Abstract
Nosocomial fungal infections are an emerging global public health threat that requires urgent attention and proper management. With the limited availability of treatment options, it has become necessary to understand the emerging epidemiological trends, mechanisms, and risk factors. However, very limited surveillance reports are available in the Latvian and broader European context. We therefore conducted a retrospective analysis of laboratory data (2017–2020) from Pauls Stradinš Clinical University Hospital (PSCUH), Riga, Latvia, which is one of the largest public multispecialty hospitals in Latvia. A total of 2278 fungal isolates were analyzed during the study period, with Candida spp. comprising 95% of the isolates, followed by Aspergillus spp. and Geotrichum spp. Amongst the Candida spp., C. albicans and C. glabrata made up about 75% of the isolates. The Department of Lung Diseases and Thoracic Surgery had the highest caseload followed by Intensive Care Department. Majority of the fungal isolates were collected from the bronchoalveolar lavage (37%), followed by urine (19%) and sputum (18%) samples. A total of 34 cases of candidemia were noted during the study period with C. albicans being the most common candidemia pathogen. Proper surveillance of emerging epidemiological trends serve as the most reliable and powerful cornerstone towards tackling this emerging threat.
1. Introduction
Antifungal resistance (AFR) or antifungal tolerance is an emerging clinical issue globally, which has historically been long been neglected, mainly due to focused attention towards the management and control of resistant bacteria, parasites, and viruses [1,2]. However, with a significant increase in the reported number of cases of systemic fungal infections, the issue of AFR has become a global concern [1,3]. Fungal infections, especially invasive ones, which are associated with a significantly higher mortality rate and a limited availability of treatment options, further worsen the current situation [3]. Additionally, the recent emergence of multi-drug-class-resistant fungal isolates makes the management of such infections even more difficult [3].
In general, fungal resistance can be divided into two broad types: Microbiological and Clinical. Microbiological or in vitro resistance is defined as the nonsusceptibility of a fungal isolate to the tested antifungal drug, whereby the minimum inhibitory concentration (MIC) of the drug exceeds the susceptibility breakpoint for a particular fungal species [1,3]. It can be further divided into intrinsic (or primary) and extrinsic (or acquired/secondary). Clinical or in vivo resistance, on the other hand, is defined as the inability of the antifungal drug to stop the persistence or progression of a fungal infection despite the drug having in vitro activity against the organism [1,4,5].
The development of fungal resistance amongst the isolates depends upon multiple factors, including the fungal factors, host factors, drug-related factors, and environmental factors [1,4,5,6]. Fungal factors include mutations in genetic encoding for efflux pumps [7], target metabolic enzymes [8], biofilm formation [9], modification of plasma membrane composition [8], etc. Amongst the host factors, degree of immunosuppression, site and severity of infection, and timing and dosage of antifungal agents play a critical role in determining the clinical outcome of the treatment [4,6]. Pharmacodynamics and pharmacokinetics, including the fungistatic nature of the drugs, empirical treatment (despite the etiological agent being known), inappropriate dosage, long treatment durations, drug–drug interactions, etc., comprise the drug-related factors that play an important role in the development of fungal resistance [1,7]. Finally, the widespread and indiscriminate use of fungicides in agricultural activities (e.g., the use of azole-based fungicides for cereal and grape cultivation in European member countries) have led to the increased incidence of resistant fungal strains in the environment [1,10].
Nosocomial infections (healthcare-associated infections, or HAIs) broadly refers to the infections that were acquired in a healthcare institution whilst receiving healthcare and which were not present at the time of admission [11]. Since the start of the century, an uptick in the incidence of fungal HAIs has been reported globally, which is most likely the consequence of the widespread use of aggressive treatment modalities [12]. Surgical procedures, such as hematopoietic stem cell transplantation, organ transplantation, chemotherapy, immunomodulatory treatment, and the use of invasive devices, such as intravascular central lines, etc., have been responsible for the increase in fungal HAIs [12,13]. Furthermore, exposure to airborne fungal spores and pathogens within the hospital environment, especially during construction, has further worsened the situation for immunocompromised patients [12,14]. In fact, according to Perlroth et al., the relative frequency of fungal nosocomial infections and the intensity of patient immunosuppression required to predispose a patient to fungal HAI are inversely dependent on one another [15]. For example, candidiasis is mostly seen in patients with relatively minimal immunosuppression, making it the most common nosocomial infection [15,16,17,18], compared with aspergillosis, the second most common nosocomial infection, which tends to occur in patients with moderate to severe immunosuppression [15,16,17,18].
There are three primary classes of antifungal drugs that are used for treating invasive fungal nosocomial infections [19], namely, polyenes, azoles, and echinocandins. Polyenes, such as amphotericin B (AMB) and nystatin (NYS), the latter of which was historically known as fungicidin, were amongst the first broad-spectrum, fungal-specific antibiotics on the market and are the gold standard for the treatment of fungal infections [20,21,22]. AMB, primarily used for systemic mycosis, has a complex mechanism of action that is yet to be fully elucidated. The drug is known to bind with ergosterol (a cholesterol analogue) at the fungal membrane, causing pore formation (leading to rapid depletion of intracellular ions, such as K+, Ca2+, and Mg2+) and sequestration of ergosterol [23,24,25,26]. Additionally, it can induce oxidative damage in the fungal cell [23,27]. Nystatin, due to its poor gastrointestinal absorption, is used only as a topical agent against mucosal infections, such as oral or vulvovaginal candidiasis in an ambulatory setting [28,29]. As with AMB, NYS also induces pore formation, which is accompanied by strong membrane reorganizations, thereby exerting its cytotoxic effects [30].
The azole-class antifungals, since their availability, have become the cornerstone of antifungal treatment and are divided into two subgroups. The older azoles, or imidazoles, such as ketoconazole (KET), econazole (ECO), and miconazole (MCZ), work by inhibiting the synthesis of ergosterol via the inhibition of the enzyme lanosterol demethylase [31,32]. However, as with NYS, due to poor gastrointestinal absorption and adverse side effects during systemic use, their application is limited to the treatment of superficial mycosesand in ambulatory settings [31]. The newer azoles, or triazoles, such as fluconazole (FLU), have a similar mechanism of action whilst having a superior pharmacokinetic and adverse event profile than imidazoles, making them suitable for systemic use [31]. Finally, echinocandins, such as caspofungin and micafungin, are secondary metabolites of fungi that can inhibit the biosynthesis of β-(1,3)-D-glucan, an important cell wall component needed for the maintenance of the structural integrity [33,34]. Apart from these three primary antifungal drug classes, there are two more classes—flucytosine and allylamine—which are used for the management of fungal infections. Flucytosine (FCT) is a pyrimidine analogue that inhibits DNA synthesis. It is converted to 5-fluorouracil which integrates itself during RNA synthesis, leading to early chain termination [34,35]. Allylamines, such as terbinafine, on the other hand, work by inhibiting the enzyme squalene epoxidase, which is involved in the ergosterol synthesis pathway [34,36].
In our recent study, we described the antimicrobial resistance (AMR) rates in Gram-negative bacteria in our hospital from 2017–2020 [37]. Subsequently, in the present study, we aim to discuss and present the epidemiological surveillance data rates during the same period (2017–2020) in our hospital. Due to constant changes in the epidemiology amongst nosocomial infectious agents, it is essential to develop and maintain an updated record of such infectious agents to improve patient care and to develop and put in place proper infection control and prophylaxis strategies.
2. Materials and Methods
2.1. Study Location and Design
The present study was conducted at the Pauls Stradinš Clinical University Hospital (PSCUH), Riga, Latvia, from 2017 to 2020 (data for full year from January to December was analyzed). A retrospective analysis of the epidemiological data of nosocomial fungal infections, along with the antifungal resistance rates, was completed. The data were collected from the storage server of the Joint Microbiology Laboratory, PSCUH. Microbiological data were collected for patients who fulfilled the ECDC (European Centre for Disease Prevention and Control) criteria for nosocomial infections [38], irrespective of nationality, gender, age, etc. Ethical permission for the present study was granted by the Clinical Research Ethics Committee of the PSCUH, vide no. 290421-16L, dated 29 April 2021.
2.2. Sample Collection and Determination of Antifungal Resistance Rates
Clinicians and nurses in their respective departments collected and delivered the patient samples to the Joint Laboratory for microbiological investigations. Specimens collected included abscess material, bronchoalveolar lavage, tracheal aspirate, sputum, blood, urine, etc. Specimen collection was performed based on local and EU guidelines. Specimens with inadequate sample material or improper labelling were removed from the present study. Repeated or different specimens from the same patient which showed the same fungal species were considered as a single isolate in one calendar year (if less than 21 days apart). Specimen handling was performed in accordance with the latest EUCAST (European Committee on Antimicrobial Susceptibility Testing) guidelines [39]. For non-Candida spp., species identification was done using a Vitek2 analyzer or MALDI-TOF MS (matrix-assisted laser desorption ionization-time of flight mass spectrometry). For Candida spp., the Candifast test (ElitechGroup, Puteaux, France, https://www.elitechgroup.com/product/candifast, accessed on 20 September 2021) was used for species.
2.3. Data Collection and Analysis
The laboratory database was perused for data regarding antifungal resistance, sample types, species, and the department from where the samples were collected. The data was then downloaded for the period 2017–2020.
3. Results
3.1. Candida spp. Were the Most Frequently Isolated Nosocomial Fungal Species
During the study period, a total of 2278 (567 in 2017, 533 in 2018, 629 in 2019, and 549 in 2020) distinct fungal samples were collected which belonged to 10 different fungal genera (Table 1). Candida spp. was the most isolated nosocomial fungal species, representing more than 95% of the total isolates in the study period. Aspergillus spp. (2.59%) and Geotrichum spp. (1.28%) were the second and third most isolated species, respectively. Since the isolated samples of Aspergillus, Fusarium, Cryptococcus, etc., were extremely sporadic and isolated (only 104 isolates over 4 years, which was on average two cases per month for all species other than Candida) and no identifiable clustered outbreak in the hospital was noticed, an empirical standard treatment regimen based on local and EU guidelines was followed for these patients. Hence, we concentrated only on the Candida spp. in the results section, whilst a brief overview of other fungal agents is provided in the discussion section.

Table 1.
Prevalence of different nosocomial fungal genera from 2017–2020.
3.2. Characterization of Candida spp. Isolates
Further, as shown in Table 2, amongst the Candida spp., C. albicans represented 68.37% of the isolates, followed by C. glabrata (8.46%) and C. tropicalis (8.14%), respectively. In the case of Aspergillus spp., A. fumigatus was more frequently isolated than A. niger.

Table 2.
Prevalence of different Candida species from 2017–2020.
3.3. Distribution of Candida Isolates Based on Department and Patient Specimen Collected
The highest number of nosocomial fungal isolates were cumulatively collected from the Department of Lung Diseases and Thoracic Surgery (Figure 1). Although the department saw a decrease in the number of cases in 2020 when compared with 2017, it still accounted for 28% of all samples collected in 2017 and 2020. The Intensive Care Department was the department with the second-highest caseload of nosocomial fungal infections (20%).
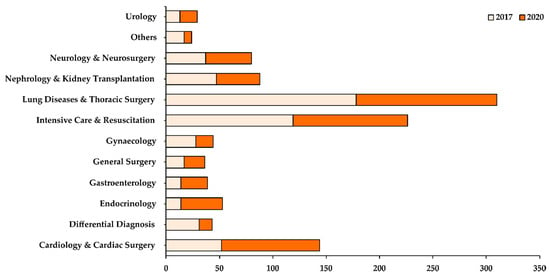
Figure 1.
Distribution of fungal samples isolated based on different departments in 2017 (light orange) and 2020 (dark orange). The X-axis shows the total number of samples collected while the Y-axis shows the different departments in the hospital.
Whilst most of the departments reported a decrease in their caseload in 2020 when compared with 2017, some departments, such as Urology, Cardiology & Cardiac Surgery, Endocrinology, and Gastroenterology, showed an increase in their caseload in 2020 (Figure 1). Based on the patient specimens collected (Figure 2), most of the fungal isolates were collected from the bronchoalveolar lavage (37%), followed by urine (19%) and sputum (18%) samples. A small number of fungal isolates were collected from the blood, abscess, and pleural fluid of patients.
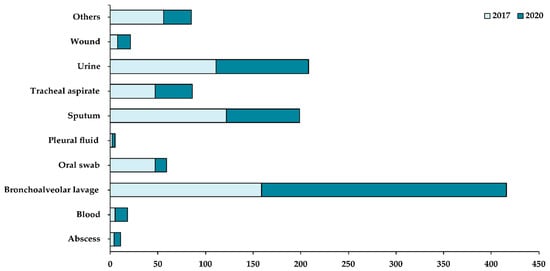
Figure 2.
Distribution of fungal samples isolated based on patient specimens collected in 2017 (light blue) and 2020 (dark blue). X-axis shows the total number of samples collected while the Y-axis shows the different patient specimens collected in the hospital.
3.4. Distribution of Candida Isolates Based on Cases of Candidemia
A total of 34 cases were reported for fungal blood infections (Table 3) in the study period. Amongst the Candida spp., in terms of the causative agents of invasive fungal infections (blood infections), C. albicans was the most common causative fungal pathogen, followed by C. glabrata and C. tropicalis. Apart from candidemia, one case of C. neoformans and two cases of S. cerevisiae in 2019 were also reported as causative agents for invasive fungal infections.

Table 3.
Number of cases of candidemia based on species from 2017–2020.
4. Discussion
Nosocomial fungal infections are an emerging global threat that leads to additional hospitalization costs, longer treatment durations, and are associated with higher mortality rates [40]. According to recent estimates in 2017, fungal infections create an economic burden of more than USD 7.2 billion (United States Dollars) on the healthcare system in the USA alone [41]. Further, in an educational survey conducted by the CDC (Centers for Disease Control and Prevention), more than two-thirds of the respondents failed to recognize any of the six common fungal infections listed in the survey [42]. This prompted the CDC to launch the “Think Fungus” yearly campaign to educate and spread awareness regarding various fungal infections [43]. Fungal diseases usually present with symptoms that are similar to those of other infections, which makes it difficult to establish the proper diagnosis, leading to delayed treatment, poor patient outcomes, and unnecessary medical costs [15,41]. Furthermore, nonidentification of etiological agents leads to the prescription of empirical treatments and/or the initiation of prophylactic treatments for high-risk group patients [15]. Finally, antifungal drugs usually target both human and fungal cells (both cells are eukaryotic) which leads to multiple adverse side effects in patients, and limits the available targets for the development of newer drugs [44,45,46]. All these reasons make the proper management of microbiological AFR the need of the hour.
Since comprehensive international or national databases are usually not available for most of the fungal infections, analyses of databases of large hospitals and laboratories can enable us to predict and estimate the national and local caseload besides examining the shifting trends in AFR amongst fungal isolates. Hence, studies akin to the present one provide an informative overview of the present situation and highlight the need for the establishment of comprehensive surveillance protocols and treatment guidelines. Furthermore, the present study can be utilized for spreading awareness amongst the hospital staff and management regarding various fungal infections and etiological agents.
4.1. Candida spp. as Nosocomial Fungal Agents
Amongst the over 200 recognized Candida spp., many species can cause candidiasis, a broad term referring to infections ranging from superficial cutaneous and mucosal infections to deep-seated organ infections [47]. Candidiasis can occur at any age and usually occurs in conditions with identifiable infection risk factors [47]. As part of the normal gastrointestinal and genitourinary microflora, many members of the genus Candida are known to cause opportunistic infections, especially in the setting of immunosuppression [44,48]. The members are known to be the causative agents of vaginitis, oral candidiasis, cutaneous candidiasis, candidemia (bloodstream infection), and other systemic infections [49]. C. albicans is the most identified nosocomial fungal agent, followed by C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei. Together it is estimated that these five members are responsible for up to 90% of invasive infections, although their distribution and caseload vary based on geographical region, patient population, and clinical settings [50,51,52].
In our present study, as shown in Table 2, we found that whilst C. albicans was the most isolated Candida spp. (68%), the burden of C. glabrata and C. tropicalis was rather similar (about 8% each). Apart from these five members, which are also found in normal healthy individuals, the emergence of other species, such as C. kefyr and C. dubliniensis, has also been reported worldwide [51,53]. In fact, in our hospital, they together comprised about 4% of the total Candida isolates and showed an increasing trend of incidence in 2020 when compared with 2017. There are multiple risk factors for the spread of nosocomial candidiasis, including overuse of broad-spectrum antibiotics, immunosuppression, chronic malignancy, surgical intervention, parenteral feeding, burns, premature neonate, diabetes, and/or prolonged hospitalization [54]. Their ability to colonize and survive in various habitats makes it easy to spread the infection, especially within the hospital environment, where Candida’s members have been reported to survive for up to 4 months [55]. Additionally, another group of researchers reported that infection can also spread via the hand-to-hand contact route due to their ability to survive for about 45 min on peoples’ hands after inoculation [56].
The primary event for candidiasis is the colonization of the host by the yeast cells. The cells usually adhere to the host cells and produce hydrolytic enzymes [57]. Adherence prevents (or at least slows down) cell clearance, while the hydrolytic enzymes facilitate adherence, tissue penetration, invasion, and subsequent delivery of toxins into the host cell [45,58]. Biofilm production additionally provides protection from the host’s defense response besides conferring antifungal resistance [59]. Apart from the above virulence factors, it is difficult to identify specific Candida spp. diagnostically. For example, the specialized culture media and many commercially available analysis equipment do not readily differentiate many Candida spp. [60,61,62], thereby delaying the initiation of precise antifungal treatment, a leading cause of poor patient outcomes with nosocomial fungal infections.
4.2. Aspergillus spp. as Nosocomial Fungal Agents
As with the genus Candida, genus Aspergillus comprises over 185 members, about 20 of which are implicated in human disease and infection [63]. A. fumigatus is mostly isolated in patients with invasive infection, while A. flavus is mostly associated with sinusitis [63]. Other emerging nosocomial Aspergillus species include A. terreus and A. niger [63,64,65], in line with the findings from our hospital (A. fumigatus was more isolated than A. niger). Though primarily found in decaying vegetation, outdoor soil, bird droppings, and hay barns, A. fumigatus can also be found in human habitations. Dust particles, infrequently cleaned places, potted flowers, shutters, hard to reach and clean places, such as attics, ventilation ducts, ceilings, etc., are common places of habitation [63]. Some studies have also demonstrated colonization of foodstuff, such as peppers, biscuits, fruits, tobacco, marijuana, etc., by A. fumigatus [66,67]. Additionally, construction, renovation, or demolition work in the ward or near the hospital campus can passively disseminate fungal conidia and then be transported by wind and convection currents [68,69].
Airborne transmission and subsequent inhalation of fungal conidia is the main route of infection [70,71]. Due to its small size, the fungal spores colonize the upper and lower respiratory tract, especially the pulmonary alveolar spaces, which serves as the optimum environment for the spores to germinate and form hyphae, ultimately leading to pulmonary aspergillosis [69,72]. Apart from the inhalation route, reports of direct contamination of the wounds or skin in burn patients or low-birth-weight babies have also been published in the literature [66,73]. Whilst transmission by contaminated water and/or aerosolization of spores remains a subject of debate [74], direct contact with contaminated adhesive tapes, gauze, and intravascular catheters provides other routes of nosocomial transmission [66,75].
4.3. Geotrichum spp. as Nosocomial Fungal Agents
Geotrichum capitatum (Blastoschizomyces capitatus), previously known as Trichosporon capitatum, is an opportunistic invasive fungal infection-causing nosocomial fungal agent. The fungus is part of the normal digestive, respiratory, and cutaneous microflora, which act as ports of entry for the fungus to cause opportunistic infections [76,77]. The genus, although reported rarely globally, is relatively more common in Europe, especially in and around the Mediterranean region (suspected geographical domination in the region is due to climatic factors that favor fungal growth) [78]. However, cases have been also reported from the USA and southern India [78,79]. As with other fungal infectious agents, immunosuppression is the key risk factor, with some authors linking contaminated milk and polytrauma as potential risk factors for infection [80,81,82,83]. Geotrichosis clinically presents similarly to invasive candidiasis, except that the focal point of infection is the lungs. Pulmonary infections and pneumonia are the hallmarks of geotrichosis, which are relatively uncommon in invasive candidiasis [84]. Diagnosis and identification of geotrichosis are standardly based on a positive GM (galactomannan) assay test and pulmonary lesions if fungal isolates are recovered from respiratory cultures [83]. There is usually cross-reactivity with the aspergillus GM assay test, making a diagnosis of invasive geotrichosis even harder [85].
4.4. Other Nosocomial Fungal Agents
Amongst other nosocomial fungal agents reported in the present study, Cryptococcus spp., represents one of the traditional fungal agents, including Candida spp. and Aspergillus spp. C. neoformans is an encapsulated fungus that usually spreads using an airborne route, with the infection being usually asymptomatic [86,87]. Naturally, the fungus is found in the soil throughout the world and clinically manifests (as pneumonia and meningitis) typically when the latent infection gets reactivated due to immunosuppression [86,88]. In our hospital, all cases were reported from the Department of Lung Diseases and Thoracic Surgery. Curvularia spp. is a genus of filamentous pigmented molds that generally colonize the soil and vegetation [89]. The mold can cause infections in both immunosuppressed and non-immunosuppressed patients. In the latter group of patients, Curvularia spp. is known to cause infections of the paranasal sinus, skin, nails, nail beds, and soft tissue [90,91,92]. Nosocomial infections, however, can range from invasive and allergic sinusitis, bronchopulmonary disease, ocular infections, peritonitis, and postsurgical endocarditis [89,93].
Fusarium spp. is an opportunistic pathogen that causes a wide variety of diseases in humans. In normal healthy individuals, it has been implicated in causing superficial infections, such as skin infections, keratitis, and onychomycosis [94,95,96]. In patients with severe immunosuppression, Fusarium spp. can cause invasive infections (both local and disseminated) [96]. Among its virulence factors, the production of mycotoxins stands out. This enables the fungus to suppress humoral and cellular immunity, whilst also causing local tissue breakdown [97]. In the hospital environment, Fusarium spp. has been isolated in and around the water distribution system, including water storage tanks, showerheads, sink drains and faucets, etc. [98]. Pichia spp. represent another genus of opportunistic pathogens that are normally found to be part of the normal microbiota of the skin, the throat, and the gastrointestinal tract [99]. As a rarely described clinical nosocomial agent, Pichia spp. are usually associated with invasive infections and fungaemia in neonates and immunosuppressed individuals [99,100,101]. Standard fungal risk factors, such as prematurity, low birth weight, long duration of hospital stay, prior use of antibiotics, intravenous catheterization, intravenous drug abuse, etc., are associated with Pichia infection [101].
Rhodotorula mucilaginosa is another opportunistic nosocomial fungal species, with most infections associated with central venous catheters in immunosuppressed hematological patients [102,103]. Some estimates put the incidence rate of Rhodotorula fungaemia at around 0.5% to 2.3% in western countries [104], in line with the findings of the present study. Apart from fungaemia, less invasive localized infections include endophthalmitis, onychomycosis, meningitis, prosthetic joint infections, and peritonitis in both immunocompromised and non-immunocompromised patients [103]. In the environment, R. mucilaginosa is commonly isolated from various food items and beverages, including peanuts, apple cider, cherries, fresh fruits, fruit juice, cheese, sausages, etc. [103].
Saccharomyces cerevisiae, commonly known as Baker’s yeast or Brewer’s yeast, is usually considered a safe, nonpathogenic organism, with widespread use in the baking, fermenting, and wine industries. However, with the recent advent in molecular detection techniques and increase in the number of immunocompromised patients, the incidence of S. cerevisiae as a nosocomial fungal agent has only increased [105]. The infections caused can range from vaginitis and cutaneous infections in healthy individuals to systemic invasive infections in compromised patients (elderly, premature babies, HIV positive patients, etc.) [106,107]. Unlike its phylogenetically close relative, Candida spp., S. cerevisiae shows low adherence to the host tissue and can only cause infections if the integrity of the epithelial/mucosal barrier is compromised [108]. Finally, Trichoderma spp., which are rapidly growing molds, are extremely rare yet emerging nosocomial fungal agents. The infection usually presents with nodular pulmonary infiltrations, peritonitis, cutaneous lesions, and CNS infections, primarily in immunocompromised patients [109].
4.5. Antifungal Treatment Principles
The first and most critical step towards proper management of nosocomial fungal infections is the determination of whether the antifungal agents being prescribed are meant for treating mucosal or systemic infections [48]. Whilst superficial infections can be effectively managed using topical preparations, systemic infections require either oral or intravenous (IV) preparations. This is especially important since some antifungal agents are available only for IV infusions (e.g., echinocandins, amphotericin B), or only as oral agents (e.g., flucytosine) [48]. Some drugs can be administered using both routes, however, and therefore the choice would be dictated by considerations of drug solubility (e.g., azoles) [48,110]. The next and most crucial step is to understand the route of clearance of the antifungal agent. For example, fluconazole is excreted in its active form in urine, making it an appropriate drug for the treatment of urinary tract fungal infections [48].
Broadly, amphotericin B remains the gold standard for treatment for most of the mycoses, although its association with nephrotoxicity and the possibility of only systemic administration limits its potential [23]. However, newer formulations of AMB, such as liposomal AMB and lipid complex AMB, have shown promising results with lower toxicity. However, their use is restricted by their high cost [23]. The next in line is the azoles, especially KET and MCZ, which provide a viable and effective alternative to AMB [111]. The triazoles are, however, now preferred over imidazoles due to their low cost, superior efficacy, and better toleration [112]. However, azoles themselves are associated with hepatotoxicity and are victims of increasing antifungal resistance [44]. The newer generation of azoles, such as voriconazole and posaconazole, have been developed and approved for clinical use. These are broad-spectrum antifungal agents which inhibit fungal cytochrome P450-mediated 14-alpha lanosterol demethylation, causing structural damage and a loss of cell membrane function [113]. Both are recommended as first-line prophylaxis against invasive Candida and Aspergillus infections, while the are second-in-line for treating fusariosis (in the case of intolerance to amphotericin B) [113].
Another treatment strategy includes using antifungal combination therapy, especially with flucytosine, due to its role in hepatic impairment, interference with bone marrow function, and the rapid occurrence of resistance amongst isolates [114,115]. Combination with FCT reduces its toxic effects and is generally clinically used with AMB and FLU. Combination with non-antifungal drugs, such as calcineurin inhibitors, proton pump inhibitors, immunomodulators, etc., have shown promising results. Cyclosporin A, for example, increases the susceptibility of fungal infections to fluconazole by deletion of efflux pumps or alteration of cellular stress responses [116]. The development of newer drug classes, including echinocandins, allylamines, etc., provides a viable alternative to these traditional antifungal agents. The development of ibrexafungerp (formerly known as SCY-078) has shown promising results for the treatment of echinocandin-resistant fungal isolates, especially C. glabrata-caused vulvovaginal candidiasis [117]. It is the first oral (1-3)-β-D-glucan synthase inhibitor (GSI) which works by hindering fungal cell wall synthesis [118].
4.6. Antifungal Resistance Mechanisms
Antifungal drug resistance (AFR) in fungal isolates is based on different mechanisms depending on the antifungal drug being overused (only a short overview is provided here). Resistance to azoles is governed by three different mechanisms [48]. The first mechanism is the reduction of the concentrations of the drug that is accumulated intracellularly. This is achieved by a gain-of-function mutation in transcription factors that control efflux pump activity (TAC1 and MRR1 in C. albicans; PDR1 in C. glabrata; Cdr1p homologue in C. neoformans; AfuMdr1p and AfuMdr2p in Aspergillus spp.) [119,120,121,122]. The second mechanism is decreased affinity for the drug target, for example, mutations in the ERG11 gene (encoding for lanosterol 14-α-demethylase) which increase resistance to azoles [48,123]. The third and final mechanism is counteracting the effects of the drug, which might include an increase in target protein concentrations and/or alterations in the protein structure [123,124].
Although resistance to polyenes (AMB; NYS) is minimum in comparison to azoles, it is achieved by using the reverse mechanism to that used against azoles. An acquired loss-of-function mutation in ERG3 or ERG6 genes, both involved in the biosynthesis pathways of ergosterol, leads to a decrease in concentrations of the target protein, thereby providing resistance [125,126]. Resistance to flucytosine is akin to that of polyene and is based on the inactivation of different enzymes in the pyrimidine pathway [48]. Point mutations in FUR1 and FCY1 genes lead to disruption of the pyrimidine pathway, thereby conferring resistance [126]. An alternative mechanism to resistance to FCT is via upregulation of efflux pumps due to mutations in the FCY2 gene [126].
4.7. Antifungal Stewardship, Infection Control, and Future Strategies
Planning, development, and implementation of a nationwide (or at least at the level of the hospital), comprehensive antifungal stewardship program (AFSP) is required to tackle the emerging threat posed by the increase in nosocomial fungal infections, along with increasing resistance and the emergence of new infectious agents. An AFSP based on the recently released core recommendations for antifungal stewardship by the Mycoses Study Group Education and Research Consortium [127] will be planned and implemented in the hospital. The plan will include the core “essential” recommendations from the consortium, including [127]:
- (i)
- The development of institutional treatment guidelines for prophylaxis and empiric therapy, including the identification of high-risk patients, the estimation of a proper dosage, the timely identification of the agent, etc.;
- (ii)
- The development of targeted education programs for appropriate diagnosis and treatment for clinicians, specialists, nurses, etc.;
- (iii)
- An antifungal prescription review for drug–drug interactions, including the identification of over-prescribed agents and the rationalization of prescription strategies;
- (iv)
- The development, encouragement, and adoption of an intravenous-to-oral antifungal drug transition program;
- (v)
- Local surveillance and reporting of invasive fungal diseases to prescribers, management, and other relevant health monitoring bodies at the national and EU/EEA level to contribute towards a comprehensive national database.
Our laboratory and hospital provide reference and educational/training services to multiple regional microbiological laboratories and clinics. The findings of the present study will be shared with such interested laboratories, along with the provision of an option to participate in the AFSP program. Within our hospital, the findings will be shared with laboratory specialists, infectious disease specialists, hospital managers, etc., during our regular meetings to formulate further plans. Apart from pharmacological control, nonpharmacological hygienic techniques will be strictly enforced, which can help us go a long way in the prevention of infection spread and decrease antifungal resistance rates.
Proper handwashing techniques (i.e., the six-step handwash), trimmed nails, regular bathing, and full drying of the skin, use of nonocclusive shoes, absorbent socks, powder, and avoidance of sharing of toiletries, beddings, etc., [128] will be strictly enforced and encouraged to prevent the inter- and intra-departmental spread of infection. Since the present study represents the first of its kind in Latvia, it will aid in the establishment of baseline caseload and resistance rates for future studies and surveillance reports.
4.8. Limitations of the Present Study
The results obtained in the present study are limited by several of the following limitations. Firstly, we did not perform antifungal resistance analysis. Although Candifast test was used for identification of resistance, the susceptibility profile and clinical practice application of this commercial kit is not well established. Furthermore, multiple studies have advised avoiding the kit for antifungal resistance profiling [129,130]. Secondly, data analysis on fungal isolates from outpatient departments was not performed (to identify community epidemiology rates). Thirdly, being a single-center study, the results may not be completely reflective of the on-ground national situation. Finally, the present study used Candifast test for susceptibility detection, use of which remains controversial in the literature and hence, we suggest the readers to interpret the results of the present study regarding AFR rates with appropriate caution. However, our study marks a beginning and provides other healthcare institutions and laboratories with the means to investigate and report their respective data, which could aid in the curation of a national database for fungal infections. Monitoring of such a curated database could prove to be instrumental in improving patient care by fast-tracking diagnosis and the implementation of proper infection control measures.
5. Conclusions
The curation of comprehensive national and international surveillance databases concerning nosocomial fungal infections is the cornerstone to the emerging threat of nosocomial infections.
Candida spp. remained the most isolated nosocomial fungal species, followed by Aspergillus spp. and Geotrichum spp. C. albicans was the most common species amongst Candida spp., while a comparable burden of C. tropicalis and C. glabrata was noted in our hospital.
Author Contributions
The present study was conceptualized and designed by N.J. and A.R. Patient specimen collection and processing was done by D.S., I.J., and T.O. Data analysis, statistics, literature search, and visualization of the data was done by N.J. Project supervision was done by R.S., J.M., D.S., and A.R. Initial draft was written by N.J., while reviewing and editing was done by N.J., R.S., J.M., D.S., and A.R. All authors have read and agreed to the published version of the manuscript.
Funding
The present study received no external funding.
Institutional Review Board Statement
Ethical permission for the present study was provided by the Clinical Research Ethics Committee of the PSCUH, vide no. 290421-16L, dated 29 April 2021. The study protocol was designed per the Declaration of Helsinki guidelines.
Informed Consent Statement
Patient consent was waived as the study was part of assessment of quality control for internal institutional infection control, prevention, and education of staff members.
Data Availability Statement
All data analyzed in the present study are presented in a summarized manner in the results section of the manuscript. Patient data cannot be provided due to confidentiality and privacy concerns.
Acknowledgments
The authors are extremely grateful of the management of Pauls Stradinš Clinical University Hospital (PSCUH) and the supporting hospital and laboratory staff for their support in the present study.
Conflicts of Interest
The authors declare no competing interest in the present study. Furthermore, the hospital administration had no role in the design of the study, in the collection, analysis, or the interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
References
- Pai, V.; Ganavalli, A.; Kikkeri, N.N. Antifungal Resistance in Dermatology. Indian J. Dermatol. 2018, 63, 361–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kapil, A. (Ed.) Ananthanarayan & Paniker’s Textbook of Microbiology, 9th ed.; University Press Private Limited: Hyderabad, India, 2013; pp. 589–615. [Google Scholar]
- Berman, J.; Krysan, D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020, 18, 319–331, Erratum in 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rossi, N.M.; Peres, N.T.; Rossi, A. Antifungal resistance mechanisms in dermatophytes. Mycopathologia 2008, 166, 369–383. [Google Scholar] [CrossRef] [PubMed]
- White, T.C.; Marr, K.A.; Bowden, R.A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 1998, 11, 382–402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perlin, D.S.; Shor, E.; Zhao, Y. Update on Antifungal Drug Resistance. Curr. Clin. Microbiol. Rep. 2015, 2, 84–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vandeputte, P.; Ferrari, S.; Coste, A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012, 2012, 713687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costa-Orlandi, C.B.; Sardi, J.C.; Santos, C.T.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J. In vitro characterization of Trichophyton rubrum and, T. mentagrophytes biofilms. Biofouling 2014, 30, 719–727. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, J.W.; Camps, S.M.; Kampinga, G.A.; Arends, J.P.; Debets-Ossenkopp, Y.J.; Haas, P.J.; Rijnders, B.J.; Kuijper, E.J.; van Tiel, F.H.; Varga, J.; et al. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 2013, 57, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Zahra, F. Nosocomial Infections. [Updated 2021 July 7]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559312 (accessed on 30 July 2021).
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2016, 30, 1023–1052. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A.; Miceli, M.H.; Alangaden, G. Invasive fungal infections in transplant recipients. Ther. Adv. Infect. Dis. 2013, 1, 85–105. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.S.; Reyes, C.M.; Stolpman, M.; Speckman, J.; Allen, K.; Beney, J. The direct cost and incidence of systemic fungal infections. Value Health 2002, 5, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, W.R. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 1995, 20, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, W.R.; Martone, W.J. Predominant pathogens in hospital infections. J. Antimicrob. Chemother. 1992, 29 (Suppl. A), 19–24. [Google Scholar] [CrossRef] [PubMed]
- Krysan, D.J. The unmet clinical need of novel antifungal drugs. Virulence 2017, 8, 135–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes-Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hazen, E.L.; Brown, R. Fungicidin, an antibiotic produced by a soil actinomycete. Proc. Soc. Exp. Biol. Med. 1951, 76, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Zotchev, S.B. Polyene macrolide antibiotics and their applications in human therapy. Curr. Med. Chem. 2003, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kinsky, S.C. Antibiotic interaction with model membranes. Annu. Rev. Pharmacol. 1970, 10, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Palacios, D.S.; Dailey, I.; Siebert, D.M.; Wilcock, B.C.; Burke, M.D. Synthesis-enabled functional group deletions reveal key underpinnings of amphotericin B ion channel and antifungal activities. Proc. Natl. Acad. Sci. USA 2011, 108, 6733–6738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gray, K.C.; Palacios, D.S.; Dailey, I.; Endo, M.M.; Uno, B.E.; Wilcock, B.C.; Burke, M.D. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. USA 2012, 109, 2234–2239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.D. The discovery and development of amphotericin, B. Dis. Chest 1968, 54 (Suppl. S1), 296–298. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.G.; Marquês, J.T.; Carreira, A.C.; Castro, I.R.; Viana, A.S.; Mingeot-Leclercq, M.P.; de Almeida, R.F.M.; Silva, L.C. The molecular mechanism of Nystatin action is dependent on the membrane biophysical properties and lipid composition. Phys. Chem. Chem. Phys. 2017, 19, 30078–30088. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti-Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.J.; Hitchcock, C.A.; Sibley, C.M. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 1999, 12, 40–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappelletty, D.; Eiselstein-McKitrick, K. The echinocandins. Pharmacotherapy 2007, 27, 369–388. [Google Scholar] [CrossRef] [PubMed]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on Current Status of Echinocandins Use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Odds, F.C.; Brown, A.J.; Gow, N.A. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Pegu, R.; Borah, R.; Pratihar, S. Synthetic Compounds for Antifungal Chemotherapy. In Recent Trends in Antifungal Agents and Antifungal Therapy; Basak, A., Chakraborty, R., Mandal, S.M., Eds.; Springer: New Delhi, India, 2016; pp. 191–215. [Google Scholar]
- Jain, N.; Jansone, I.; Obidenova, T.; Simanis, R.; Meisters, J.; Straupmane, D.; Reinis, A. Antimicrobial Resistance in Nosocomial Isolates of Gram-Negative Bacteria: Public Health Implications in the Latvian Context. Antibiotics 2021, 10, 791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control EU Case Definitions. 2021. Available online: https://www.ecdc.europa.eu/en/surveillance-and-disease-data/eu-case-definitions (accessed on 21 April 2021).
- The European Committee on Antimicrobial Susceptibility Testing Antifungal Agents Breakpoint Tables for Interpretation of MICs. Version 9.0; valid from Feb 2018. 2021. Available online: http://www.eucast.org (accessed on 5 July 2021).
- European Antimicrobial Resistance Surveillance Network (EARS-Net). 2017–2019. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/report (accessed on 5 July 2021).
- Benedict, K.; Jackson, B.R.; Chiller, T.; Beer, K.D. Estimation of Direct Healthcare Costs of Fungal Diseases in the United States. Clin. Infect. Dis. 2019, 68, 1791–1797. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benedict, K.; Molinari, N.A.; Jackson, B.R. Public Awareness of Invasive Fungal Diseases—United States, 2019. MMWR Morb. Mortal Wkly. Rep. 2020, 69, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Think Fungus: Fungal Disease Awareness Week. Available online: https://www.cdc.gov/fungal/awareness-week.html (accessed on 30 July 2021).
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.D.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and Therapeutic Strategies: Mechanisms of Action for Traditional and Alternative Agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62 Pt 1, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Paramythiotou, E.; Frantzeskaki, F.; Flevari, A.; Armaganidis, A.; Dimopoulos, G. Invasive fungal infections in the ICU: How to approach, how to treat. Molecules 2014, 19, 1085–1119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wächtler, B.; Citiulo, F.; Jablonowski, N.; Förster, S.; Dalle, F.; Schaller, M.; Wilson, D.; Hube, B. Candida albicans-epithelial interactions: Dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS ONE 2012, 7, e36952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maccallum, D.M. Hosting infection: Experimental models to assay Candida virulence. Int. J. Microbiol. 2012, 2012, 363764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Martínez, R. Candidosis, a new challenge. Clin. Dermatol. 2010, 28, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Jahagirdar, V.L.; Davane, M.S.; Aradye, S.C.; Nagoba, B.S. Candida species as potential nosocomial pathogens—A review. Electron. J. Gen. Med. 2018, 15, em05. [Google Scholar] [CrossRef]
- Ha, J.F.; Italiano, C.M.; Heath, C.H.; Shih, S.; Rea, S.; Wood, F.M. Candidemia and invasive candidiasis: A review of the literature for the burns surgeon. Burns 2011, 37, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Frausto, M.S.; Houston, A.K.; Bale, M.J.; Fu, C.; Wenzel, R.P. An experimental model for study of Candida survival and transmission in human volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 1994, 13, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef] [PubMed]
- Deorukhkar, S.C.; Saini, S.; Mathew, S. Virulence Factors Contributing to Pathogenicity of Candida tropicalis and Its Antifungal Susceptibility Profile. Int. J. Microbiol. 2014, 2014, 456878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deorukhkar, S.; Saini, S. Evaluation of phospholipase activity in biofilm forming Candida species isolated from intensive care unit patients. Br. Microbiol. Res. J. 2013, 3, 440–447. [Google Scholar] [CrossRef]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin. Infect. Dis. 2006, 43, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Righi, E.; Ansaldi, F.; Merelli, M.; Trucchi, C.; De Pascale, G.; Diaz-Martin, A.; Luzzati, R.; Rosin, C.; Lagunes, L.; et al. A multicenter study of septic shock due to candidemia: Outcomes and predictors of mortality. Intensive Care Med. 2014, 40, 839–845, Erratum in 2014, 40, 1186. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, M.C.; Benet, T.; Vanhems, P. Aspergillosis: Nosocomial or community-acquired? Med. Mycol. 2011, 49 (Suppl. S1), S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Mandell, G.L.; Benett, J.E.; Dolin, R. Aspergillus Species, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 6th ed.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2005; pp. 2958–2973. [Google Scholar]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Voss, A.; Verweij, P.E. Hospital sources of Aspergillus: New routes of transmission? Rev. Iberoam. Micol. 2001, 18, 156–162. [Google Scholar] [PubMed]
- Verweij, P.E.; Kerremans, J.J.; Voss, A.; Meis, J.F. Fungal contamination of tobacco and marijuana. JAMA 2000, 284, 2875. [Google Scholar] [CrossRef] [PubMed]
- Rhame, F.S. Prevention of nosocomial aspergillosis. J. Hosp. Infect. 1991, 18 (Suppl. A), 466–472. [Google Scholar] [CrossRef] [PubMed]
- National Guidelines for the Prevention of Nosocomial Invasive Aspergillosis during Construction/Renovation Activities/Developed by a Sub-Committee of the Scientific Advisory Committee of the National Disease Surveillance Centre. National Disease Surveillance Centre (NDSC) [Lenus—The Irish Health Repository]. Available online: http://hdl.handle.net/10147/43715 (accessed on 31 July 2021).
- Partridge-Hinckley, K.; Liddell, G.M.; Almyroudis, N.G.; Segal, B.H. Infection control measures to prevent invasive mould diseases in hematopoietic stem cell transplant recipients. Mycopathologia 2009, 168, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.H. Aspergillosis. N. Engl. J. Med. 2009, 360, 1870–1884. [Google Scholar] [CrossRef] [PubMed]
- Einsele, H.; Quabeck, K.; Müller, K.D.; Hebart, H.; Rothenhöfer, I.; Löffler, J.; Schaefer, U.W. Prediction of invasive pulmonary aspergillosis from colonisation of lower respiratory tract before marrow transplantation. Lancet 1998, 352, 1443. [Google Scholar] [CrossRef] [PubMed]
- VandenBergh, M.F.; Verweij, P.E.; Voss, A. Epidemiology of nosocomial fungal infections: Invasive aspergillosis and the environment. Diagn. Microbiol. Infect. Dis. 1999, 34, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Anaissie, E.J.; Costa, S.F. Nosocomial aspergillosis is waterborne. Clin. Infect. Dis. 2001, 33, 1546–1548. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J. Primary cutaneous aspergillosis--an emerging infection among immunocompromised patients. Clin. Infect. Dis. 1998, 27, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Martino, P.; Venditti, M.; Micozzi, A.; Morace, G.; Polonelli, L.; Mantovani, M.P.; Petti, M.C.; Burgio, V.L.; Santini, C.; Serra, P.; et al. Blastoschizomyces capitatus: An emerging cause of invasive fungal disease in leukemia patients. Rev. Infect. Dis. 1990, 12, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Fouassier, M.; Joly, D.; Cambon, M.; Peigue-Lafeuille, H.; Condat, P. Infection à Geotrichum capitatum chez un patient neutropénique. A propos d’un cas et revue de la littérature [Geotrichum capitatum infection in a neutropenic patient. Apropos of a case and review of the literature]. Rev. Med. Interne 1998, 19, 431–433. (In French) [Google Scholar] [CrossRef] [PubMed]
- Mazzocato, S.; Marchionni, E.; Fothergill, A.W.; Sutton, D.A.; Staffolani, S.; Gesuita, R.; Skrami, E.; Fiorentini, A.; Manso, E.; Barchiesi, F. Epidemiology and outcome of systemic infections due to saprochaete capitata: Case report and review of the literature. Infection 2015, 43, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pamidimukkala, U.; Kancharla, A.; Sudhaharan, S.; Gundeti, S.; Mandarapu, S.; Nagalla, V.K.; Raju, S.B.; Karanam, S.D. Isolation of the Rare Opportunistic Yeast Saprochaete capitata from Clinical Samples-Experience from a Tertiary Care Hospital in Southern India and a Brief Review of the Literature. J. Clin. Diagn. Res. 2017, 11, DC36–DC42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Girmenia, C.; Pagano, L.; Martino, B.; D’Antonio, D.; Fanci, R.; Specchia, G.; Melillo, L.; Buelli, M.; Pizzarelli, G.; Venditti, M.; et al. Invasive infections caused by Trichosporon species and Geotrichum capitatum in patients with hematological malignancies: A retrospective multicenter study from Italy and review of the literature. J. Clin. Microbiol. 2005, 43, 1818–1828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurgui, M.; Sanchez, F.; March, F.; Lopez-Contreras, J.; Martino, R.; Cotura, A.; Galvez, M.L.; Roig, C.; Coll, P. Nosocomial outbreak of Blastoschizomyces capitatus associated with contaminated milk in a haematological unit. J. Hosp. Infect. 2011, 78, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Radic, M.; Goic Barisic, I.; Kuscevic, D.; Novak, A.; Tonkic, M.; Rubic, Z. Geotrichum capitatum respiratory tract infection in a patient with polytrauma. Infez. Med. 2015, 23, 270–274. [Google Scholar] [PubMed]
- Hajar, Z.; Medawar, W.; Rizk, N. Saprochaete capitata (Geotrichum capitatum), an emerging fungal infection in kidney transplant recipients. J. Mycol. Med. 2018, 28, 387–389. (In French) [Google Scholar] [CrossRef] [PubMed]
- Ulu-Kilic, A.; Atalay, M.A.; Metan, G.; Cevahir, F.; Koç, N.; Eser, B.; Çetin, M.; Kaynar, L.; Alp, E. Saprochaete capitata as an emerging fungus among patients with haematological malignencies. Mycoses 2015, 58, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bonini, A.; Capatti, C.; Parmeggiani, M.; Gugliotta, L.; Micozzi, A.; Gentile, G.; Capria, S.; Girmenia, C. Galactomannan detection in Geotrichum capitatum invasive infections: Report of 2 new cases and review of diagnostic options. Diagn. Microbiol. Infect. Dis. 2008, 62, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Haselow, D.; Lloyd, S.; Lockhart, S.; Moulton-Meissner, H.; Lester, L.; Wheeler, G.; Gladden, L.; Garner, K.; Derado, G.; et al. Cluster of Cryptococcus neoformans Infections in Intensive Care Unit, Arkansas, USA, 2013. Emerg. Infect. Dis. 2015, 21, 1719–1724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ellis, D.H.; Pfeiffer, T.J. Ecology, life cycle, and infectious propagule of Cryptococcus neoformans. Lancet 1990, 336, 923–925. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.C.; Goldman, D.L.; Shao, X.; Casadevall, A.; Husain, S.; Limaye, A.P.; Lyon, M.; Somani, J.; Pursell, K.; Pruett, T.L.; et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin. Vaccine Immunol. 2007, 14, 1550–1554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wnamalil, S.S.; Nagdeo, N.N.; Thombare, V.R.; Mathurkar, H. Curvularia A Most Common Missed Occulomycosis in Ocular Trauma. J. Med. Sci. Clin. Res. (JMSCR) 2014, 6, 1344–1348. Available online: https://jmscr.igmpublication.org/v2-i6/12%20jmscr.pdf (accessed on 10 August 2021).
- Ismail, Y.; Johnson, R.H.; Wells, M.V.; Pusavat, J.; Douglas, K.; Arsura, E.L. Invasive sinusitis with intracranial extension caused by Curvularia lunata. Arch. Intern. Med. 1993, 153, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Noyola, D.E.; Rossmann, S.N.; Edwards, M.S. Cutaneous phaeohyphomycosis caused by Curvularia lunata and a review of Curvularia infections in pediatrics. Pediatr. Infect. Dis. J. 1999, 18, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A. Curvularia--favorable response to oral itraconazole therapy in two patients with locally invasive phaeohyphomycosis. Clin. Microbiol. Infect. 2003, 9, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.G.; Phillips, P.; Schwartz, J.G.; Winn, R.E.; Holt, G.R.; Shagets, F.W.; Elrod, J.; Nishioka, G.; Aufdemorte, T.B. Human Curvularia infections. Report of five cases and review of the literature. Diagn. Microbiol. Infect. Dis. 1987, 6, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Fusarium infections in immunocompromised patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Diepeningen, A.D.; Brankovics, B.; Iltes, J.; van der Lee, T.A.; Waalwijk, C. Diagnosis of Fusarium Infections: Approaches to Identification by the Clinical Mycology Laboratory. Curr. Fungal Infect. Rep. 2015, 9, 135–143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nucci, M.; Anaissie, E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: Implications for diagnosis and management. Clin. Infect. Dis. 2002, 35, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anaissie, E.J.; Kuchar, R.T.; Rex, J.H.; Francesconi, A.; Kasai, M.; Müller, F.M.; Lozano-Chiu, M.; Summerbell, R.C.; Dignani, M.C.; Chanock, S.J.; et al. Fusariosis associated with pathogenic fusarium species colonization of a hospital water system: A new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Dis. 2001, 33, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Paula, C.R.; Krebs, V.L.; Auler, M.E.; Ruiz, L.S.; Matsumoto, F.E.; Silva, E.H.; Diniz, E.M.; Vaz, F.A. Nosocomial infection in newborns by Pichia anomala in a Brazilian intensive care unit. Med. Mycol. 2006, 44, 479–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, N.; Buchanan, C.R.; Damjanovic, V.; Whitaker, R.; Hart, C.A.; Cooke, R.W. Infection and colonisation of neonates by Hansenula anomala. Lancet 1986, 1, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M.; Cerikcioğlu, N.; Tirtir, A.; Berrak, S.; Ozek, E.; Canpolat, C. Pichia anomala fungaemia in immunocompromised children. Mycoses 2004, 47, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, L.W.; Aquino, V.R.; Zimerman, R.A.; Goldani, L.Z. Epidemiology and outcome of Rhodotorula fungemia in a tertiary care hospital. Clin. Infect. Dis. 2006, 43, e60–e63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wirth, F.; Goldani, L.Z. Epidemiology of Rhodotorula: An emerging pathogen. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 465717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Almeida, G.M.; Costa, S.F.; Melhem, M.; Motta, A.L.; Szeszs, M.W.; Miyashita, F.; Pierrotti, L.C.; Rossi, F.; Burattini, M.N. Rhodotorula spp. isolated from blood cultures: Clinical and microbiological aspects. Med. Mycol. 2008, 46, 547–556. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pérez-Torrado, R.; Querol, A. Opportunistic Strains of Saccharomyces cerevisiae: A Potential Risk Sold in Food Products. Front. Microbiol. 2016, 6, 1522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Enache-Angoulvant, A.; Hennequin, C. Invasive Saccharomyces infection: A comprehensive review. Clin. Infect. Dis. 2005, 41, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Bouza, E.; Cuenca-Estrella, M.; Eiros, J.M.; Pérez, M.J.; Sánchez-Somolinos, M.; Rincón, C.; Hortal, J.; Peláez, T. Saccharomyces cerevisiae fungemia: An emerging infectious disease. Clin. Infect. Dis. 2005, 40, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrado, R.; Llopis, S.; Jespersen, L.; Fernández-Espinar, T.; Querol, A. Clinical Saccharomyces cerevisiae isolates cannot cross the epithelial barrier in vitro. Int. J. Food Microbiol. 2012, 157, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Groll, A.; Hiemenz, J.; Fleming, R.; Roilides, E.; Anaissie, E. Infections due to emerging and uncommon medically important fungal pathogens. Clin. Microbiol. Infect. 2004, 10 (Suppl. S1), 48–66. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.S.D.; Lewis, R.; Lewis, J.S.; Martin, C.; Andes, D. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 2006, 43, S28–S39. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Rafati, H.; Ilkit, M.; Tolooe, A.; Hedayati, M.T.; Verweij, P. Systemic Antifungal Agents: Current Status and Projected Future Developments. Methods Mol. Biol. 2017, 1508, 107–139. [Google Scholar] [CrossRef] [PubMed]
- Dismukes, W.E. Introduction to antifungal drugs. Clin. Infect. Dis. 2000, 30, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, B.M.; Łapiński, Ł.; Wiela-Hojeńska, A. Comparison of clinical pharmacology of voriconazole and posaconazole. Contemp. Oncol. 2016, 20, 365–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, R.; Shah, A.H.; Rawal, M.K. Antifungals: Mechanism of action and drug resistance. In Yeast Membrane Transporter Advances in Experimental Medicine and Biology; Ramos, J., Sychrová, H., Kschischo, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 327–349. [Google Scholar]
- Nett, J.E.; Andes, D.R. Antifungal Agents: Spectrum of Activity, Pharmacology, and Clinical Indications. Infect. Dis. Clin. N. Am. 2016, 30, 51–83. [Google Scholar] [CrossRef] [PubMed]
- Onyewu, C.; Eads, E.; Schell, W.A.; Perfect, J.R.; Ullmann, Y.; Kaufman, G.; Horwitz, B.A.; Berdicevsky, I.; Heitman, J. Targeting the calcineurin pathway enhances ergosterol biosynthesis inhibitors against Trichophyton mentagrophytes in vitro and in a human skin infection model. Antimicrob. Agents Chemother. 2007, 51, 3743–3746. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gamal, A.; Chu, S.; McCormick, T.S.; Borroto-Esoda, K.; Angulo, D.; Ghannoum, M.A. Ibrexafungerp, a Novel Oral Triterpenoid Antifungal in Development: Overview of Antifungal Activity Against Candida glabrata. Front. Cell Infect. Microbiol. 2021, 11, 642358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghannoum, M.; Arendrup, M.C.; Chaturvedi, V.P.; Lockhart, S.R.; McCormick, T.S.; Chaturvedi, S.; Berkow, E.L.; Juneja, D.; Tarai, B.; Azie, N.; et al. Ibrexafungerp: A Novel Oral Triterpenoid Antifungal in Development for the Treatment of Candida auris Infections. Antibiotics 2020, 9, 539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva Ferreira, M.E.; Capellaro, J.L.; dos Reis Marques, E.; Malavazi, I.; Perlin, D.; Park, S.; Anderson, J.B.; Colombo, A.L.; Arthington-Skaggs, B.A.; Goldman, M.H.; et al. In vitro evolution of itraconazole resistance in Aspergillus fumigatus involves multiple mechanisms of resistance. Antimicrob. Agents Chemother. 2004, 48, 4405–4413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coste, A.T.; Karababa, M.; Ischer, F.; Bille, J.; Sanglard, D. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004, 3, 1639–1652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sanglard, D. Resistance of human fungal pathogens to antifungal drugs. Curr. Opin. Microbiol. 2002, 5, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; White, T.C. Genetic Basis of Antifungal Drug Resistance. Curr. Fungal Infect. Rep. 2009, 3, 163–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noël, T. The cellular and molecular defense mechanisms of the Candida yeasts against azole antifungal drugs. J. Mycol. Med. 2012, 22, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Geber, A.; Miyazaki, H.; Falconer, D.; Parkinson, T.; Hitchcock, C.; Grimberg, B.; Nyswaner, K.; Bennett, J.E. Cloning, sequencing, expression and allelic sequence diversity of ERG3 (C-5 sterol desaturase gene) in Candida albicans. Gene 1999, 236, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Manning, N.J.; Loeffler, J.; Hebart, H.; Schumacher, U.; Einsele, H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 1997, 400, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A. Mechanisms of resistance to antifungal agents: Yeasts and filamentous fungi. Rev. Iberoam. Micol. 2008, 25, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Lewis, R.E.; Dodds Ashley, E.S.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R.; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; Cornely, O.A.; et al. Core Recommendations for Antifungal Stewardship: A Statement of the Mycoses Study Group Education and Research Consortium. J. Infect. Dis. 2020, 222 (Suppl. S3), S175–S198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seebacher, C.; Bouchara, J.P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; Romano, L.; Sanguinetti, M.; Masucci, L.; Morace, G.; Fadda, G. Commercial systems for fluconazole susceptibility testing of yeasts: Comparison with the broth microdilution method. Diagn. Microbiol. Infect. Dis. 2000, 38, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.L.; Pfaller, M.A.; Brown, S.D.; Espinel-Ingroff, A.; Ghannoum, M.A.; Knapp, C.; Rennie, R.P.; Rex, J.H.; Rinaldi, M.G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 2000, 38, 3457–3459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).