Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function: Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Transcatheter Arterial Chemoembolization
2.3. Study End Point
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
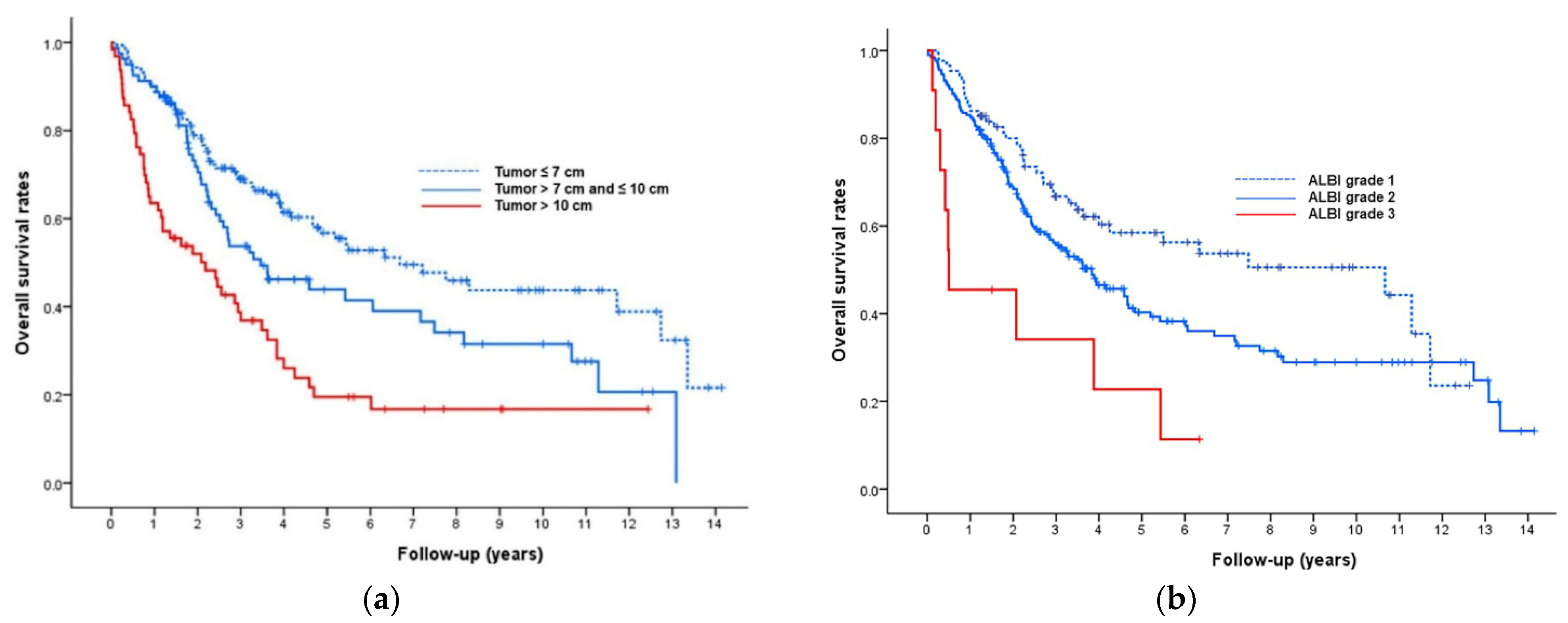
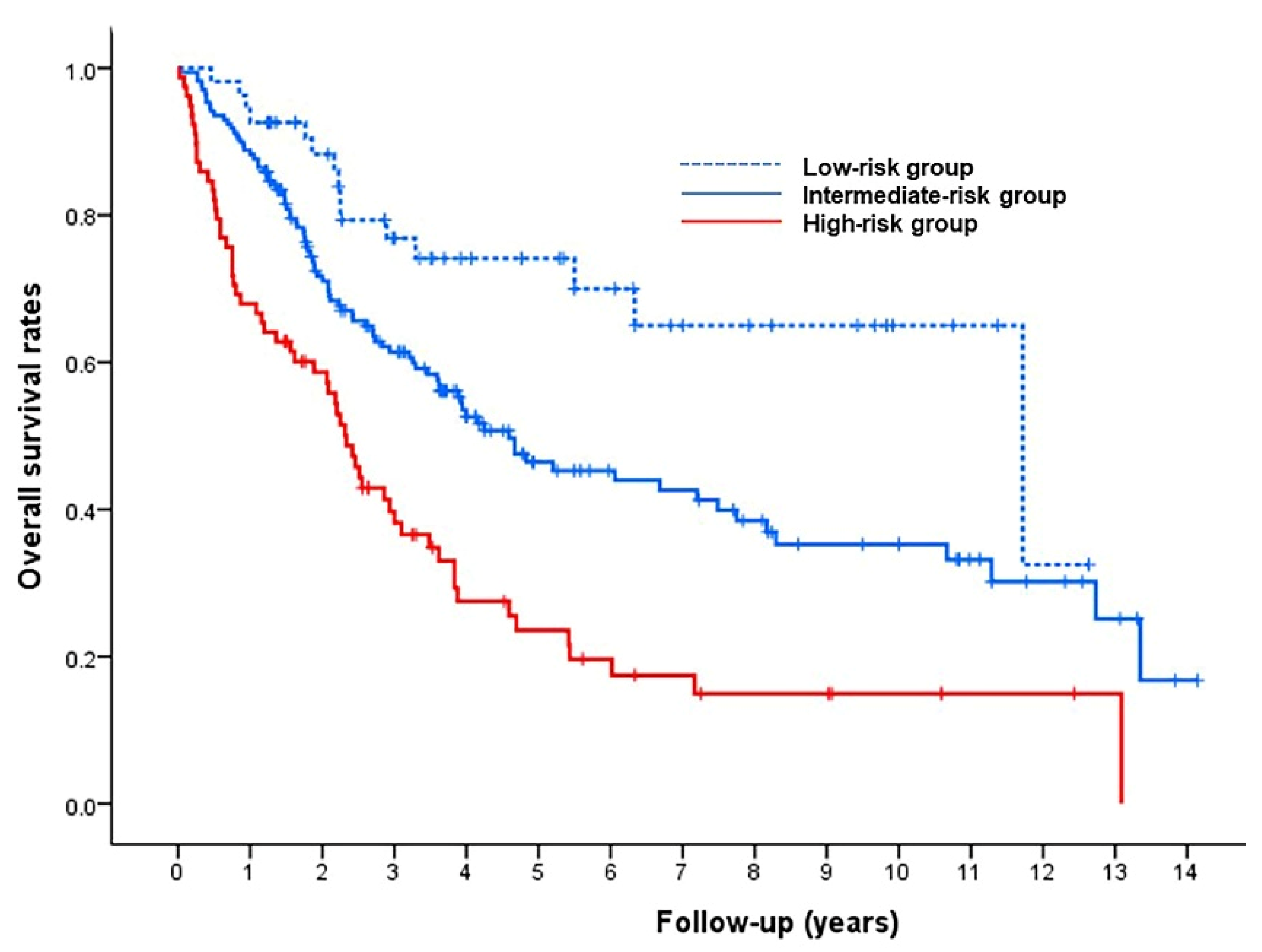
3.2. Model for Prediction of Overall Survival
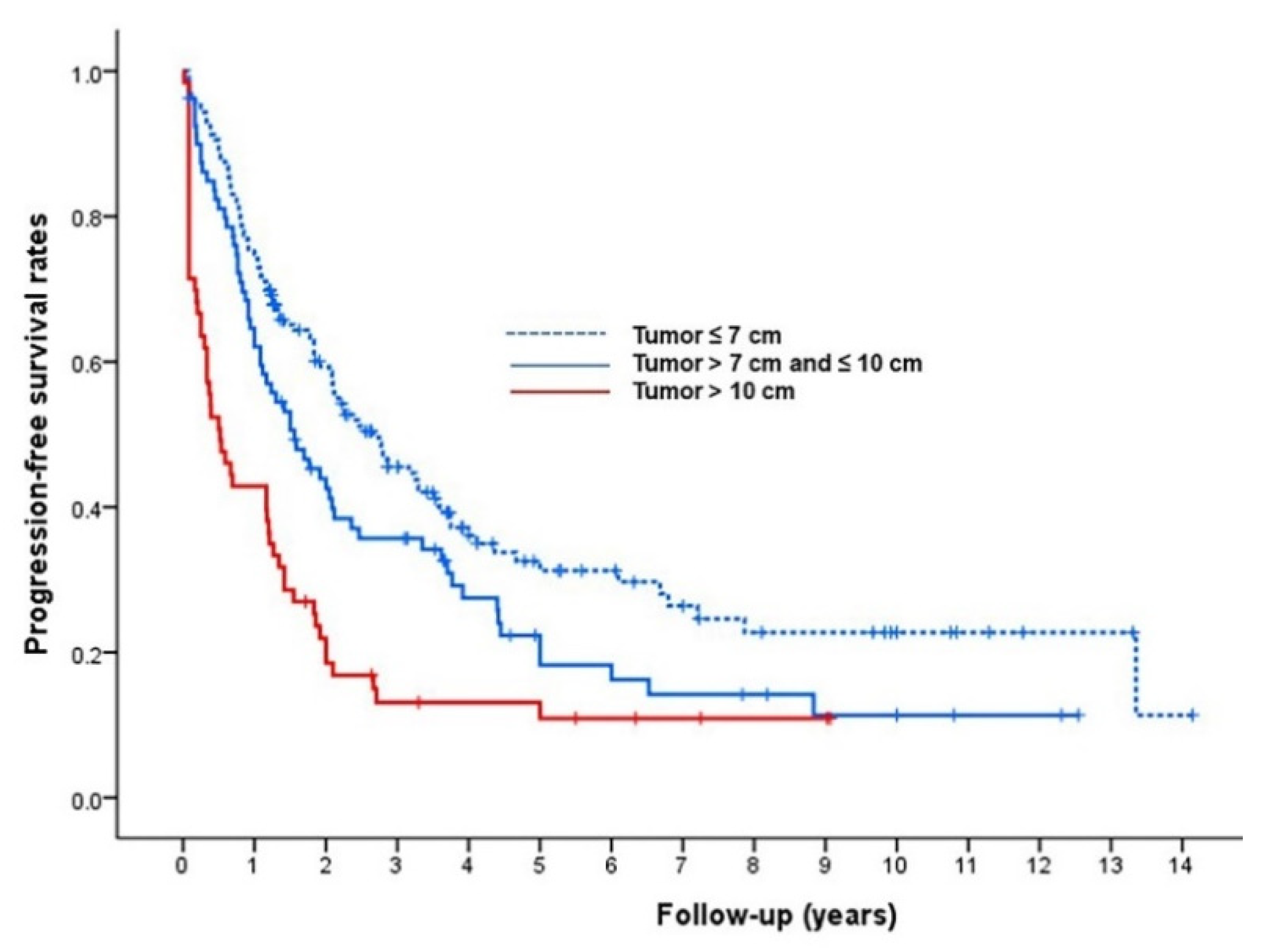
3.3. Radiologic Tumor Response, Progression-Free Survival, and Major Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar]
- Bruix, J.; Gores, G.J.; Mazzaferro, V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut 2014, 63, 844–855. [Google Scholar] [CrossRef]
- Vitale, A.; Burra, P.; Frigo, A.C.; Trevisani, F.; Farinati, F.; Spolverato, G.; Volk, M.; Giannini, E.G.; Ciccarese, F.; Piscaglia, F.; et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: A multicentre study. J. Hepatol. 2015, 62, 617–624. [Google Scholar] [CrossRef]
- Wang, Q.; Xia, D.; Bai, W.; Wang, E.; Sun, J.; Huang, M.; Mu, W.; Yin, G.; Li, H.; Zhao, H.; et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J. Hepatol. 2019, 70, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Centonze, L.; Di Sandro, S.; Lauterio, A.; De Carlis, R.; Frassoni, S.; Rampoldi, A.; Tuscano, B.; Bagnardi, V.; Vanzulli, A.; De Carlis, L. Surgical Resection vs. Percutaneous Ablation for Single Hepatocellular Carcinoma: Exploring the Impact of Li-RADS Classification on Oncological Outcomes. Cancers 2021, 13, 1671. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.T.; Kim, J.H.; Lee, L.S.; Kim, K.A.; Ko, G.Y.; Yoon, H.K.; Sung, K.B.; Gwon, D.I.; Shin, J.H.; Song, H.Y. Chemoembolization for hepatocellular carcinoma: Multivariate analysis of predicting factors for tumor response and survival in a 362-patient cohort. J. Vasc. Interv. Radiol. 2011, 22, 917–923. [Google Scholar] [CrossRef]
- Iezzi, R.; Bilhim, T.; Crocetti, L.; Peynircioglu, B.; Goldberg, S.; Bilbao, J.I.; Sami, A.; Akhan, O.; Scalise, P.; Giuliante, F.; et al. “Primum Non Nocere” in Interventional Oncology for Liver Cancer: How to Reduce the Risk for Complications? Life 2020, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Lee, D.H.; Cho, Y.; Yu, S.J.; Lee, J.H.; Yoon, J.H.; Lee, H.S.; Kim, H.C.; Yi, N.J.; Lee, K.W.; et al. Comparison of transarterial chemoembolization and hepatic resection for large solitary hepatocellular carcinoma: A propensity score analysis. J. Vasc. Interv. Radiol. 2015, 26, 651–659. [Google Scholar] [CrossRef]
- Gaba, R.C.; Lokken, R.P.; Hickey, R.M.; Lipnik, A.J.; Lewandowski, R.J.; Salem, R.; Brown, D.B.; Walker, T.G.; Silberzweig, J.E.; Baerlocher, M.O.; et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. J. Vasc. Interv. Radiol. 2017, 28, 1210–1223.e3. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.K.; Shim, J.H.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.M.; Lim, Y.S.; Han, K.H.; Lee, H.C. Risk prediction for patients with hepatocellular carcinoma undergoing chemoembolization: Development of a prediction model. Liver Int. 2016, 36, 92–99. [Google Scholar] [CrossRef]
- Lee, I.C.; Hung, Y.W.; Liu, C.A.; Lee, R.C.; Su, C.W.; Huo, T.I.; Li, C.P.; Chao, Y.; Lin, H.C.; Hou, M.C.; et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int. 2019, 39, 1704–1712. [Google Scholar] [CrossRef]
- Pinato, D.J.; Kaneko, T.; Saeed, A.; Pressiani, T.; Kaseb, A.; Wang, Y.; Szafron, D.; Jun, T.; Dharmapuri, S.; Naqash, A.R.; et al. Immunotherapy in Hepatocellular Cancer Patients with Mild to Severe Liver Dysfunction: Adjunctive Role of the ALBI Grade. Cancers 2020, 12, 1862. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Kim, P.N.; Kim, S.Y.; Lim, Y.S.; Park, S.H.; Ko, H.K.; Lee, S.G. Surgical resection versus radiofrequency ablation very early-stage HCC (≤2 cm Single HCC): A propensity score analysis. Liver Int. 2019, 39, 2397–2407. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Chu, H.H.; Kim, J.H.; Kim, S.Y.; Alrashidi, I.; Gwon, D.I.; Yoon, H.K.; Kim, N. Clinical Significance of the Initial and Best Responses after Chemoembolization in the Treatment of Intermediate-Stage Hepatocellular Carcinoma with Preserved Liver Function. J. Vasc. Interv. Radiol. 2020, 31, 1998–2006.e1. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.D.; Katz, A. Progression-free survival and time to progression as primary end points in advanced breast cancer: Often used, sometimes loosely defined. Ann. Oncol. 2009, 20, 460–464. [Google Scholar] [CrossRef]
- Brown, D.B.; Gould, J.E.; Gervais, D.A.; Goldberg, S.N.; Murthy, R.; Millward, S.F.; Rilling, W.S.; Geschwind, J.F.; Salem, R.; Vedantham, S.; et al. Transcatheter therapy for hepatic malignancy: Standardization of terminology and reporting criteria. J. Vasc. Interv. Radiol. 2009, 20, S425–S434. [Google Scholar] [CrossRef]
- Uno, H.; Cai, T.; Tian, L.; Wei, L.J. Evaluating Prediction Rules for t-Year Survivors With Censored Regression Models. J. Am. Stat. Assoc. 2007, 102, 527–537. [Google Scholar] [CrossRef]
- Chu, H.H.; Kim, J.H.; Yoon, H.K.; Ko, H.K.; Gwon, D.I.; Kim, P.N.; Sung, K.B.; Ko, G.Y.; Kim, S.Y.; Park, S.H. Chemoembolization Combined with Radiofrequency Ablation for Medium-Sized Hepatocellular Carcinoma: A Propensity-Score Analysis. J. Vasc. Interv. Radiol. 2019, 30, 1533–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, T.; Le, F.; Chen, R.; Xie, X.; Zhang, L.; Ge, N.; Chen, Y.; Wang, Y.; Zhang, B.; Ye, S.; et al. Transarterial chemoembolization for huge hepatocellular carcinoma with diameter over ten centimeters: A large cohort study. Med. Oncol. 2015, 32, 64. [Google Scholar] [CrossRef] [PubMed]
- Miyayama, S.; Kikuchi, Y.; Yoshida, M.; Yamashiro, M.; Sugimori, N.; Ikeda, R.; Okimura, K.; Sakuragawa, N.; Ueda, T.; Sanada, T.; et al. Outcomes of conventional transarterial chemoembolization for hepatocellular carcinoma ≥10 cm. Hepatol. Res. 2019, 49, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.C.; Kao, W.Y.; Su, C.W.; Chen, P.C.; Lee, P.C.; Huang, Y.H.; Huo, T.I.; Chang, C.C.; Hou, M.C.; Lin, H.C.; et al. The Prognosis of Single Large Hepatocellular Carcinoma Was Distinct from Barcelona Clinic Liver Cancer Stage A or B: The Role of Albumin-Bilirubin Grade. Liver Cancer 2018, 7, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Berhane, S.; Toyoda, H.; Bettinger, D.; Elshaarawy, O.; Chan, A.W.H.; Kirstein, M.; Mosconi, C.; Hucke, F.; Palmer, D.; et al. Prediction of Survival Among Patients Receiving Transarterial Chemoembolization for Hepatocellular Carcinoma: A Response-Based Approach. Hepatology 2020, 72, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Choe, A.R.; Sinn, D.H.; Lee, J.H.; Kim, H.Y.; Yu, S.J.; Kim, Y.J.; Yoon, J.H.; Lee, J.M.; Chung, J.W.; et al. A differential risk assessment and decision model for Transarterial chemoembolization in hepatocellular carcinoma based on hepatic function. BMC Cancer 2020, 20, 504. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, J.H.; Kim, P.H.; Kim, S.Y.; Gwon, D.I.; Chu, H.H.; Park, M.; Hur, J.; Kim, J.Y.; Kim, D.J. Imaging Predictors of Survival in Patients with Single Small Hepatocellular Carcinoma Treated with Transarterial Chemoembolization. Korean J. Radiol. 2021, 22, 213–224. [Google Scholar] [CrossRef]
- Sieghart, W.; Hucke, F.; Peck-Radosavljevic, M. Transarterial chemoembolization: Modalities, indication, and patient selection. J. Hepatol. 2015, 62, 1187–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.L.; Zhong, J.H.; Ke, Y.; Ma, L.; You, X.M.; Li, L.Q. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 9630–9637. [Google Scholar] [CrossRef]
- Min, Y.W.; Lee, J.H.; Gwak, G.Y.; Paik, Y.H.; Lee, J.H.; Rhee, P.L.; Koh, K.C.; Paik, S.W.; Yoo, B.C.; Choi, M.S. Long-term survival after surgical resection for huge hepatocellular carcinoma: Comparison with transarterial chemoembolization after propensity score matching. J. Gastroenterol. Hepatol. 2014, 29, 1043–1048. [Google Scholar] [CrossRef]
- Bogdanovic, A.; Bulajic, P.; Masulovic, D.; Bidzic, N.; Zivanovic, M.; Galun, D. Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: A propensity score matched analysis. Sci. Rep. 2021, 11, 4493. [Google Scholar] [CrossRef]
- Wei, C.Y.; Chen, P.C.; Chau, G.Y.; Lee, R.C.; Chen, P.H.; Huo, T.I.; Huang, Y.H.; Su, Y.H.; Hou, M.C.; Wu, J.C.; et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann. Transl. Med. 2020, 8, 238. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, Y.J.; Kim, K.H.; Ahn, C.S.; Moon, D.B.; Ha, T.Y.; Song, G.W.; Jung, D.H.; Lee, S.G. Long-Term Outcome After Resection of Huge Hepatocellular Carcinoma ≥ 10 cm: Single-Institution Experience with 471 Patients. World J. Surg. 2015, 39, 2519–2528. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.D.; Lu, L.; Wu, H.; Yu, J.J.; Zhang, W.G.; Pawlik, T.M.; Zhang, Y.M.; Zhou, Y.H.; Gu, W.M.; et al. Preoperative transcatheter arterial chemoembolization for surgical resection of huge hepatocellular carcinoma (≥ 10 cm): A multicenter propensity matching analysis. Hepatol. Int. 2019, 13, 736–747. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Qian, Y.W.; Cao, Z.Y.; Wu, M.C.; Cong, W.M. Postoperative adjuvant transcatheter arterial chemoembolization improves the prognosis of patients with huge hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2021, 20, 232–239. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, M.S.; Chang, J.S.; Han, K.H.; Kim, D.Y.; Seong, J. Therapeutic benefit of radiotherapy in huge (≥10 cm) unresectable hepatocellular carcinoma. Liver Int. 2014, 34, 784–794. [Google Scholar] [CrossRef]
- Vitale, A.; Trevisani, F.; Farinati, F.; Cillo, U. Treatment of Hepatocellular Carcinoma in the Precision Medicine Era: From Treatment Stage Migration to Therapeutic Hierarchy. Hepatology 2020, 72, 2206–2218. [Google Scholar] [CrossRef]
- Di Sandro, S.; Centonze, L.; Pinotti, E.; Lauterio, A.; De Carlis, R.; Romano, F.; Gianotti, L.; De Carlis, L. Surgical and oncological outcomes of hepatic resection for BCLC-B hepatocellular carcinoma: A retrospective multicenter analysis among 474 consecutive cases. Updates Surg. 2019, 71, 285–293. [Google Scholar] [CrossRef]
- Vitale, A.; Farinati, F.; Pawlik, T.M.; Frigo, A.C.; Giannini, E.G.; Napoli, L.; Ciccarese, F.; Rapaccini, G.L.; Di Marco, M.; Caturelli, E.; et al. The concept of therapeutic hierarchy for patients with hepatocellular carcinoma: A multicenter cohort study. Liver Int. 2019, 39, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Bucci, L.; Garuti, F.; Brunacci, M.; Lenzi, B.; Valente, M.; Caturelli, E.; Cabibbo, G.; Piscaglia, F.; Virdone, R.; et al. Patients with advanced hepatocellular carcinoma need a personalized management: A lesson from clinical practice. Hepatology 2018, 67, 1784–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelizzaro, F.; Penzo, B.; Peserico, G.; Imondi, A.; Sartori, A.; Vitale, A.; Cillo, U.; Giannini, E.G.; Forgione, A.; Ludovico Rapaccini, G.; et al. Monofocal hepatocellular carcinoma: How much does size matter? Liver Int. 2021, 41, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Sinn, D.H.; Yu, S.J.; Gwak, G.Y.; Kim, J.H.; Yoo, Y.J.; Jun, D.W.; Kim, T.Y.; Lee, H.Y.; Cho, E.J.; et al. Survival Analysis of Single Large (>5 cm) Hepatocellular Carcinoma Patients: BCLC A versus B. PLoS ONE 2016, 11, e0165722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.H.; Su, C.W.; Hsu, C.Y.; Hsia, C.Y.; Lee, Y.H.; Huang, Y.H.; Lee, R.C.; Lin, H.C.; Huo, T.I. Solitary Large Hepatocellular Carcinoma: Staging and Treatment Strategy. PLoS ONE 2016, 11, e0155588. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.K.; Jung, C.H.; Seo, Y.S.; Kim, J.H.; Kim, T.H.; Yoo, Y.J.; Kang, S.H.; Yim, S.Y.; Suh, S.J.; An, H.; et al. BCLC stage B is a better designation for single large hepatocellular carcinoma than BCLC stage A. J. Gastroenterol. Hepatol. 2016, 31, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Chapiro, J.; Geschwind, J.F. Hepatocellular carcinoma: Have we finally found the ultimate staging system for HCC? Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 334–336. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Lee, Y.H.; Hsia, C.Y.; Huang, Y.H.; Su, C.W.; Lin, H.C.; Lee, R.C.; Chiou, Y.Y.; Lee, F.Y.; Huo, T.I. Performance status in patients with hepatocellular carcinoma: Determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology 2013, 57, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Golfieri, R.; Bargellini, I.; Spreafico, C.; Trevisani, F. Patients with Barcelona Clinic Liver Cancer Stages B and C Hepatocellular Carcinoma: Time for a Subclassification. Liver Cancer 2019, 8, 78–91. [Google Scholar] [CrossRef] [PubMed]


| All Study Patients | Tumor Size ≤ 7 cm | Tumor Size 7–10 cm | Tumor Size > 10 cm | p-Value | |
|---|---|---|---|---|---|
| (n = 302) | (n = 159) | (n = 80) | (n = 63) | ||
| Age, mean ± SD, years | 63 ± 112 | 62 ± 11 | 64 ± 11 | 64 ± 13 | 0.400 |
| Gender (%) | 0.950 | ||||
| Male | 253 (84) | 134 (84) | 67 (84) | 52 (83) | |
| Female | 49 (16) | 25 (16) | 13 (16) | 11 (17) | |
| Etiology (%) | 0.730 | ||||
| HBV | 208 (69) | 110 (69) | 57 (71) | 41 (65) | |
| Others | 94 (31) | 49 (31) | 23 (29) | 22 (35) | |
| Tumor type | 0.737 | ||||
| Nodular | 290 (96) | 154 (97) | 76 (95) | 60 (95) | |
| Infiltrative | 12 (4) | 5 (3) | 4 (5) | 3 (5) | |
| Tumor involvement (%) | <0.001 | ||||
| Unilobar | 284 (94) | 156 (98) | 79 (99) | 49 (78) | |
| Bilobar | 18 (6) | 3 (2) | 1 (1) | 14 (22) | |
| Albumin (g/dL, mean ± SD) | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.5 | 3.5 ± 0.5 | 0.052 |
| Bilirubin (mg/dL, mean ± SD) | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.4 | 0.690 |
| ALBI grade | 0.283 | ||||
| 1 | 87 (29) | 53 (33) | 18 (22) | 16 (26) | |
| 2 | 204 (67) | 102 (64) | 59 (74) | 43 (68) | |
| 3 | 11 (4) | 4 (3) | 3 (4) | 4 (6) | |
| AFP ≥ 400 ng/mL (%) | 86 (28) | 33 (21) | 23 (29) | 30 (48) | <0.001 |
| Presence of portal hypertension | 0.005 | ||||
| Yes | 77 (25) | 42 (26) | 28 (35) | 7 (11) | |
| No | 225 (75) | 117 (74) | 52 (65) | 56 (89) | |
| ECOG PS | <0.001 | ||||
| 0 | 255 (84) | 148 (93) | 69 (86) | 38 (60) | |
| 1 | 47 (16) | 11 (7) | 11 (14) | 25 (40) |
| Univariable Regression Analysis | Multivariable Regression Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | HR | 95% CI | p-Value | Beta Coefficients | Risk Point | ||
| Tumor size | <0.001 | 0.001 | ||||||||
| ≤7 cm | 1 | 1 | ||||||||
| 7–10 cm | 1.55 | 1.07 | 2.24 | 1.43 | 0.98 | 2.09 | 0.36 | 1 | ||
| >10 cm | 2.55 | 1.75 | 3.71 | 2.16 | 1.44 | 3.25 | 0.77 | 2 | ||
| Portal hypertension | 1.24 | 0.89 | 1.74 | 0.210 | ||||||
| Infiltrative tumor type | 2.41 | 1.13 | 5.17 | 0.020 | 2.21 | 1.10 | 4.41 | 0.030 | 0.79 | 2 |
| Bilobar involvement | 2.24 | 1.30 | 3.89 | 0.004 | 1.45 | 0.77 | 2.71 | 0.250 | ||
| ECOG PS 1 | 1.96 | 1.36 | 2.83 | <0.001 | 1.51 | 1.01 | 2.27 | 0.045 | 0.41 | 1 |
| AFP ≥ 400 ng/mL | 1.56 | 1.13 | 2.16 | 0.007 | 1.20 | 0.85 | 1.71 | 0.310 | ||
| Albumin ≤ 3.5 g/dL | 1.61 | 1.18 | 2.19 | 0.002 | 1.20 | 0.82 | 1.75 | 0.350 | ||
| Bilirubin (mg/dL) | 1.17 | 0.85 | 1.60 | 0.330 | ||||||
| ALBI grade | 0.001 | 0.005 | ||||||||
| 1 | 1 | 1 | ||||||||
| 2 | 1.49 | 1.04 | 2.16 | 1.30 | 0.90 | 1.89 | 0.26 | 1 | ||
| 3 | 3.76 | 1.82 | 7.80 | 3.41 | 1.63 | 7.13 | 1.22 | 3 | ||
| Age (years) | 1.01 | 0.99 | 1.03 | 0.080 | ||||||
| Male sex | 0.79 | 0.52 | 1.19 | 0.250 | ||||||
| HBV etiology | 0.85 | 0.61 | 1.19 | 0.350 | ||||||
| All Study Patients | Tumor Size ≤ 7 cm | Tumor Size 7–10 cm | Tumor Size > 10 cm | p-Value | ||
|---|---|---|---|---|---|---|
| (n = 302) | (n = 159) | (n = 80) | (n = 63) | |||
| Initial response (%) | CR or PR | 239 (79) | 146 (92) | 70 (88) | 23 (37) | < 0.001 |
| SD or PD | 63 (21) | 13 (8) | 10 (12) | 40 (63) | ||
| Best overall response (%) | CR or PR | 260 (86) | 150 (94) | 72 (90) | 38 (60) | < 0.001 |
| SD or PD | 42 (14) | 9 (6) | 8 (10) | 25 (40) |
| Major Complications | Number |
|---|---|
| Persistent fever > 7 days | 8 |
| Hepatic abscess | 5 |
| Sepsis | 4 |
| TACE-related cholecystitis | 3 |
| Hepatic failure | 2 |
| Tumor lysis syndrome | 2 |
| Biloma | 2 |
| Contrast agent-associated ARF | 1 |
| Spontaneous bacterial peritonitis | 1 |
| Tumor rupture | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.H.; Kim, J.H.; Shim, J.H.; Ko, H.-K.; Chu, H.H.; Shin, J.H.; Yoon, H.-K.; Ko, G.-Y.; Gwon, D.I. Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function: Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort. Life 2021, 11, 840. https://doi.org/10.3390/life11080840
Kim GH, Kim JH, Shim JH, Ko H-K, Chu HH, Shin JH, Yoon H-K, Ko G-Y, Gwon DI. Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function: Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort. Life. 2021; 11(8):840. https://doi.org/10.3390/life11080840
Chicago/Turabian StyleKim, Gun Ha, Jin Hyoung Kim, Ju Hyun Shim, Heung-Kyu Ko, Hee Ho Chu, Ji Hoon Shin, Hyun-Ki Yoon, Gi-Young Ko, and Dong Il Gwon. 2021. "Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function: Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort" Life 11, no. 8: 840. https://doi.org/10.3390/life11080840
APA StyleKim, G. H., Kim, J. H., Shim, J. H., Ko, H.-K., Chu, H. H., Shin, J. H., Yoon, H.-K., Ko, G.-Y., & Gwon, D. I. (2021). Chemoembolization for Single Large Hepatocellular Carcinoma with Preserved Liver Function: Analysis of Factors Predicting Clinical Outcomes in a 302 Patient Cohort. Life, 11(8), 840. https://doi.org/10.3390/life11080840







