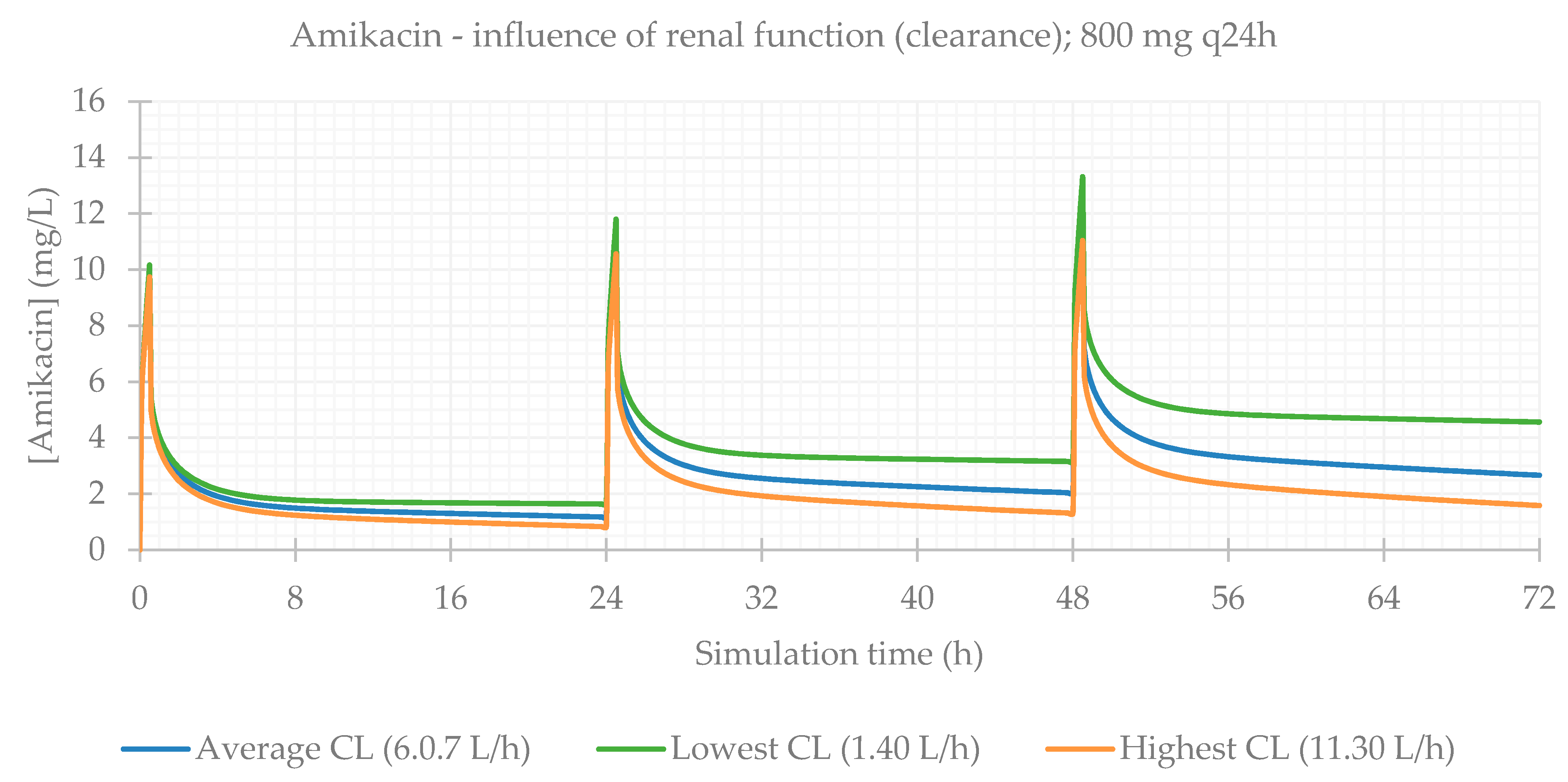
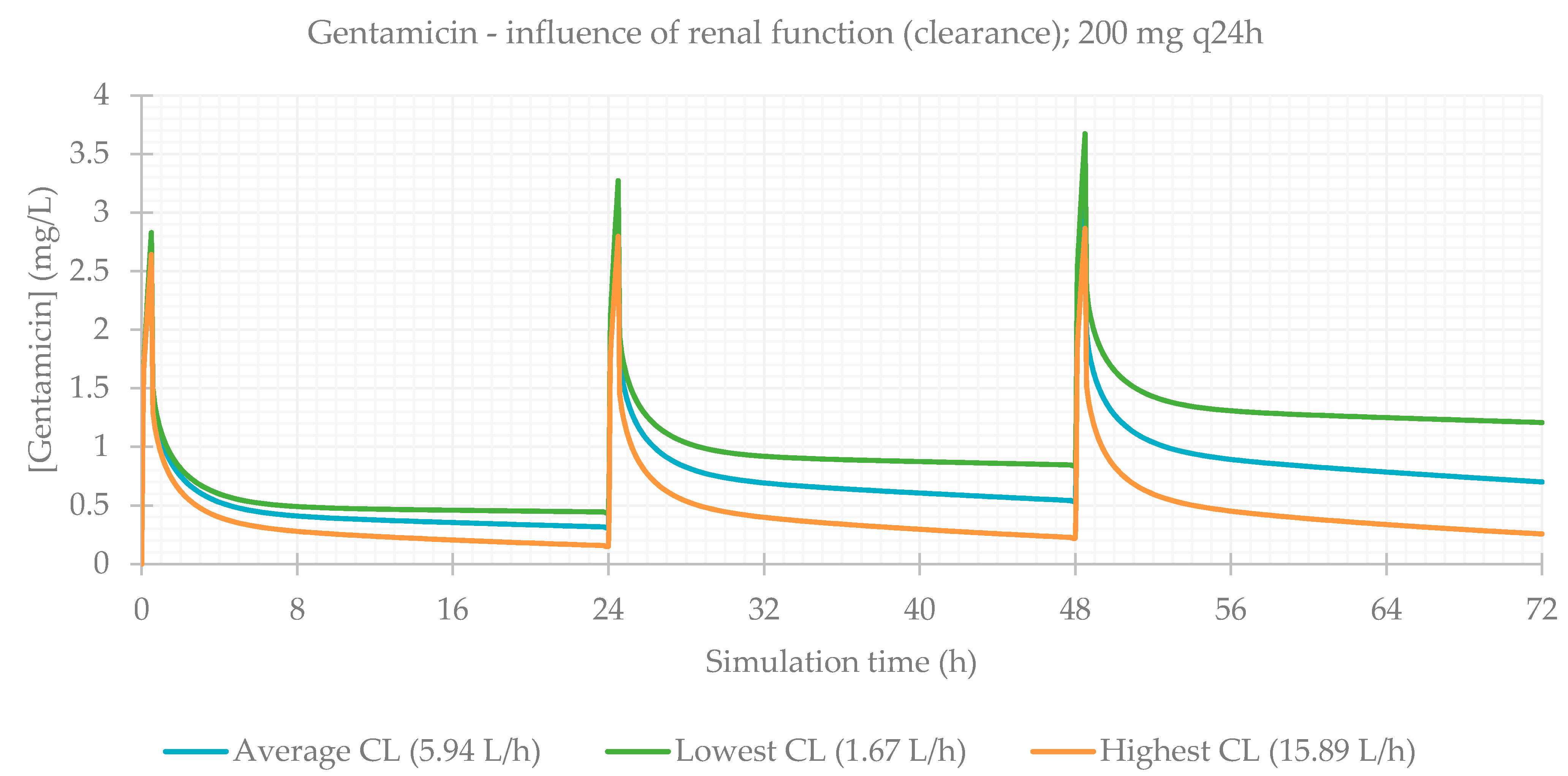
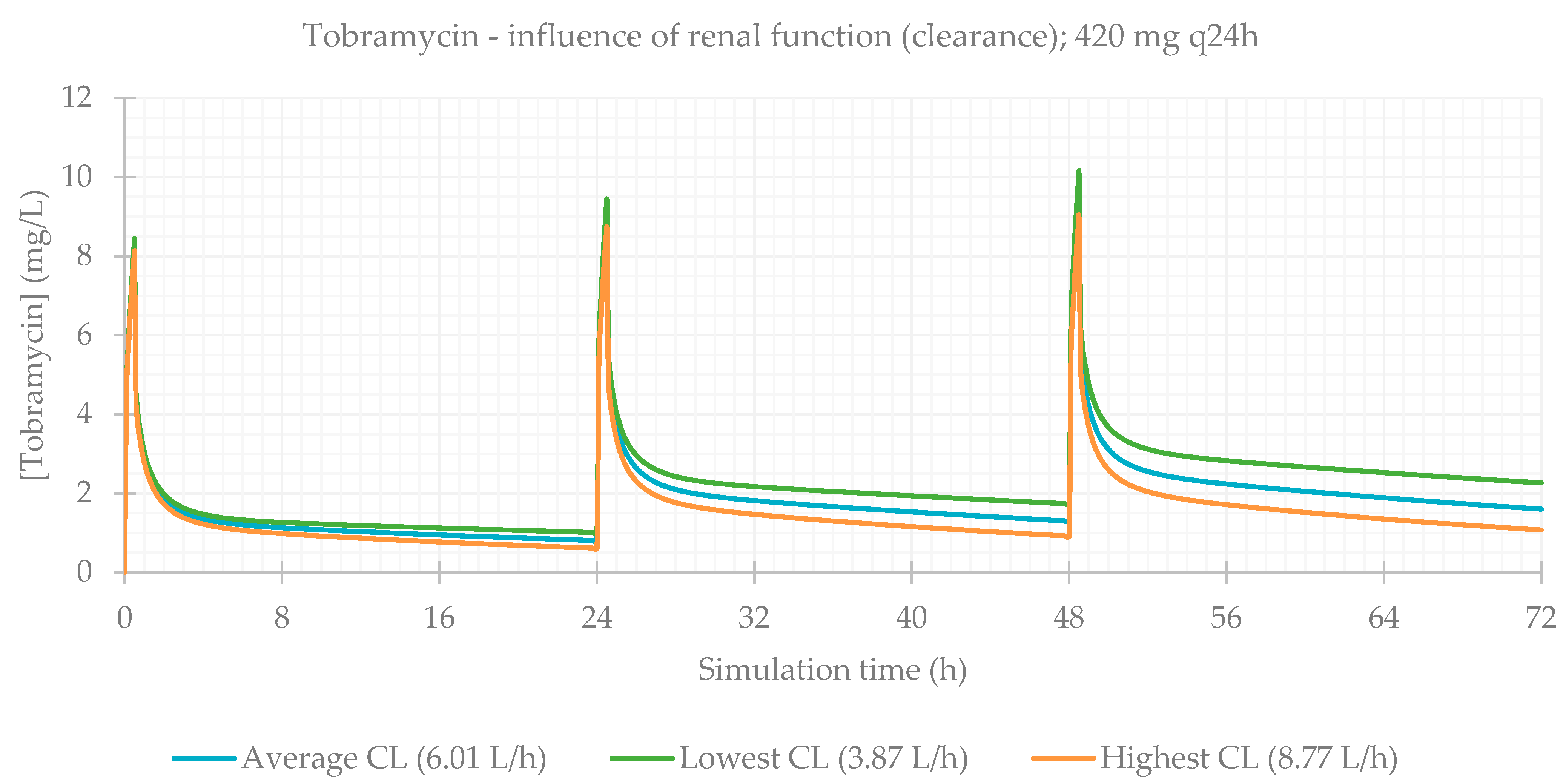
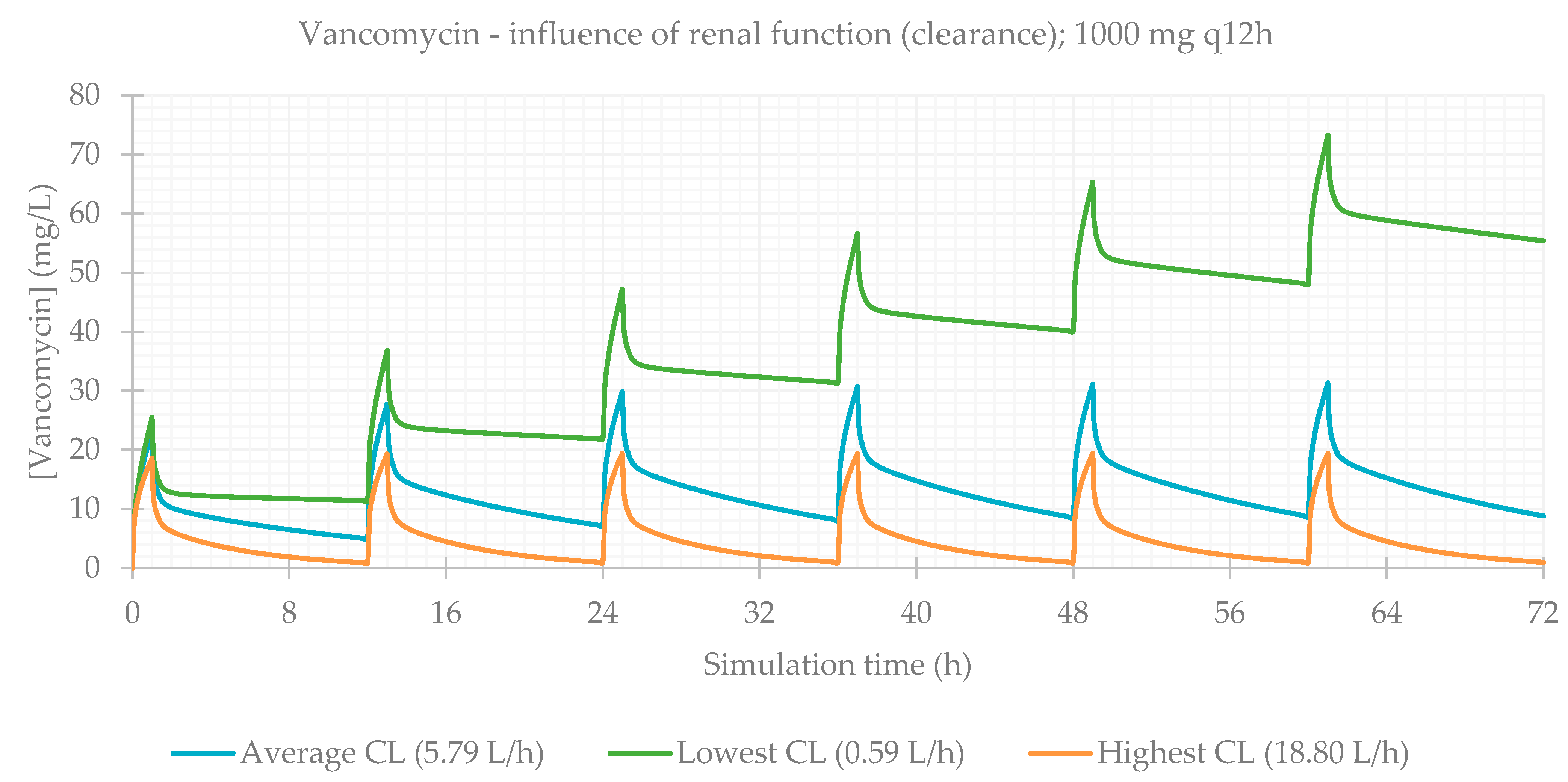
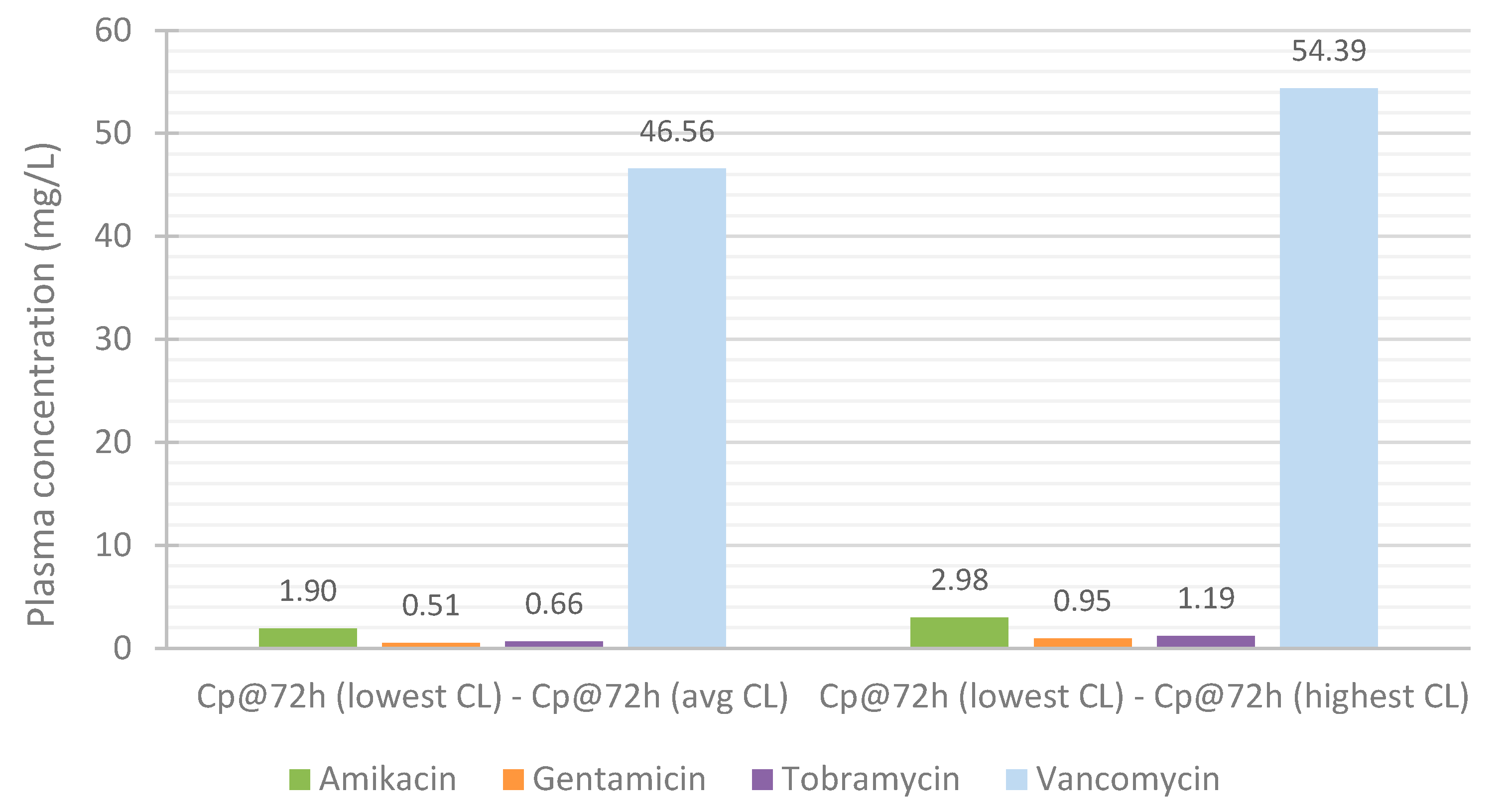
3.3. Influence of Renal Function
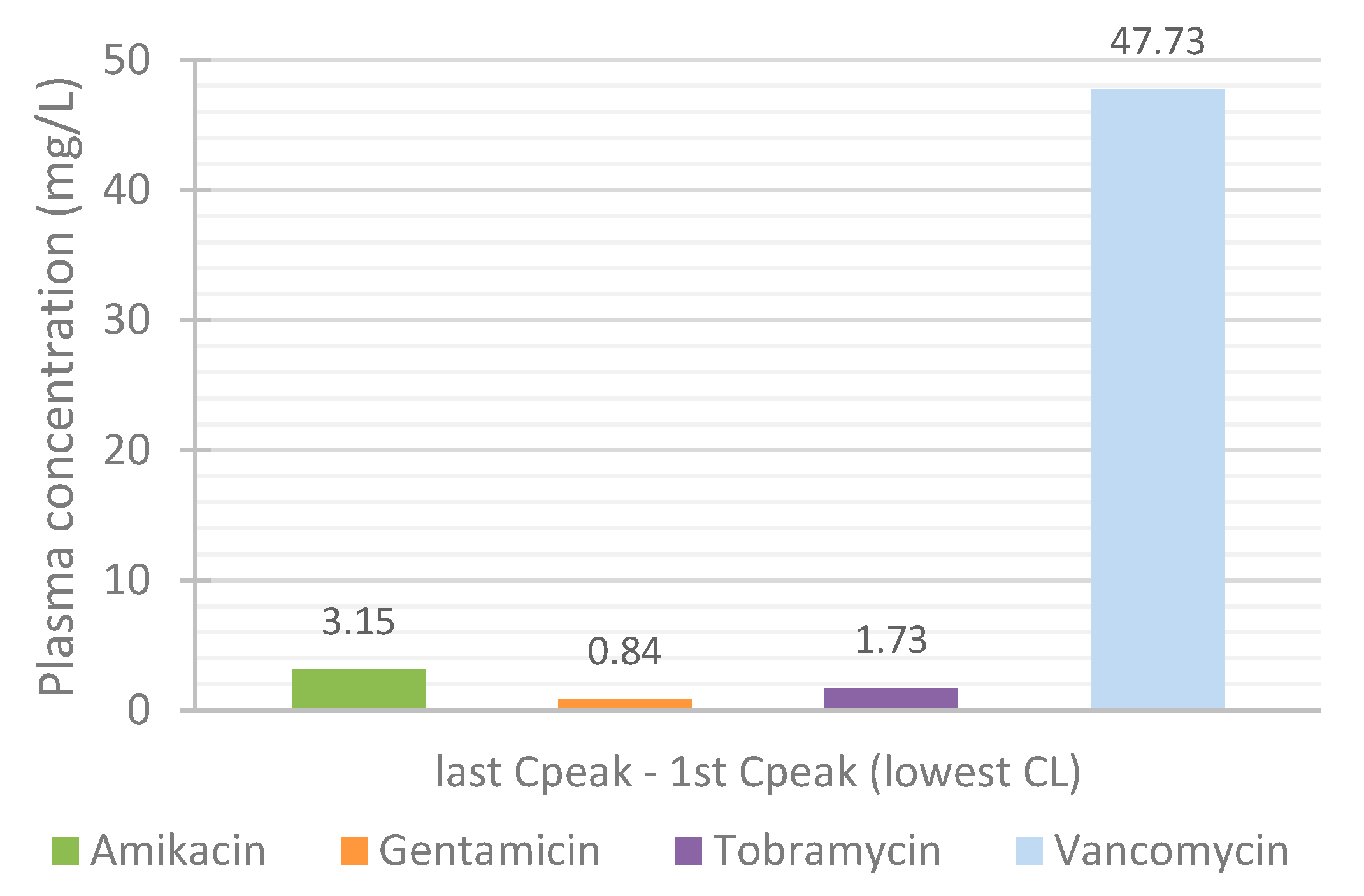
3.4. Effect of Renal Function on the Accumulation of Drugs in Plasma over Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, W.; Dasgupta, A. Clinical Challenges in Therapeutic Drug Monitoring: Special Populations, Physiological Conditions and Pharmacogenomics; Elsevier Science: Amsterdam, The Netherlands, 2016; ISBN 9780128020524. [Google Scholar]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Buclin, T.; Thoma, Y.; Widmer, N.; André, P.; Guidi, M.; Csajka, C.; Decosterd, L.A. The Steps to Therapeutic Drug Monitoring: A Structured Approach Illustrated With Imatinib. Front. Pharmacol. 2020, 11, 177. [Google Scholar] [CrossRef]
- Widmer, N.; Bardin, C.; Chatelut, E.; Paci, A.; Beijnen, J.; Levêque, D.; Veal, G.; Astier, A. Review of therapeutic drug monitoring of anticancer drugs part two-Targeted therapies. Eur. J. Cancer 2014, 50, 2020–2036. [Google Scholar] [CrossRef]
- Muller, A.E.; Huttner, B.; Huttner, A. Therapeutic Drug Monitoring of Beta-Lactams and Other Antibiotics in the Intensive Care Unit: Which Agents, Which Patients and Which Infections? Drugs 2018, 78, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Maitre, T.; Muret, P.; Blot, M.; Waldner, A.; Duong, M.; Si-Mohammed, A.; Chavanet, P.; Aho, S.; Piroth, L. Benefits and Limits of Antiretroviral Drug Monitoring in Routine Practice. Curr. HIV Res. 2019, 17, 190–197. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S. Therapeutic drug monitoring in patients on biologics: Lessons from gastroenterology. Curr. Opin. Rheumatol. 2020, 32, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017; a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General Principles of Antimicrobial Therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Mabilat, C.; Gros, M.F.; Nicolau, D.; Mouton, J.W.; Textoris, J.; Roberts, J.A.; Cotta, M.O.; van Belkum, A.; Caniaux, I. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 791–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicha, S.G.; Märtson, A.G.; Nielsen, E.I.; Koch, B.C.P.; Friberg, L.E.; Alffenaar, J.W.; Minichmayr, I.K. From Therapeutic Drug Monitoring to Model-Informed Precision Dosing for Antibiotics. Clin. Pharmacol. Ther. 2021, 109, 928–941. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Woo, H.I.; Park, H.D.; Lee, S.Y. A nationwide utilization survey of therapeutic drug monitoring for five antibiotics in South Korea. Infect. Drug Resist. 2019, 12, 2163–2173. [Google Scholar] [CrossRef]
- Begg, E.J.; Barclay, M.L.; Kirkpatrick, C.M. The therapeutic monitoring of antimicrobial agents. Br. J. Clin. Pharmacol. 2001, 52 (Suppl. 1), S35–S43. [Google Scholar] [CrossRef]
- Reeves, D.; Lovering, A.; Thomson, A. Therapeutic drug monitoring in the past 40 years of the Journal of Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2016, 71, 3330–3332. [Google Scholar] [CrossRef] [Green Version]
- Cunha, B.A. Antibiotic side effects. Med. Clin. N. Am. 2001, 85, 149–185. [Google Scholar] [CrossRef]
- Yılmaz, Ç.; Özcengiz, G. Antibiotics: Pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem. Pharmacol. 2017, 133, 43–62. [Google Scholar] [CrossRef]
- Möhlmann, J.E.; van Luin, M.; Mascini, E.M.; van Leeuwen, H.J.; de Maat, M.R. Monitoring of tobramycin serum concentrations in selected critically ill patients receiving selective decontamination of the digestive tract: A retrospective evaluation. Eur. J. Clin. Pharmacol. 2019, 75, 831–836. [Google Scholar] [CrossRef]
- Coulthard, K.P.; Peckham, D.G.; Conway, S.P.; Smith, C.A.; Bell, J.; Turnidge, J. Therapeutic drug monitoring of once daily tobramycin in cystic fibrosis--caution with trough concentrations. J. Cyst. Fibros. 2007, 6, 125–130. [Google Scholar] [CrossRef]
- Ye, Z.K.; Tang, H.L.; Zhai, S.D. Benefits of therapeutic drug monitoring of vancomycin: A systematic review and meta-analysis. PLoS ONE 2013, 8, e77169. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 42 (Suppl. 1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, A.; Thomson, A.H.; Brown, N.M.; Semple, Y.; Sluman, C.; MacGowan, A.; Lovering, A.M.; Wiffen, P.J. Amikacin use and therapeutic drug monitoring in adults: Do dose regimens and drug exposures affect either outcome or adverse events? A systematic review. J. Antimicrob. Chemother. 2016, 71, 2754–2759. [Google Scholar] [CrossRef] [Green Version]
- Kovačević, T.; Avram, S.; Milaković, D.; Špirić, N.; Kovačević, P. Therapeutic monitoring of amikacin and gentamicin in critically and noncritically ill patients. J. Basic Clin. Pharm. 2016, 7, 65–69. [Google Scholar] [CrossRef] [Green Version]
- de Velde, F.; Mouton, J.W.; de Winter, B.C.M.; van Gelder, T.; Koch, B.C.P. Clinical applications of population pharmacokinetic models of antibiotics: Challenges and perspectives. Pharmacol. Res. 2018, 134, 280–288. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Janssen, J.M.; de Jong, M.D.; Mathôt, R.A.; Juffermans, N.P.; van Hest, R.M. Therapeutic Drug Monitoring of Gentamicin Peak Concentrations in Critically Ill Patients. Ther. Drug Monit. 2017, 39, 522–530. [Google Scholar] [CrossRef]
- Drennan, P.G.; Thoma, Y.; Barry, L.; Matthey, J.; Sivam, S.; van Hal, S.J. Bayesian Forecasting for Intravenous Tobramycin Dosing in Adults With Cystic Fibrosis Using One Versus Two Serum Concentrations in a Dosing Interval. Ther. Drug Monit. 2021, 43, 505–511. [Google Scholar] [CrossRef]
- Gottlieb, T.; Nimmo, G.R. Antibiotic resistance is an emerging threat to public health: An urgent call to action at the Antimicrobial Resistance Summit 2011. Med. J. Aust. 2011, 194, 281–283. [Google Scholar] [CrossRef]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDougall, C.; Polk, R.E. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005, 18, 638–656. [Google Scholar] [CrossRef] [Green Version]
- Michaels, K.; Mahdavi, M.; Krug, A.; Kuper, K. Implementation of an antimicrobial stewardship program in a community hospital: Results of a three-year analysis. Hosp. Pharm. 2012, 47, 608–616. [Google Scholar] [CrossRef]
- Pollack, L.A.; Srinivasan, A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S97–S100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.; Martins, H.; Oliveira, J.C.; Lapa, R.; Vale, N. In silico pharmacokinetic study of vancomycin using PBPK modeling and therapeutic drug monitoring. Curr. Drug Metab. 2021, 22, 150–162. [Google Scholar] [CrossRef]
- Ferreira, A.; Lapa, R.; Vale, N. Combination of Gemcitabine with Cell-Penetrating Peptides: A Pharmacokinetic Approach Using In Silico Tools. Biomolecules 2019, 9, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Lobato, L.; Vale, N. Clinical pharmacokinetic study of latrepirdine via in silico sublingual administration. In Silico Pharmacol. 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Amikacin. Available online: https://go.drugbank.com/drugs/DB00479 (accessed on 1 February 2021).
- Gentamicin. Available online: https://go.drugbank.com/drugs/DB00798 (accessed on 1 February 2021).
- Gonçalves-Pereira, J.; Martins, A.; Póvoa, P. Pharmacokinetics of gentamicin in critically ill patients: Pilot study evaluating the first dose. Clin. Microbiol. Infect. 2010, 16, 1258–1263. [Google Scholar] [CrossRef] [Green Version]
- Tobramycin. Available online: https://go.drugbank.com/drugs/DB00684 (accessed on 1 February 2021).
- Aminimanizani, A.; Beringer, P.M.; Kang, J.; Tsang, L.; Jelliffe, R.W.; Shapiro, B.J. Distribution and elimination of tobramycin administered in single or multiple daily doses in adult patients with cystic fibrosis. J. Antimicrob. Chemother. 2002, 50, 553–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobramycin for Injection. Available online: https://reference.medscape.com/drug/nebcin-injection-tobramycin-342521#10 (accessed on 1 February 2021).
- Vancomycin. Available online: https://go.drugbank.com/drugs/DB00512 (accessed on 1 February 2021).
- Miller, N.A.; Reddy, M.B.; Heikkinen, A.T.; Lukacova, V.; Parrott, N. Physiologically Based Pharmacokinetic Modelling for First-In-Human Predictions: An Updated Model Building Strategy Illustrated with Challenging Industry Case Studies. Clin. Pharmacokinet. 2019, 58, 727–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, C.; Zhao, P.; Pan, Y.; Hsu, V.; Grillo, J.; Huang, S.M.; Sinha, V. Application of Physiologically Based Pharmacokinetic (PBPK) Modeling to Support Dose Selection: Report of an FDA Public Workshop on PBPK. CPT Pharmacomet. Syst. Pharmacol. 2015, 4, 226–230. [Google Scholar] [CrossRef]
- Kovar, L.; Schräpel, C.; Selzer, D.; Kohl, Y.; Bals, R.; Schwab, M.; Lehr, T. Physiologically-Based Pharmacokinetic (PBPK) Modeling of Buprenorphine in Adults, Children and Preterm Neonates. Pharmaceutics 2020, 12, 578. [Google Scholar] [CrossRef] [PubMed]
- Fuhr, L.M.; Marok, F.Z.; Hanke, N.; Selzer, D.; Lehr, T. Pharmacokinetics of the CYP3A4 and CYP2B6 Inducer Carbamazepine and Its Drug–Drug Interaction Potential: A Physiologically Based Pharmacokinetic Modeling Approach. Pharmaceutics 2021, 13, 270. [Google Scholar] [CrossRef]
- Zapke, S.E.; Willmann, S.; Grebe, S.-O.; Menke, K.; Thürmann, P.A.; Schmiedl, S. Comparing Predictions of a PBPK Model for Cyclosporine With Drug Levels From Therapeutic Drug Monitoring. Front. Pharmacol. 2021, 12, 1134. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Wu, J.; Bao, X.; Sanai, N. Physiologically based pharmacokinetic modeling of central nervous system pharmacokinetics of CDK4/6 inhibitors to guide selection of drug and dosing regimen for brain cancer treatment. Clin. Pharmacol. Ther. 2021, 109, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yao, X.; Zhang, M.; Liu, D.; Gao, Y.; Sahasranaman, S.; Ou, Y.C. Comprehensive PBPK model to predict drug interaction potential of Zanubrutinib as a victim or perpetrator. CPT Pharmacomet. Syst. Pharmacol. 2021, 10, 441–454. [Google Scholar] [CrossRef] [PubMed]
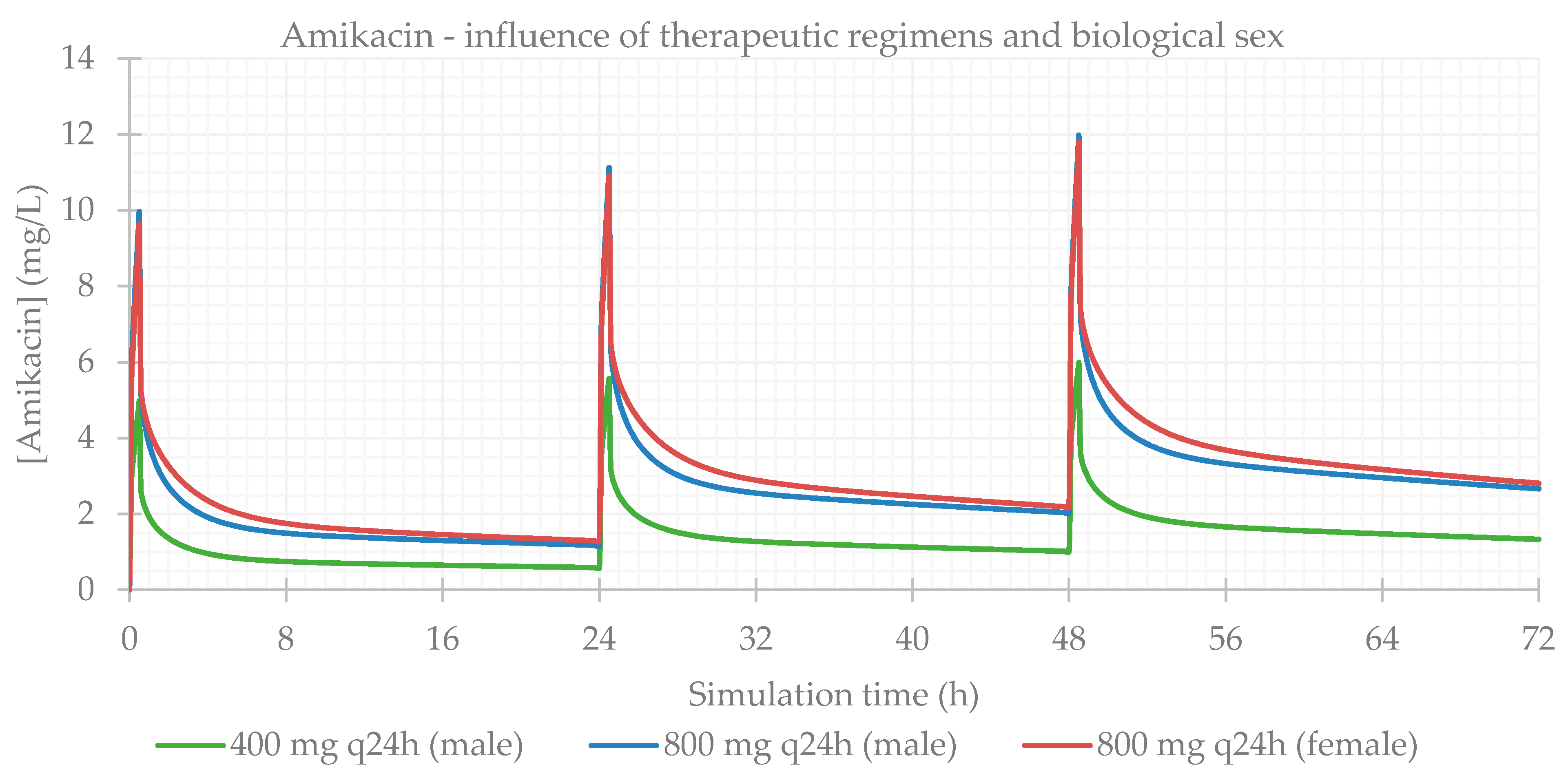
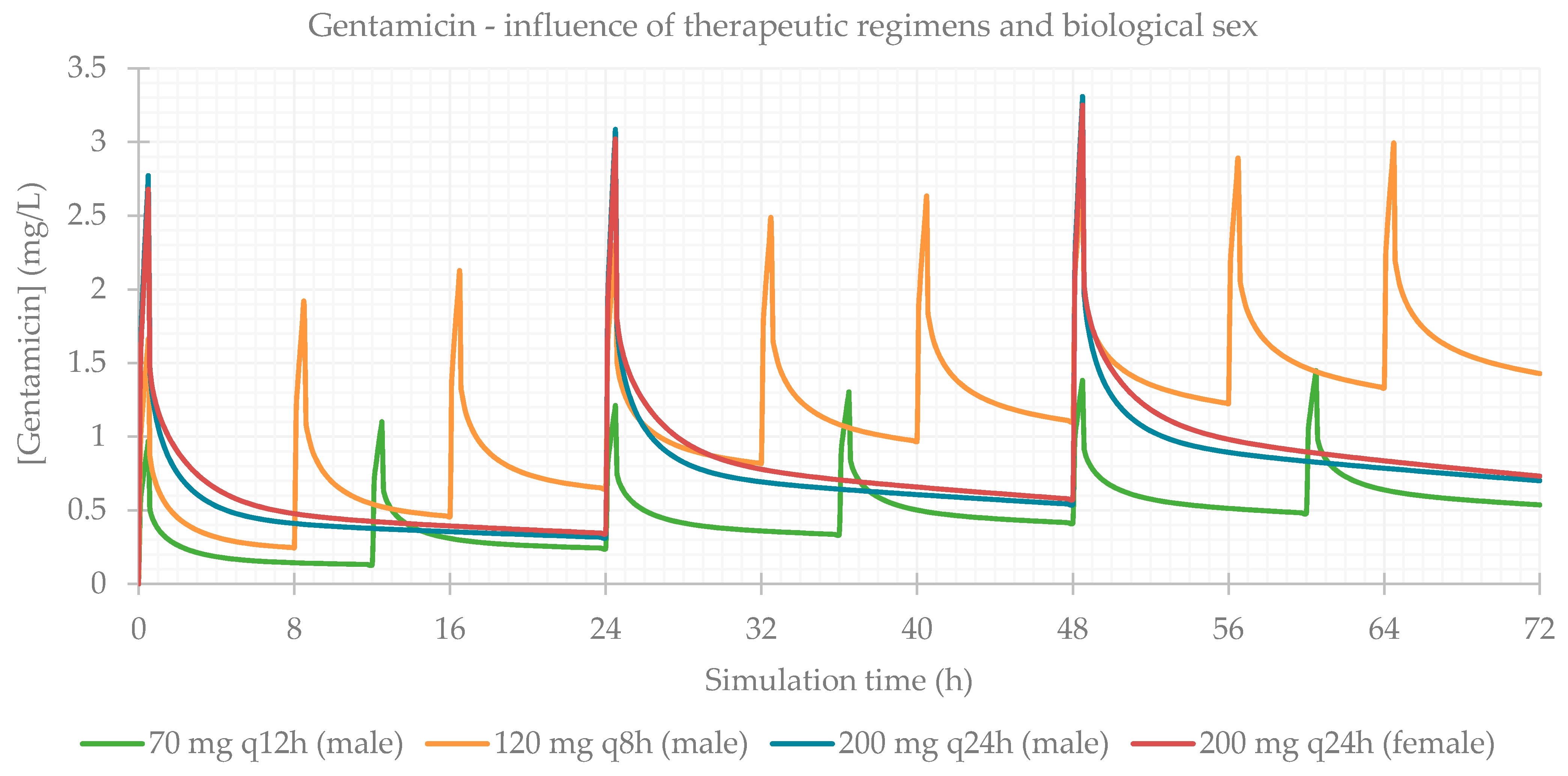
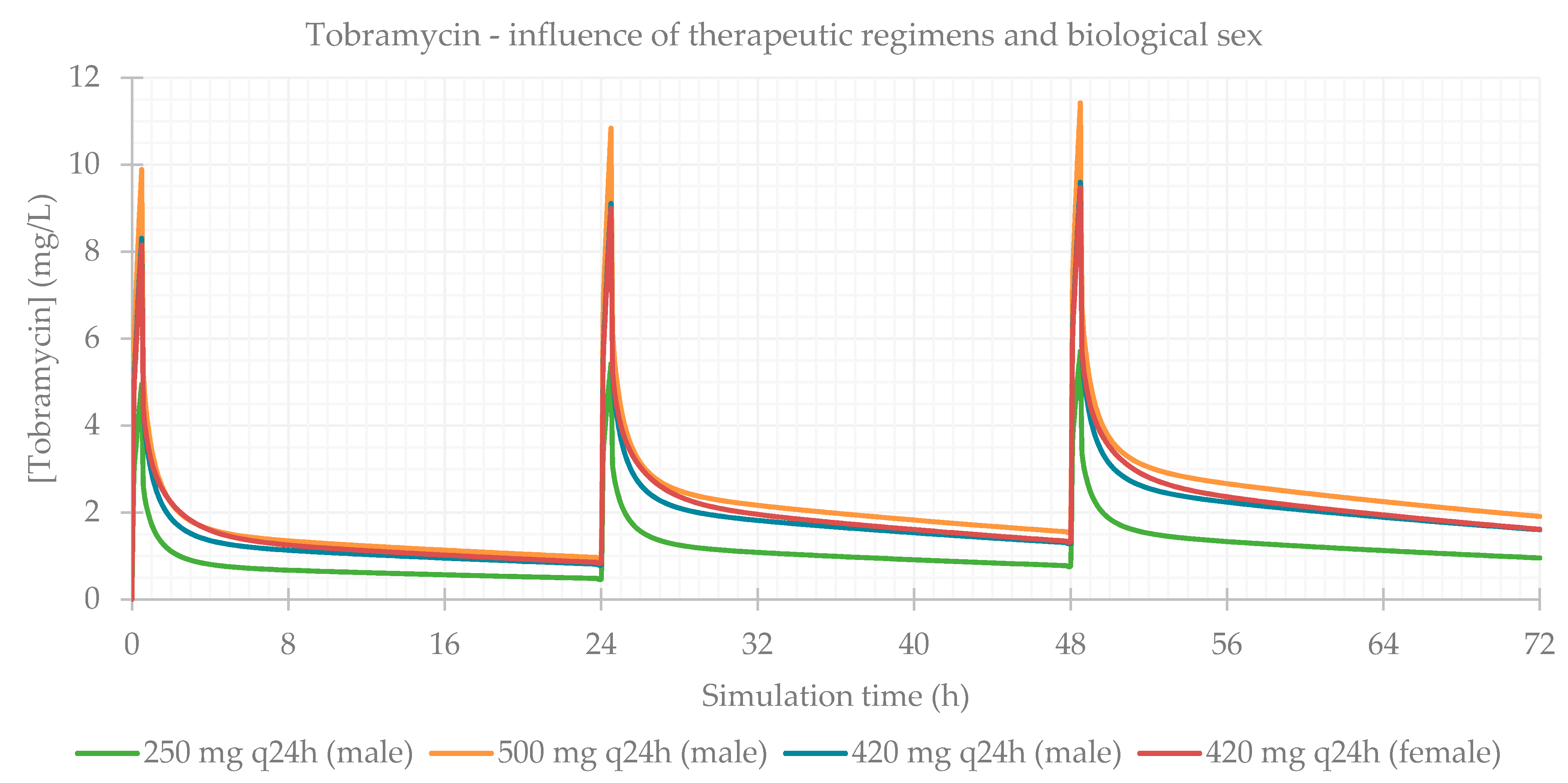
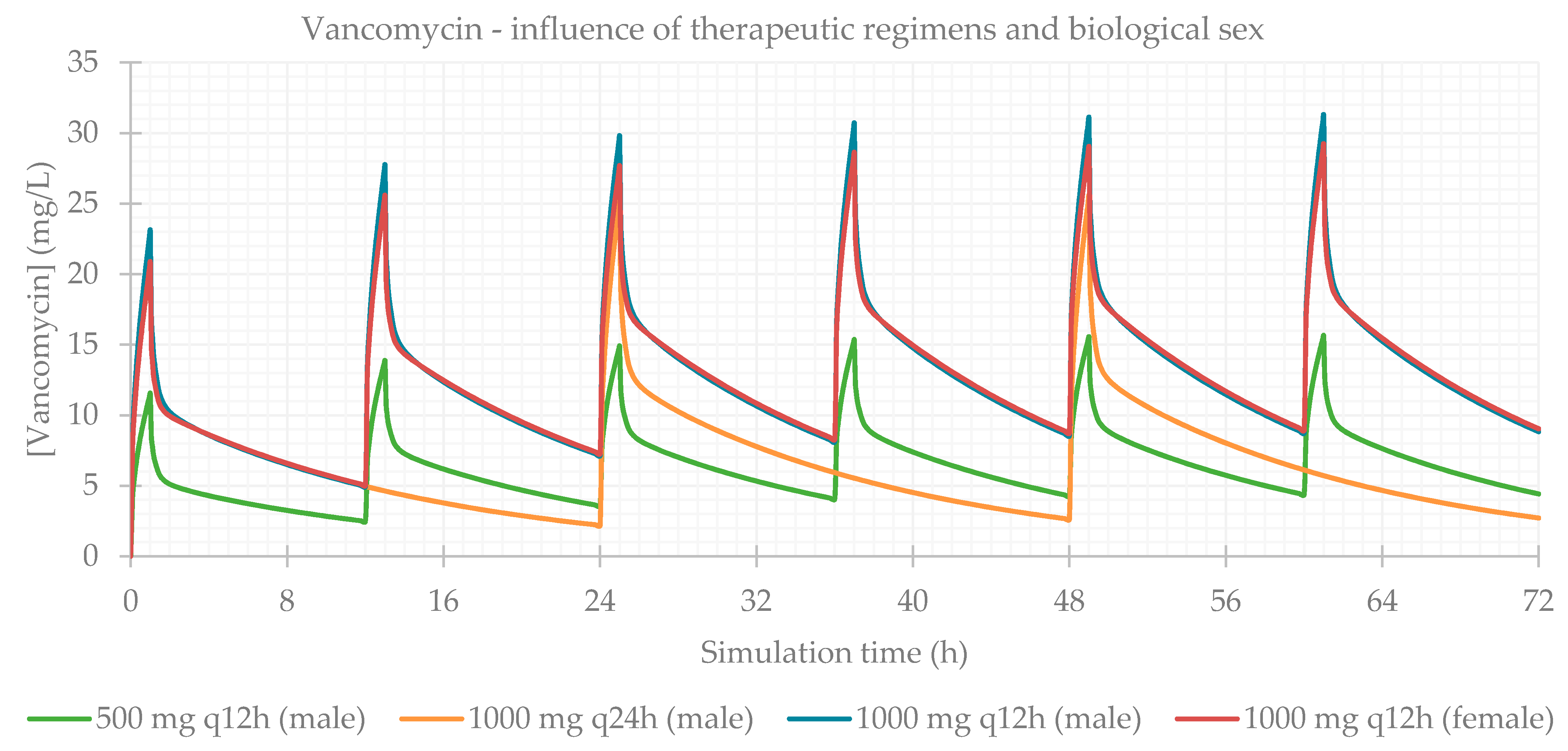
| Amikacin | Gentamicin | Tobramycin | Vancomycin | |
|---|---|---|---|---|
| nt (nsex) | 8 (F: 1; M: 7) | 22 (F: 8; M: 14) | 5 (F: 4; M: 1) | 47 (F: 21; M: 26) |
| Age (years) | 14–87 (avg 57) | 7–88 (avg 58) | 13–19 (avg 15) | 19–93 (avg 63) |
| Weight (kg) | 50.0–92.5 (avg 66.0) | 15.5–85.0 (avg 66.0) | 25.8–44.5 (avg 33.0) | 29.0–140. (avg 69.0) |
| Height (cm) | 163–180 (avg 169) | 108–185 (avg 165) | 130–158 (avg 146) | 147–185 (avg 163) |
| Cmin (mg/L) | 0.30–16.40 (avg 3.70) (ref 1–8) | 0.20–4.80 (avg 1.05) (ref 0.5–2) | 0.06–0.23 (avg 0.17) (ref 0.5–2; <1 CF) | 4.50–45.60 (avg 16.34) (ref 15–20) |
| Cmax (mg/L) | 19.70–87.80 (avg 38.97) (ref 20–30) | 2.90–19.50 (avg 9.22) (ref 5–10) | 16.32–36.12 (avg 27.05) (ref 5–10; 20–30 CF) | 11.20–60.10 (avg 25.99) (ref < 40) |
| [Cr] (mg/dL) | 0.47–1.58 (avg 0.93) | 0.29–1.89 (avg 0.83) | 0.35–0.64 (avg 0.49) | 0.27–4.78 (avg 1.00) |
| CL (L/h) * | 1.40–11.30 (avg 6.07) | 1.67–15.89 (avg 5.94) | 3.87–8.77 (avg 6.01) | 0.59–18.80 (avg 5.79) |
| Amikacin | Gentamicin | Tobramycin | Vancomycin | |
|---|---|---|---|---|
| Molecular weight | 585.61 | 477.61 | 467.52 | 1449.28 |
| logP | −5 1 | −1.79 1; −3.1 2 | −4.8 1; −5.8 2 | 2.48 1; −3.1 2 |
| pKa | 8.1 2 | 12.55; 10.18 2 | 12.54; 9.83 2 | 2.99; 9.93 2 |
| Solubility | 166.49 g/L, pH = 11.73 1; 50 g/L 2 | 56.54 g/L, pH = 11.51 1; 12.6 g/L 2 | 59.47 g/L, pH = 11.34 1; freely soluble 2 | 0.26 g/L, pH = 8.17 1; 0.225 g/L 2 |
| Diffusion Coefficient | 0.53 cm2/s × 105 1 | 0.56 cm2/s × 105 1 | 0.6 cm2/s × 105 1 | 0.32 cm2/s × 105 1 |
| Drug particle density | 1.2 g/mL | 1.2 g/mL | 1.2 g/mL | 1.2 g/mL |
| Mean particle radius | 25 | 25 | 25 | 25 |
| Peff | 0.0202 cm/s × 10−4 1 | 0.0891 cm/s × 10−4 1 | 0.0368 cm/s × 10−4 1 | 0.0747 cm/s × 10−4 1 |
| Fup | 98.75% 1; >90% 2 | 85.4% 1; >70% 2 | 100% 1; >70% 2 | 21.36% 1; ~50% 2 |
| Blood/plasma ratio | 1.23 1 | 1.11 1 | 1.31 1 | 0.68 1 |
| T1/2 | 2–3 h | 2–3 h | 2–3 h | ~6 h (4–11 h) |
| Vc | 0.24 1; ~0.34 L/kg 2 | 0.38 1; 0.2–0.3 L/kg | 0.3 1; 0.2–0.3 L/kg | 0.4–1 L/kg |
| Clearance | 6.00 L/h | 3.42 L/h | 8.48 L/h | 4.03 L/h |
| Typical dosing for susceptible infections | 7.5 mg/kg q12h 15 mg/kg q24h | 1 mg/kg q8h | 1 mg/kg q8h | 1000 mg q12h |
| Common dosing in CHUP | Variable: 400–800 mg q24h or q48h | 120 mg q8h 200 mg q24h 70 mg q12h | 70 mg q8h 420 mg q24h | 1 g q12h 1 g q24h |
| Drug & Posology | Fa (%) 1 | FDp (%) 2 | F (%) 3 | Cmax (mg/L) 4 | Tmax (h) 5 | AUC0-inf (µg∙h/mL) 6 | AUC0-t (µg∙h/mL) 7 | Cmax liver (mg/L) 8 |
|---|---|---|---|---|---|---|---|---|
| Amikacin | ||||||||
| 400 mg q24h | 99.980 | 99.964 | 99.964 | 5.981 | 48.5 | 197.570 | 94.836 | 64.761 |
| 800 mg q24h | 99.980 | 99.964 | 99.964 | 11.962 | 48.5 | 395.150 | 189.670 | 129.520 |
| 800 mg q24h female | 99.978 | 99.960 | 99.960 | 11.790 | 48.5 | 395.060 | 209.520 | 128.610 |
| Gentamicin | ||||||||
| 70 mg q12h | 99.859 | 99.834 | 99.834 | 1.446 | 60.5 | 68.971 | 32.731 | 14.831 |
| 120 mg q8h | 99.864 | 99.838 | 99.838 | 2.981 | 64.5 | 163.850 | 81.287 | 31.975 |
| 200 mg q24h | 99.845 | 99.822 | 99.822 | 3.304 | 48.5 | 100.650 | 51.345 | 31.876 |
| 200 mg q24h female | 99.829 | 99.806 | 99.806 | 3.246 | 48.5 | 100.630 | 56.321 | 31.524 |
| Tobramycin | ||||||||
| 250 mg q24h | 99.971 | 99.971 | 99.971 | 5.702 | 48.5 | 124.780 | 78.230 | 62.346 |
| 500 mg q24h | 99.971 | 99.971 | 99.971 | 11.403 | 48.5 | 249.570 | 156.460 | 124.690 |
| 420 mg q24h | 99.971 | 99.971 | 99.971 | 9.579 | 48.5 | 209.640 | 131.430 | 104.740 |
| 420 mg q24h female | 99.969 | 99.969 | 99.969 | 9.460 | 48.5 | 209.640 | 139.840 | 104.770 |
| Vancomycin | ||||||||
| 500 mg q12h | 99.929 | 99.921 | 99.921 | 15.632 | 61.0 | 517.990 | 452.870 | 30.871 |
| 1000 mg q24h | 99.926 | 99.921 | 99.921 | 25.547 | 49.0 | 518.000 | 477.990 | 50.086 |
| 1000 mg q12h | 99.929 | 99.921 | 99.921 | 31.263 | 61.0 | 1036.000 | 905.740 | 61.742 |
| 1000 mg q12h female | 99.930 | 99.921 | 99.921 | 29.202 | 61.0 | 1036.000 | 901.460 | 57.773 |
| Drug & Posology | Fa (%) 1 | FDp (%) 2 | F (%) 3 | Cmax (mg/L) 4 | Tmax (h) 5 | AUC0-inf (µg∙h/mL) 6 | AUC0-t (µg∙h/mL) 7 | Cmax liver (mg/L) 8 |
|---|---|---|---|---|---|---|---|---|
| Amikacin | ||||||||
| 800 mg q24h BW avg (66.0 kg) | 99.980 | 99.964 | 99.964 | 11.962 | 48.5 | 395.150 | 189.670 | 129.520 |
| 800 mg q24h BW min (50.0 kg) | 99.981 | 99.965 | 99.965 | 14.813 | 48.5 | 395.250 | 223.520 | 161.780 |
| 800 mg q24h BW max (92.5 kg) | 99.982 | 99.966 | 99.966 | 9.161 | 48.5 | 394.940 | 151.390 | 97.834 |
| Gentamicin | ||||||||
| 200 mg q24h BW avg (66.0 kg) | 99.845 | 99.822 | 99.822 | 3.304 | 48.5 | 100.650 | 51.345 | 31.876 |
| 200 mg q24h BW min (15.5 kg) | 99.910 | 99.905 | 99.905 | 9.555 | 48.5 | 100.890 | 90.418 | 97.446 |
| 200 mg q24h BW max (85.0 kg) | 99.680 | 99.656 | 99.656 | 9.546 | 48.5 | 100.630 | 90.200 | 97.126 |
| Tobramycin | ||||||||
| 420 mg q24h BW avg (33.0 kg) | 99.971 | 99.971 | 99.971 | 9.579 | 48.5 | 209.640 | 131.430 | 104.740 |
| 420 mg q24h BW min (25.8 kg) | 99.973 | 99.973 | 99.973 | 11.479 | 48.5 | 209.660 | 147.220 | 127.010 |
| 420 mg q24h BW max (44.5 kg) | 99.970 | 99.970 | 99.970 | 7.646 | 48.5 | 209.600 | 111.740 | 82.333 |
| Vancomycin | ||||||||
| 1000 mg q12h BW avg (69.0 kg) | 99.929 | 99.921 | 99.921 | 31.263 | 61.0 | 1036.000 | 905.740 | 61.742 |
| 1000 mg q12h BW min (29.0 kg) | 99.956 | 99.954 | 99.954 | 48.459 | 61.0 | 1036.300 | 1005.000 | 95.749 |
| 1000 mg q12h BW max (140.0 kg) | 99.936 | 99.926 | 99.926 | 22.535 | 61.0 | 1035.800 | 736.330 | 44.587 |
| Drug & Posology | Fa (%) 1 | FDp (%) 2 | F (%) 3 | Cmax (mg/L) 4 | Tmax (h) 5 | AUC0-inf (µg∙h/mL) 6 | AUC0-t (µg∙h/mL) 7 | Cmax liver (mg/L) 8 |
|---|---|---|---|---|---|---|---|---|
| Amikacin | ||||||||
| 800 mg q24h CL avg (6.068 L/h) | 99.980 | 99.964 | 99.964 | 11.962 | 48.5 | 395.150 | 189.670 | 129.520 |
| 800 mg q24h CL min (1.401 L/h) | 99.974 | 99.950 | 99.950 | 13.293 | 48.5 | 1705.900 | 258.260 | 147.360 |
| 800 mg q24h CL max (11.298 L/h) | 99.985 | 99.973 | 99.973 | 11.015 | 48.5 | 212.320 | 142.930 | 117.190 |
| Gentamicin | ||||||||
| 200 mg q24h CL avg (5.941 L/h) | 99.845 | 99.822 | 99.822 | 3.304 | 48.5 | 100.650 | 51.345 | 31.876 |
| 200 mg q24h CL min (1.667 L/h) | 99.789 | 99.751 | 99.751 | 3.667 | 48.5 | 355.600 | 69.999 | 36.212 |
| 200 mg q24h CL max (15.887 L/h) | 99.908 | 99.899 | 99.899 | 2.860 | 48.5 | 37.744 | 30.152 | 26.809 |
| Tobramycin | ||||||||
| 420 mg q24h CL avg (6.007 L/h) | 99.971 | 99.971 | 99.971 | 9.579 | 48.5 | 209.640 | 131.430 | 104.740 |
| 420 mg q24h CL min (3.868 L/h) | 99.965 | 99.965 | 99.965 | 10.149 | 48.5 | 325.460 | 158.850 | 111.740 |
| 420 mg q24h CL max (8.771 L/h) | 99.977 | 99.977 | 99.977 | 9.032 | 48.5 | 143.600 | 106.330 | 98.209 |
| Vancomycin | ||||||||
| 1000 mg q12h CL avg (5.787 L/h) | 99.929 | 99.921 | 99.921 | 31.263 | 61.0 | 1036.000 | 905.740 | 61.742 |
| 1000 mg q12h CL min (0.590 L/h) | 99.789 | 99.743 | 99.743 | 73.192 | 61.0 | 10090.000 | 2659.400 | 146.400 |
| 1000 mg q12h CL max (18.796 L/h) | 99.976 | 99.974 | 99.974 | 19.356 | 61.0 | 319.140 | 313.790 | 38.136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.; Martins, H.; Oliveira, J.C.; Lapa, R.; Vale, N. PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice. Life 2021, 11, 1130. https://doi.org/10.3390/life11111130
Ferreira A, Martins H, Oliveira JC, Lapa R, Vale N. PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice. Life. 2021; 11(11):1130. https://doi.org/10.3390/life11111130
Chicago/Turabian StyleFerreira, Abigail, Helena Martins, José Carlos Oliveira, Rui Lapa, and Nuno Vale. 2021. "PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice" Life 11, no. 11: 1130. https://doi.org/10.3390/life11111130
APA StyleFerreira, A., Martins, H., Oliveira, J. C., Lapa, R., & Vale, N. (2021). PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice. Life, 11(11), 1130. https://doi.org/10.3390/life11111130







