Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Serum Extraction and Blood Chemistry Analysis
2.3. Measurement of Body Composition and BMD
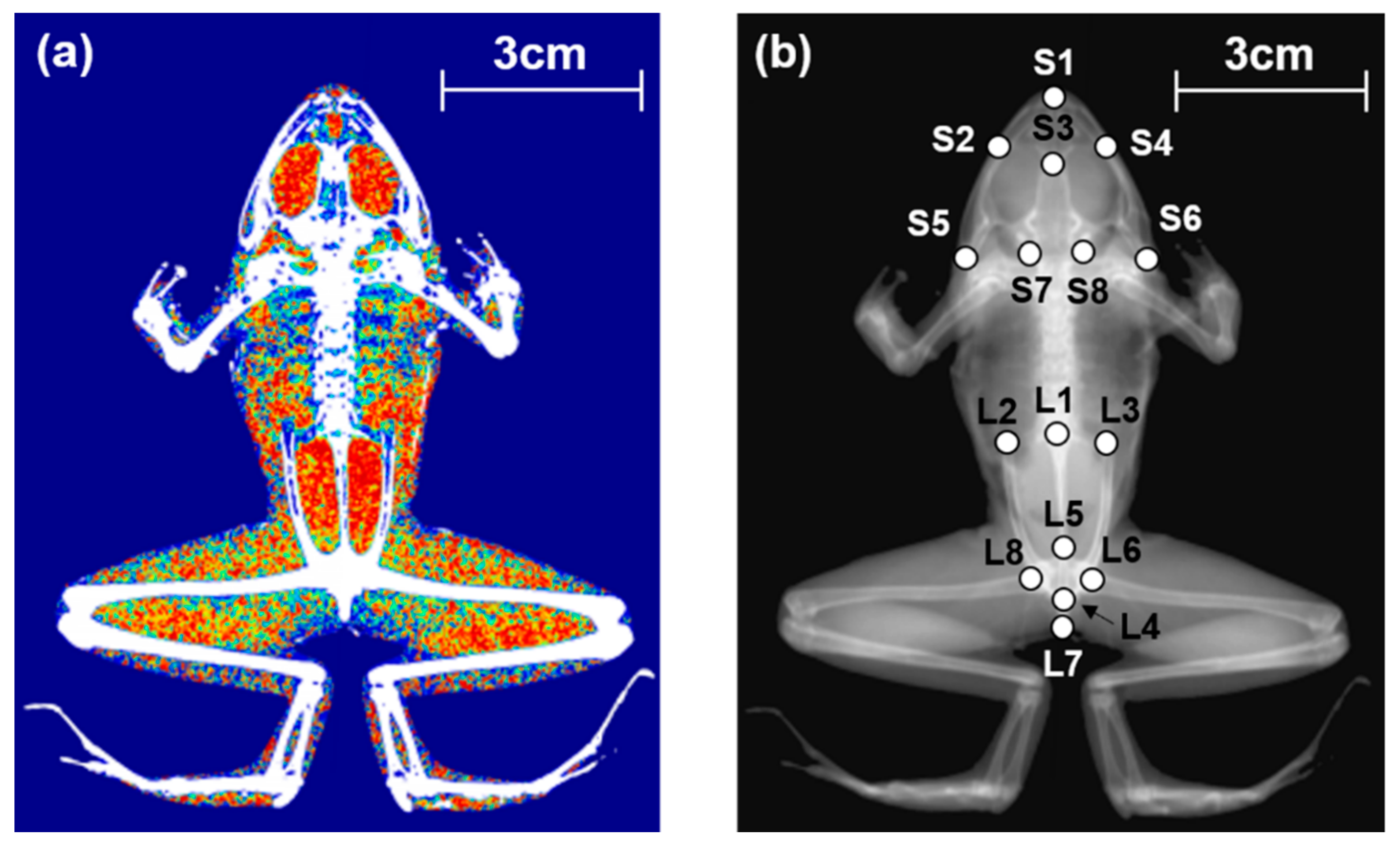
2.4. Analysis of Skeletal Shape
2.5. Statistical Analysis
3. Results
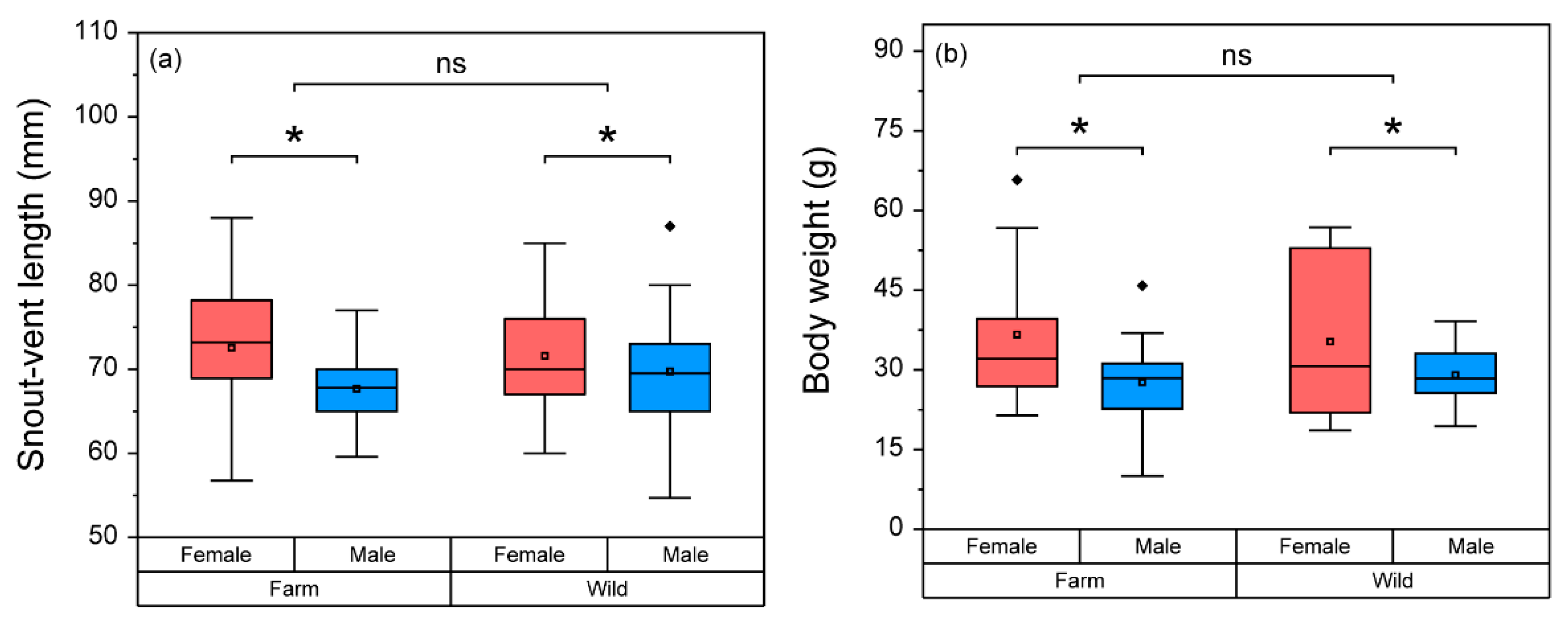
3.1. Basic Physical, Nutritional, and Physiological Status of Frogs
3.2. Comparison of Body Composition and BMD among Frogs
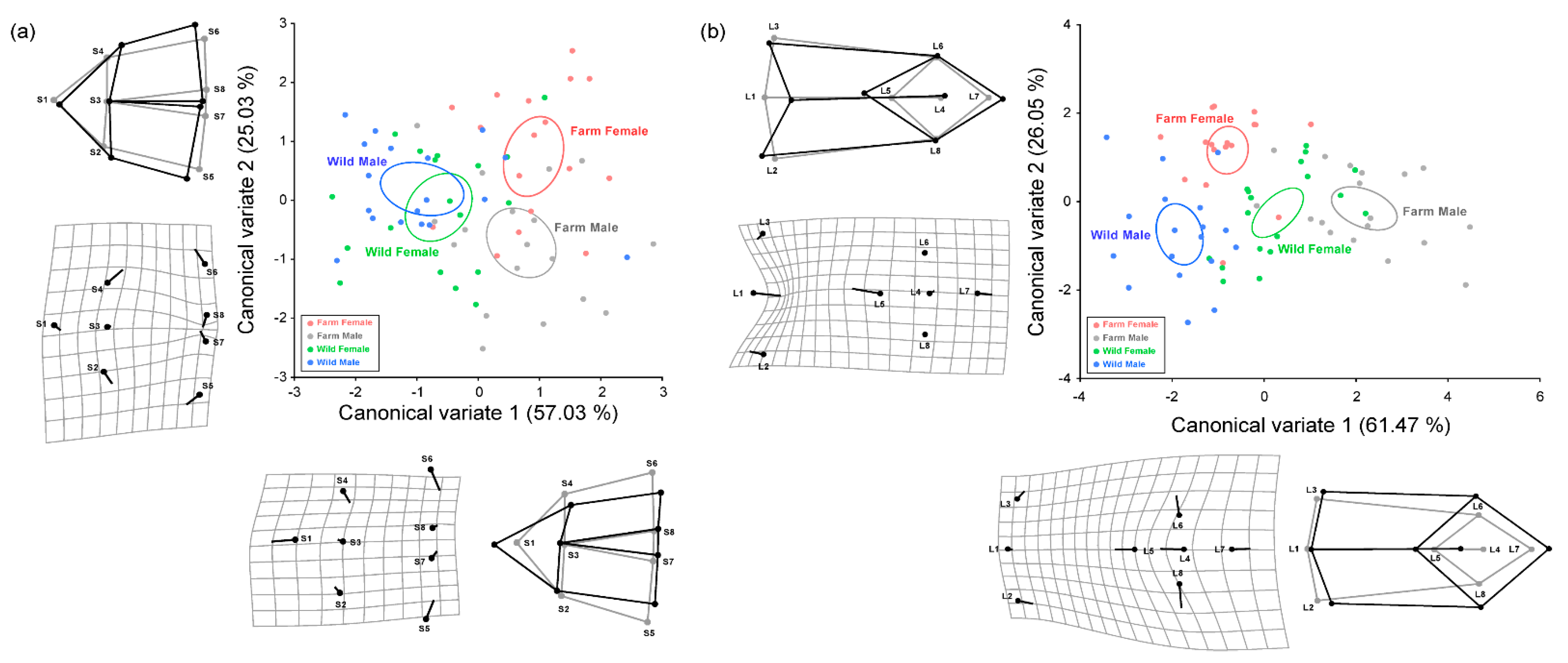
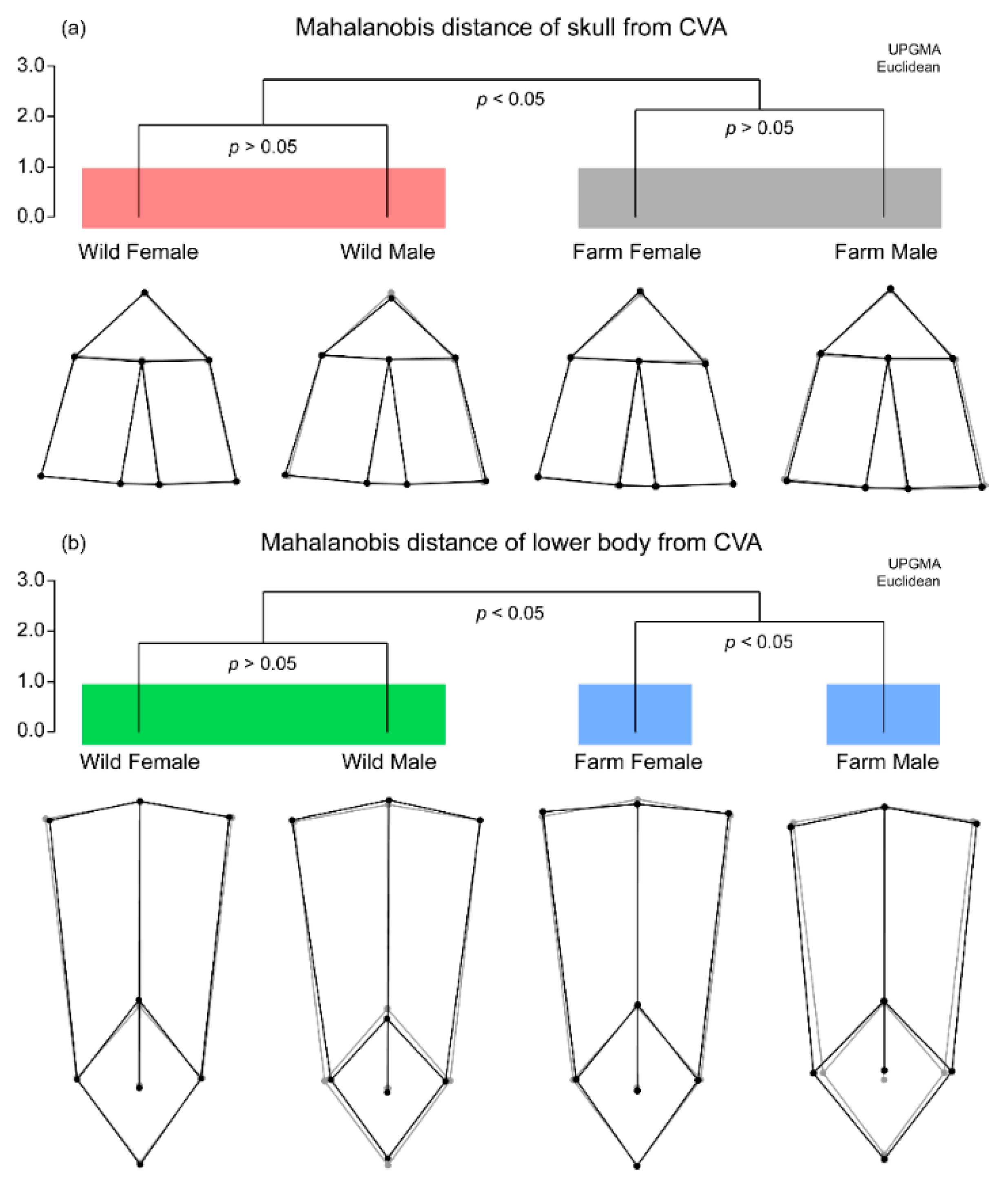
3.3. Skeletal Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wickins-Dražilová, D. Zoo animal welfare. J. Agric. Environ. Ethics 2006, 19, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Fraser, D. Understanding animal welfare. Acta Vet. Scand. 2008, 50, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Bostock, S.C. Zoos and Animal Rights: The Ethics of Keeping Animals; Routledge: London, UK, 1993. [Google Scholar]
- Wright, K.M.; Whitaker, B.R. Amphibian Medicine and Captive Husbandry; Krieger Publishing Company: Malabar, FL, USA, 2001. [Google Scholar]
- Clauss, M.; Franz-Odendaal, T.A.; Brasch, J.; Castell, J.C.; Kaiser, T. Tooth wear in captive giraffes (Giraffa camelopardalis): Mesowear analysis classifies free-ranging specimens as browsers but captive ones as grazers. J. Zoo Wildl. Med. 2007, 38, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Mason, G.J. Species differences in responses to captivity: Stress, welfare and the comparative method. Trends Ecol. Evol. 2010, 25, 713–721. [Google Scholar] [CrossRef] [Green Version]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S. Assessment of welfare in zoo animals: Towards optimum quality of life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [Green Version]
- Andreone, F.; Mercurio, V.; Mattioli, F. Between environmental degradation and international pet trade: Conservation strategies for the threatened amphibians of Madagascar. Natura 2006, 95, 81–96. [Google Scholar]
- Auliya, M.; García-Moreno, J.; Schmidt, B.R.; Schmeller, D.S.; Hoogmoed, M.S.; Fisher, M.C.; Pasmans, F.; Henle, K.; Bickford, D.; Martel, A. The global amphibian trade flows through Europe: The need for enforcing and improving legislation. Biodivers. Conserv. 2016, 25, 2581–2595. [Google Scholar] [CrossRef]
- Carpenter, A.I.; Andreone, F.; Moore, R.D.; Griffiths, R.A. A review of the international trade in amphibians: The types, levels and dynamics of trade in CITES-listed species. Oryx 2014, 48, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Enge, K.M. Commercial harvest of amphibians and reptiles in Florida for the pet trade. In Amphibians and Reptiles: Status and Conservation in Florida; Meshaka, W.E., Jr., Babbitt, K.J., Eds.; Krieger Publishing Company: Malabar, FL, USA, 2005; pp. 198–211. [Google Scholar]
- Dawson, J.; Patel, F.; Griffiths, R.A.; Young, R.P. Assessing the global zoo response to the amphibian crisis through 20-year trends in captive collections. Conserv. Biol. 2016, 30, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pessier, A.P.; Baitchman, E.J.; Crump, P.; Wilson, B.; Griffith, E.; Ross, H. Causes of mortality in anuran amphibians from an ex situ survival assurance colony in Panama. Zoo Biol. 2014, 33, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Zippel, K.; Johnson, K.; Gagliardo, R.; Gibson, R.; McFadden, M.; Browne, R.; Martinez, C.; Townsend, E. The Amphibian Ark: A global community for ex situ conservation of amphibians. Herpetol. Conserv. Biol. 2011, 6, 340–352. [Google Scholar]
- Tapley, B.; Bradfield, K.S.; Michaels, C.; Bungard, M. Amphibians and conservation breeding programmes: Do all threatened amphibians belong on the ark? Biodivers. Conserv. 2015, 24, 2625–2646. [Google Scholar] [CrossRef]
- Zippel, K. Zoos play a vital role in amphibian conservation. In AmphibiaWeb, Information on Amphibian Biology and Conservation; AmphibiaWeb: Berkeley, CA, USA, 2006; Available online: http://amphibiaweb.org/declines/zoo/index.html (accessed on 1 May 2021).
- Garner, T.W.; Stephen, I.; Wombwell, E.; Fisher, M.C. The amphibian trade: Bans or best practice? Ecohealth 2009, 6, 148–151. [Google Scholar] [CrossRef]
- Stuart, S.N.; Chanson, J.S.; Cox, N.A.; Young, B.E.; Rodrigues, A.S.; Fischman, D.L.; Waller, R.W. Status and trends of amphibian declines and extinctions worldwide. Science 2004, 306, 1783–1786. [Google Scholar] [CrossRef] [Green Version]
- King, J.D.; Muhlbauer, M.C.; James, A. Radiographic diagnosis of metabolic bone disease in captive bred mountain chicken frogs (Leptodactylus fallax). Zoo Biol. 2011, 30, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Huang, X.M.; Xia, R.; Xu, Y.C.; Dahmer, T.D. Use of femur bone density to segregate wild from farmed Dybowski’s frog (Rana dybowskii). Forensic Sci. Int. 2011, 207, 61–65. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, D.A. Nutrition Recommendations for Some Captive Amphibian Species (Anura and Caudata); Can. Assoc. Zoo Aquariums Nutr. Advis. Res. Group: Vanier Dr, ON, Canada, 2008. [Google Scholar]
- Kelly, W.R. Veterinary Clinical Diagnosis; Bailliere Tindall: London, UK, 1984. [Google Scholar]
- Kaneko, J.J.; Harvey, J.W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- Kerr, M.G. Veterinary Laboratory Medicine: Clinical Biochemistry and Haematology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Radostits, O.M.; Mayhew, I.G.; Houston, D.M. Veterinary Clinical Examination and Diagnosis; W.B. Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Divers, S.J.; Cooper, J.E. Reptile hepatic lipidosis. Semin. Avian Exot. Pet Med. 2000, 9, 153–164. [Google Scholar] [CrossRef]
- Hellbach, K.; Yaroshenko, A.; Willer, K.; Pritzke, T.; Baumann, A.; Hesse, N.; Auweter, S.; Reiser, M.F.; Eickelberg, O.; Pfeiffer, F. Facilitated diagnosis of pneumothoraces in newborn mice using x-ray dark-field radiography. Investig. Radiol. 2016, 51, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zotti, A.; Selleri, P.; Carnier, P.; Morgante, M.; Bernardini, D. Relationship between metabolic bone disease and bone mineral density measured by dual-energy X-ray absorptiometry in the green iguana (Iguana iguana). Vet. Radiol. Ultrasound 2004, 45, 10–16. [Google Scholar] [CrossRef]
- Park, J.K.; Do, Y. Assessment of body condition in amphibians using radiography: Relationship between bone mineral density and food resource availability. Korean J. Ecol. Environ. 2019, 52, 358–365. [Google Scholar] [CrossRef]
- Park, J.K.; Do, Y. Evaluating the physical condition of Hyla japonica using radiographic techniques. Sci. Total Environ. 2020, 726, 138596. [Google Scholar] [CrossRef]
- Nauwelaerts, S.; Ramsay, J.; Aerts, P. Morphological correlates of aquatic and terrestrial locomotion in a semi-aquatic frog, Rana esculenta: No evidence for a design conflict. J. Anat. 2007, 210, 304–317. [Google Scholar] [CrossRef]
- Vega-Trejo, R.; Zúniga-Vega, J.J.; Langerhans, R.B. Morphological differentiation among populations of Rhinella marina (Amphibia: Anura) in western Mexico. Evol. Ecol. 2014, 28, 69–88. [Google Scholar] [CrossRef]
- Buttimer, S.M.; Stepanova, N.; Womack, M.C. Evolution of the unique anuran pelvic and hind limb skeleton in relation to microhabitat, locomotor mode, and jump performance. Integr. Comp. Biol. 2020, 60, 1330–1345. [Google Scholar] [CrossRef]
- Paluh, D.J.; Stanley, E.L.; Blackburn, D.C. Evolution of hyperossification expands skull diversity in frogs. Proc. Natl. Acad. Sci. USA 2020, 117, 8554–8562. [Google Scholar] [CrossRef] [Green Version]
- Medler, S. Anesthetic MS-222 eliminates nerve and muscle activity in frogs used for physiology teaching laboratories. Adv. Physiol. Educ. 2019, 43, 69–75. [Google Scholar] [CrossRef]
- Lillywhite, H.B.; Shine, R.; Jacobson, E.; Denardo, D.F.; Gordon, M.S.; Navas, C.A.; Wang, T.; Seymour, R.S.; Storey, K.B.; Heatwole, H. Anesthesia and euthanasia of amphibians and reptiles used in scientific research: Should hypothermia and freezing be prohibited? Bioscience 2017, 67, 53–61. [Google Scholar] [CrossRef]
- Salazar, J.H. Overview of urea and creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef] [Green Version]
- Casey, A.; Greenhaff, P.L. Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance? Am. J. Clin. Nutr. 2000, 72, 607S–617S. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Barreira, T.V.; Tseh, W. The effects of acute water ingestion on body composition analyses via Dual-Energy X-Ray Absorptiometry. Clin. Nutr. 2020, 39, 3836–3838. [Google Scholar] [CrossRef] [PubMed]
- Rohlf, F.J. tpsDig, Version 2.10. 2006. Available online: http://life.bio.sunysb.edu/morph/index.html (accessed on 17 February 2021).
- McPhee, M.E. Generations in captivity increases behavioral variance: Considerations for captive breeding and reintroduction programs. Biol. Conserv. 2004, 115, 71–77. [Google Scholar] [CrossRef]
- Emerson, S.B. Allometry and jumping in frogs: Helping the twain to meet. Evolution 1978, 32, 551–564. [Google Scholar] [CrossRef]
- Zug, G.R. Anuran locomotion: Structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia 1972, 613–624. [Google Scholar] [CrossRef]
- Emerson, S.B. Jumping and leaping. In Functional Vertebrate Morphology; Hildebrand, M., Bramble, D.M., Liem, K.F., Wake, D.B., Eds.; Harvard University Press: Cambridge, MA, USA, 2013; pp. 198–211. [Google Scholar]
- Relyea, R.A. The lasting effects of adaptive plasticity: Predator-induced tadpoles become long-legged frogs. Ecology 2001, 82, 1947–1955. [Google Scholar] [CrossRef]
- Wells, K.D. Anuran Vocal Communication. The Ecology and Behavior of Amphibians; Wells, K.D., Ed.; University of Chicago Press: Chicago, IL, USA, 2007; pp. 268–337. [Google Scholar]
- Wells, K.D.; Schwartz, J.J. The behavioral ecology of anuran communication. In Hearing and Sound Communication in Amphibians; Narins, M., Feng, A.S., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2007; pp. 44–86. [Google Scholar]
- Moreno-Rueda, G.; Requena-Blanco, A.; Zamora-Camacho, F.J.; Comas, M.; Pascual, G. Morphological determinants of jumping performance in the Iberian green frog. Curr. Zool. 2020, 66, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, G.R.; Brodie, E.D., Jr.; Neuman-Lee, L.A.; Mohammadi, S.; Brusch IV, G.A.; Hopkins, Z.M.; French, S.S. Physiological responses to salinity vary with proximity to the ocean in a coastal amphibian. Physiol. Biochem. Zool. 2016, 89, 322–330. [Google Scholar] [CrossRef] [Green Version]
- Narayan, E.J.; Cockrem, J.F.; Hero, J.-M. Sight of a predator induces a corticosterone stress response and generates fear in an amphibian. PLoS ONE 2013, 8, e73564. [Google Scholar] [CrossRef] [Green Version]
- Troïanowski, M.; Mondy, N.; Dumet, A.; Arcanjo, C.; Lengagne, T. Effects of traffic noise on tree frog stress levels, immunity, and color signaling. Conserv. Biol. 2017, 31, 1132–1140. [Google Scholar] [CrossRef]
- Ragy, M.M. Effect of exposure and withdrawal of 900-MHz-electromagnetic waves on brain, kidney and liver oxidative stress and some biochemical parameters in male rats. Electromagn. Biol. Med. 2015, 34, 279–284. [Google Scholar] [CrossRef]
- Shukla, P.K.; Meena, A.S.; Dalal, K.; Canelas, C.; Samak, G.; Pierre, J.F.; Rao, R. Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef]
- De Sanctis, V.M.; Soliman, A.; Yassin, M.; Garofalo, P. Cortisol levels in central adrenal insufficiency: Light and shade. Pediatr. Endocr. Rev. 2015, 12, 213–219. [Google Scholar]
- Zaytsoff, S.J.; Brown, C.L.; Montina, T.; Metz, G.A.; Abbott, D.W.; Uwiera, R.R.; Inglis, G.D. Corticosterone-mediated physiological stress modulates hepatic lipid metabolism, metabolite profiles, and systemic responses in chickens. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Ma, Y.; Zhang, Y.H. Oxidative stress and hepatotoxicity in the frog, Rana chensinensis, when exposed to low doses of trichlorfon. J. Environ. Sci. Health B 2017, 52, 476–482. [Google Scholar] [CrossRef]
- Van Meter, R.J.; Glinski, D.A.; Purucker, S.T.; Henderson, W.M. Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci. Total Environ. 2018, 624, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Cathers, T.; Lewbart, G.A.; Correa, M.; Stevens, J.B. Serum chemistry and hematology values for anesthetized American bullfrogs (Rana catesbeiana). J. Zoo Wildl. Med. 1997, 28, 171–174. [Google Scholar] [PubMed]
- Wilson, S.; Felt, S.; Torreilles, S.; Howard, A.; Behan, C.; Moorhead, R.; Green, S. Serum clinical biochemical and hematologic reference ranges of laboratory-reared and wild-caught Xenopus laevis. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 635–640. [Google Scholar]
- Ryan, M.J.; Bartholomew, G.A.; Rand, A.S. Energetics of Reproduction in a Neotropical Frog, Physalaemus Pustulosus. Ecology 1983, 64, 1456–1462. [Google Scholar] [CrossRef] [Green Version]
- Ryser, J. Weight loss, reproductive output, and the cost of reproduction in the common frog, Rana temporaria. Oecologia 1989, 78, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Bruscalupi, G.; Castellano, F.; Scapin, S.; Trentalance, A. Cholesterol metabolism in frog (Rana esculenta) liver: Seasonal and sex-related variations. Lipids 1989, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Iela, L.; Esposito, V.; Rastogi, R.K.; Chieffi, G. Annual variations in the total lipid and protein content of the liver, fat body, ovary and plasma of the female frog (Rana esculenta L.). J. Endocrinol. 1978, 78, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Sinha, R.C. Seasonal changes in energy reserves in the common frog, Rana tigrina. Jpn. J. Physiol. 1989, 39, 969–973. [Google Scholar] [CrossRef] [Green Version]
- Park, J.K.; Kim, J.B.; Do, Y. Reference intervals in combined veterinary clinical examinations of male black-spotted pond frogs (Pelophylax nigromaculatus). Animals 2021, 11, 1407. [Google Scholar] [CrossRef] [PubMed]
- Farver, T.B. Concepts of Normality in Clinical Biochemistry. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 1–19. [Google Scholar]

| Contents | Farm | Wild | ||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| Glucose (mg/dL) | 21.75 ± 8.98 | 19.13 ± 9.60 | 21.74 ± 24.69 | 24.71 ± 17.70 |
| AST (U/L) | 283.27 ± 230.15 | 305.23 ± 254.23 | 533.90 ± 346.96 | 389.44 ± 268.94 |
| ALT (U/L) | 51.25 ± 66.58 | 56.14 ± 58.82 | 120.73 ± 81.79 | 59.39 ± 54.42 |
| BUN (mg/dL) | 13.83 ± 17.01 | 17.00 ± 18.47 | 12.04 ± 10.69 | 10.32 ± 11.62 |
| Creatinine (mg/dL) | 0.15 ± 0.08 | 0.14 ± 0.07 | 0.14 ± 0.05 | 0.10 ± 0.05 |
| TP (g/dL) | 3.32 ± 0.84 | 2.50 ± 0.63 | 3.80 ± 0.80 | 2.04 ± 0.37 |
| Albumin (g/dL) | 0.94 ± 0.26 | 0.69 ± 0.21 | 1.00 ± 0.26 | 0.56 ± 0.11 |
| Calcium (mg/dL) | 10.05 ± 4.39 | 6.08 ± 1.37 | 14.17 ± 6.66 | 6.56 ± 1.46 |
| Phosphorus (mg/dL) | 6.76 ± 2.87 | 6.55 ± 3.10 | 11.05 ± 4.58 | 9.32 ± 3.29 |
| Contents | Main Factor | df | Mean Square | F Value | p-Value | Summary |
|---|---|---|---|---|---|---|
| Glucose (mg/dL) | Group | 1 | 137.594 | 0.497 | 0.483 | Farm = Wild |
| Sex | 1 | 0.526 | 0.002 | 0.965 | Female = Male | |
| Group*Sex | 1 | 138.078 | 0.499 | 0.482 | NS | |
| AST (U/L) | Group | 1 | 497,215.951 | 6.379 | 0.014 | Farm < Wild |
| Sex | 1 | 66,557.517 | 0.854 | 0.359 | Female = Male | |
| Group*Sex | 1 | 122,821.288 | 1.576 | 0.214 | NS | |
| ALT (U/L) | Group | 1 | 23,455.741 | 5.348 | 0.024 | Farm < Wild |
| Sex | 1 | 14,130.106 | 3.222 | 0.077 | Female > Male | |
| Group*Sex | 1 | 19,457.491 | 4.437 | 0.039 | WF > FF, FM, WM | |
| BUN (mg/dL) | Group | 1 | 317.233 | 1.450 | 0.233 | Farm = Wild |
| Sex | 1 | 9.311 | 0.043 | 0.837 | Female = Male | |
| Group*Sex | 1 | 105.807 | 0.484 | 0.489 | NS | |
| Creatinine (mg/dL) | Group | 1 | 0.012 | 2.680 | 0.106 | Farm = Wild |
| Sex | 1 | 0.011 | 2.452 | 0.122 | Female = Male | |
| Group*Sex | 1 | 0.004 | 0.920 | 0.341 | NS | |
| TP (g/dL) | Group | 1 | 0.002 | 0.004 | 0.951 | Farm = Wild |
| Sex | 1 | 29.546 | 63.117 | <0.001 | Female > Male | |
| Group*Sex | 1 | 3.854 | 8.233 | 0.005 | FF, WF > FM, WM | |
| Albumin (g/dL) | Group | 1 | 0.023 | 0.490 | 0.486 | Farm = Wild |
| Sex | 1 | 2.103 | 44.859 | <0.001 | Female > Male | |
| Group*Sex | 1 | 0.166 | 3.544 | 0.064 | NS | |
| Calcium (mg/dL) | Group | 1 | 93.803 | 5.554 | 0.021 | Farm < Wild |
| Sex | 1 | 594.782 | 35.218 | <0.001 | Female > Male | |
| Group*Sex | 1 | 59.233 | 3.507 | 0.065 | NS | |
| Phosphorus (mg/dL) | Group | 1 | 221.103 | 17.714 | <0.001 | Farm < Wild |
| Sex | 1 | 16.790 | 1.345 | 0.250 | Female = Male | |
| Group*Sex | 1 | 10.199 | 0.817 | 0.369 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-K.; Kim, J.B.; Do, Y. Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus). Life 2021, 11, 1089. https://doi.org/10.3390/life11101089
Park J-K, Kim JB, Do Y. Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus). Life. 2021; 11(10):1089. https://doi.org/10.3390/life11101089
Chicago/Turabian StylePark, Jun-Kyu, Jeong Bae Kim, and Yuno Do. 2021. "Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus)" Life 11, no. 10: 1089. https://doi.org/10.3390/life11101089
APA StylePark, J.-K., Kim, J. B., & Do, Y. (2021). Examination of Physiological and Morphological Differences between Farm-Bred and Wild Black-Spotted Pond Frogs (Pelophylax nigromaculatus). Life, 11(10), 1089. https://doi.org/10.3390/life11101089






