Perspective on Stem Cell Therapy in Organ Fibrosis: Animal Models and Human Studies
Abstract
1. Introduction
2. Cellular and Molecular Basis of Tissue Fibrosis
3. Stem Cell Types for Fibrotic Disorders Therapy
3.1. Pulmonary Fibrosis
3.2. Liver Fibrosis
3.3. Cardiac Fibrosis
3.4. Renal Fibrosis
3.5. Uterine Fibrosis (Asherman Syndrome)
3.6. Skin Fibrosis
| Organ | Disease | Stem Cell Type | Number of Cells | Delivery Route | Effects | References |
|---|---|---|---|---|---|---|
| Lung | Idiopathic pulmonary fibrosis | Allogeneic BM-MSCs | 2 × 108 cells/infusion (4 infusions in 3 month intervals, total 1.6 × 109) | Intravenous | Improved lung function based on FVC, DLCO, and 6MWT | [82] |
| Idiopathic pulmonary fibrosis | Allogeneic BM-MSCs | 2 × 107 or 1 × 108 | Intravenous | Higher cell dose alleviated fibrosis progression | [83] | |
| Idiopathic pulmonary fibrosis | Allogeneic BM-MSCs | 2 × 107, 1 × 108, or 2 × 108 | Intravenous | Improved lung function assessed by 6MWT at 36 weeks | [84] | |
| Liver | Alcoholic cirrhosis | Autologous BM-MSCs | 5 × 107/injection (2 injections at study weeks 4 and 8) | Intra-arterial (right hepatic) | Histological improvement of liver biopsy based on Laennec fibrosis scoring system; decreased collagen deposition, mRNA expression of TGFβ1, Col1, and αSMA in liver biopsy, and MELD score; improved Child–Pugh score | [107] |
| Hepatitis C-induced liver cirrhosis | Autologous BM-MSCs | 1 × 107 | Intrasplenic injection | Improved liver function assessed by decreased TBIL, AST, ALT, PT, and INR levels and increased albumin and PC levels | [105] | |
| Hepatitis C-induced liver cirrhosis | Autologous BM-MSCs | 1 × 106/kg | Intravenous | Decreased jaundice, lower limb edema, MELD score, and serum creatinine level; improved encephalopathic manifestation, ascites, serum bilirubin, and albumin levels | [106] | |
| End-stage liver failure due to chronic hepatitis C | Autologous BM-MSCs | 2 × 108 | Intrasplenic or intrahepatic injection | Improved liver function based on MELD and Child scores, fatigue impact scale, and performance status | [104] | |
| Liver failure caused by hepatitis B | Autologous BM-MSCs | N/A | Intra-arterial (hepatic) | Improved liver function assessed by ALB, TBIL, and PT levels and MELD score | [110] | |
| Liver cirrhosis | Autologous BM-MSCs | 3–5 × 107 | Intravenous (peripheral or portal vein) | Improved liver function assessed by MELD score and decreased prothrombin complex, serum creatinine, and bilirubin at 24 weeks | [108] | |
| Liver cirrhosis | Autologous ASCs | 3.3 × 105 or 6.6 × 105 cells/kg | Intra-arterial (hepatic) | Increased concentrations of serum HGF, IL-6, IL-18, M-CSF, and MIF at 1 day post treatment | [111] | |
| Decompensated liver cirrhosis | Allogeneic UC-MSC | 5 × 105 cells/k | Intravenous | Decreased hypogastric ascites volume and serum levels of plasma laminin, procollagen III, COLIV, and HA | [112] | |
| Heart | Ischemic cardiomyopathy | Allogeneic BM-MSCs | 2 × 107, 1 × 108 | Transendocardial injection | Reduced scar size and improved NYHA classification for both groups; increased ejection fraction (1 × 108 group) and proBNP (2 × 107 group) | [162] |
| Ischemic cardiomyopathy | Autologous or allogeneic BM-MSCs | 2 × 107, 1 × 108, or 2 × 108 | Transendocardial injection in 10 left ventricular sites | Reduced scar size accompanied by decreased end-diastolic and end-systolic volume, increased ejection fraction; improved sphericity index; functional improvement in autologous group assessed by 6MWT | [127] | |
| Nonischemic dilated cardiomyopathy | Autologous or allogeneic BM-MSCs | 1 × 108 | Transendocardial injection in 10 left ventricular sites | Increased ejection fraction (EF) and 6MWT in allogeneic BM-MCSc group compared to autologous group; allogeneic BM-MCSc group showed improved endothelial function, suppression of TNFα, functional capacity, and quality of life compared to allogeneic group; serious adverse events occurred in 28.2% of allogeneic group and 63.5% of autologous group | [126] | |
| Ischemic heart disease and ischemic heart failure (IHF) | Allogeneic ASCs | 1.1 × 108 | Intramyocardial injection | Decrease left ventricular end-systolic volume, increased LVEF and exercise capacity | [167] | |
| Heart failure (HF) with reduced ejection fraction (HFrEF) | Allogeneic UC-MSCs | 1 × 106/kg | Intravenous | Increased LVEF, improved NYHA classification and MLHFQ | [165] | |
| Acute myocardial infarction (AMI) | Wharton’s jelly-derived MSCs | 6 × 106 | Intracoronary infusion into the infarct artery | Improved cardiac function reflected by absolute increase in myocardial viability, perfusion within infarcted territory (SPECT); absolute increase in LVEF; absolute decreases in LV end-systolic and end-diastolic volume | [166] | |
| Kidney | Renal fibrosis on peritoneal dialysis (PD) | Autologous ASCs | 1.2 ± 0.1 × 106/kg | Intravenous (cubital vein) | Decline in rate of solute transport across peritoneum determined by PET and D/Pcr | [66] |
| Atherosclerotic renovascular disease (RVD) | Autologous ASCs | 1 × 105, 2.5 × 105/kg | Intra-arterial infusion | Increased renal tissue oxygenation and cortical blood flow | [68] | |
| Uterus | Asherman’s syndrome and endometrial atrophy | Autologous BM-MSCs | Mean 6.53 × 107 (range: 1.9 × 107 to 2 × 108) | Transmyometrial implant in subendometrial zone | Improved uterine cavity, increased endometrial thickness; improved menstrual duration and intensity and pregnancy rates | [225] |
| Asherman’s syndrome and endometrial atrophy | Autologous CD133+ BM-SCs | Mean 1.23 × 108 (range: 4.2 × 107 to 2 × 108) | Intra-arterial catherization | Improved uterine cavity and increased endometrial thickness; increased mature vessel density, duration and intensity of menses, and pregnancy rates | [229] | |
| Asherman’s syndrome | Autologous SVFs | 4.6 ± 0.7 × 106 | Intrauterine | Increased endometrial thickness and menstrual bleeding | [230] | |
| Recurrent intrauterine adhesions (IUAs) | Allogeneic UC-MSCs in collagen scaffold | 1 × 107 | Infusion in uterine wall via catheter placed in uterine cavity | Increased endometrial thickness and expression levels of ERα, Ki67, and vWF; resumed menses and increased menstrual bleeding; improved uterine cavity and pregnancy rates | [231] | |
| Asherman’s syndrome | Autologous menstrual blood-derived stromal cells (menSCs) | 1.0 × 106 | Intrauterine | Increased endometrial thickness and pregnancy rates | [232] | |
| Skin | Systemic sclerosis | Autologous CD34+ HSCs | Mean dose 5.63 × 106 /kg | Intravenous | Improved skin thickness assessed by mRSS | [251] |
| Systemic sclerosis | Autologous CD34+ HSCs | Median dose 5.6 × 106/kg | N/A (infusion) | Improved skin thickness based on mRSS | [254] | |
| Systemic sclerosis | Autologous CD34+ HSCs | ≥2 × 106/kg | N/A (infusion) | Improved skin thickness based on mRSS | [253] | |
| Systemic sclerosis | Autologous HSCs | N/A | N/A (infusion) | Improved skin thickness based on mRSS | [252] | |
| Systemic sclerosis | Autologous SVFs | Mean 3.76 ± 1.85 × 106 | Injection into subcutaneous tissue in contact with neurovascular pedicles | Reduced pain, finger circumference, and Raynaud’s severity; increased grip strength; decreased dystrophic capillaries and vascular suppression score | [256] | |
| Systemic sclerosis | Autologous ASCs | 4 × 106 to 8 × 106 | Injection into patient-specific area (face, arm, foot, limb) | Regression of dyschromia and reduced erythema; improved skin softening and sensitivity | [255] | |
| Systemic sclerosis | Autologous fat-enriched multipotent stem cells | N/A | Injection into oro-facial tissues | Improved mouth function and facial volumetric appearance | [257] | |
| Systemic sclerosis | Autologous fat-enriched multipotent stem cells | N/A | Local injection into oro-facial tissues | Increased interincisal distance and oral perimeter; improved neovascularization | [258] |
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6MWT | 6-min walk test |
| αSMA | α-smooth muscle actin |
| ACTA2 | α-smooth muscle actin |
| ADPKD | autosomal-dominant polycystic kidney disease |
| AECs | alveolar epithelial cells |
| AFSCs | amniotic fluid-derived stem cells |
| AKI | acute kidney injury |
| Akt | protein kinase B |
| ALB | serum albumin |
| ALT | alanine aminotransferase |
| AS | Asherman syndrome |
| AST | aspartate aminotransferase |
| ASCs | adipose-derived stem cells |
| ASCs-CM | conditioned medium from ASCs |
| AQP | aquaporin |
| BLM | bleomycin |
| bFGF | basic fibroblast growth factor |
| BMI | body mass index |
| BM-MSCs | bone marrow mesenchymal stem cells |
| BMPCs | bone marrow progenitor cells |
| CCl4 | carbon tetrachloride |
| COL | collagen |
| CTGF | connective tissue growth factor |
| dcSS | diffuse cutaneous systemic sclerosis |
| DDIT3 | DNA-damage inducible transcript 3 |
| Dkk-1 | Dickkopf-1 |
| DLCO | carbon monoxide diffusing capacity |
| D/P cr | dialysate-to-creatinine ratio |
| EBs | embryoid bodies |
| ECM | extracellular matrix |
| EF | ejection fraction |
| eGFR | estimated glomerular filtration rate |
| Eln | elastin |
| eMSC | endometrial MSCs |
| EMT | epithelial-to-mesenchymal transition process |
| ERα | estrogen receptor alpha |
| ERK | extracellular-signal-regulated kinase |
| ESCs | embryonic stem cells |
| Ev | extracellular vesicles |
| FN | fibronectin |
| FVC | forced vital capacity |
| G-CSF | granulocyte colony-stimulating factor |
| GDNF | glial cell line-derived neurotrophic factor |
| GFP | green fluorescent protein |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| GPSCs | germline cell-derived pluripotent stem cells |
| GTCs | tubular-like cells |
| GVHD | graft-versus-host disease |
| HA | hyaluronic acid |
| hAMSCs | human amniotic mesenchymal stromal cells |
| HBO | hyperbaric oxygen |
| HBV | hepatitis B |
| HCV | hepatitis C |
| HGF | hepatocyte growth factor |
| HLA | histocompatibility complex |
| HOCl | hypochlorous acid |
| HSCs | hematopoietic stem cells |
| IGF-1 | insulin growth factor 1 |
| IL4Rα | IL-4 receptor alpha |
| IL | interleukin |
| ILCs | insulin-producing islet-like clusters |
| INR | international normalized ratio |
| IPF | idiopathic pulmonary fibrosis |
| iPSCs | induced pluripotent stem cells |
| IUAs | intrauterine adhesions |
| JNK | JUN N-terminal kinase |
| lcSS | limited cutaneous systemic sclerosis |
| LVEF | left ventricular ejection fraction |
| MCP-1 | monocyte chemotactic protein-1 |
| MELD | model for end-stage liver disease |
| MI | myocardial infarction |
| MIF | macrophage migration inhibitory factor |
| MLHFQ | Minnesota living with heart failure questionnaire |
| MMPs | matrix metalloproteinases |
| mRSS | modified Rodnan’s skin score |
| MSCs | mesenchymal stem cells |
| MT1-MMP | membrane-bound matrix metalloproteinases |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NYHA | New York Heart Association class |
| PAI-1 | plasminogen activator inhibitor 1 |
| PCNA | proliferating cell nuclear antigen |
| PDE | phosphodiesterase |
| PET | peritoneal equilibration test |
| PF | pulmonary fibrosis |
| proBNP | pro-brain natriuretic peptide |
| PT | prothrombin time |
| PVD | portal vein diameter |
| ROCK | RHO-associated kinase |
| RPCs | renal progenitor cells |
| RVD | renovascular disease |
| SDF-1α | stromal cell-derived factor 1 alpha |
| SHH | sonic hedgehog |
| SPC | surfactant protein C |
| SPECT | single-photon emission computed tomography |
| SS | systemic sclerosis |
| SVF | stromal vascular fraction |
| TBIL | total bilirubin |
| TECs | tubular epithelial cells |
| TGF- β | transforming growth factor β |
| TGFβRI | TGFβ receptor type 1 |
| Thbs1 | thrombospondin 1 |
| TIMPs | tissue inhibitors of matrix metalloproteinases |
| TNF | tumor necrosis factor |
| TTA | thioacetamide |
| UC-MSC | umbilical cord MSCs |
| vWF | von Willebrand factor |
References
- Jun, J.I.; Lau, L.F. Resolution of organ fibrosis. J. Clin. Investig. 2018, 128, 97–107. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. Cell Physiol. 2013, 304, C216–C225. [Google Scholar] [CrossRef]
- Wynn, T.A. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004, 4, 583–594. [Google Scholar] [CrossRef]
- Muzes, G.; Sipos, F. Issues and opportunities of stem cell therapy in autoimmune diseases. World J. Stem Cells 2019, 11, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Cucullo, L. Regenerative stem cell therapy for neurodegenerative diseases: An overview. Int. J. Mol. Sci. 2021, 22, 2153. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.; McKee, C.; Bakshi, S.; Walker, K.; Hakman, E.; Halassy, S.; Svinarich, D.; Dodds, R.; Govind, C.K.; Chaudhry, G.R. Mesenchymal stem cells: Cell therapy and regeneration potential. J. Tissue Eng. Regen. Med. 2019, 13, 1738–1755. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal Stem Cells for Regenerative Medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-S.; Jeong, E.-J.; Kim, J.-Y.; Park, S.J.; Ju, W.S.; Kim, C.-H.; Kim, J.-S.; Choo, Y.-K. Application of Mesenchymal Stem Cells in Inflammatory and Fibrotic Diseases. Int. J. Mol. Sci. 2020, 21, 8366. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky, S., II; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar]
- Romanov, Y.A.; Svintsitskaya, V.A.; Smirnov, V.N. Searching for alternative sources of postnatal human mesenchymal stem cells: Candidate MSC-like cells from umbilical cord. Stem Cells 2003, 21, 105–110. [Google Scholar] [CrossRef]
- Schwab, K.E.; Gargett, C.E. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum. Reprod. 2007, 22, 2903–2911. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Dominici, M.; le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Nie, C.; Yang, D.; Xu, J.; Si, Z.; Jin, X.; Zhang, J. Locally Administered Adipose-Derived Stem Cells Accelerate Wound Healing through Differentiation and Vasculogenesis. Cell Transplant. 2011, 20, 205–216. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, J.-W.; Jun, H.-S.; Roh, J.Y.; Lee, H.-Y.; Hong, I.-S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, H.S.; Kang, J.M.; Bae, S.H.; Kim, C.; Lee, S.H.; Schwarz, J.; Kim, G.J.; Kim, J.S.; Cha, D.H.; et al. Dual Effects of Human Placenta-Derived Neural Cells on Neuroprotection and the Inhibition of Neuroinflammation in a Rodent Model of Parkinson’s Disease. Cell Transpl. 2018, 27, 814–830. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; Yan, K.; Chen, L.; Chen, X.R.; Li, P.; Chen, F.F.; Jiang, X.D. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J. Neuroinflammation 2013, 10, 106. [Google Scholar] [CrossRef]
- El Agha, E.; Kramann, R.; Schneider, R.K.; Li, X.; Seeger, W. BD Humphreys and S Bellusci. Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell 2017, 21, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.; Thannickal, V.J.; Prunotto, M.; Desmoulière, A.; Varga, J.; De Wever, O.; Mareel, M.M.; Gabbiani, G. Recent Developments in Myofibroblast Biology: Paradigms for Connective Tissue Remodeling. Am. J. Pathol. 2012, 180, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Kugler, M.C.; Wolters, P.J.; Robillard, L.; Galvez, M.G.; Brumwell, A.N.; Sheppard, D.; Chapman, H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl. Acad. Sci. USA 2006, 103, 13180–13185. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in Murine Cutaneous Fibrosis Originate From Adiponectin-Positive Intradermal Progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, S.; Graf, J.; Roderfeld, M.; Roeb, E. Expression of MMPs and TIMPs in liver fibrosis—A systematic review with special emphasis on anti-fibrotic strategies. J. Hepatol. 2007, 46, 955–975. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Benyon, R.C.; Iredale, J.P.; Goddard, S.; Winwood, P.J.; Arthur, M.J. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996, 110, 821–831. [Google Scholar] [CrossRef]
- Holmbeck, K.; Bianco, P.; Caterina, J.; Yamada, S.; Kromer, M.; Kuznetsov, S.A.; Mankani, M.; Robey, P.; Poole, A.; Pidoux, I.; et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 1999, 99, 81–92. [Google Scholar] [CrossRef]
- Grupp, C.; Troche, I.; Klass, C.; Köhler, M.; Müller, G.A. A novel model to study renal myofibroblast formation in vitro. Kidney Int. 2001, 59, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Foreman, D.; Ferguson, M. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J. Cell Sci. 1995, 108, 985–1002. [Google Scholar] [CrossRef]
- Sonnylal, S.; Denton, C.P.; Zheng, B.; Keene, D.R.; He, R.; Adams, H.P.; Vanpelt, C.S.; Geng, Y.J.; Deng, J.M.; Behringer, R.R.; et al. Postnatal induction of transforming growth factor beta signaling in fibroblasts of mice recapitulates clinical, histologic, and biochemical features of scleroderma. Arthritis Rheum. 2007, 56, 334–344. [Google Scholar] [CrossRef]
- Wojcik-Pszczola, K.; Chlon-Rzepa, G.; Jankowska, A.; Slusarczyk, M.; Ferdek, P.E.; Kusiak, A.A.; Swierczek, A.; Pociecha, K.; Koczurkiewicz-Adamczyk, P.; Wyska, E.; et al. A Novel, Pan-PDE Inhibitor Exerts Anti-Fibrotic Effects in Human Lung Fibroblasts via Inhibition of TGF-beta Signaling and Activation of cAMP/PKA Signaling. Int. J. Mol. Sci. 2020, 21, 4008. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming growth factor beta—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Palumbo, K.; Dees, C.; Bergmann, C.; Venalis, P.; Zerr, P.; Horn, A.; Kireva, T.; Beyer, C.; Zwerina, J.; et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat. Commun. 2012, 3, 735. [Google Scholar] [CrossRef]
- He, W.; Dai, C.; Li, Y.; Zeng, G.; Monga, S.P.; Liu, Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J. Am. Soc. Nephrol. 2009, 20, 765–776. [Google Scholar] [CrossRef]
- Horn, A.; Palumbo, K.; Cordazzo, C.; Dees, C.; Akhmetshina, A.; Tomcik, M.; Zerr, P.; Avouac, J.; Gusinde, J.; Zwerina, J.; et al. Hedgehog signaling controls fibroblast activation and tissue fibrosis in systemic sclerosis. Arthritis Rheum. 2012, 64, 2724–2733. [Google Scholar] [CrossRef]
- Beyer, C.; Huscher, D.; Ramming, A.; Bergmann, C.; Avouac, J.; Guiducci, S.; Meier, F.; Vettori, S.; Siegert, E.; Jaeger, V.K.; et al. Elevated serum levels of sonic hedgehog are associated with fibrotic and vascular manifestations in systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 626–628. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.X.; Chen, Y.; Li, X.F.; Li, H.D.; Huang, H.M.; Bu, F.T.; Pan, X.Y.; Yang, Y.; Huang, C.; et al. Suppression of SUN2 by DNA methylation is associated with HSCs activation and hepatic fibrosis. Cell Death Dis. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Barbu, V.; Wendum, D.; Kinnman, N.; Rey, C.; Poupon, R.; Housset, C.; Rosmorduc, O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology 2002, 35, 1010–1021. [Google Scholar] [CrossRef]
- Tang, P.M.K.; Nikolic-Paterson, D.J.; Lan, H.-Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Blanpain, C.; Fuchs, E. Plasticity of epithelial stem cells in tissue regeneration. Science 2014, 344, 6189. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Fuentes, D.E.; Fernandez-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldana, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 1–24. [Google Scholar] [CrossRef]
- Rippon, H.J.; Bishop, A.E. Embryonic stem cells. Cell Prolif. 2004, 37, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.N.; Edgar, A.J.; Samadikuchaksaraei, A.; Timson, C.M.; Romanska, H.M.; Polak, J.M.; Bishop, A.E. Derivation of Type II Alveolar Epithelial Cells from Murine Embryonic Stem Cells. Tissue Eng. 2002, 8, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Buttery, L.D.K.; Bourne, S.; Xynos, J.D.; Wood, H.; Hughes, F.J.; Hughes, S.P.F.; Episkopou, V.; Polak, J.M. Differentiation of Osteoblasts andin VitroBone Formation from Murine Embryonic Stem Cells. Tissue Eng. 2001, 7, 89–99. [Google Scholar] [CrossRef]
- Kramer, J.; Hegert, C.; Guan, K.; Wobus, A.M.; Müller, P.K.; Rohwedel, J. Embryonic stem cell-derived chondrogenic differentiation in vitro: Activation by BMP-2 and BMP-4. Mech. Dev. 2000, 92, 193–205. [Google Scholar] [CrossRef]
- Stavridis, M.P.; Smith, A.G. Neural differentiation of mouse embryonic stem cells. Biochem. Soc. Trans. 2003, 31, 45–49. [Google Scholar] [CrossRef]
- Yurugi-Kobayashi, T.; Itoh, H.; Yamashita, J.; Yamahara, K.; Hirai, H.; Kobayashi, T.; Ogawa, M.; Nishikawa, S.; Nishikawa, S.-I.; Nakao, K. Effective contribution of transplanted vascular progenitor cells derived from embryonic stem cells to adult neovascularization in proper differentiation stage. Blood 2003, 101, 2675–2678. [Google Scholar] [CrossRef]
- Lerou, P. Embryonic Stem Cell Derivation from Human Embryos. Methods Mol. Biol. 2011, 767, 31–35. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Bellin, M.; Marchetto, M.C.; Gage, F.H.; Mummery, C.L. Induced pluripotent stem cells: The new patient? Nat. Rev. Mol. Cell Biol. 2012, 13, 713–726. [Google Scholar] [CrossRef]
- Ahmed, R.P.; Haider, H.K.; Buccini, S.; Li, L.; Jiang, S.; Ashraf, M. Reprogramming of skeletal myoblasts for induction of pluripotency for tumor-free cardiomyogenesis in the infarcted heart. Circ. Res. 2011, 109, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, K.; He, J.; Taranova, O.; Liang, G.; D’Alessio, A.C.; Zhang, Y. Generation of Insulin-secreting Islet-like Clusters from Human Skin Fibroblasts. J. Biol. Chem. 2008, 283, 31601–31607. [Google Scholar] [CrossRef]
- Wernig, M.; Zhao, J.P.; Pruszak, J.; Hedlund, E.; Fu, D.; Soldner, F.; Broccoli, V.; Constantine-Paton, M.; Isacson, O.; Jaenisch, R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2008, 105, 5856–5861. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Liu, C.; Wu, J.C. Translation of Human-Induced Pluripotent Stem Cells: From Clinical Trial in a Dish to Precision Medicine. J. Am. Coll. Cardiol. 2016, 67, 2161–2176. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell. Tissue Kinet. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Gopal, S.; Masood, H.; Vivek, P.; Deb, K. Regenerative potential of dental pulp mesenchymal stem cells harvested from high caries patient’s teeth. J. Stem. Cells 2013, 8, 25–41. [Google Scholar]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; de Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemother. 2008, 35, 228–238. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, N.; Marsano, A.; Vunjak-Novakovic, G.; Zhang, Y.; Lopez, M.J. In vitro mesenchymal trilineage differentiation and extracellular matrix production by adipose and bone marrow derived adult equine multipotent stromal cells on a collagen scaffold. Stem Cell Rev. Rep. 2013, 9, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Bukowska, J.; Szostek-Mioduchowska, A.Z.; Kopcewicz, M.; Walendzik, K.; Machcinska, S.; Gawronska-Kozak, B. Adipose-Derived Stromal/Stem Cells from Large Animal Models: From Basic to Applied Science. Stem Cell Rev. Rep. 2021, 17, 719–738. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Shekarchian, S.; Najafi, I.; Moghadasali, R.; Ahmadbeigi, N.; Pourmand, M.R.; Bolurieh, T.; Jaroughi, N.; Pourmand, G.; Aghdami, N. Systemic Infusion of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells in Peritoneal Dialysis Patients: Feasibility and Safety. Cell J. 2019, 20, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Cil, N.; Yaka, M.; Unal, M.S.; Dodurga, Y.; Tan, S.; Secme, M.; Karagur, E.R.; Mete, G.A. Adipose derived mesenchymal stem cell treatment in experimental asherman syndrome induced rats. Mol. Biol. Rep. 2020, 47, 4541–4552. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Dietz, A.B.; Herrmann, S.M.S.; Hickson, L.J.; Glockner, J.F.; McKusick, M.A.; Misra, S.; Bjarnason, H.; Armstrong, A.S.; Gastineau, D.A.; et al. Autologous Mesenchymal Stem Cells Increase Cortical Perfusion in Renovascular Disease. J. Am. Soc. Nephrol. 2017, 28, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Ricardo, S.D.; Sievert, W. Cell-Based Therapies for Tissue Fibrosis. Front. Pharmacol. 2017, 8, 633. [Google Scholar] [CrossRef] [PubMed]
- Cameli, P.; Refini, R.M.; Bergantini, L.; d’Alessandro, M.; Alonzi, V.; Magnoni, C.; Rottoli, P.; Sestini, P.; Bargagli, E. Long-Term Follow-Up of Patients With Idiopathic Pulmonary Fibrosis Treated With Pirfenidone or Nintedanib: A Real-Life Comparison Study. Front. Mol. Biosci. 2020, 7, 581828. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Fell, C.D.; Huggins, J.T.; Nunes, H.; Sussman, R.; Valenzuela, C.; Petzinger, U.; Stauffer, J.L.; Gilberg, F.; Bengus, M.; et al. Safety of nintedanib added to pirfenidone treatment for idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 52, 1800230. [Google Scholar] [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 8407–8411. [Google Scholar] [CrossRef]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir. Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kotani, T.; Masutani, R.; Suzuka, T.; Oda, K.; Makino, S.; Ii, M. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci. Rep. 2017, 7, 14608. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Fonseca, L.; Gowda, S.; Chougule, B.; Hari, A.; Totey, S. Human Adipose-derived Mesenchymal Stem Cells Attenuate Early Stage of Bleomycin Induced Pulmonary Fibrosis: Comparison with Pirfenidone. Int. J. Stem. Cells 2016, 9, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Elliot, S.J.; Gerth, D.J.; Xia, X.; Pereira-Simon, S.; Choi, R.; Catanuto, P.; Shahzeidi, S.; Toonkel, R.L.; Shah, R.H.; et al. Therapeutic benefits of young, but not old, adipose-derived mesenchymal stem cells in a chronic mouse model of bleomycin-induced pulmonary fibrosis. Transl. Res. 2015, 166, 554–567. [Google Scholar] [CrossRef]
- Cargnoni, A.; Gibelli, L.; Tosini, A.; Signoroni, P.B.; Nassuato, C.; Arienti, D.; Lombardi, G.; Albertini, A.; Wengler, G.S.; Parolini, O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transpl. 2009, 18, 405–422. [Google Scholar] [CrossRef]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef]
- Zhou, Q.; Ye, X.; Sun, R.; Matsumoto, Y.; Moriyama, M.; Asano, Y.; Ajioka, Y.; Saijo, Y. Differentiation of mouse induced pluripotent stem cells into alveolar epithelial cells in vitro for use in vivo. Stem Cells Transl. Med. 2014, 3, 675–685. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Z.; Gao, Y.; Zheng, R.; Zhang, X.; Zhao, L.; Tan, M. Induced Pluripotent Stem Cells Inhibit Bleomycin-Induced Pulmonary Fibrosis in Mice through Suppressing TGF-beta1/Smad-Mediated Epithelial to Mesenchymal Transition. Front. Pharmacol. 2016, 7, 430. [Google Scholar] [CrossRef]
- Gazdhar, A.; Grad, I.; Tamo, L.; Gugger, M.; Feki, A.; Geiser, T. The secretome of induced pluripotent stem cells reduces lung fibrosis in part by hepatocyte growth factor. Stem Cell Res. Ther. 2014, 5, 123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Averyanov, A.; Koroleva, I.; Konoplyannikov, M.; Revkova, V.; Lesnyak, V.; Kalsin, V.; Danilevskaya, O.; Nikitin, A.; Sotnikova, A.; Kotova, S.; et al. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 2020, 9, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.E.; Kim, G.J.; Kyeong, N.Y.; Goldin, J.G.; Glassberg, M.K. Intravenous stem cell dose and changes in quantitative lung fibrosis and DLCO in the AETHER trial: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7568–7572. [Google Scholar] [PubMed]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Paspaliaris, V.; Koliakos, G.; Ntolios, P.; Bouros, E.; Oikonomou, A.; Zissimopoulos, A.; Boussios, N.; Dardzinski, B.; Gritzalis, D.; et al. A prospective, non-randomized, no placebo-controlled, phase Ib clinical trial to study the safety of the adipose derived stromal cells-stromal vascular fraction in idiopathic pulmonary fibrosis. J. Transl. Med. 2013, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Gines, P.; Graupera, I.; Lammert, F.; Angeli, P.; Caballeria, L.; Krag, A.; Guha, I.N.; Murad, S.D.; Castera, L. Screening for liver fibrosis in the general population: A call for action. Lancet Gastroenterol. Hepatol. 2016, 1, 256–260. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Leeming, D.J.; Karsdal, M.A.; Krag, A. Biomarkers of Extracellular Matrix Remodeling in Liver Diseases. In Biomarkers in Liver Disease. Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V., Preedy, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Marra, F. Hepatic stellate cells and the regulation of liver inflammation. J. Hepatol. 1999, 31, 1120–1130. [Google Scholar] [CrossRef]
- Rabani, V.; Shahsavani, M.; Gharavi, M.; Piryaei, A.; Azhdari, Z.; Baharvand, H. Mesenchymal stem cell infusion therapy in a carbon tetrachloride-induced liver fibrosis model affects matrix metalloproteinase expression. Cell Biol. Int. 2010, 34, 601–605. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; Atta, H.M.; Mahfouz, S.; Fouad, H.H.; Roshdy, N.K.; Ahmed, H.H.; Rashed, L.A.; Sabry, D.; Hassouna, A.A.; Hasan, N.M. Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clin. Biochem. 2007, 40, 893–899. [Google Scholar] [CrossRef]
- Zhao, D.C.; Lei, J.X.; Chen, R.; Yu, W.H.; Zhang, X.M.; Li, S.N.; Xiang, P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J. Gastroenterol. 2005, 11, 3431–3440. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, X.; Shi, Y.; Li, J.; Zheng, L.; Cui, L.; Zhang, J.; Wang, L.; Han, Z.; Han, Y.; et al. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS ONE 2013, 8, e62363. [Google Scholar] [CrossRef]
- Chang, Y.J.; Liu, J.W.; Lin, P.C.; Sun, L.Y.; Peng, C.W.; Luo, G.H.; Chen, T.M.; Lee, R.P.; Lin, S.Z.; Harn, H.J.; et al. Mesenchymal stem cells facilitate recovery from chemically induced liver damage and decrease liver fibrosis. Life Sci. 2009, 85, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, H.; Terai, S.; Taro, T.; Murata, Y.; Fujisawa, K.; Yamamoto, N.; Sakaida, I. Improvement of liver fibrosis by infusion of cultured cells derived from human bone marrow. Cell Tissue Res. 2013, 354, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.J.; Lin, S.Z.; Hung, S.H.; Subeq, Y.M.; Li, Y.S.; Syu, W.S.; Ding, D.C.; Lee, R.P.; Hsieh, D.K.; Lin, P.C.; et al. Adipose-derived stem cells can abrogate chemical-induced liver fibrosis and facilitate recovery of liver function. Cell Transplant. 2012, 21, 2753–2764. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Ma, T.; Chen, W.; Hu, J.; Bai, X.; Li, J.; Liang, T. Therapeutic potential and related signal pathway of adipose-derived stem cell transplantation for rat liver injury. Hepatol. Res. 2009, 39, 822–832. [Google Scholar] [CrossRef]
- Munoz, M.F.; Arguelles, S.; Guzman-Chozas, M.; Guillen-Sanz, R.; Franco, J.M.; Pintor-Toro, J.A.; Cano, M.; Ayala, A. Cell tracking, survival, and differentiation capacity of adipose-derived stem cells after engraftment in rat tissue. J. Cell Physiol. 2018, 233, 6317–6328. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Shin, H.P.; Lee, S.; Lim, Y.J.; Hwang, S.H.; Han, H.; Park, H.K.; Chung, J.H.; Yim, S.V. Effect of human umbilical cord blood-derived mesenchymal stem cells in a cirrhotic rat model. Liver Int. 2009, 29, 898–909. [Google Scholar] [CrossRef]
- Tsai, P.C.; Fu, T.W.; Chen, Y.M.; Ko, T.L.; Chen, T.H.; Shih, Y.H.; Hung, S.C.; Fu, Y.S. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton’s jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009, 15, 484–495. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Wang, B.; Qian, H.; Zhang, X.; Shen, L.; Wang, M.; Zhou, Y.; Zhu, W.; Li, W.; et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013, 22, 845–854. [Google Scholar] [CrossRef]
- Carvalho, A.B.; Quintanilha, L.F.; Dias, J.V.; Paredes, B.D.; Mannheimer, E.G.; Carvalho, F.G.; Asensi, K.D.; Gutfilen, B.; Fonseca, L.M.; Resende, C.M.; et al. Bone marrow multipotent mesenchymal stromal cells do not reduce fibrosis or improve function in a rat model of severe chronic liver injury. Stem Cells 2008, 26, 1307–1314. [Google Scholar] [CrossRef]
- Mannheimer, E.G.; Quintanilha, L.F.; Carvalho, A.B.; Paredes, B.D.; de Carvalho, F.G.; Takyia, C.M.; Resende, C.M.; Rezende, G.F.d.; de Carvalho, A.C.C.; Schanaider, A.; et al. Bone marrow cells obtained from cirrhotic rats do not improve function or reduce fibrosis in a chronic liver disease model. Clin. Transpl. 2011, 25, 54–60. [Google Scholar] [CrossRef]
- Amer, M.E.; El-Sayed, S.Z.; El-Kheir, W.A.; Gabr, H.; Gomaa, A.A.; El-Noomani, N.; Hegazy, M. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur. J. Gastroenterol. Hepatol. 2011, 23, 936–941. [Google Scholar] [CrossRef]
- Amin, M.A.; Sabry, D.; Rashed, L.A.; Aref, W.M.; el-Ghobary, M.A.; Farhan, M.S.; Fouad, H.A.; Youssef, Y.A. Short-term evaluation of autologous transplantation of bone marrow-derived mesenchymal stem cells in patients with cirrhosis: Egyptian study. Clin. Transplant. 2013, 27, 607–612. [Google Scholar] [CrossRef]
- El-Ansary, M.; Abdel-Aziz, I.; Mogawer, S.; Abdel-Hamid, S.; Hammam, O.; Teaema, S.; Wahdan, M. Phase II trial: Undifferentiated versus differentiated autologous mesenchymal stem cells transplantation in Egyptian patients with HCV induced liver cirrhosis. Stem Cell Rev. Rep. 2012, 8, 972–981. [Google Scholar] [CrossRef]
- Jang, Y.O.; Kim, Y.J.; Baik, S.K.; Kim, M.Y.; Eom, Y.W.; Cho, M.Y.; Park, H.J.; Park, S.Y.; Kim, B.R.; Kim, J.W.; et al. Histological improvement following administration of autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: A pilot study. Liver Int. 2014, 34, 33–41. [Google Scholar] [CrossRef]
- Kharaziha, P.; Hellstrom, P.M.; Noorinayer, B.; Farzaneh, F.; Aghajani, K.; Jafari, F.; Telkabadi, M.; Atashi, A.; Honardoost, M.; Zali, M.R.; et al. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: A phase I-II clinical trial. Eur. J. Gastroenterol. Hepatol. 2009, 21, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Mohamadnejad, M.; Alimoghaddam, K.; Bagheri, M.; Ashrafi, M.; Abdollahzadeh, L.; Akhlaghpoor, S.; Bashtar, M.; Ghavamzadeh, A.; Malekzadeh, R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013, 33, 1490–1496. [Google Scholar] [CrossRef]
- Peng, L.; Xie, D.Y.; Lin, B.L.; Liu, J.; Zhu, H.P.; Xie, C.; Zheng, Y.B.; Gao, Z.L. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology 2011, 54, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Takamura, M.; Seki, A.; Sunagozaka, H.; Terashima, T.; Komura, T.; Yamato, M.; Miyazawa, M.; Kawaguchi, K.; Nasti, A.; et al. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen. Ther. 2017, 6, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27, 112–120. [Google Scholar] [CrossRef]
- Li, C.; Kong, Y.; Wang, H.; Wang, S.; Yu, H.; Liu, X.; Yang, L.; Jiang, X.; Li, L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J. Hepatol. 2009, 50, 1174–1183. [Google Scholar] [CrossRef]
- Russo, F.P.; Alison, M.R.; Bigger, B.W.; Amofah, E.; Florou, A.; Amin, F.; Bou-Gharios, G.; Jeffery, R.; Iredale, J.P.; Forbes, S.J. The bone marrow functionally contributes to liver fibrosis. Gastroenterology 2006, 130, 1807–1821. [Google Scholar] [CrossRef]
- Hassan, S.; Barrett, C.J.; Crossman, D.J. Imaging tools for assessment of myocardial fibrosis in humans: The need for greater detail. Biophys. Rev. 2020, 12, 969–987. [Google Scholar] [CrossRef]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef]
- Okyere, A.D.; Tilley, D.G. Leukocyte-Dependent Regulation of Cardiac Fibrosis. Front. Physiol. 2020, 11, 301. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumari, R.; Verma, S.K. Cardiac Fibroblasts and Cardiac Fibrosis: Precise Role of Exosomes. Front. Cell Dev. Biol. 2019, 7, 318. [Google Scholar] [CrossRef]
- Fan, Z.; Guan, J. Antifibrotic therapies to control cardiac fibrosis. Biomater. Res. 2016, 20, 13. [Google Scholar] [CrossRef]
- Sweeney, M.; Corden, B.; Cook, S.A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: Mirage or miracle? EMBO Mol. Med. 2020, 12, e10865. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Zhang, M.; Ji, R.; Wei, J.; Xin, Y.; Jiang, X. Radiation-induced myocardial fibrosis: Mechanisms underlying its pathogenesis and therapeutic strategies. J. Cell Mol. Med. 2020, 24, 7717–7729. [Google Scholar] [CrossRef]
- Webber, M.; Jackson, S.P.; Moon, J.C.; Captur, G. Myocardial Fibrosis in Heart Failure: Anti-Fibrotic Therapies and the Role of Cardiovascular Magnetic Resonance in Drug Trials. Cardiol. Ther. 2020, 9, 363–376. [Google Scholar] [CrossRef]
- Golpanian, S.; Wolf, A.; Hatzistergos, K.E.; Hare, J.M. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol. Rev. 2016, 96, 1127–1168. [Google Scholar] [CrossRef]
- Mias, C.; Lairez, O.; Trouche, E.; Roncalli, J.; Calise, D.; Seguelas, M.H.; Ordener, C.; Piercecchi-Marti, M.D.; Auge, N.; Salvayre, A.N.; et al. Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells 2009, 27, 2734–2743. [Google Scholar] [CrossRef]
- Pei, Z.; Zeng, J.; Song, Y.; Gao, Y.; Wu, R.; Chen, Y.; Li, F.; Li, W.; Zhou, H.; Yang, Y. In vivo imaging to monitor differentiation and therapeutic effects of transplanted mesenchymal stem cells in myocardial infarction. Sci. Rep. 2017, 7, 6296. [Google Scholar] [CrossRef]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; Velazquez, D.L.D.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The POSEIDON randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef]
- Kudo, M.; Wang, Y.; Wani, M.A.; Xu, M.; Ayub, A.; Ashraf, M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J. Mol. Cell. Cardiol. 2003, 35, 1113–1119. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Li, Y.; Yu, B.; Xu, Y.; Zhao, S.; Guan, Z. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl. Int. 2008, 21, 1181–1189. [Google Scholar] [CrossRef]
- Nagaya, N.; Kangawa, K.; Itoh, T.; Iwase, T.; Murakami, S.; Miyahara, Y.; Fujii, T.; Uematsu, M.; Ohgushi, H.; Yamagishi, M.; et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation 2005, 112, 1128–1135. [Google Scholar] [CrossRef]
- Tang, X.L.; Rokosh, G.; Sanganalmath, S.K.; Yuan, F.; Sato, H.; Mu, J.; Dai, S.; Li, C.; Chen, N.; Peng, Y.; et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010, 121, 293–305. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Z.; Xu, Y.; Cui, G. Selective down-regulation of extracellular matrix gene expression by bone marrow derived stem cell transplantation into infarcted myocardium. Circ. J. 2005, 69, 1275–1283. [Google Scholar] [CrossRef][Green Version]
- Du, Y.Y.; Zhou, S.H.; Zhou, T.; Su, H.; Pan, H.W.; Du, W.H.; Liu, B.; Liu, Q.M. Immuno-inflammatory regulation effect of mesenchymal stem cell transplantation in a rat model of myocardial infarction. Cytotherapy 2008, 10, 469–478. [Google Scholar] [CrossRef]
- Ohnishi, S.; Yanagawa, B.; Tanaka, K.; Miyahara, Y.; Obata, H.; Kataoka, M.; Kodama, M.; Ishibashi-Ueda, H.; Kangawa, K.; Kitamura, S.; et al. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J. Mol. Cell. Cardiol. 2007, 42, 88–97. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 420–428. [Google Scholar] [CrossRef]
- Kishore, R.; Verma, S.K.; Mackie, A.R.; Vaughan, E.E.; Abramova, T.V.; Aiko, I.; Krishnamurthy, P. Bone marrow progenitor cell therapy-mediated paracrine regulation of cardiac miRNA-155 modulates fibrotic response in diabetic hearts. PLoS ONE 2013, 8, e60161. [Google Scholar] [CrossRef]
- Ohnishi, S.; Sumiyoshi, H.; Kitamura, S.; Nagaya, N. Mesenchymal stem cells attenuate cardiac fibroblast proliferation and collagen synthesis through paracrine actions. FEBS Lett. 2007, 581, 3961–3966. [Google Scholar] [CrossRef]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef]
- Gray, W.D.; French, K.M.; Ghosh-Choudhary, S.; Maxwell, J.T.; Brown, M.E.; Platt, M.O.; Searles, C.D.; Davis, M.E. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 2015, 116, 255–263. [Google Scholar] [CrossRef]
- Chen, H.; Xia, R.; Li, Z.; Zhang, L.; Xia, C.; Ai, H.; Yang, Z.; Guo, Y. Mesenchymal Stem Cells Combined with Hepatocyte Growth Factor Therapy for Attenuating Ischaemic Myocardial Fibrosis: Assessment using Multimodal Molecular Imaging. Sci. Rep. 2016, 6, 33700. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Chen, W.; Xie, L.; Zhao, Z.A.; Yang, J.; Lei, W.; Shen, Z. MicroRNA-133 overexpression promotes the therapeutic efficacy of mesenchymal stem cells on acute myocardial infarction. Stem Cell Res. Ther. 2017, 8, 268. [Google Scholar] [CrossRef]
- Mangi, A.A.; Noiseux, N.; Kong, D.; He, H.; Rezvani, M.; Ingwall, J.S.; Dzau, V.J. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003, 9, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.N.; Souza, B.S.F.; Azevedo, C.M.; Vasconcelos, J.F.; de Jesus, P.G.; Feitoza, M.S.; Meira, C.S.; Carvalho, G.B.; Cavalcante, B.R.; Ribeiro-Dos-Santos, R.; et al. IGF-1-Overexpressing Mesenchymal Stem/Stromal Cells Promote Immunomodulatory and Proregenerative Effects in Chronic Experimental Chagas Disease. Stem Cells Int. 2018, 2018, 9108681. [Google Scholar] [CrossRef]
- Hoke, N.N.; Salloum, F.N.; Kass, D.A.; Das, A.; Kukreja, R.C. Preconditioning by phosphodiesterase-5 inhibition improves therapeutic efficacy of adipose-derived stem cells following myocardial infarction in mice. Stem Cells 2012, 30, 326–335. [Google Scholar] [CrossRef]
- Khan, M.; Meduru, S.; Mohan, I.K.; Kuppusamy, M.L.; Wisel, S.; Kulkarni, A.; Rivera, B.K.; Hamlin, R.L.; Kuppusamy, P. Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J. Mol. Cell. Cardiol. 2009, 47, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Uemura, R.; Xu, M.; Ahmad, N.; Ashraf, M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ. Res. 2006, 98, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Wisel, S.; Khan, M.; Kuppusamy, M.L.; Mohan, I.K.; Chacko, S.M.; Rivera, B.K.; Sun, B.C.; Hideg, K.; Kuppusamy, P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J. Pharmacol. Exp. Ther. 2009, 329, 543–550. [Google Scholar] [CrossRef]
- Ceccaldi, C.; Bushkalova, R.; Alfarano, C.; Lairez, O.; Calise, D.; Bourin, P.; Frugier, C.; Rouzaud-Laborde, C.; Cussac, D.; Parini, A.; et al. Evaluation of polyelectrolyte complex-based scaffolds for mesenchymal stem cell therapy in cardiac ischemia treatment. Acta Biomater. 2014, 10, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Fiumana, E.; Pasquinelli, G.; Foroni, L.; Carboni, M.; Bonafe, F.; Orrico, C.; Nardo, B.; Tsivian, M.; Neri, F.; Arpesella, G.; et al. Localization of mesenchymal stem cells grafted with a hyaluronan-based scaffold in the infarcted heart. J. Surg. Res. 2013, 179, e21–e29. [Google Scholar] [CrossRef]
- Sun, C.K.; Zhen, Y.Y.; Leu, S.; Tsai, T.H.; Chang, L.T.; Sheu, J.J.; Chen, Y.L.; Chua, S.; Chai, H.T.; Lu, H.I.; et al. Direct implantation versus platelet-rich fibrin-embedded adipose-derived mesenchymal stem cells in treating rat acute myocardial infarction. Int. J. Cardiol. 2014, 173, 410–423. [Google Scholar] [CrossRef]
- Xia, Y.; Zhu, K.; Lai, H.; Lang, M.; Xiao, Y.; Lian, S.; Guo, C.; Wang, C. Enhanced infarct myocardium repair mediated by thermosensiive copolymer hydrogel-based stem cell transplantation. Exp. Biol. Med. 2015, 240, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Boheler, K.R.; Czyz, J.; Tweedie, D.; Yang, H.T.; Anisimov, S.V.; Wobus, A.M. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 2002, 91, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K. Akt-mTOR Pathway Inhibits Apoptosis and Fibrosis in Doxorubicin-Induced Cardiotoxicity Following Embryonic Stem Cell Transplantation. Cell Transpl. 2015, 24, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Ahmed, A.; Singla, R.; Yan, B. Embryonic stem cells improve cardiac function in Doxorubicin-induced cardiomyopathy mediated through multiple mechanisms. Cell Transpl. 2012, 21, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Lyons, G.E.; Kamp, T.J. Transplanted embryonic stem cells following mouse myocardial infarction inhibit apoptosis and cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H1308–H1314. [Google Scholar] [CrossRef]
- Glass, C.; Singla, D.K. Overexpression of TIMP-1 in embryonic stem cells attenuates adverse cardiac remodeling following myocardial infarction. Cell Transpl. 2012, 21, 1931–1944. [Google Scholar] [CrossRef]
- Burt, R.K.; Chen, Y.H.; Verda, L.; Lucena, C.; Navale, S.; Johnson, J.; Han, X.; Lomasney, J.; Baker, J.M.; Ngai, K.L.; et al. Mitotically inactivated embryonic stem cells can be used as an in vivo feeder layer to nurse damaged myocardium after acute myocardial infarction: A preclinical study. Circ. Res. 2012, 111, 1286–1296. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.; Benedict, C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Bagno, L.; Hatzistergos, K.E.; Balkan, W.; Hare, J.M. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol. Ther. 2018, 26, 1610–1623. [Google Scholar] [CrossRef]
- Boudoulas, K.D.; Hatzopoulos, A.K. Cardiac repair and regeneration: The Rubik’s cube of cell therapy for heart disease. Dis. Model. Mech. 2009, 2, 344–358. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H.; Landin, A.M.; et al. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients with Ischemic Cardiomyopathy (The TRIDENT Study). Circ. Res. 2017, 121, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Michler, R.E. The current status of stem cell therapy in ischemic heart disease. J. Card. Surg. 2018, 33, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, J.; Verdugo, F.J.; Gonzalez, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar] [PubMed]
- Gao, L.R.; Chen, Y.; Zhang, N.K.; Yang, X.L.; Liu, H.L.; Wang, Z.G.; Yan, X.Y.; Wang, Y.; Zhu, Z.M.; Li, T.C.; et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: Double-blind, randomized controlled trial. BMC Med. 2015, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Kastrup, J.; Haack-Sorensen, M.; Juhl, M.; Sondergaard, R.H.; Follin, B.; Lund, L.D.; Johansen, E.M.; Qayyum, A.A.; Mathiasen, A.B.; Jorgensen, E.; et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef]
- Caspi, O.; Huber, I.; Kehat, I.; Habib, M.; Arbel, G.; Gepstein, A.; Yankelson, L.; Aronson, D.; Beyar, R.; Gepstein, L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J. Am. Coll. Cardiol. 2007, 50, 1884–1893. [Google Scholar] [CrossRef]
- Vernon, M.A.; Mylonas, K.J.; Hughes, J. Macrophages and renal fibrosis. Semin. Nephrol. 2010, 30, 302–317. [Google Scholar] [CrossRef] [PubMed]
- Bulow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Brennan, E.P.; Cacace, A.; Godson, C. Specialized pro-resolving mediators in renal fibrosis. Mol. Asp. Med. 2017, 58, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Francois, H.; Chatziantoniou, C. Renal fibrosis: Recent translational aspects. Matrix Biol. 2018, 68–69, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Klinkhammer, B.M.; Goldschmeding, R.; Floege, J.; Boor, P. Treatment of Renal Fibrosis-Turning Challenges into Opportunities. Adv. Chronic Kidney Dis. 2017, 24, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, J.S.; Lee, H.K.; Park, E.J.; Jeon, H.W.; Kang, Y.J.; Lee, T.Y.; Kim, K.S.; Bae, S.C.; Park, J.H.; et al. Mesenchymal Stem Cells Ameliorate Renal Inflammation in Adriamycin-induced Nephropathy. Immune Netw. 2019, 19, e36. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.; Oliveira, M.; Martins, M.; Silva, C.; Carvalho, S.; Stumbo, A.C.; Cortez, E.; Verdoorn, K.; Einicker-Lamas, M.; Thole, A.; et al. Transplantation of bone marrow-derived MSCs improves renal function and Na(+)+K(+)-ATPase activity in rats with renovascular hypertension. Cell Tissue Res. 2017, 369, 287–301. [Google Scholar] [CrossRef]
- Semedo, P.; Correa-Costa, M.; Cenedeze, M.A.; Malheiros, D.M.A.C.; Reis, M.A.d.; Shimizu, M.H.; Seguro, A.C.; Pacheco-Silva, A.; Camara, N.O.S. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells 2009, 27, 3063–3073. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Yiu, W.H.; Li, R.X.; Wong, D.W.; Leung, J.C.; Chan, L.Y.; Zhang, Y.; Lian, Q.; Lin, M.; Tse, H.F.; et al. Mesenchymal stem cells modulate albumin-induced renal tubular inflammation and fibrosis. PLoS ONE 2014, 9, e90883. [Google Scholar] [CrossRef]
- Alfarano, C.; Roubeix, C.; Chaaya, R.; Ceccaldi, C.; Calise, D.; Mias, C.; Cussac, D.; Bascands, J.L.; Parini, A. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transpl. 2012, 21, 2009–2019. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, G.; Sun, M.; Guan, G.; Chen, B.; Li, X. Functional, histological and biochemical consequences of renal lymph disorder in mononephrectomized rats. J. Nephrol. 2009, 22, 109–116. [Google Scholar]
- Boor, P.; Floege, J. Renal allograft fibrosis: Biology and therapeutic targets. Am. J. Transpl. 2015, 15, 863–886. [Google Scholar] [CrossRef]
- Canaud, G.; Bonventre, J.V. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol. Dial. Transpl. 2015, 30, 575–583. [Google Scholar] [CrossRef]
- Zhu, F.; Shin, O.L.S.C.L.; Pei, G.; Hu, Z.; Yang, J.; Zhu, H.; Wang, M.; Mou, J.; Sun, J.; Wang, Y.; et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget 2017, 8, 70707–70726. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transpl. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Kholia, S.; Sanchez, M.B.H.; Cedrino, M.; Papadimitriou, E.; Tapparo, M.; Deregibus, M.C.; Bruno, S.; Antico, F.; Brizzi, M.F.; Quesenberry, P.J.; et al. Mesenchymal Stem Cell Derived Extracellular Vesicles Ameliorate Kidney Injury in Aristolochic Acid Nephropathy. Front. Cell Dev. Biol. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Tritta, S.; Tapparo, M.; Cedrino, M.; Tetta, C.; Camussi, G.; Brizzi, M.F. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Sci. Rep. 2019, 9, 4468. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.F.; Fu, G.P.; Guan, C.; Zhang, X.; Yang, D.G.; Shi, Y.C. Protective effect of miRNA-containing extracellular vesicles derived from mesenchymal stromal cells of old rats on renal function in chronic kidney disease. Stem Cell Res. Ther. 2020, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Zhang, X.; Jiang, K.; Krier, J.D.; Zhu, X.; Conley, S.; Lerman, A.; Lerman, L.O. Adjunctive mesenchymal stem/stromal cells augment microvascular function in poststenotic kidneys treated with low-energy shockwave therapy. J. Cell Physiol. 2020, 235, 9806–9818. [Google Scholar] [CrossRef]
- Eirin, A.; Zhang, X.; Zhu, X.Y.; Tang, H.; Jordan, K.L.; Grande, J.P.; Dietz, A.B.; Lerman, A.; Textor, S.C.; Lerman, L.O. Renal vein cytokine release as an index of renal parenchymal inflammation in chronic experimental renal artery stenosis. Nephrol. Dial. Transpl. 2014, 29, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, X.; Zhang, L.; Ferguson, C.M.; Song, T.; Jiang, K.; Conley, S.M.; Krier, J.D.; Tang, H.; Saadiq, I.; et al. Mesenchymal Stem/Stromal Cells and their Extracellular Vesicle Progeny Decrease Injury in Poststenotic Swine Kidney Through Different Mechanisms. Stem Cells Dev. 2020, 29, 1190–1200. [Google Scholar] [CrossRef]
- Choi, H.Y.; Lee, H.G.; Kim, B.S.; Ahn, S.H.; Jung, A.; Lee, M.; Lee, J.E.; Kim, H.J.; Ha, S.K.; Park, H.C. Mesenchymal stem cell-derived microparticles ameliorate peritubular capillary rarefaction via inhibition of endothelial-mesenchymal transition and decrease tubulointerstitial fibrosis in unilateral ureteral obstruction. Stem Cell Res. Ther. 2015, 6, 18. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Li, S.; Zuo, B.; Zhang, X.; Wang, F.; Sun, D. Exosomes derived from GDNF-modified human adipose mesenchymal stem cells ameliorate peritubular capillary loss in tubulointerstitial fibrosis by activating the SIRT1/eNOS signaling pathway. Theranostics 2020, 10, 9425–9442. [Google Scholar] [CrossRef]
- Ozbek, E.; Adas, G.; Otunctemur, A.; Duruksu, G.; Koc, B.; Polat, E.C.; Sarvan, A.K.; Okcu, A.; Kamali, G.; Subasi, C.; et al. Role of Mesenchymal Stem Cells Transfected With Vascular Endothelial Growth Factor in Maintaining Renal Structure and Function in Rats with Unilateral Ureteral Obstruction. Exp. Clin. Transpl. 2015, 13, 262–272. [Google Scholar]
- Wang, B.; Yao, K.; Huuskes, B.M.; Shen, H.H.; Zhuang, J.; Godson, C.; Brennan, E.P.; Wilkinson-Berka, J.L.; Wise, A.F.; Ricardo, S.D. Mesenchymal Stem Cells Deliver Exogenous MicroRNA-let7c via Exosomes to Attenuate Renal Fibrosis. Mol. Ther. 2016, 24, 1290–1301. [Google Scholar] [CrossRef]
- Xie, M.; Wan, J.; Zhang, F.; Zhang, R.; Zhou, Z.; You, D. Influence of hepatocyte growth factor-transfected bone marrow-derived mesenchymal stem cells towards renal fibrosis in rats. Indian J. Med. Res. 2019, 149, 508–516. [Google Scholar] [PubMed]
- Huuskes, B.M.; Wise, A.F.; Cox, A.J.; Lim, E.X.; Payne, N.L.; Kelly, D.J.; Samuel, C.S.; Ricardo, S.D. Combination therapy of mesenchymal stem cells and serelaxin effectively attenuates renal fibrosis in obstructive nephropathy. FASEB J. 2015, 29, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lv, S.; Liu, J.; Liu, S.; Wang, Y.; Liu, G. Mesenchymal stem cells modified with angiotensin-converting enzyme 2 are superior for amelioration of glomerular fibrosis in diabetic nephropathy. Diabetes Res. Clin. Pract. 2020, 162, 108093. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Nakashima, A.; Doi, S.; Kimura, T.; Yoshida, K.; Maeda, S.; Ishiuchi, N.; Yamada, Y.; Ike, T.; Doi, T.; et al. Interferon-gamma enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci. Rep. 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Saberi, K.; Pasbakhsh, P.; Omidi, A.; Borhani-Haghighi, M.; Nekoonam, S.; Omidi, N.; Ghasemi, S.; Kashani, I.R. Melatonin preconditioning of bone marrow-derived mesenchymal stem cells promotes their engraftment and improves renal regeneration in a rat model of chronic kidney disease. J. Mol. Histol. 2019, 50, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Y.; Wang, Z.; Wang, J.; Liu, C.; Sun, D. Transplantation of Amniotic Fluid-Derived Stem Cells Preconditioned with Glial Cell Line-Derived Neurotrophic Factor Gene Alleviates Renal Fibrosis. Cell Transpl. 2019, 28, 65–78. [Google Scholar] [CrossRef]
- Li, H.; Rong, P.; Ma, X.; Nie, W.; Chen, Y.; Zhang, J.; Dong, Q.; Yang, M.; Wang, W. Mouse Umbilical Cord Mesenchymal Stem Cell Paracrine Alleviates Renal Fibrosis in Diabetic Nephropathy by Reducing Myofibroblast Transdifferentiation and Cell Proliferation and Upregulating MMPs in Mesangial Cells. J. Diabetes Res. 2020, 2020, 3847171. [Google Scholar] [CrossRef]
- Liu, B.; Ding, F.; Hu, D.; Zhou, Y.; Long, C.; Shen, L.; Zhang, Y.; Zhang, D.; Wei, G. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-kappaB signaling pathway in vivo and in vitro. Stem Cell Res. Ther. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wu, Y.; Xu, Y.; Sun, L.; Zhang, X. Human umbilical mesenchymal stem cells attenuate the progression of focal segmental glomerulosclerosis. Am. J. Med. Sci. 2013, 346, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Park, J.; Hwang, S.H.; Han, H.; Ha, H. Delayed treatment with human umbilical cord blood-derived stem cells attenuates diabetic renal injury. Transplant. Proc. 2012, 44, 1123–1126. [Google Scholar] [CrossRef] [PubMed]
- Xiang, E.; Han, B.; Zhang, Q.; Rao, W.; Wang, Z.; Chang, C.; Zhang, Y.; Tu, C.; Li, C.; Wu, D. Human umbilical cord-derived mesenchymal stem cells prevent the progression of early diabetic nephropathy through inhibiting inflammation and fibrosis. Stem Cell Res. Ther. 2020, 11, 336. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, D.; Zhou, Y.; Xiang, H.; Liu, B.; Shen, L.; Long, C.; Liu, X.; Lin, T.; He, D.; et al. Human umbilical cord mesenchymal stem cell attenuates renal fibrosis via TGF-beta/Smad signaling pathways in vivo and in vitro. Eur. J. Pharmacol. 2020, 883, 173343. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tanaka, H.; Kuwana, H.; Inoshita, S.; Teraoka, H.; Sasaki, S.; Terada, Y. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem. Biophys. Res. Commun. 2005, 336, 585–595. [Google Scholar] [CrossRef]
- Narayanan, K.; Schumacher, K.M.; Tasnim, F.; Kandasamy, K.; Schumacher, A.; Ni, M.; Gao, S.; Gopalan, B.; Zink, D.; Ying, J.Y. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013, 83, 593–603. [Google Scholar] [CrossRef]
- Geng, X.D.; Zheng, W.; Wu, C.M.; Wang, S.Q.; Hong, Q.; Cai, G.Y.; Chen, X.M.; Wu, D. Embryonic Stem Cells-loaded Gelatin Microcryogels Slow Progression of Chronic Kidney Disease. Chin. Med. J. 2016, 129, 392–398. [Google Scholar] [CrossRef]
- De Chiara, L.; Fagoonee, S.; Ranghino, A.; Bruno, S.; Camussi, G.; Tolosano, E.; Silengo, L.; Altruda, F. Renal cells from spermatogonial germline stem cells protect against kidney injury. J. Am. Soc. Nephrol. 2014, 25, 316–328. [Google Scholar] [CrossRef]
- Ciampi, O.; Iacone, R.; Longaretti, L.; Benedetti, V.; Graf, M.; Magnone, M.C.; Patsch, C.; Xinaris, C.; Remuzzi, G.; Benigni, A.; et al. Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res. 2016, 17, 130–139. [Google Scholar] [CrossRef]
- Qian, T.; Hernday, S.E.; Bao, X.; Olson, W.R.; Panzer, S.E.; Shusta, E.V.; Palecek, S.P. Directed Differentiation of Human Pluripotent Stem Cells to Podocytes under Defined Conditions. Sci. Rep. 2019, 9, 2765. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.C.; Lojudice, F.H.; Fernandes-Charpiot, I.M.M.; Baptista, M.; Araujo, S.D.; Mendes, G.E.F.; Sogayar, M.C.; Abbud-Filho, M.; Caldas, H.C. Therapeutic potential of human induced pluripotent stem cells and renal progenitor cells in experimental chronic kidney disease. Stem Cell Res. Ther. 2020, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Caldas, H.C.; Lojudice, F.H.; Dias, C.; Fernandes-Charpiot, I.M.M.; Baptista, M.; Kawasaki-Oyama, R.S.; Sogayar, M.C.; Takiya, C.M.; Abbud-Filho, M. Induced Pluripotent Stem Cells Reduce Progression of Experimental Chronic Kidney Disease but Develop Wilms’ Tumors. Stem Cells Int. 2017, 2017, 7428316. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2018, 29, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Makhlough, A.; Shekarchian, S.; Moghadasali, R.; Einollahi, B.; Hosseini, S.E.; Jaroughi, N.; Bolurieh, T.; Baharvand, H.; Aghdami, N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res. Ther. 2017, 8, 116. [Google Scholar] [CrossRef]
- Evans-Hoeker, E.A.; Young, S.L. Endometrial receptivity and intrauterine adhesive disease. Semin. Reprod. Med. 2014, 32, 392–401. [Google Scholar] [PubMed]
- Yu, D.; Wong, Y.M.; Cheong, Y.; Xia, E.; Li, T.C. Asherman syndrome—One century later. Fertil. Steril. 2008, 89, 759–779. [Google Scholar] [CrossRef]
- March, C.M. Asherman’s syndrome. Semin. Reprod. Med. 2011, 29, 83–94. [Google Scholar] [CrossRef]
- Chen, F.P.; Soong, Y.K.; Hui, Y.L. Successful treatment of severe uterine synechiae with transcervical resectoscopy combined with laminaria tent. Hum. Reprod. 1997, 12, 943–947. [Google Scholar] [CrossRef]
- Hooker, A.B.; de Leeuw, R.; van de Ven, P.M.; Bakkum, E.A.; Thurkow, A.L.; Vogel, N.E.A.; van Vliet, H.; Bongers, M.Y.; Emanuel, M.H.; Verdonkschot, A.E.M.; et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: Short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil. Steril. 2017, 107, 1223.e3–1231.e3. [Google Scholar] [CrossRef]
- Lin, X.N.; Zhou, F.; Wei, M.L.; Yang, Y.; Li, Y.; Li, T.C.; Zhang, S.Y. Randomized, controlled trial comparing the efficacy of intrauterine balloon and intrauterine contraceptive device in the prevention of adhesion reformation after hysteroscopic adhesiolysis. Fertil. Steril. 2015, 104, 235–240. [Google Scholar] [CrossRef]
- Schenker, J.G. Etiology of and therapeutic approach to synechia uteri. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 65, 109–113. [Google Scholar] [CrossRef]
- Alawadhi, F.; Du, H.; Cakmak, H.; Taylor, H.S. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS ONE 2014, 9, e96662. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Shekhar, B.; Mohanty, S.; Kumar, S.; Seth, T.; Girish, B. Autologous Bone Marrow-Derived Stem Cell Therapy for Asherman’s Syndrome and Endometrial Atrophy: A 5-Year Follow-up Study. J. Hum. Reprod. Sci. 2020, 13, 31–37. [Google Scholar] [CrossRef]
- Cervello, I.; Gil-Sanchis, C.; Santamaria, X.; Cabanillas, S.; Diaz, A.; Faus, A.; Pellicer, A.; Simon, C. Human CD133(+) bone marrow-derived stem cells promote endometrial proliferation in a murine model of Asherman syndrome. Fertil. Steril. 2015, 104, 1552.e1-3–1560.e1-3. [Google Scholar] [CrossRef] [PubMed]
- Domnina, A.; Novikova, P.; Obidina, J.; Fridlyanskaya, I.; Alekseenko, L.; Kozhukharova, I.; Lyublinskaya, O.; Zenin, V.; Nikolsky, N. Human mesenchymal stem cells in spheroids improve fertility in model animals with damaged endometrium. Stem Cell Res. Ther. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Q.; Sun, J.; Lai, D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res. Ther. 2019, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, X.; Cabanillas, S.; Cervello, I.; Arbona, C.; Raga, F.; Ferro, J.; Palmero, J.; Remohi, J.; Pellicer, A.; Simon, C. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: A pilot cohort study. Hum. Reprod. 2016, 31, 1087–1096. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, J.E.; Kwon, H.; Choi, D.H.; Kim, J.H. Effect of Autologous Adipose-Derived Stromal Vascular Fraction Transplantation on Endometrial Regeneration in Patients of Asherman’s Syndrome: A Pilot Study. Reprod. Sci. 2020, 27, 561–568. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, H.; Zhu, H.; Zhu, X.; Tang, X.; Yan, G.; Wang, J.; Bai, D.; Wang, L.; Zhou, Q.; et al. Allogeneic cell therapy using umbilical cord MSCs on collagen scaffolds for patients with recurrent uterine adhesion: A phase I clinical trial. Stem Cell Res. Ther. 2018, 9, 192. [Google Scholar] [CrossRef]
- Tan, J.; Li, P.; Wang, Q.; Li, Y.; Li, X.; Zhao, D.; Xu, X.; Kong, L. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum. Reprod. 2016, 31, 2723–2729. [Google Scholar] [CrossRef]
- Canady, J.; Karrer, S.; Fleck, M.; Bosserhoff, A.K. Fibrosing connective tissue disorders of the skin: Molecular similarities and distinctions. J. Dermatol. Sci. 2013, 70, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Christner, P.J.; Artlett, C.M.; Conway, R.F.; Jimenez, S.A. Increased numbers of microchimeric cells of fetal origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis Rheum. 2000, 43, 2598–2605. [Google Scholar] [CrossRef]
- Servettaz, A.; Goulvestre, C.; Kavian, N.; Nicco, C.; Guilpain, P.; Chereau, C.; Vuiblet, V.; Guillevin, L.; Mouthon, L.; Weill, B.; et al. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J. Immunol. 2009, 182, 5855–5864. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T. Animal model of systemic sclerosis. J. Dermatol. 2010, 37, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Melichian, D.; Komura, K.; Hinchcliff, M.; Lam, A.P.; Lafyatis, R.; Gottardi, C.J.; MacDougald, O.A.; Varga, J. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: A novel mouse model for scleroderma? Arthritis. Rheum. 2011, 63, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Medsger, T.A., Jr. Epidemiology of systemic sclerosis. Clin. Dermatol. 1994, 12, 207–216. [Google Scholar] [CrossRef]
- Gilliam, A.C. Scleroderma. Curr. Dir. Autoimmun. 2008, 10, 258–279. [Google Scholar]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Lorenz, H.P.; Adzick, N.S. Scarless Skin Wound Repair in the Fetus. West. J. Med. 1993, 159, 350–355. [Google Scholar]
- Merkel, J.R.; DiPaolo, B.R.; Hallock, G.G.; Rice, D.C. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc. Soc. Exp. Biol Med. 1988, 187, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. Hypertrophic scars and keloids: Overview of the evidence and practical guide for differentiating between these abnormal scars. Exp. Dermatol. 2021, 30, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, D.; Moshaverinia, A.; Liu, D.; Kou, X.; Yu, W.; Yang, R.; Sun, L.; Shi, S. Mesenchymal stem cell transplantation in tight-skin mice identifies miR-151-5p as a therapeutic target for systemic sclerosis. Cell Res. 2017, 27, 559–577. [Google Scholar] [CrossRef]
- Maria, A.T.; Toupet, K.; Bony, C.; Pirot, N.; Vozenin, M.C.; Petit, B.; Roger, P.; Batteux, F.; le Quellec, A.; Jorgensen, C.; et al. Antifibrotic, Antioxidant, and Immunomodulatory Effects of Mesenchymal Stem Cells in HOCl-Induced Systemic Sclerosis. Arthritis Rheumatol. 2016, 68, 1013–1025. [Google Scholar] [CrossRef]
- Okamura, A.; Matsushita, T.; Komuro, A.; Kobayashi, T.; Maeda, S.; Hamaguchi, Y.; Takehara, K. Adipose-derived stromal/stem cells successfully attenuate the fibrosis of scleroderma mouse models. Int. J. Rheum. Dis. 2020, 23, 216–225. [Google Scholar] [CrossRef]
- Rubio, G.A.; Elliot, S.J.; Wikramanayake, T.C.; Xia, X.; Pereira-Simon, S.; Thaller, S.R.; Glinos, G.D.; Jozic, I.; Hirt, P.; Pastar, I.; et al. Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J. Cell Physiol. 2018, 233, 5503–5512. [Google Scholar] [CrossRef]
- Domergue, S.; Bony, C.; Maumus, M.; Toupet, K.; Frouin, E.; Rigau, V.; Vozenin, M.C.; Magalon, G.; Jorgensen, C.; Noel, D. Comparison between Stromal Vascular Fraction and Adipose Mesenchymal Stem Cells in Remodeling Hypertrophic Scars. PLoS ONE 2016, 11, e0156161. [Google Scholar] [CrossRef]
- Liu, J.; Ren, J.; Su, L.; Cheng, S.; Zhou, J.; Ye, X.; Dong, Y.; Sun, S.; Qi, F.; Liu, Z.; et al. Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 2018, 44, 370–385. [Google Scholar] [CrossRef]
- Henrique-Neto, A.; Vasconcelos, M.Y.K.; Dias, J.B.E.; de Moraes, D.A.; Goncalves, M.S.; Zanin-Silva, D.C.; Zucoloto, T.G.; de Oliveira, M.F.C.; Dotoli, G.M.; Weffort, L.F.; et al. Hematopoietic stem cell transplantation for systemic sclerosis: Brazilian experience. Adv. Rheumatol. 2021, 61, 9. [Google Scholar] [CrossRef]
- Burt, R.K.; Shah, S.J.; Dill, K.; Grant, T.; Gheorghiade, M.; Schroeder, J.; Craig, R.; Hirano, I.; Marshall, K.; Ruderman, E.; et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): An open-label, randomised phase 2 trial. Lancet 2011, 378, 498–506. [Google Scholar] [CrossRef]
- van Laar, J.M.; Farge, D.; Sont, J.K.; Naraghi, K.; Marjanovic, Z.; Larghero, J.; Schuerwegh, A.J.; Marijt, E.W.; Vonk, M.C.; Schattenberg, A.V.; et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: A randomized clinical trial. JAMA 2014, 311, 2490–2498. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, N.; Ceccarelli, S.; Onesti, M.G.; Fioramonti, P.; Guidi, C.; Romano, F.; Frati, L.; Angeloni, A.; Marchese, C. Human adipose-derived stromal cells for cell-based therapies in the treatment of systemic sclerosis. Cell Transpl. 2013, 22, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Granel, B.; Daumas, A.; Jouve, E.; Harle, J.R.; Nguyen, P.S.; Chabannon, C.; Colavolpe, N.; Reynier, J.C.; Truillet, R.; Mallet, S.; et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: An open-label phase I trial. Ann. Rheum Dis. 2015, 74, 2175–2182. [Google Scholar] [CrossRef]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Del Papa, N.; Caviggioli, F.; Sambataro, D.; Zaccara, E.; Vinci, V.; di Luca, G.; Parafioriti, A.; Armiraglio, E.; Maglione, W.; Polosa, R.; et al. Autologous fat grafting in the treatment of fibrotic perioral changes in patients with systemic sclerosis. Cell Transpl. 2015, 24, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Murry, C.E.; Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: Lessons from embryonic development. Cell 2008, 132, 661–680. [Google Scholar] [CrossRef] [PubMed]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Prokhorova, T.A.; Harkness, L.M.; Frandsen, U.; Ditzel, N.; Schroder, H.D.; Burns, J.S.; Kassem, M. Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev. 2009, 18, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Gold, J.; Xu, C.; Hassanipour, M.; Rosler, E.; Police, S.; Muskheli, V.; Murry, C.E. Formation of human myocardium in the rat heart from human embryonic stem cells. Am. J. Pathol. 2005, 167, 663–671. [Google Scholar] [CrossRef]
- Meyer, J.R. The significance of induced pluripotent stem cells for basic research and clinical therapy. J. Med. Ethics 2008, 34, 849–851. [Google Scholar] [CrossRef]
- Kiskinis, E.; Eggan, K. Progress toward the clinical application of patient-specific pluripotent stem cells. J. Clin. Investig. 2010, 120, 51–59. [Google Scholar] [CrossRef]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.M.; Fries, J.W.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Park, J.S.; Tkebuchava, T.; Luedeman, C.; Losordo, D.W. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation 2004, 109, 3154–3157. [Google Scholar] [CrossRef]
- Portalska, K.J.; Leferink, A.; Groen, N.; Fernandes, H.; Moroni, L.; van Blitterswijk, C.; de Boer, J. Endothelial differentiation of mesenchymal stromal cells. PLoS ONE 2012, 7, e46842. [Google Scholar]
- Oswald, J.; Boxberger, S.; Jorgensen, B.; Feldmann, S.; Ehninger, G.; Bornhauser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Marriott, S.; Baskir, R.S.; Gaskill, C.; Menon, S.; Carrier, E.J.; Williams, J.; Talati, M.; Helm, K.; Alford, C.E.; Kropski, J.A.; et al. ABCG2pos lung mesenchymal stem cells are a novel pericyte subpopulation that contributes to fibrotic remodeling. Am. J. Physiol. Cell Physiol. 2014, 307, C684–C698. [Google Scholar] [CrossRef]
- Schneider, R.K.; Mullally, A.; Dugourd, A.; Peisker, F.; Hoogenboezem, R.; van Strien, P.M.H.; Bindels, E.M.; Heckl, D.; Busche, G.; Fleck, D.; et al. Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell 2017, 20, 785–800.e8. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.O.; Han, J.W.; Kim, J.M.; Cho, H.J.; Park, C.; Lee, N.; Kim, D.W.; Yoon, Y.S. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
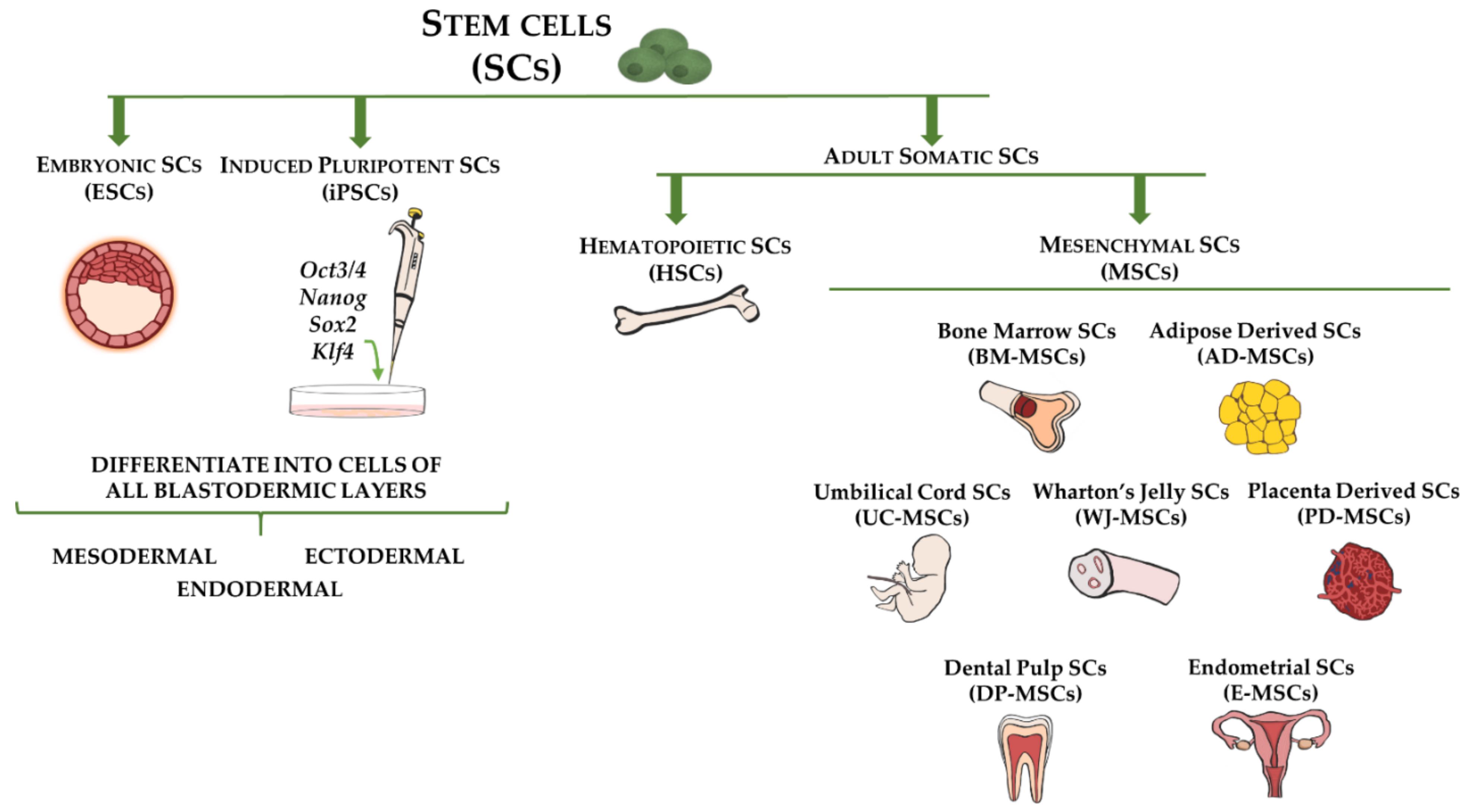
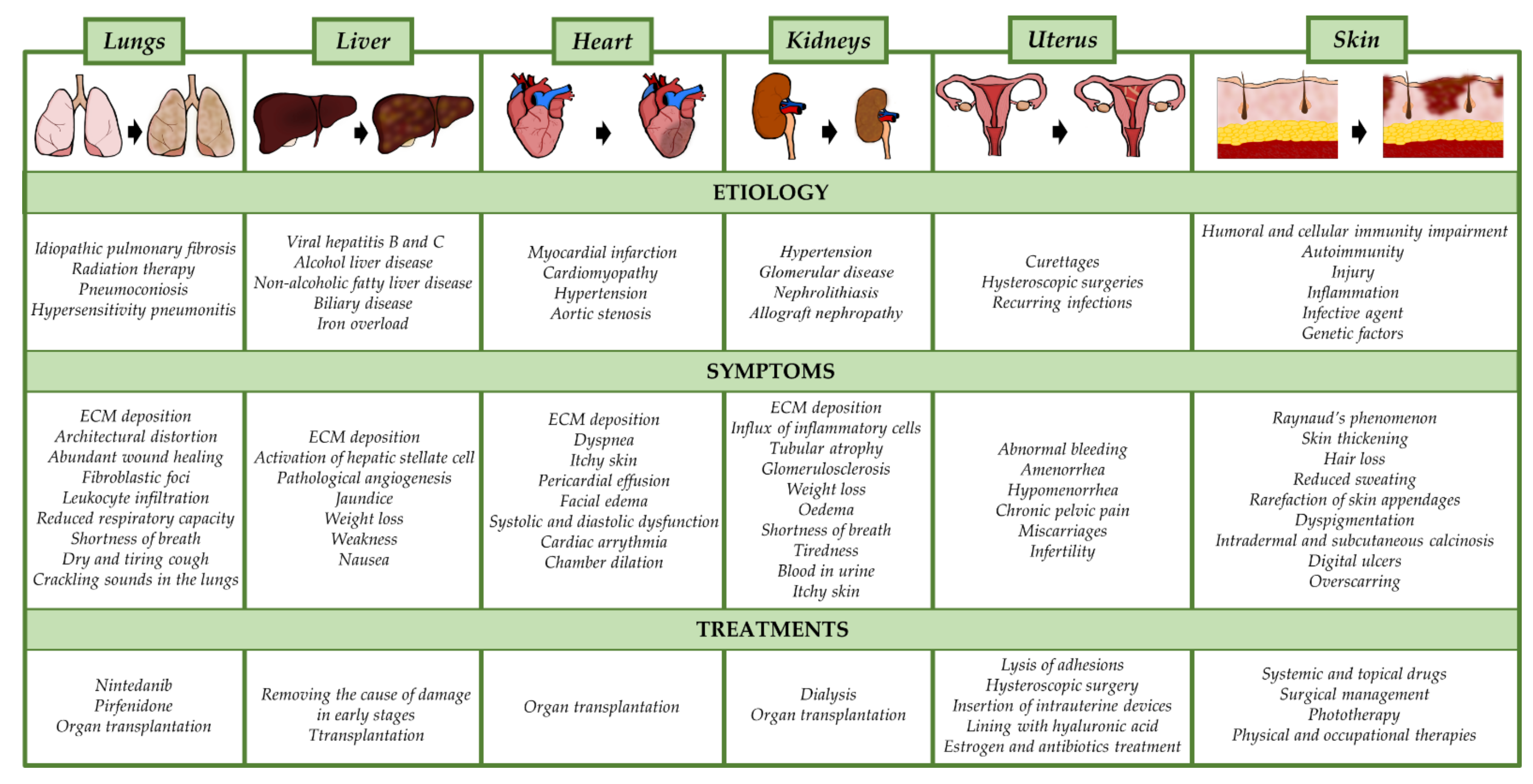
| No. | Patent Name | Patent Number | Applicant |
|---|---|---|---|
| 1. | Mesenchymal stromal cells and extracellular vesicles for treating viral infections, inflammation, and tissue fibrosis | WO2021181399A1 | Exostem Biotec Ltd. (Petah Tikva, Israel) |
| 2. | Biomarker composition for predicting the therapeutic efficacy of mesenchymal stem cells in renal fibrosis | KR102242286B1 | Corestem Co Ltd. (Seongnam, South Korea); Chungbunk National University (Cheongju, South Korea) |
| 3. | Generation of quiescent cardiac fibroblasts from human induced pluripotent stem cells for in vitro modeling of cardiac fibrosis | WO2020264308A1 | Leland Stanford Junior University (Stanford, CA, United States) |
| 4. | Mesenchymal stem cells and application thereof to treatment of acute lung injury, acute respiratory distress or pulmonary fibrosis | CN111904980A | IPM Biopharm Co Ltd. (Hangzhou, China) |
| 5. | Method for improving living quality of patient with liver cirrhosis by applying stem cell therapy | CN111135192A | CAR-T (Shanghai, China) Bitechnology Co Ltd. (Guangzhou, China) |
| 6. | Mesenchymal stem cell anti-liver fibrosis injection | CN110755454A | Qingdao re Store Biotechnology Co Ltd. (Qingdao, China) |
| 7. | Composition for preventing or treating of liver fibrosis comprising exosomes derived from adipose stem cells as an active ingredient | KR102159791B1 | ExoStemTech (Ansan, South Korea) |
| 8. | Treatment of pulmonary fibrosis by atomizing inhalation of stem cell active peptide and preparation of atomizing agent | CN110403958A | Chenxin Shanghai Medical Tech Co Ltd. (Shanghai, China) |
| 9. | Use of umbilical mesenchymal stem cells for treating pulmonary fibrosis | TW201838636A | National Yang Ming University (Taipei, Taiwan); Fu Yu Show (Taipei, Taiwan) |
| 10. | Application of endometrial stem cells in preparation of drugs for preventing or treating pulmonary fibrosis | CN108969539A | Southern Medical University (Guangzhou, China); Guangdong Shengsai Biological Tech Co Ltd. (Guangzhou, China) |
| 11. | Mesenchymal stem cell line useful for developing fibrosis therapeutic agent | KR20180093396A | Osteoneurogen (Seoul, South Korea) |
| 12. | Production method of enhanced hepatocyte regenerating mesenchymal stem cell and Composition for treating liver cirrhosis using the same | KR101903964B1 | Catholic Kwandong University (Gangneung, South Korea) |
| 13. | Human adipose-derived mesenchymal stem cell anti-hepatic fibrosis injection and preparation method thereof | CN107496456A | Qingdao re Store Biotechnology Co Ltd. (Qingdao, China) |
| 14. | Treatment of fibrosis using deep tissue heating and stem cell therapy | US2017232276A1 | Primegen Biotech Llc (Santa Ana, CA, USA) |
| 15. | Stem cell preparation capable of resisting hepatic fibrosis and preparation method of stem cell preparation | CN106038596A | Shenzhen Istem Regenerative Medicine Sci-Tech Co Ltd. (Shenzhen, China) |
| 16. | Composition for preventing and treating liver fibrosis or liver cirrhosis, containing, as active ingredient, mesenchymal stem cells derived from human embryonic stem cells | US2016151420A1 | Seoul National University Hospital (Seoul, South Korea) |
| 17. | Application of exosome derived from human mesenchymal stem cells to resistance to tissue fibrosis and scar forming | CN105477016A | PLA Second Military Medical University (Shanghai, China) |
| 18. | Application of umbilical cord mesenchymal stem cells in preparation of pharmaceutical preparation for treating PF (pulmonary fibrosis) | CN104666347B | The First Affiliated Hospital of Guangzhou Medical University (Guangzhou, China); Shenzhen Beike Biotechnology Co Ltd. (Shenzhen, China) |
| 19. | Stem cell preparation for treating hepatic fibrosis | CN104622902B | Hangzhou S-Evans Ketuo Stem Cell Technology Res Co Ltd. (Hangzhou, China) |
| 20. | Application of gene modified mesenchymal stem cell in pulmonary fibrosis treatment | CN103203025A | INST Radiation Med AMMS PLA (Beijing, China) |
| 21. | Human umbilical cord mesenchymal stem cell (HUMSC) anti-hepatic fibrosis injection and preparation method thereof | CN102008507B | Tianjin Heze Stem Cells Technology Co Ltd. (Tianjin, China) |
| 22. | Pharmaceutical composition for preventing and treating liver fibrosis or hepatic cirrhosis comprising mesenchymal stem cell | EP3878432A1 | Seoul National University Foundation (Seoul, South Korea) RNL Bio Co Ltd. (Seoul, South Korea) |
| 23. | Use of stroma stem cell derived from bone marrow in preparing formulation for treating hepatic fibrosis | CN1803192A | Sun Yat-sen University (Guangzhou, China) |
| 24. | Stem Cell Therapy in endometrial pathologies | AU2015275798B2 | Igenomix S.L. (Paterna, Spain) |
| 25. | Compositions and methods for ameliorating tissue injury, enhancing liver regeneration and stem cell therapies | WO2020005891A1 | University of Southern California (Los Angeles, CA, USA) |
| 26. | ROR-1-positive mesenchymal stem cell-containing pharmaceutical composition for preventing or treating disease associated with fibrosis, method for preparing same, and method for preventing or treating disease associated with fibrosis using ROR-1-positive mesenchymal stem cells | WO2018164228A1 | Rohto Pharmaceutical Co Ltd. (Osaka, Japan) |
| 27. | Composition containing adipose stem cell-derived exosomes as active ingredient for preventing or treating liver fibrosis | WO2018093233 | ExoStemTech Co Ltd. (Ansan, South Korea) |
| 28. | Pluripotent stem cells that induces repair and regeneration after myocardial infarction | EP3659612 | Clio Inc. (Tokyo, Japan); Gifu University (Gifu, Japan); Tohoku University (Sendai, Japan) |
| 29. | Prophylactic or therapeutic agent for organ fibrosis | WO2005082402A1 | Tohoku University (Sendai, Japan); Life Science Institute, Inc. (Tokyo, Japan) |
| 30. | Stem cells for treating lung diseases | US20090274665A1 | Cell Therapy Technologies Inc. (Vancouver, BC, Canada); Thebaud Bernard (Canada) |
| 31. | Tissue repair by activated cells | CA3111750A1 | Technion Research and Development Foundation Limited (Haifa, Israel) |
| 32. | Compositions and methods directed to treating liver fibrosis | US2021087218A1 | Alnylam Pharmaceuticals Inc. (Cambridge, MA, USA) |
| 33. | Methods of reversing liver fibrosis using stem cells therapy | CN111281885A | CAR-T (Shanghai) Biotechnology Co Ltd. (Shanghai, China) |
| 34. | Composition for preventing or treating pulmonary fibrosis comprising exosomes extracted form adipose-derived stem cells | CN109562129A | Hanyang University ERICA Industry-University Cooperation Foundation (Hanyang, China) |
| 35. | Mesenchymal stem cell line useful for development of fibrosis therapeutic agent | WO2018147504A1 | Osteoneurogen (South Korea) |
| 36. | Umbilical cord mesenchymal stem cells for treating lung diseases and preparation method thereof | CN111518758A | Shenzhen Hornectorn Biotechnology Co Ltd. (Shenzhen, China) |
| 37. | Stem cell preparation for treating liver cirrhosis | CN106109497A | Shenzhen Istem Regenerative Medicine Sci-Tech Co Ltd. (Shenzhen, China) |
| 38. | Use of umbilical cord mesenchymal stem cells for the treatment of pulmonary fibrosis | CN108324736A | Fu Yuxiu (China) |
| 39. | Use of human fat-derived mesenchymal stem cells in treatment of diseases in kidney and ocular fundus | CN102048756A | Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences (Beijing, China) |
| 40. | Mesenchymal stem cells for use in improving pulmonary function | EP3653217A1 | Mesoblast International Sárl (Switzerland) |
| 41. | Uterine blood stem cells and exosomes for treating intrauterine adhesion | CN111979199A | Zhejiang Puhui Medical Technology Co Ltd. (Hangzhou, China) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, J.; Sadowska, A.; Wójtowicz, A.; Słyszewska, M.; Szóstek-Mioduchowska, A. Perspective on Stem Cell Therapy in Organ Fibrosis: Animal Models and Human Studies. Life 2021, 11, 1068. https://doi.org/10.3390/life11101068
Wiśniewska J, Sadowska A, Wójtowicz A, Słyszewska M, Szóstek-Mioduchowska A. Perspective on Stem Cell Therapy in Organ Fibrosis: Animal Models and Human Studies. Life. 2021; 11(10):1068. https://doi.org/10.3390/life11101068
Chicago/Turabian StyleWiśniewska, Joanna, Agnieszka Sadowska, Anna Wójtowicz, Magda Słyszewska, and Anna Szóstek-Mioduchowska. 2021. "Perspective on Stem Cell Therapy in Organ Fibrosis: Animal Models and Human Studies" Life 11, no. 10: 1068. https://doi.org/10.3390/life11101068
APA StyleWiśniewska, J., Sadowska, A., Wójtowicz, A., Słyszewska, M., & Szóstek-Mioduchowska, A. (2021). Perspective on Stem Cell Therapy in Organ Fibrosis: Animal Models and Human Studies. Life, 11(10), 1068. https://doi.org/10.3390/life11101068



.png)




