Histocompatibility Antigen, Class I, G (HLA-G)’s Role during Pregnancy and Parturition: A Systematic Review of the Literature
Abstract
1. Introduction
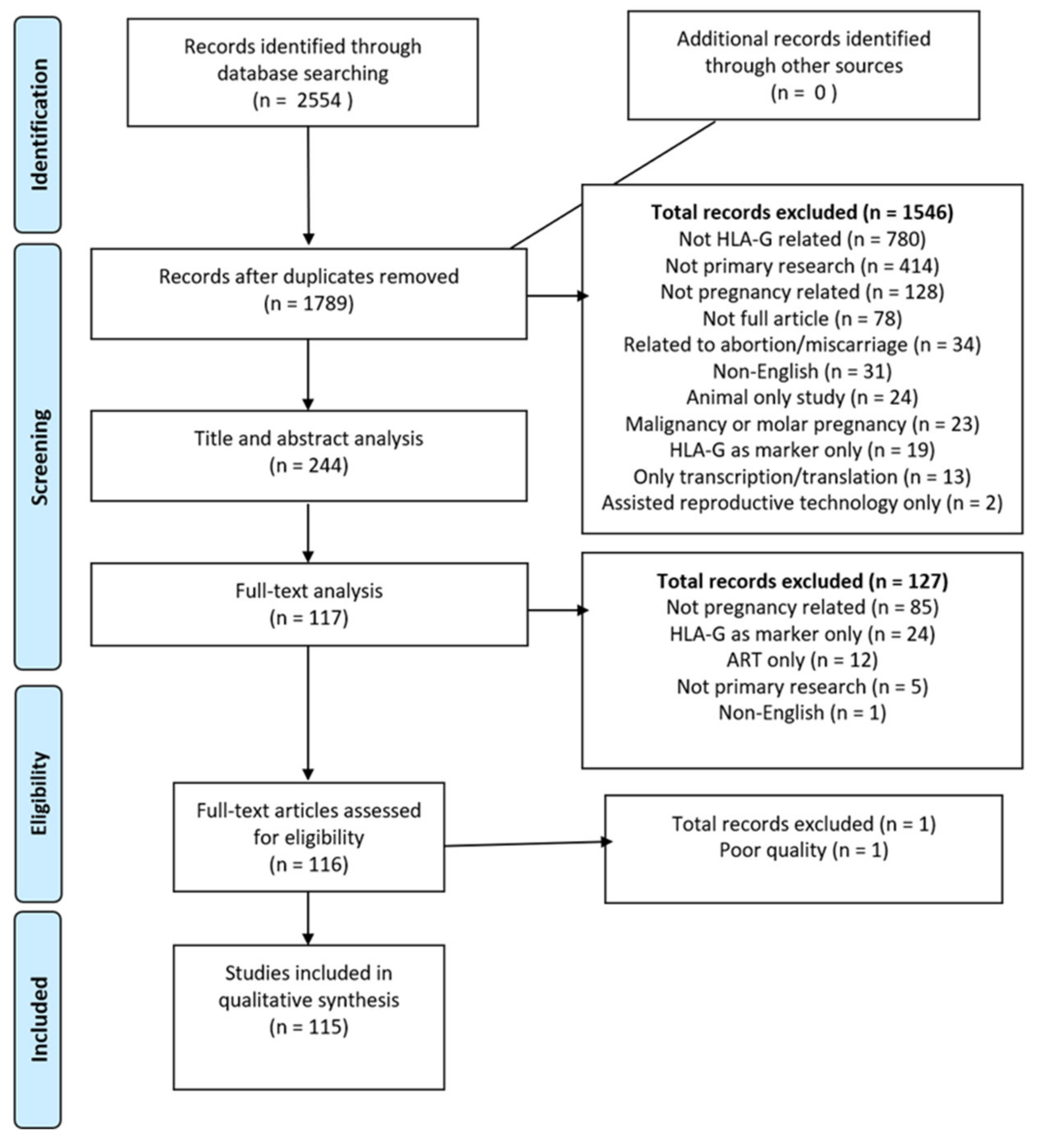
2. Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Collection and Analysis
2.4. Data Synthesis
3. Results
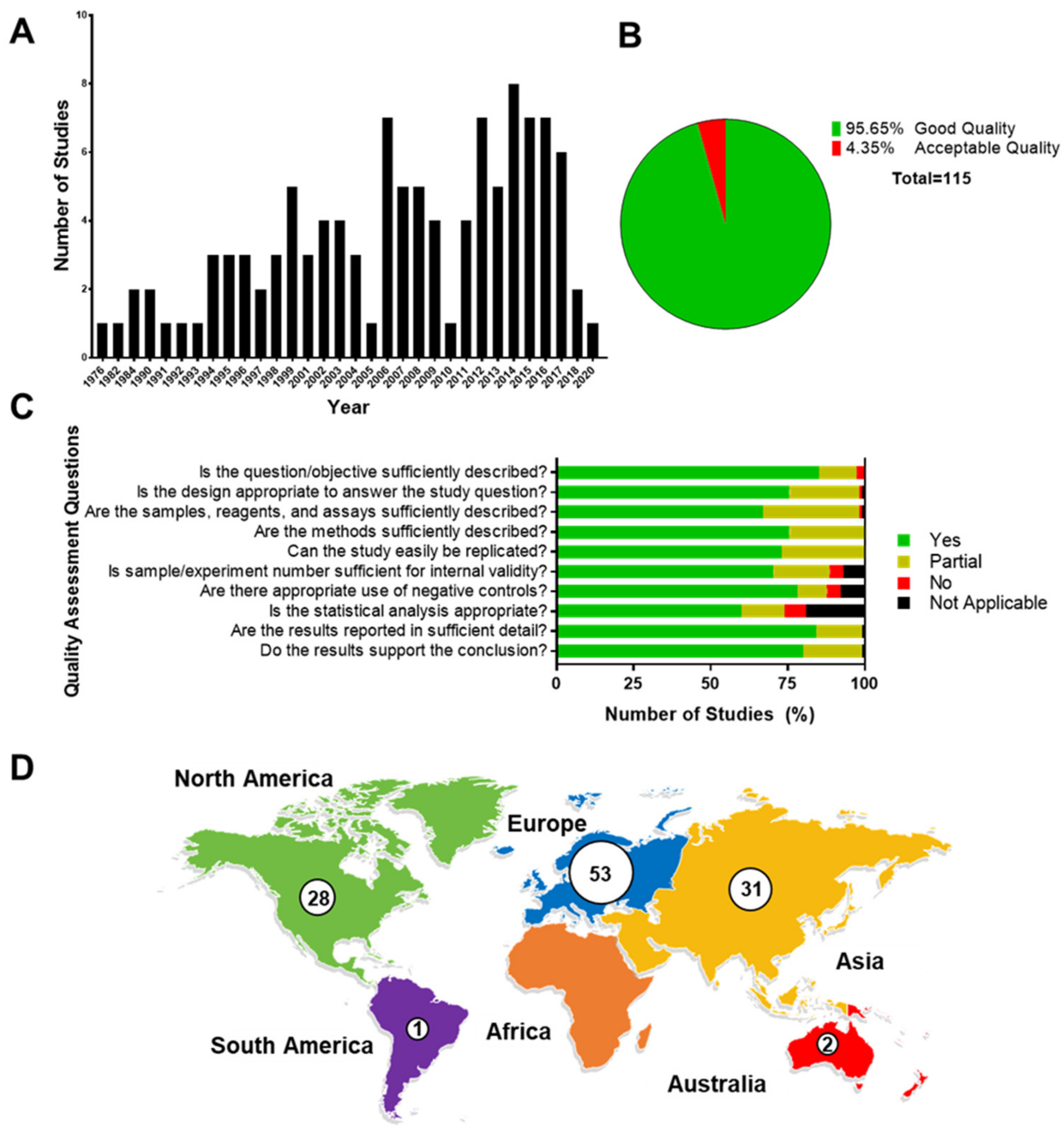
3.1. Quality Assessment
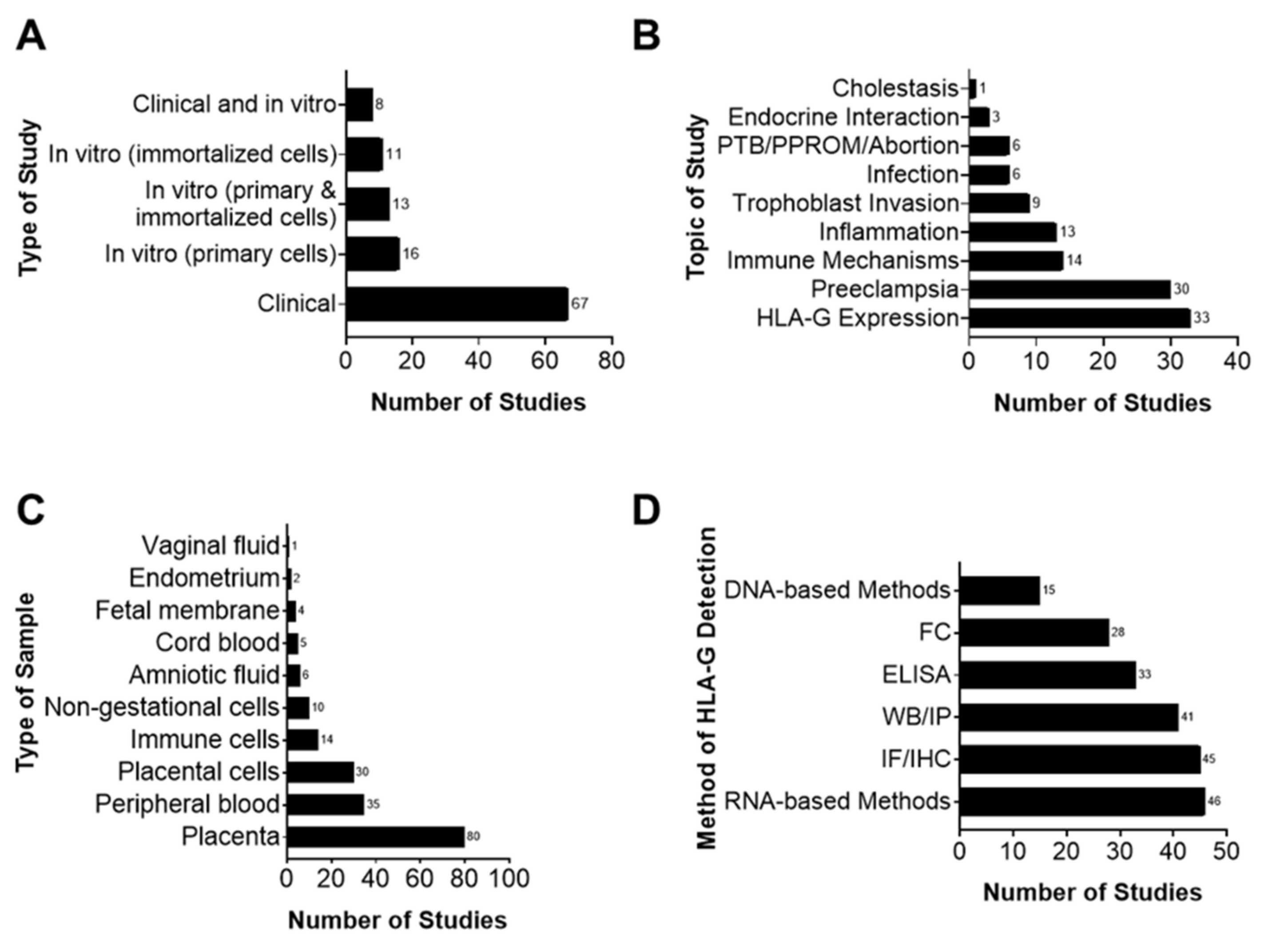
3.2. Main Characteristics of Studies
3.3. Methods Used to Detect HLA-G Expression and Interactions
3.4. Main Findings
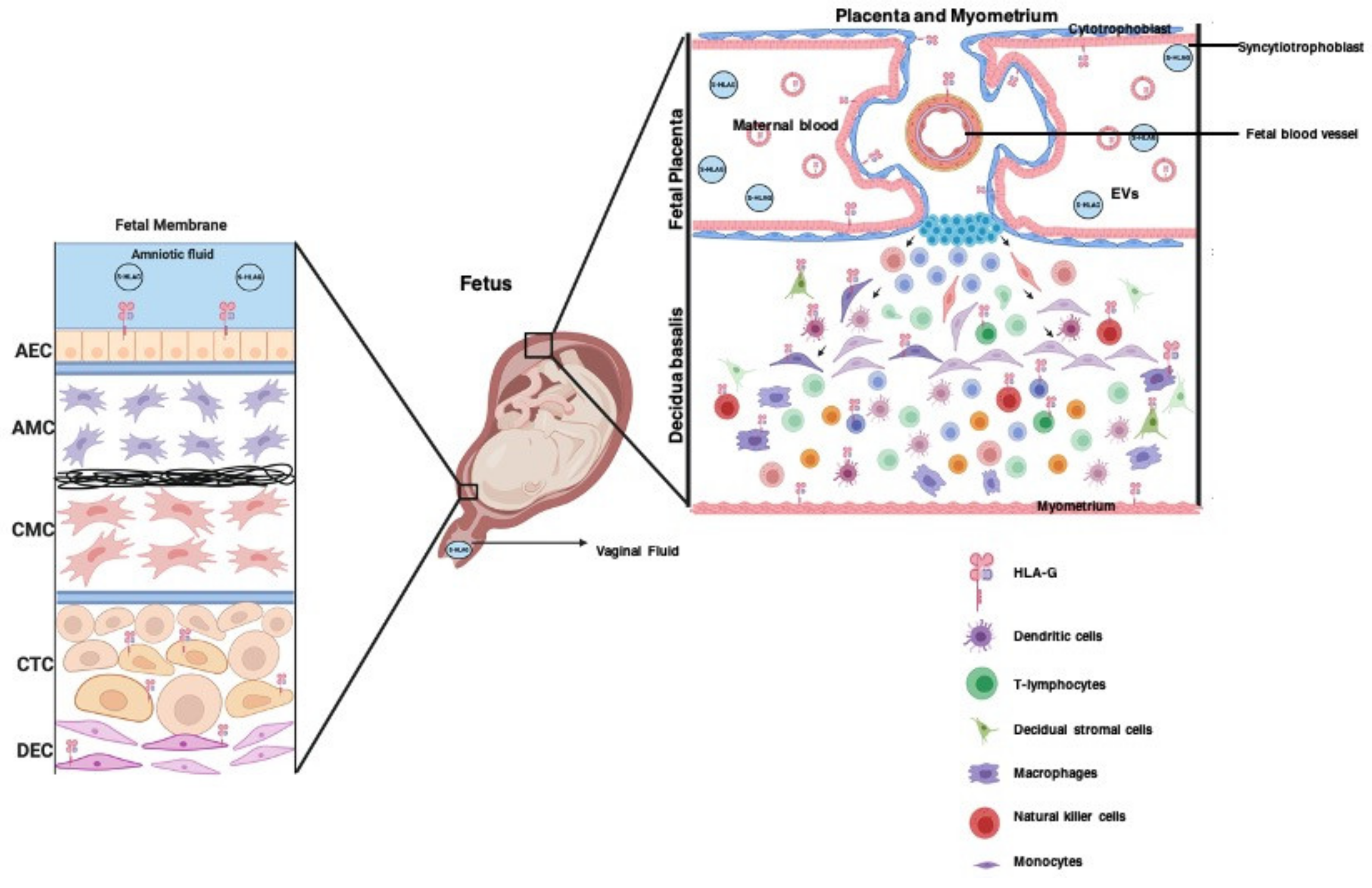
3.5. The Role of HLA-G in Placentation
3.6. The Role of HLA-G in Promoting Maternal–Fetal Tolerance in Pregnancy
3.7. The Role of HLA-G in the Pathophysiology of Pregnancy Complications
3.8. Extracellular Vesicles Package HLA-G during Pregnancy
4. Discussion
5. Future Directions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medawar, P. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp. Soc. Exp. Biol. 1953, 7, 320–337. [Google Scholar]
- Sankaran, S. Creasy and Resnik’s maternal–fetal medicine: Principles and practice sixth edition. Obstet. Med. 2012, 5, 88–89. [Google Scholar] [CrossRef]
- Stites, D.P.; Pavia, C.S.; Clemens, L.E.; Kuhn, R.W.; Siiteri, P.K. Immunologic regulation in pregnancy. Arthritis Rheum. 1979, 22, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Formby, B. Immunologic response in pregnancy: Its role in endocrine disorders of pregnancy and influence on the course of maternal autoimmune diseases. Endocrinol. Metab. Clin. N. Am. 1995, 24, 187–205. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; DeMars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef]
- Le Bouteiller, P.; Mallet, V. HLA-G and pregnancy. Rev. Reprod. 1997, 2, 7–13. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; McIntire, R.H.; Ober, C. HLA-G and immune tolerance in pregnancy. FASEB J. 2005, 19, 681–693. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Petroff, M.G.; Perchellet, A. B7 family molecules as regulators of the maternal immune system in pregnancy. Am. J. Reprod. Immunol. 2010, 63, 506–519. [Google Scholar] [CrossRef]
- Orr, H.T.; Bach, F.H.; Ploegh, H.L.; Strominger, J.L.; Kavathas, P.; DeMars, R. Use of HLA loss mutants to analyse the structure of the human major histocompatibility complex. Nature 1982, 296, 454–456. [Google Scholar] [CrossRef]
- Bodmer, J.G.; Marsh, S.G.; Albert, E. Nomenclature for factors of the HLA system, 1989. Immunol. Today 1990, 11, 3–10. [Google Scholar] [CrossRef]
- Redman, C.W.; McMichael, A.J.; Stirrat, G.M.; Sunderland, C.A.; Ting, A. Class 1 major histocompatibility complex antigens on human extra-villous trophoblast. Immunology 1984, 52, 457–468. [Google Scholar] [PubMed]
- Le Discorde, M.; Moreau, P.; Sabatier, P.; Legeais, J.-M.; Carosella, E.D. Expression of HLA-G in human cornea, an immune-privileged tissue. Hum. Immunol. 2003, 64, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, V.; Zalatan, J.; McMaster, M.; Prinsen, R.; Salomon, D.R.; Ricordi, C.; Torbett, B.E.; Meda, P.; Crisa, L. The class I HLA repertoire of pancreatic islets comprises the nonclassical class Ib antigen HLA-G. Diabetes 2006, 55, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Mallet, V.; Blaschitz, A.; Crisa, L.; Schmitt, C.; Fournel, S.; King, A.; Loke, Y.W.; Dohr, G.; Le Bouteiller, P. HLA-G in the human thymus: A subpopulation of medullary epithelial but not CD83+ dendritic cells expresses HLA-G as a membrane-bound and soluble protein. Int. Immunol. 1999, 11, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Cabestre, F.A.; Lefebvre, S.; Moreau, P.; Rouas-Friess, N.; Dausset, J.; Carosella, E.D.; Paul, P. HLA-G expression: Immune privilege for tumour cells? Semin. Cancer Biol. 1999, 9, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tilburgs, T.; Evans, J.H.; Crespo, Â.C.; Strominger, J.L. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2015, 112, 13312–13317. [Google Scholar] [CrossRef] [PubMed]
- Chumbley, G.; King, A.; Robertson, K.; Holmes, N.; Loke, Y.W. Resistance of HLA-G and HLA-A2 transfectants to lysis by decidual NK cells. Cell. Immunol. 1994, 155, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Long, E.O. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. J. Exp. Med. 1999, 189, 1093–1100. [Google Scholar] [CrossRef]
- Pazmany, L.; Mandelboim, O.; Valés-Gómez, M.; Davis, D.M.; Reyburn, H.T.; Strominger, J.L. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science 1996, 274, 792–795. [Google Scholar] [CrossRef]
- Kirszenbaum, M.; Moreau, P.; Teyssier, M.; Lafon, C.; Gluckman, E.; Dausset, J.; Carosella, E. Evidence for the presence of the alternatively spliced HLA-G mRNA forms in human mononuclear cells from peripheral blood and umbilical cord blood. Hum. Immunol. 1995, 43, 237–241. [Google Scholar] [CrossRef]
- Fujii, T.; Ishitani, A.; Geraghty, D.E. A soluble form of the HLA-G antigen is encoded by a messenger ribonucleic acid containing intron 4. J. Immunol. 1994, 153, 5516–5524. [Google Scholar]
- Ishitani, A.; Geraghty, D.E. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc. Natl. Acad. Sci. USA 1992, 89, 3947–3951. [Google Scholar] [CrossRef]
- HoWangYin, K.-Y.; Loustau, M.; Wu, J.; Alegre, E.; Daouya, M.; Caumartin, J.; Sousa, S.; Horuzsko, A.; Carosella, E.D.; LeMaoult, J. Multimeric structures of HLA-G isoforms function through differential binding to LILRB receptors. Cell. Mol. Life Sci. 2012, 69, 4041–4049. [Google Scholar] [CrossRef]
- Rizzo, R.; Gabrielli, L.; Bortolotti, D.; Gentili, V.; Piccirilli, G.; Chiereghin, A.; Pavia, C.; Bolzani, S.; Guerra, B.; Simonazzi, G.; et al. Study of soluble HLA-G in congenital human cytomegalovirus infection. J. Immunol. Res. 2016, 2016, 3890306. [Google Scholar] [CrossRef]
- Park, G.M.; Lee, S.; Park, B.; Kim, E.; Shin, J.; Cho, K.; Ahn, K. Soluble HLA-G generated by proteolytic shedding inhibits NK-mediated cell lysis. Biochem. Biophys. Res. Commun. 2004, 313, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, V.; Morandi, F.; Wang, X.; Ferrone, S. Soluble HLA-G: Are they clinically relevant? Semin. Cancer Biol. 2007, 17, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.M.R.; Meissner, T.B.; Tilburgs, T.; Strominger, J.L. HLA-G: At the interface of maternal-fetal tolerance. Trends Immunol. 2017, 38, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human Leucocyte Antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39. [Google Scholar] [CrossRef]
- Sipak, O.; Rył, A.; Grzywacz, A.; Laszczyńska, M.; Zimny, M.; Karakiewicz, B.; Rotter, I.; Kosik-Bogacka, D.; Cybulski, C. The relationship between the HLA-G polymorphism and sHLA-G levels in parental pairs with high-risk pregnancy. Int. J. Environ. Res. Public Health 2019, 16, 1546. [Google Scholar] [CrossRef] [PubMed]
- Knafel, A.; Basta, P.; Pitynski, K.; Mach, P.; Bednarek, W.; Klimek, M.; Zietek, J.; Zajac, K.; Dancewicz, L.; Iwaniec, M.; et al. Soluble HLA-G changes in maternal blood serum during the progression of labor. Neuro-Endocrinol. Lett. 2009, 30, 67–73. [Google Scholar] [PubMed]
- Papuchova, H.; Kshirsagar, S.; Xu, L.; Hanna, A.; Gomes, B.; Li, Q.; Iyer, V.; Norwitz, E.; Strominger, J.; Tilburgs, T. Three types of HLA-G+ extravillous trophoblasts that have distinct immune regulatory properties. Proc. Natl. Acad. Sci. USA 2020, 117, 15772–15777. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Richardson, L.; Martin, L.; Jin, J.; Menon, R. Systematic review of p38 mitogen-activated kinase and its functional role in reproductive tissues. Am. J. Reprod. Immunol. 2018, 80, e13047. [Google Scholar] [CrossRef]
- Lavu, N.; Richardson, L.; Bonney, E.; Menon, R. Glycogen Synthase Kinase (GSK) 3 in pregnancy and parturition: A systematic review of literature. J. Matern.-Fetal Neonatal Med. 2020, 33, 1946–1957. [Google Scholar] [CrossRef]
- Hadley, E.E.; Richardson, L.S.; Torloni, M.R.; Menon, R. Gestational tissue inflammatory biomarkers at term labor: A systematic review of literature. Am. J. Reprod. Immunol. 2018, 79, e12776. [Google Scholar] [CrossRef]
- Hackmon, R.; Pinnaduwage, L.; Zhang, J.; Lye, S.J.; Geraghty, D.E.; Dunk, C.E. Definitive class I human leukocyte antigen expression in gestational placentation: HLA-F, HLA-E, HLA-C, and HLA-G in extravillous trophoblast invasion on placentation, pregnancy, and parturition. Am. J. Reprod. Immunol. 2017, 77, e12643. [Google Scholar] [CrossRef]
- Sageshima, N.; Ishitani, A.; Omura, M.; Akasaki, M.; Umekage, H.; Katabuchi, H.; Okamura, H.; Hatake, K. Necrotic feature of the trophoblasts lacking HLA-G expression in normal and pre-eclamptic placentas. Am. J. Reprod. Immunol. 2003, 49, 174–182. [Google Scholar] [CrossRef]
- Guo, Y.; Lee, C.-L.; So, K.-H.; Gao, J.; Yeung, W.S.B.; Yao, Y.; Lee, K.F. Soluble human leukocyte antigen-g5 activates extracellular signal-regulated protein kinase signaling and stimulates trophoblast invasion. PLoS ONE 2013, 8, e76023. [Google Scholar]
- McCormick, J.; Whitley, G.S.J.; Le Bouteiller, P.; Cartwright, J.E. Soluble HLA-G regulates motility and invasion of the trophoblast-derived cell line SGHPL-4. Hum. Reprod. 2009, 24, 1339–1345. [Google Scholar] [CrossRef][Green Version]
- Choi, J.H.; Jung, J.; Na, K.-H.; Cho, K.J.; Yoon, T.K.; Kim, G.J. Effect of mesenchymal stem cells and extracts derived from the placenta on trophoblast invasion and immune responses. Stem Cells Dev. 2014, 23, 132–145. [Google Scholar] [CrossRef]
- Komatsu, T.; Konishi, I.; Mandai, M.; Mori, T.; Hiai, H.; Fukumoto, M. Expression of class I Human Leukocyte Antigen (HLA) and beta2-microglobulin is associated with decidualization of human endometrial stromal cells. Hum. Reprod. 1998, 13, 2246–2251. [Google Scholar] [CrossRef]
- Kanai, T.; Fujii, T.; Kozuma, S.; Miki, A.; Yamashita, T.; Hyodo, H.; Unno, N.; Yoshida, S.; Taketani, Y. A subclass of soluble HLA-G1 modulates the release of cytokines from mononuclear cells present in the decidua additively to membrane-bound HLA-G1. J. Reprod. Immunol. 2003, 60, 85–96. [Google Scholar] [CrossRef]
- Lombardelli, L.; Aguerre-Girr, M.; Logiodice, F.; Kullolli, O.; Casart, Y.; Polgar, B.; Berrebi, A.; Romagnani, S.; Maggi, E.; Le Bouteiller, P.; et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4+ T cells and macrophages. J. Immunol. 2013, 191, 3651–3662. [Google Scholar] [CrossRef] [PubMed]
- Gorvel, L.; ben Amara, A.; Ka, M.B.; Textoris, J.; Gorvel, J.-P.; Mege, J.-L. Myeloid decidual dendritic cells and immunoregulation of pregnancy: Defective responsiveness to Coxiella burnetii and Brucella abortus. Front. Cell. Infect. Microbiol. 2014, 4, 179. [Google Scholar] [CrossRef] [PubMed]
- Rieger, L.; Hofmeister, V.; Probe, C.; Dietl, J.; Weiss, E.H.; Steck, T.; Kämmerer, U. Th1- and Th2-like cytokine production by first trimester decidual large granular lymphocytes is influenced by HLA-G and HLA-E. Mol. Hum. Reprod. 2002, 8, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kanai, T.; Fujii, T.; Unno, N.; Yamashita, T.; Hyodo, H.; Miki, A.; Hamai, Y.; Kozuma, S.; Taketani, Y. Human leukocyte antigen-G-expressing cells differently modulate the release of cytokines from mononuclear cells present in the decidua versus peripheral blood. Am. J. Reprod. Immunol. 2001, 45, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.; Santner-Nanan, B.; Dahlstrom, J.E.; Fadia, M.; Chandra, A.; Peek, M.; Nanan, R. Altered decidual DC-SIGN+ antigen-presenting cells and impaired regulatory T-cell induction in preeclampsia. Am. J. Pathol. 2012, 181, 2149–2160. [Google Scholar] [CrossRef]
- Köstlin, N.; Ostermeir, A.-L.; Spring, B.; Schwarz, J.; Marmé, A.; Walter, C.B.; Poets, C.F.; Gille, C. HLA-G promotes myeloid-derived suppressor cell accumulation and suppressive activity during human pregnancy through engagement of the receptor ILT4. Eur. J. Immunol. 2017, 47, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Marlin, R.; Duriez, M.; Berkane, N.; de Truchis, C.; Madec, Y.; Rey-Cuille, M.A.; Cummings, J.S.; Cannou, C.; Quillay, H.; Barré-Sinoussi, F.; et al. Dynamic shift from CD85j/ILT-2 to NKG2D NK receptor expression pattern on human decidual NK during the first trimester of pregnancy. PLoS ONE 2012, 7, e30017. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.L.; Han, Y.; Chen, J.H.; Yao, Y.Q. Down-regulation of HLA-G boosted natural killer cell-mediated cytolysis in JEG-3 cells cultured in vitro. Fertil. Steril. 2008, 90, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Han, Z.Q.; Zhou, H.; Zou, L.; Zou, P. Inhibition of HLA-G expression via RNAi abolishes resistance of extravillous trophoblast cell line TEV-1 to NK lysis. Placenta 2010, 31, 519–527. [Google Scholar] [CrossRef]
- Lindaman, A.; Dowden, A.; Zavazava, N. Soluble HLA-G molecules induce apoptosis in natural killer cells. Am. J. Reprod. Immunol. 2006, 56, 68–76. [Google Scholar] [CrossRef]
- Han, M.; Jiang, Y.; Lao, K.; Xu, X.; Zhan, S.; Wang, Y. sHLA-G involved in the apoptosis of decidual natural killer cells following Toxoplasma gondii infection. Inflammation 2014, 37, 1718–1727. [Google Scholar] [CrossRef]
- Djurisic, S.; Skibsted, L.; Hviid, T.V.F. A phenotypic analysis of regulatory T cells and uterine NK cells from first trimester pregnancies and associations with HLA-G. Am. J. Reprod. Immunol. 2015, 74, 427–444. [Google Scholar] [CrossRef]
- Steinborn, A.; Varkonyi, T.; Scharf, A.; Bahlmann, F.; Klee, A.; Sohn, C. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. Am. J. Reprod. Immunol. 2007, 57, 277–286. [Google Scholar] [CrossRef]
- Rokhafrooz, S.; Ghadiri, A.; Ghandil, P.; Ghafourian, M.; Hossaini, S.H.; Daraei, N.; Najafian, M.; Rouhizadeh, A. Association between HLA-G 14bp gene polymorphism and serum sHLA-G protein concentrations in preeclamptic patients and normal pregnant women. Immunol. Investig. 2018, 47, 558–568. [Google Scholar] [CrossRef]
- Marozio, L.; Garofalo, A.; Berchialla, P.; Tavella, A.M.; Salton, L.; Cavallo, F.; Benedetto, C. Low expression of soluble human leukocyte antigen G in early gestation and subsequent placenta-mediated complications of pregnancy. J. Obstet. Gynaecol. Res. 2017, 43, 1391–1396. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Kolarz, B.; Rolinski, J.; Leszczynska-Gorzelak, B.; Oleszczuk, J. The concentrations of soluble HLA-G protein are elevated during mid-gestation and decreased in pre-eclampsia. Folia Histochem. Cytobiol. 2012, 50, 286–291. [Google Scholar] [CrossRef][Green Version]
- Hsu, P.; Santner-Nanan, B.; Joung, S.; Peek, M.J.; Nanan, R. Expansion of CD4+ HLA-G+ T cell in human pregnancy is impaired in pre-eclampsia. Am. J. Reprod. Immunol. 2014, 71, 217–228. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Li, H.; Peng, T.; Gu, W.; Li, X. Hypermethylation of the HLA-G promoter is associated with preeclampsia. Mol. Hum. Reprod. 2015, 21, 736–744. [Google Scholar] [CrossRef]
- Hviid, T.V. HLA-G genotype is associated with fetoplacental growth. Hum. Immunol. 2004, 65, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Zhou, Y.; Janatpour, M.; McMaster, M.; Bass, K.; Chun, S.H.; Fisher, S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997, 151, 1809–1818. [Google Scholar] [PubMed]
- Nevalainen, J.; Skarp, S.; Savolainen, E.-R.; Ryynänen, M.; Järvenpää, J. Intrauterine growth restriction and placental gene expression in severe preeclampsia, comparing early-onset and late-onset forms. J. Perinat. Med. 2017, 45, 869–877. [Google Scholar] [CrossRef]
- Goldman-Wohl, D.S.; Ariel, I.; Greenfield, C.; Hochner-Celnikier, D.; Cross, J.; Fisher, S.; Yagel, S. Lack of human leukocyte antigen-G expression in extravillous trophoblasts is associated with pre-eclampsia. Mol. Hum. Reprod. 2000, 6, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Fujii, T.; Yamashita, T.; Kozuma, S.; Okai, T.; Taketani, Y. Altered expression of human leukocyte antigen G (HLA-G) on extravillous trophoblasts in preeclampsia: Immunohistological demonstration with anti-HLA-G specific antibody “87G” and anti-cytokeratin antibody “CAM5.2”. Am. J. Reprod. Immunol. 1996, 36, 349–358. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, G.; Wang, J.; Lu, S.; Cao, J.; Sun, L. A novel bridge between oxidative stress and immunity: The interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, 447. [Google Scholar] [CrossRef]
- Aldrich, C.; Verp, M.S.; Walker, M.A.; Ober, C. A null mutation in HLA-G is not associated with preeclampsia or intrauterine growth retardation. J. Reprod. Immunol. 2000, 47, 41–48. [Google Scholar] [CrossRef]
- Emmery, J.; Christiansen, O.B.; Nilsson, L.L.; Dahl, M.; Skovbo, P.; Møller, A.M.; Steffensen, R.; Hviid, T.V.F. Associations between fetal HLA-G genotype and birth weight and placental weight in a large cohort of pregnant women—Possible implications for HLA diversity. J. Reprod. Immunol. 2017, 120, 8–14. [Google Scholar] [CrossRef]
- Dahl, M.; Klitkou, L.; Christiansen, O.B.; Djurisic, S.; Piosik, Z.M.; Skovbo, P.; Møller, A.M.; Steffensen, R.; Hviid, T.V. Human Leukocyte Antigen (HLA)-G during pregnancy part II: Associations between maternal and fetal HLA-G genotypes and soluble HLA-G. Hum. Immunol. 2015, 76, 260–271. [Google Scholar] [CrossRef]
- Mandò, C.; Pileri, P.; Mazzocco, M.I.; Lattuada, D.; Zolin, A.; Plebani, M.; Massari, M.; Calabrese, S.; Milani, S.; Cetin, I. Maternal and fetal HLA-G 14 bp gene polymorphism in pregnancy-induced hypertension, preeclampsia, intrauterine growth restricted and normal pregnancies. J. Matern.-Fetal Neonatal Med. 2016, 29, 1509–1514. [Google Scholar] [CrossRef]
- Iversen, A.-C.; Nguyen, O.T.D.; Tømmerdal, L.F.; Eide, I.P.; Landsem, V.M.; Acar, N.; Myhre, R.; Klungland, H.; Austgulen, R. The HLA-G 14bp gene polymorphism and decidual HLA-G 14bp gene expression in pre-eclamptic and normal pregnancies. J. Reprod. Immunol. 2008, 78, 158–165. [Google Scholar] [CrossRef]
- Bıyık, I. Maternal serum soluble HLA-G in complicated pregnancies. J. Matern.-Fetal Neonatal Med. 2014, 27, 381–384. [Google Scholar] [CrossRef]
- Beneventi, F.; Locatelli, E.; de Amici, M.; Simonetta, M.; Cavagnoli, C.; Bellingeri, C.; Scancarello, C.; Ierullo, A.; Martinetti, M.; Spinillo, A. Soluble HLA-G concentrations in maternal blood and cervical vaginal fluid of pregnant women with preterm premature rupture of membranes. J. Reprod. Immunol. 2016, 116, 76–80. [Google Scholar] [CrossRef]
- Stout, M.J.; Cao, B.; Landeau, M.; French, J.; Macones, G.A.; Mysorekar, I.U. Increased human leukocyte antigen-G expression at the maternal-fetal interface is associated with preterm birth. J. Matern.-Fetal Neonatal Med. 2015, 28, 454–459. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Romero, R.; Jodicke, C.; Mazaki-Tovi, S.; Vaisbuch, E.; Erez, O.; Mittal, P.; Gotsch, F.; Chaiworapongsa, T.; Edwin, S.S.; et al. Amniotic fluid soluble human leukocyte antigen-G in term and preterm parturition, and intra-amniotic infection/inflammation. J. Matern.-Fetal Neonatal Med. 2009, 22, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Robert-Gangneux, F.; Gangneux, J.-P.; Vu, N.; Jaillard, S.; Guiguen, C.; Amiot, L. High level of soluble HLA-G in amniotic fluid is correlated with congenital transmission of Toxoplasma gondii. Clin. Immunol. 2011, 138, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Orozco, A.F.; Jorgez, C.J.; Ramos-Perez, W.D.; Popek, E.J.; Yu, X.; Kozinetz, C.A.; Bischoff, F.Z.; Lewis, D.E. Placental release of distinct DNA-associated micro-particles into maternal circulation: Reflective of gestation time and preeclampsia. Placenta 2009, 30, 891–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hadley, E.E.; Sheller-Miller, S.; Saade, G.; Salomon, C.; Mesiano, S.; Taylor, R.N.; Menon, R. Amnion epithelial cell–derived exosomes induce inflammatory changes in uterine cells. Am. J. Obstet. Gynecol. 2018, 219, 478.e1–478.e21. [Google Scholar] [CrossRef] [PubMed]
- Menon, R. Initiation of human parturition: Signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet. Gynecol. Sci. 2019, 62, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Mesiano, S.; Taylor, R.N. Programmed fetal membrane senescence and exosome-mediated signaling: A mechanism associated with timing of human parturition. Front. Endocrinol. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Trivedi, J.; Yellon, S.M.; Menon, R. Exosomes cause preterm birth in mice: Evidence for paracrine signaling in pregnancy. Sci. Rep. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Shahin, H.I.; Radnaa, E.; Tantengco, O.A.G.; Kechichian, T.; Kammala, A.K.; Sheller-Miller, S.; Taylor, B.D.; Menon, R. Microvesicles and exosomes released by amnion epithelial cells under oxidative stress cause inflammatory changes in uterine cells. Biol. Reprod. 2021, 7, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Yie, S.; Xiao, R.; Librach, C.L. Progesterone regulates HLA-G gene expression through a novel progesterone response element. Hum. Reprod. 2006, 21, 2538–2544. [Google Scholar] [CrossRef]
- Ivanova-Todorova, E.; Mourdjeva, M.; Kyurkchiev, D.; Bochev, I.; Stoyanova, E.; Dimitrov, R.; Timeva, T.; Yunakova, M.; Bukarev, D.; Shterev, A.; et al. HLA-G expression is up-regulated by progesterone in mesenchymal stem cells. Am. J. Reprod. Immunol. 2009, 62, 25–33. [Google Scholar] [CrossRef]
- Moreau, P.; Adrian-Cabestre, F.; Menier, C.; Guiard, V.; Gourand, L.; Dausset, J.; Carosella, E.D.; Paul, P. IL-10 selectively induces HLA-G expression in human trophoblasts and monocytes. Int. Immunol. 1999, 11, 803–811. [Google Scholar] [CrossRef]
- Hladunewich, M.; Karumanchi, S.A.; Lafayette, R. Pathophysiology of the clinical manifestations of preeclampsia. Clin. J. Am. Soc. Nephrol. 2007, 2, 543–549. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin stimulates angiogenesis through VEGF and expression of HLA-G in first-trimester human placental trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantengco, O.A.G.; Richardson, L.; Lee, A.; Kammala, A.; Silva, M.d.C.; Shahin, H.; Sheller-Miller, S.; Menon, R. Histocompatibility Antigen, Class I, G (HLA-G)’s Role during Pregnancy and Parturition: A Systematic Review of the Literature. Life 2021, 11, 1061. https://doi.org/10.3390/life11101061
Tantengco OAG, Richardson L, Lee A, Kammala A, Silva MdC, Shahin H, Sheller-Miller S, Menon R. Histocompatibility Antigen, Class I, G (HLA-G)’s Role during Pregnancy and Parturition: A Systematic Review of the Literature. Life. 2021; 11(10):1061. https://doi.org/10.3390/life11101061
Chicago/Turabian StyleTantengco, Ourlad Alzeus G., Lauren Richardson, Alan Lee, Ananthkumar Kammala, Mariana de Castro Silva, Hend Shahin, Samantha Sheller-Miller, and Ramkumar Menon. 2021. "Histocompatibility Antigen, Class I, G (HLA-G)’s Role during Pregnancy and Parturition: A Systematic Review of the Literature" Life 11, no. 10: 1061. https://doi.org/10.3390/life11101061
APA StyleTantengco, O. A. G., Richardson, L., Lee, A., Kammala, A., Silva, M. d. C., Shahin, H., Sheller-Miller, S., & Menon, R. (2021). Histocompatibility Antigen, Class I, G (HLA-G)’s Role during Pregnancy and Parturition: A Systematic Review of the Literature. Life, 11(10), 1061. https://doi.org/10.3390/life11101061







