Association between Time to Local Tumor Control and Treatment Outcomes Following Repeated Loco-Regional Treatment Session in Patients with Hepatocellular Carcinoma: A Retrospective, Single-Center Study
Abstract
1. Introduction
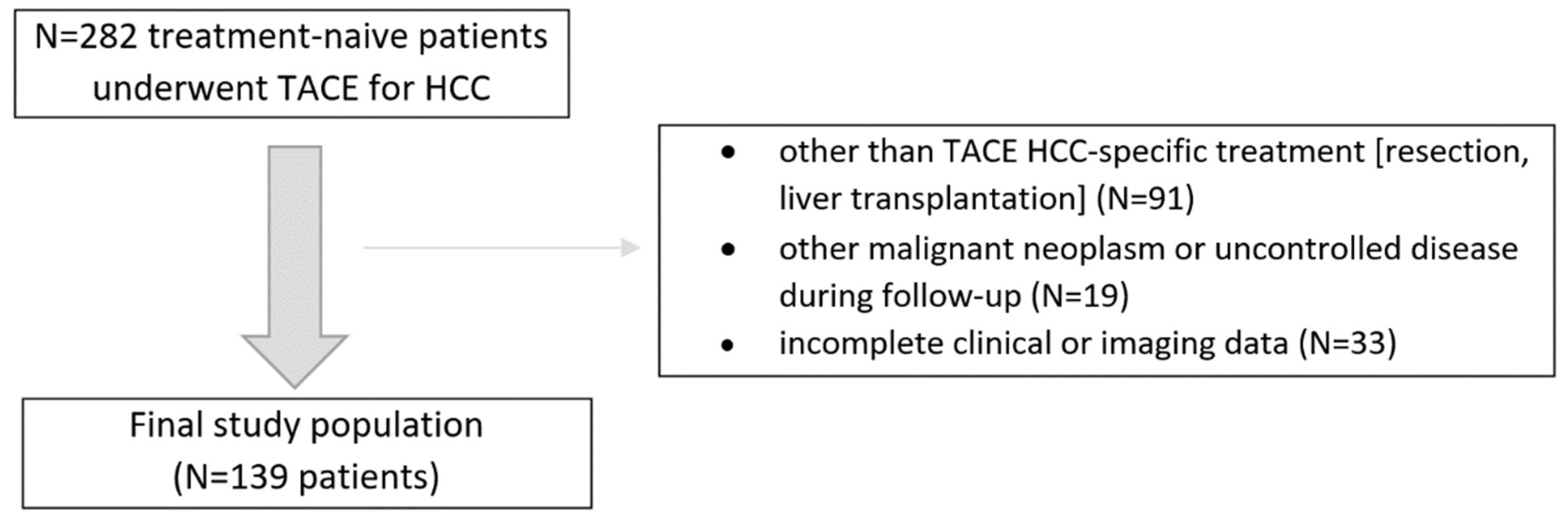
2. Materials and Methods
3. Results
3.1. Patient Characteristics
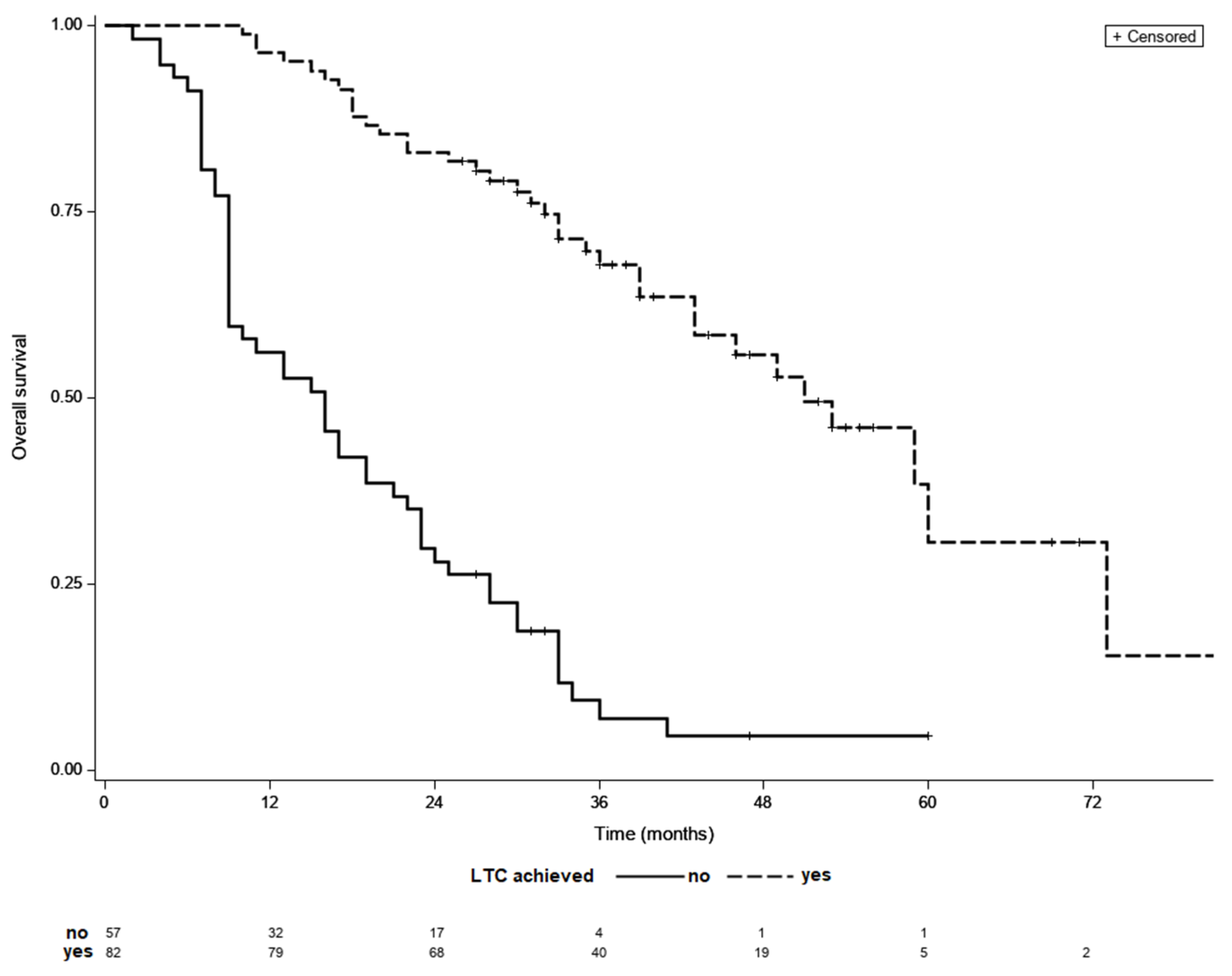
3.2. Treatment Outcomes in Patients with No LTC
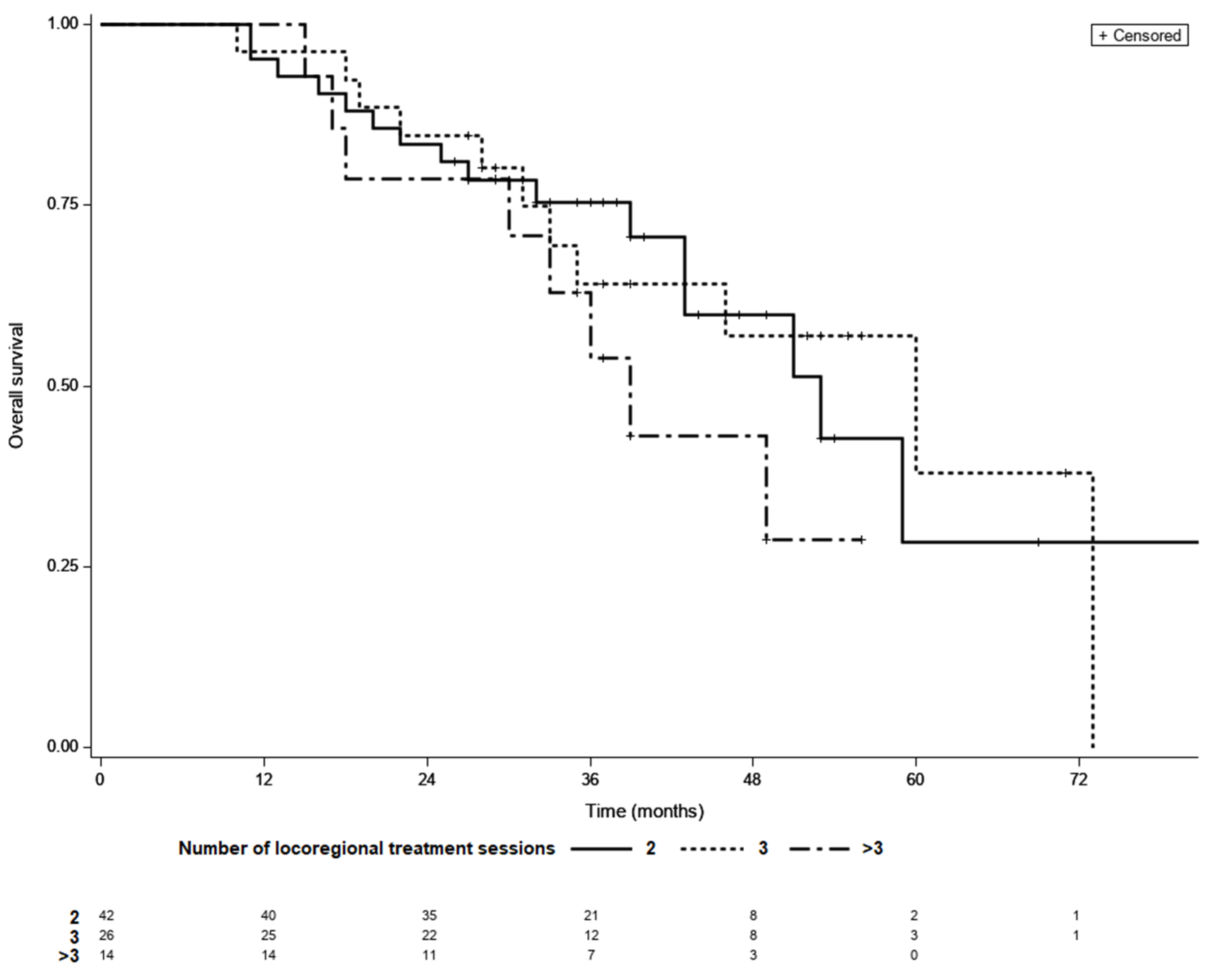
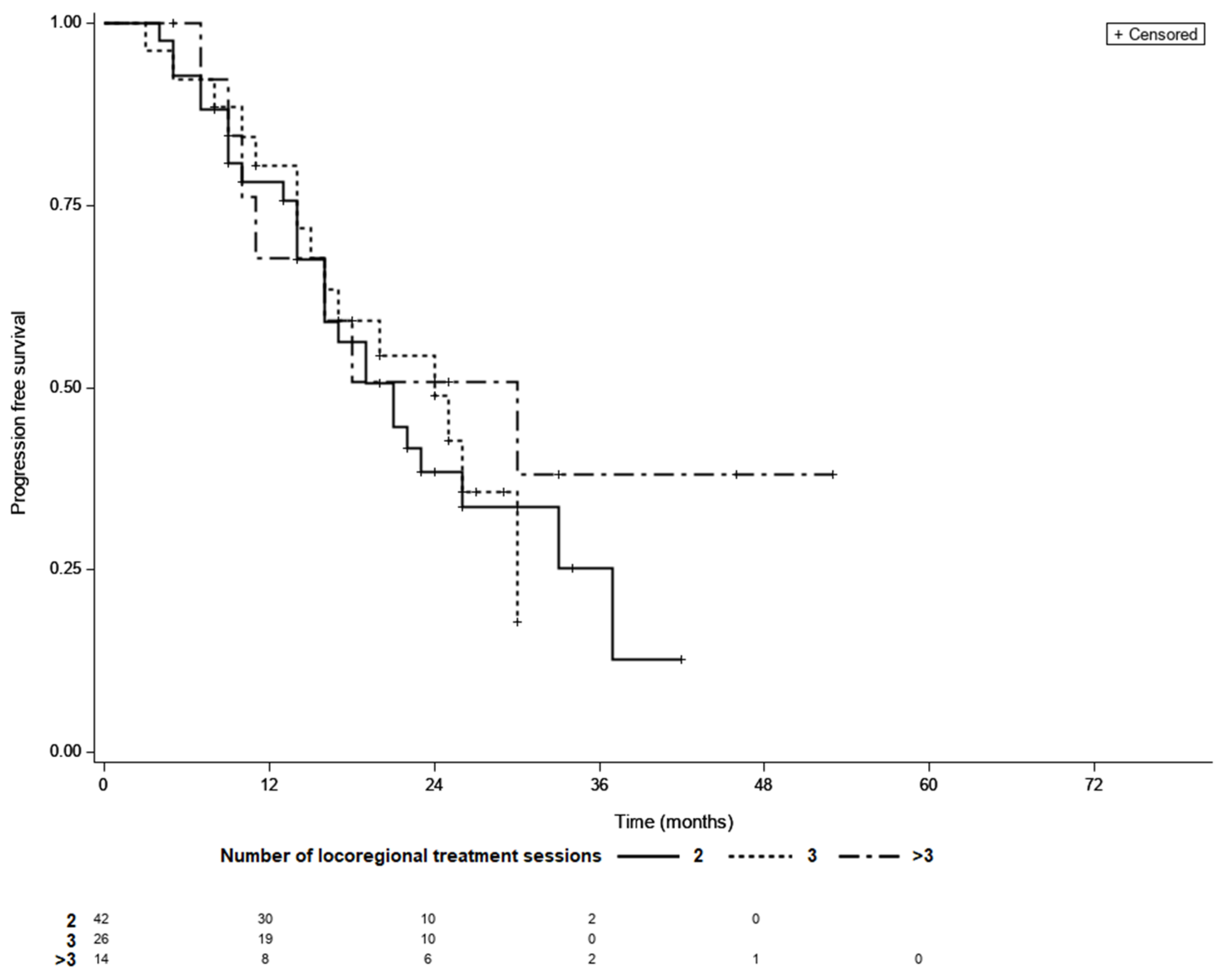
3.3. Treatment Outcomes in Patients LTC
3.4. Time to LTC as a Prognostic Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv238–iv255. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Bashir, M.R.; Corwin, M.T.; Cruite, I.; Dietrich, C.F.; Do, R.K.G.; Ehman, E.C.; Fowler, K.J.; Hussain, H.K.; Jha, R.C.; et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: A systematic review. Radiology 2018, 286, 29–48. [Google Scholar] [CrossRef]
- Forner, A.; Ayuso, C.; Varela, M.; Rimola, J.; Hessheimer, A.J.; De Lope, C.R.; Reig, M.; Bianchi, L.; Llovet, J.M.; Bruix, J. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: Are response evaluation criteria in solid tumors reliable? Cancer 2009, 115, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mähringer-Kunz, A.; Wagner, F.; Hahn, F.; Weinmann, A.; Brodehl, S.; Schotten, S.; Hinrichs, J.B.; Düber, C.; Galle, P.R.; dos Santos, D.P.; et al. Predicting survival after transarterial chemoembolization for hepatocellular carcinoma using a neural network: A Pilot Study. Liver Int. 2020, 40, 694–703. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, W.; Han, G. Response assessment for HCC patients treated with repeated TACE: The optimal time-point is still an open issue. J. Hepatol. 2015, 63, 1530–1531. [Google Scholar] [CrossRef]
- Georgiades, C.; Geschwind, J.F.; Harrison, N.; Hines-Peralta, A.; Liapi, E.; Hong, K.; Wu, Z.; Kamel, I.; Frangakis, C. Lack of response after initial chemoembolization for hepatocellular carcinoma: Does it predict failure of subsequent treatment? Radiology 2012, 265, 115–123. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.U.; Kim, K.A.; Chung, Y.E.; Kim, M.J.; Park, M.S.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, M.D.; et al. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J. Hepatol. 2015, 62, 1304–1310. [Google Scholar] [CrossRef]
- Gillmore, R.; Stuart, S.; Kirkwood, A.; Hameeduddin, A.; Woodward, N.; Burroughs, A.K.; Meyer, T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J. Hepatol. 2011, 55, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.J.; Kim, H.J.; Park, J.H.; Park, D., II; Cho, Y.K.; Sohn, C., II; Jeon, W.K.; Kim, B.I.; Kim, M.J. Radiologic response to transcatheter hepatic arterial chemoembolization and clinical outcomes in patients with hepatocellular carcinoma. Liver Int. 2014, 34, 305–312. [Google Scholar] [CrossRef]
- Tacher, V.; Lin, M.; Duran, R.; Yarmohammadi, H.; Lee, H.; Chapiro, J.; Chao, M.; Wang, Z.; Frangakis, C.; Sohn, J.H.; et al. Comparison of Existing Response Criteria in Patients with Hepatocellular Carcinoma Treated with Transarterial Chemoembolization Using a 3D Quantitative Approach. Radiology 2016, 278, 275–284. [Google Scholar] [CrossRef]
- Kim, J.H.; Sinn, D.H.; Shin, S.W.; Cho, S.K.; Kang, W.; Gwak, G.Y.; Paik, Y.H.; Lee, J.H.; Koh, K.C.; Paik, S.W.; et al. The role of scheduled second TACE in early-stage hepatocellular carcinoma with complete response to initial TACE. Clin. Mol. Hepatol. 2017, 23, 42–50. [Google Scholar] [CrossRef][Green Version]
- Jianyong, L.; Jinjing, Z.; Yefang, L.; Lunan, Y.; Jinqiang, Z.; Wentao, W.; Bo, L.; Tianfu, W.; Jiaying, Y. Response to transarterial chemoembolization may serve as selection criteria for hepatocellular carcinoma liver transplantation. Oncotarget 2017, 8, 91328–91342. [Google Scholar] [CrossRef]
- Ciftciler, R.; Demiroglu, H.; Haznedaroglu, I.C.; Sayınalp, N.; Aksu, S.; Ozcebe, O.; Goker, H.; Aydın, M.S.; Buyukasık, Y. Impact of Time between Induction Chemotherapy and Complete Remission on Survival Outcomes in Patients with Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2019, 19, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Sandmaier, B.M.; Storer, B.E.; Godwin, C.D.; Buckley, S.A.; Pagel, J.M.; Sorror, M.L.; Deeg, H.J.; Storb, R.; Ap-pelbaum, F.R. Number of Courses of Induction Therapy Independently Predicts Outcome after Allogeneic Transplantation for Acute Myeloid Leukemia in First Morphological Remission. Biol. Blood Marrow Transplant. 2015, 21, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: Imaging of hepatocellular carcinoma in at-risk pa-tients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef]
- Berenguer, M.; Herreras, J.; Di Maira, T.; Vinaixa, C.; Juan, F.S.; Rubín, Á. Milan-out Criteria and Worse Intention-to-Treat Outcome Postliver Transplantation. Transplantation 2019, 5, e487. [Google Scholar] [CrossRef]
- Wehling, C.; Dill, M.T.; Olkus, A.; Springfeld, C.; Chang, D.-H.; Naumann, P.; Longerich, T.; Kratochwil, C.; Mehrabi, A.; Merle, U.; et al. Treatment stage migration and treatment sequences in patients with hepatocellular carcinoma: Drawbacks and opportunities. J. Cancer Res. Clin. Oncol. 2021, 147, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach—The albi grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Kadalayil, L.; Benini, R.; Pallan, L.; O’Beirne, J.; Marelli, L.; Yu, D.; Hackshaw, A.; Fox, R.; Johnson, P.; Burroughs, A.K.; et al. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann. Oncol. 2013, 24, 2565–2570. [Google Scholar] [CrossRef]
- Park, C.; Chu, H.H.; Kim, J.H.; Kim, S.Y.; Alrashidi, I.; Gwon, D., II; Yoon, H.; Kim, N. Clinical significance of the initial and best responses after chemoembolization in the treatment of intermediate-stage hepatocellular carcinoma with pre-served liver function. J. Vasc. Interv. Radiol. 2020, 31, 1998–2006.e1. [Google Scholar] [CrossRef] [PubMed]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Sgrazzutti, C.; Ciulli, C.; Vella, I.; Vicentin, I.; Incarbone, N.; Bagnardi, V.; et al. A retrospective single-centre analysis of the oncological impact of LI-RADS classification applied to Metroticket 2.0 calculator in liver transplantation: Every nodule matters. Transpl. Int. 2021, 34, 1712–1721. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Chen, H.; Zhao, Y.; Bai, W.; Yin, Z.; He, C.; Jia, J.; Yang, M.; Xia, J.; et al. EASL- and mRECIST-Evaluated Responses to Combination Therapy of Sorafenib with Transarterial Chemoembolization Predict Survival in Patients with Hepato-cellular Carcinoma. Clin. Cancer Res. 2014, 20, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lee, H.C.; Kim, S.O.; Shin, Y.M.; Kim, K.M.; Lim, Y.S.; Suh, D.J. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 2012, 262, 708–718. [Google Scholar] [CrossRef]
- Bartnik, K.; Podgórska, J.; Rosiak, G.; Korzeniowski, K.; Giziński, J.; Sajdek, M.; Wróblewski, T.; Zieniewicz, K.; Nyckowski, P.; Rowiński, O. Performance of initial LI-RADS 2018 treatment response in predicting survival of patients with hepatocellular carcinoma following TACE: A retrospective, single-center cohort study. J. Cancer Res. Clin. Oncol. 2021. [Google Scholar] [CrossRef]
- Biolato, M.; Gallusi, G.; Iavarone, M.; Cabibbo, G.; Racco, S.; De Santis, A.; Corte, C.D.; Maida, M.; Attili, A.F.; Sangio-Vanni, A.; et al. Prognostic ability of BCLC-B Subclassification in Patients with Hepatocellular Carcinoma Undergoing Transarteri-al Chemoembolization. Ann. Hepatol. 2018, 17, 110–118. [Google Scholar] [CrossRef]
- Burrel, M.; Reig, M.; Forner, A.; Barrufet, M.; de Lope, C.R.; Tremosini, S.; Ayuso, C.; Llover, J.M.; Real, M.I.; Bruix, J. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Im-plications for clinical practice and trial design. J. Hepatol. 2012, 56, 1330–1335. [Google Scholar] [CrossRef]
- Han, G.; Berhane, S.; Toyoda, H.; Bettinger, D.; Elshaarawy, O.; Chan, A.W.H.; Kirstein, M.; Mosconi, C.; Hucke, F.; Palmer, D.; et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology 2020, 72, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shen, L.; Zhao, L.; Guan, Z.; Chen, Q.; Li, W. Combined transarterial chemoembolization and microwave ablation versus transarterial chemoembolization in BCLC stage B hepatocellular carcinoma. Diagn. Interv. Radiol. 2018, 24, 219–224. [Google Scholar] [CrossRef]
- Yen, C.; Sharma, R.; Rimassa, L.; Arizumi, T.; Bettinger, D.; Choo, H.Y.; Pressiani, T.; Burlone, M.E.; Pirisi, M.; Giordano, L.; et al. Treatment Stage Migration Maximizes Survival Outcomes in Patients with Hepatocellular Carcinoma Treated with Soraf-enib: An Observational Study. Liver Cancer 2017, 6, 313–324. [Google Scholar] [CrossRef]
| Variable | No LTC | LTC | p Value |
|---|---|---|---|
| N of patients | 57 | 82 | - |
| Age (years) | |||
| <60 | 17 (30%) | 21 (26%) | 0.56 |
| >60 | 39 (70%) | 61 (74%) | |
| Gender | |||
| Male | 46 (81%) | 58 (71%) | 0.23 |
| Female | 11 (19%) | 24 (29%) | |
| BCLC stage | |||
| A | 16 (28%) | 51 (62%) | <0.001 |
| B | 41 (72%) | 31 (38%) | |
| Child Turcotte Pugh Class | |||
| A | 48 (84%) | 75 (91%) | 0.27 |
| B | 9 (16%) | 7 (9%) | |
| Serum AFP | |||
| <200 ng/mL | 32 (56%) | 64 (78%) | 0.01 |
| ≥200 ng/mL | 25 (44%) | 18 (22%) | |
| ALBI | |||
| 1 | 27 (47%) | 51 (62%) | 0.16 |
| 2 | 26 (46%) | 29 (35%) | |
| 3 | 4 (7%) | 2 (2%) | |
| Albumin, g/L | 3.95 (2.5–5.0) | 4.1 (2.0–5.2) | 0.17 |
| Creatinine | 0.87 (0.56–1.56) | 0.91 (0.54–1.97) | 0.65 |
| Total bilirubin (umol/L) | 0.98 (0.3–5.6) | 1.05 (0.21–4.04) | 0.14 |
| INR | 1.15 (0.91-1.66) | 1.15 (0.92–2.45) | 0.82 |
| ALT, IU/L | 47 (12–348) | 49 (19–303) | 0.69 |
| AST, IU/L | 65 (18–408) | 50 (20–423) | 0.02 |
| N of treated HCC lesions | |||
| 1 | 22 (39%) | 55 (67%) | 0.003 |
| 2 | 17 (30%) | 19 (23%) | |
| ≥3 | 18 (31%) | 4 (10%) | |
| Tumor size | |||
| <30 mm | 9 (16%) | 32 (39%) | 0.002 |
| 30–50 mm | 20 (35%) | 31 (38%) | |
| >50 mm | 28 (49%) | 19 (23%) | |
| Fulfilled Milan criteria | |||
| Yes | 6 (11%) | 21 (26%) | 0.03 |
| No | 51 (89%) | 61 (74%) |
| Variable | 2 Sessions | 3 Sessions | >3 Sessions | p Value |
|---|---|---|---|---|
| N of patients | 42 | 26 | 14 | - |
| Age (years) | ||||
| <60 | 13 (31%) | 5 (19%) | 3 (21%) | 0.59 |
| >60 | 29 (69%) | 21 (81%) | 11 (79%) | |
| Gender | ||||
| Male | 27 (64%) | 22 (85%) | 9 (64%) | 0.16 |
| Female | 15 (36%) | 4 (15%) | 5 (36%) | |
| Child Turcotte Pugh Class | ||||
| A | 40 (95%) | 24 (92%) | 11 (79%) | 0.16 |
| B | 2 (5%) | 2 (8%) | 3 (23%) | |
| BCLC Stage | ||||
| A | 30 (71%) | 16 (62%) | 5 (36%) | 0.06 |
| B | 12 (29%) | 10 (38%) | 9 (64%) | |
| Serum AFP | ||||
| <200 ng/mL | 32 (76%) | 21 (81%) | 11 (79%) | 0.94 |
| ≥200 ng/mL | 10 (24%) | 5 (19%) | 3 (21%) | |
| ALBI | ||||
| 1 | 27 (64%) | 17 (65%) | 7 (50%) | 0.73 |
| 2 | 14(33%) | 8 (31%) | 7 (50%) | |
| 3 | 1 (3%) | 1 (4%) | 0 (0%) | |
| Albumin, g/L | 4.15 (3.0–5.2) | 4.3 (2.0–5.2) | 3.9 (2.8–4.6) | 0.16 |
| Creatinine | 0.89 (0.54–1.97) | 0.92 (0.65–1.35) | 0.89 (0.59–1.42) | 0.81 |
| Total bilirubin (umol/L) | 0.85 (0.24–4.04) | 0.98 (0.43–2.0) | 1.01 (0.42–3.0) | 0.76 |
| INR | 1.15 (0.92–2.45) | 1.16 (1.01–1.69) | 1.17 (0.97–1.43) | 0.97 |
| ALT, IU/L | 49 (19–200) | 49 (20–303) | 55 (23–264) | 0.6 |
| AST, IU/L | 42 (20–189) | 50 (22–243) | 72 (22–193) | 0.14 |
| N of treated HCC lesions | ||||
| 1 | 32 (76%) | 16 (62%) | 7 (50%) | 0.31 |
| 2 | 7 (17%) | 8 (31%) | 4 (29%) | |
| ≥3 | 3 (7%) | 2 (7%) | 3 (21%) | |
| Tumor size | ||||
| <30 mm | 19 (45%) | 9 (35%) | 4 (29%) | 0.07 |
| 30–50 mm | 18 (43%) | 10 (38%) | 3 (21%) | |
| >50 mm | 5 (12%) | 7 (27%) | 7 (50%) |
| Variable | Hazard Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Overall survival | |||
| LTC × 2 sessions | 1.00 | reference | |
| LTC × 3 sessions | 0.96 | 0.76–2.36 | 0.41 |
| LTC × >3 sessions | 1.74 | 1.00–1.01 | 0.16 |
| Time to LTC (weeks) | 1.03 | 1.01–1.6 | 0.04 |
| BCLC A | 1.00 | reference | |
| BCLC B | 2.06 | 1.06–4.02 | 0.03 |
| CPS A | 1.00 | reference | |
| CPS B | 1.87 | 0.64–5.38 | 0.25 |
| ALBI grade 1 | 1.00 | reference | |
| ALBI grade 2 | 1.73 | 0.87–3.44 | 0.93 |
| ALBI grade 3 | 3.25 | 0.42–24.84 | 0.38 |
| Progression free survival | |||
| LTC × 2 sessions | 1.00 | reference | |
| LTC × 3 sessions | 0.92 | 0.49–1.75 | 0.76 |
| LTC × >3 sessions | 0.69 | 0.30–1.61 | 0.43 |
| Time to LTC (weeks) | 1.05 | 1.02–1.09 | 0.004 |
| BCLC A | 1.00 | reference | |
| BCLC B | 1.86 | 1.04–3.35 | 0.04 |
| CPS A | 1.00 | reference | |
| CPS B | 2.02 | 0.79–5.16 | 0.14 |
| ALBI grade 1 | 1.00 | reference | |
| ALBI grade 2 | 1.48 | 0.82–2.67 | 0.68 |
| ALBI grade 3 | 3.16 | 0.74–13.41 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartnik, K.; Hołówko, W.; Rowiński, O. Association between Time to Local Tumor Control and Treatment Outcomes Following Repeated Loco-Regional Treatment Session in Patients with Hepatocellular Carcinoma: A Retrospective, Single-Center Study. Life 2021, 11, 1062. https://doi.org/10.3390/life11101062
Bartnik K, Hołówko W, Rowiński O. Association between Time to Local Tumor Control and Treatment Outcomes Following Repeated Loco-Regional Treatment Session in Patients with Hepatocellular Carcinoma: A Retrospective, Single-Center Study. Life. 2021; 11(10):1062. https://doi.org/10.3390/life11101062
Chicago/Turabian StyleBartnik, Krzysztof, Wacław Hołówko, and Olgierd Rowiński. 2021. "Association between Time to Local Tumor Control and Treatment Outcomes Following Repeated Loco-Regional Treatment Session in Patients with Hepatocellular Carcinoma: A Retrospective, Single-Center Study" Life 11, no. 10: 1062. https://doi.org/10.3390/life11101062
APA StyleBartnik, K., Hołówko, W., & Rowiński, O. (2021). Association between Time to Local Tumor Control and Treatment Outcomes Following Repeated Loco-Regional Treatment Session in Patients with Hepatocellular Carcinoma: A Retrospective, Single-Center Study. Life, 11(10), 1062. https://doi.org/10.3390/life11101062





