Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality of Assessment
2.5. Statistical Analyses
3. Results
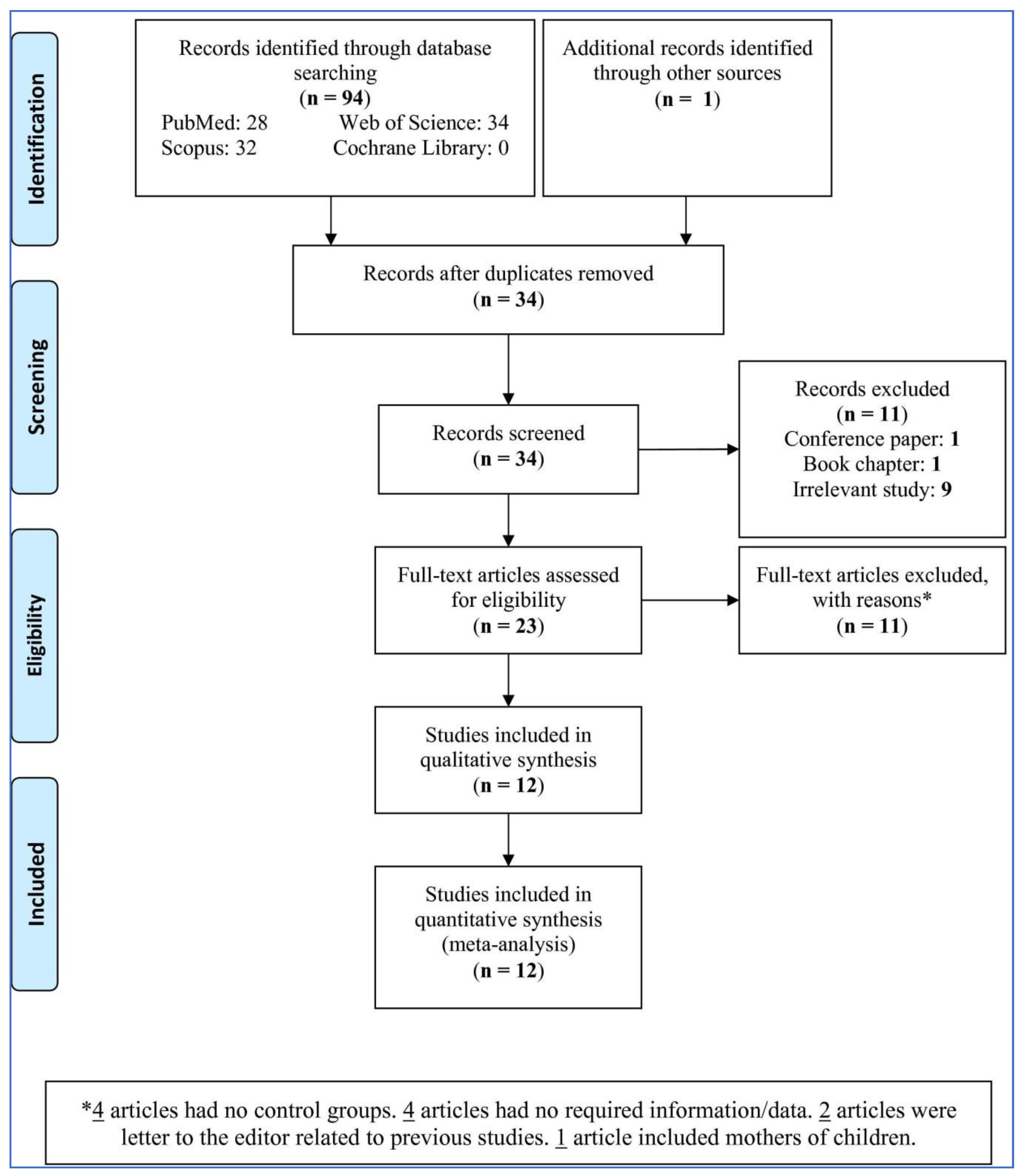
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
3.4. Pooled Analysis
3.5. Subgroup Analysis
3.6. Meta-Regression
3.7. Sensitivity Analysis
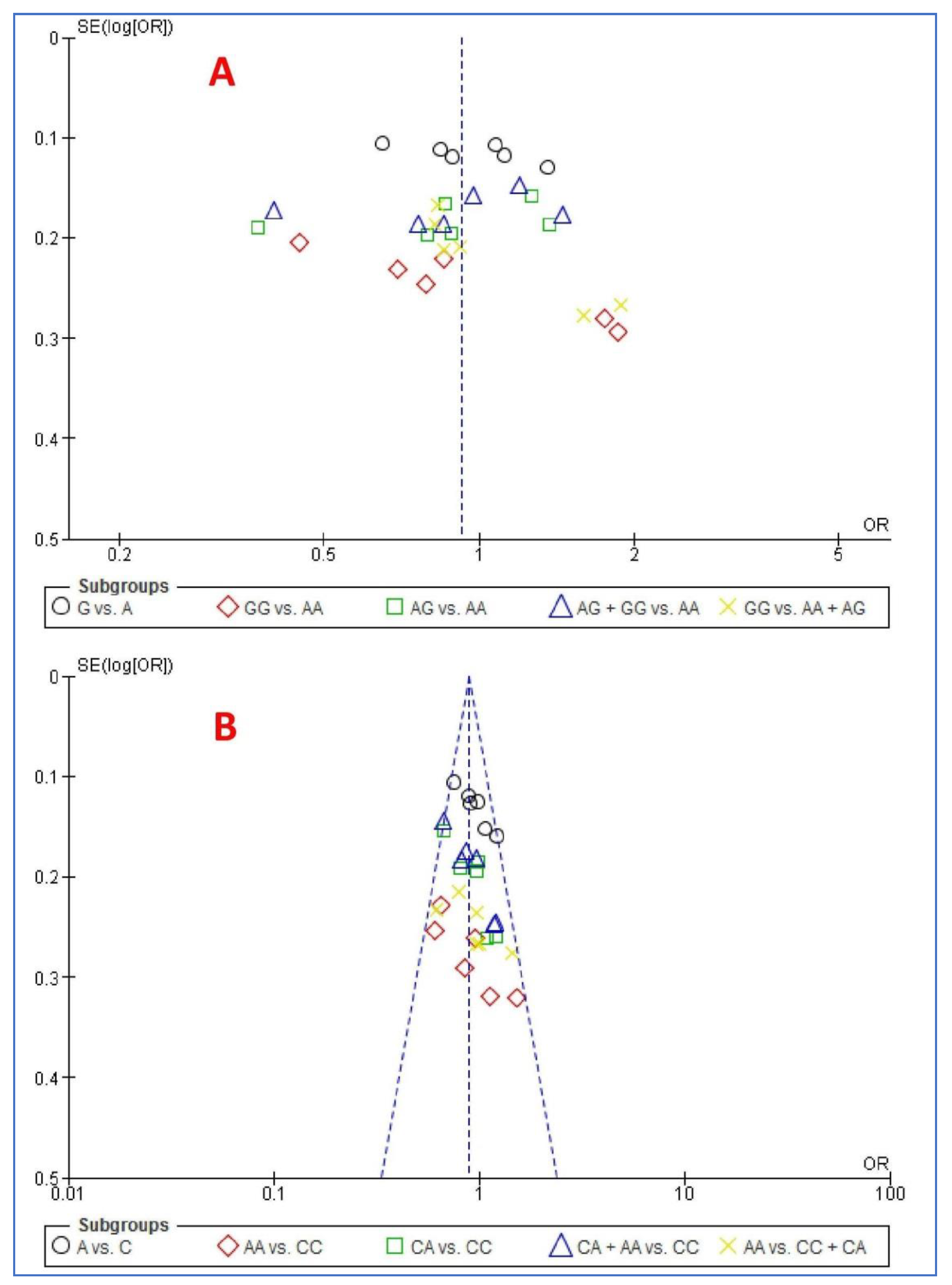
3.8. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA4 | ATP-binding cassette, sub-family A, member 4 |
| CI | Confidence interval |
| GWAS | genome-wide association |
| NSCL/P | Non-syndromic cleft lip/palate |
| OR | Odds ratio |
| ARHGAP29 | Rho GTPase Activating Protein 29 |
| IRF6 | Interferon Regulatory Factor 6 |
References
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Mossey, P.A.; Modell, B. Epidemiology of oral clefts 2012: An international perspective. Front. Oral Biol. 2012, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.J.; Marazita, M.L.; Beaty, T.H.; Murray, J.C. Cleft lip and palate: Understanding genetic and environmental influences. Nat. Rev. Genet. 2011, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.F.; Oliveira, G.H.M.; Soares, C.D.; Cardoso, M.L.; Ururahy, M.A.G.; Neto, F.P.F.; Lima-Neto, L.G.; Luchessi, A.D.; Silbiger, V.N.; Fajardo, C.M.; et al. Genetic and non-genetic factors that increase the risk of non-syndromic cleft lip and/or palate development. Oral Dis. 2015, 21, 393–399. [Google Scholar] [CrossRef]
- Perillo, L.; Isola, G.; Esercizio, D.; Iovane, M.; Triolo, G.; Matarese, G. Differences in craniofacial characteristics in Southern Italian children from Naples: A retrospective study by cephalometric analysis. Eur. J. Paediatr. Dent. 2013, 14, 195–198. [Google Scholar]
- Leonardi, R.; Aboulazm, K.; Giudice, A.L.; Ronsivalle, V.; D’Antò, V.; Lagravère, M.; Isola, G. Evaluation of mandibular changes after rapid maxillary expansion: A CBCT study in youngsters with unilateral posterior crossbite using a surface-to-surface matching technique. Clin. Oral Investig. 2020, 1–11. [Google Scholar] [CrossRef]
- Velázquez-Aragón, J.A.; Alcántara-Ortigoza, M.A.; Estandia-Ortega, B.; Reyna-Fabián, M.E.; Méndez-Adame, C.; Angel, A.G.-D. Gene Interactions Provide Evidence for Signaling Pathways Involved in Cleft Lip/Palate in Humans. J. Dent. Res. 2016, 95, 1257–1264. [Google Scholar] [CrossRef]
- Wu, N.; Lu, Y.; Liu, K.; Li, Z.; Liu, Q.; Lu, L. Associations of ABCA4 and MAFB with Nonsyndromic Cleft Lip with or without Cleft Palate in a Northeastern Chinese Population. J. Hard Tissue Biol. 2018, 27, 181–184. [Google Scholar] [CrossRef]
- Ludwig, K.U.; Wahle, P.; Reutter, H.; Paredes-Zenteno, M.; Munoz-Jimenez, S.G.; Ortiz-López, R.; Böhmer, A.C.; Tessmann, P.; Nowak, S.; Nöthen, M.M.; et al. Evaluating eight newly identified susceptibility loci for nonsyndromic cleft lip with or without cleft palate in a Mesoamerican population. Birth Defects Res. Part A Clin. Mol. Teratol. 2013, 100, 43–47. [Google Scholar] [CrossRef]
- Böhmer, A.C.; Gölz, L.; Kreusch, T.; Kramer, F.J.; Pötzsch, B.; Nöthen, M.M.; Jäger, A.; Mangold, E.; Knapp, M.; Ludwig, K.U. Investigation of dominant and recessive inheritance models in GWAS data ofnonsyndromic cleft lip with or without cleft palate. Birth Defects Res. 2018, 110, 336–341. [Google Scholar] [CrossRef]
- Beaty, T.H.; Taub, M.A.; Scott, A.F.; Murray, J.C.; Marazita, M.L.; Schwender, H.; Parker, M.M.; Hetmanski, J.B.; Balakrishnan, P.; Mansilla, M.A.; et al. Confirming genes influencing risk to cleft lip with/without cleft palate in a case-parent trio study. Hum. Genet. 2013, 132, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.J.; Mansilla, M.A.; Biggs, L.C.; Schuette, K.; Bullard, S.; Cooper, M.; Dunnwald, M.; Lidral, A.C.; Marazita, M.L.; Beaty, T.H.; et al. Expression and mutation analyses implicate ARHGAP29 as the etiologic gene for the cleft lip with or without cleft palate locus identified by genome-wide association on chromosome 1p22. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 934–942. [Google Scholar] [CrossRef]
- Howe, L.J.; Lee, M.K.; Sharp, G.C.; Smith, G.D.; Pourcain, B.S.; Shaffer, J.R.; Ludwig, K.U.; Mangold, E.; Marazita, M.L.; Feingold, E.; et al. Investigating the shared genetics of non-syndromic cleft lip/palate and facial morphology. PLoS Genet. 2018, 14, e1007501. [Google Scholar] [CrossRef] [PubMed]
- Molday, R.S.; Zhong, M.; Quazi, F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim. Biophys. Acta 2009, 1791, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Molday, R.S.; Nathans, J. Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J. Biol. Chem. 1999, 274, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Swider, M.; Aleman, T.S.; Tsybovsky, Y.; Schwartz, S.B.; Windsor, E.A.; Roman, A.J.; Sumaroka, A.; Steinberg, J.D.; Jacobson, S.G.; et al. ABCA4 disease progression and a proposed strategy for gene therapy. Hum. Molec. Genet. 2009, 18, 931–941. [Google Scholar] [CrossRef]
- Bhongsatiern, J.; Ohtsuki, S.; Tachikawa, M.; Hori, S.; Terasaki, T. Retinal-specific ATP-binding cassette transporter (ABCR/ABCA4) is expressed at the choroid plexus in rat brain. J. Neurochem. 2005, 92, 1277–1280. [Google Scholar] [CrossRef]
- Násser, L.S.; Martelli, D.R.B.; Swerts, M.S.O.; Popoff, D.A.V.; De Barros, L.M.; Martelli, H., Jr. Ophthalmic changes in cleft lip and palate. Rev. Bras. Talmol. 2016, 75, 94–98. [Google Scholar] [CrossRef]
- Beaty, T.H.; Murray, J.C.; Marazita, M.L.; Munger, R.G.; Ruczinski, I.; Hetmanski, J.B.; Liang, K.Y.; Wu, T.; Murray, T.; Fallin, M.D.; et al. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat. Genet. 2010, 42, 525–529. [Google Scholar] [CrossRef]
- Yu, Y.; Zuo, X.; He, M.; Gao, J.; Fu, Y.; Qin, C.; Meng, L.; Wang, W.; Song, Y.; Cheng, Y.; et al. Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nat. Commun. 2017, 8, 14364. [Google Scholar] [CrossRef]
- Ludwig, K.U.; Mangold, E.; Herms, S.; Nowak, S.; Reutter, H.; Paul, A.; Becker, J.; Herberz, R.; AlChawa, T.; Nasser, E.; et al. Genome-wide meta-analyses of nonsyndromic cleft lip with or without cleft palate identify six new risk loci. Nat. Genet. 2012, 44, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-H.; Chang, N.-C.; Chen, K.-T.; Lu, J.-J.; Chang, P.-Y.; Chang, S.-C.; Wu-Chou, Y.-H.; Chou, Y.-T.; Phang, W.; Cheng, P.-J. Nonsynonymous variants in MYH9 and ABCA4 are the most frequent risk loci associated with nonsyndromic orofacial cleft in Taiwanese population. BMC Med. Genet. 2016, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Do Rego Borges, A.; Sá, J.; Hoshi, R.; Viena, C.S.; Mariano, L.C.; De Castro Veiga, P.; Medrado, A.P.; Machado, R.A.; De Aquino, S.N.; Messetti, A.C.; et al. Genetic risk factors for nonsyndromic cleft lip with or without cleft palate in a Brazilian population with high African ancestry. Am. J. Med. Genet. Part A 2015, 167, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Robertson, J.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (Nos) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2011; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 January 2016).
- Lei, S.; Huang, L.; Liu, Y.; Xu, L.; Wang, D.; Yang, L. Association between polymorphisms of heat-shock protein 70 genes and noise-induced hearing loss: A meta-analysis. PLoS ONE 2017, 12, e0188539. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, W.; Du, Y.; Tong, N.; Han, Y.; Zhang, H.; Wang, M.; Ma, J.; Wan, L.; Wang, L. Different roles of two novel susceptibility loci for nonsyndromic orofacial clefts in a Chinese Han population. Am. J. Med. Genet. Part A 2011, 155, 2180–2185. [Google Scholar] [CrossRef]
- Huang, E.; Cheng, H.; Xu, M.; Shu, S.; Tang, S. Association between single-nucleotide polymorphisms on chromosome 1p22 and 20q12 and nonsyndromic cleft lip with or without cleft palate: New data in Han Chinese and meta-analysis. Birth Defects Res. Part A Clin. Mol. Teratol. 2012, 94, 469–476. [Google Scholar] [CrossRef]
- Zhou, Z.-W.; Yang, X.; Wan, Y.-B.; Xin, Y.-H.; Zhai, K.; Ma, J.; Huang, Y.-Q.; Jiang, M.; Wang, Y.-R. Associations of chromosomes 17q22, 10q25.3 and ABCA4 gene polymorphisms with non-syndromic cleft lip/palate in Ningxia Hui and Han population. J. Shandong Univ. 2013, 51, 103–108. [Google Scholar]
- Mi, N.; Hao, Y.; Jiao, X.; Zheng, X.; Shi, J.; Chen, Y. A polymorphic marker associated with non-syndromic cleft lip with or without cleft palate in a population in Heilongjiang Province, northern China. Arch. Oral Biol. 2015, 60, 357–361. [Google Scholar] [CrossRef]
- Bagordakis, E.; Paranaíba, L.M.R.; Brito, L.A.; De Aquino, S.N.; Messetti, A.C.; Martelli-Júnior, H.; Swerts, M.S.O.; Graner, E.; Passos-Bueno, M.R.; Coletta, R.D. Polymorphisms at regions 1p22.1 (rs560426) and 8q24 (rs1530300) are risk markers for nonsyndromic cleft lip and/or palate in the Brazilian population. Am. J. Med. Genet. Part A 2013, 161, 1177–1180. [Google Scholar] [CrossRef]
- Fontoura, C.; Silva, R.M.; Granjeiro, J.M.; Letra, A. Further evidence of association of the ABCA4 gene with cleft lip/palate. Eur. J. Oral Sci. 2012, 120, 553–557. [Google Scholar] [CrossRef]
- Mostowska, A.; Hozyasz, K.K.; Wójcicka, K.; Biedziak, B.; Jagodziński, P.P. Polymorphic variants at 10q25.3 and 17q22 loci and the risk of non-syndromic cleft lip and palate in the Polish population. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 94, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Gurramkonda, V.B.; Syed, A.H.; Murthy, J.; Chaubey, G.; Bhaskar Lakkakula, V.K. Polymorphic variants near 1p22 and 20q11.2 loci and the risk of non-syndromic cleft lip and palate in South Indian population. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 2389–2393. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Blanton, S.H.; Hecht, J.T. Association of ABCA4 and MAFB with non-syndromic cleft lip with or without cleft palate. Am. J. Med. Genet. Part A 2011, 155, 1469–1471. [Google Scholar] [CrossRef]
- Weng, J.; Mata, N.L.; Azarian, S.M.; Tzekov, R.T.; Birch, D.G.; Travis, G.H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in Abcr knockout mice. Cell 1999, 98, 13–23. [Google Scholar] [CrossRef]
- Lennon, C.J.; Birkeland, A.C.; Nuñez, J.A.; Su, G.H.; Lanzano, P.; Guzman, E.; Celis, K.; Eisig, S.; Hoffman, D.; Rendon, M.R.G.; et al. Association of candidate genes with nonsyndromic clefts in Honduran and Colombian populations. Laryngoscope 2012, 122, 2082–2087. [Google Scholar] [CrossRef]
- Liu, H.; Leslie, E.J.; Carlson, J.C.; Beaty, T.H.; Marazita, M.L.; Lidral, A.C.; Cornell, R.A. Identification of common non-coding variants at 1p22 that are functional for non-syndromic orofacial clefting. Nat. Commun. 2017, 8, 14759. [Google Scholar] [CrossRef]
| First Author, (Year) | Ethnic Group | Source of Controls | Mean Age, Year (NSCL/P Patients to Controls) | No. of Males, (NSCL/P Patients to Controls) | ABCA4 rs560426 | ABCA4 rs481931 | Genotyping Method | p-Value for HWE in Controls | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AA/AG/GG | CC/CA/AA | |||||||||
| Case | Control | Case | Control | |||||||
| Pan et al. (2011) [27] | Asian | HB | 5.54 to 5.49 | 246 to 242 | 145/175/51 | 167/160/57 | NA | NA | TaqMan | 0.071 |
| Fontoura et al. (2012) [32] | European descent | HB | 17.3 to 24.8 | 252 to 165 | 116/118/86 | 74/203/123 | 184/155/44 | 154/192/57 | TaqMan | 0.542/0.818 |
| Huang et al. (2012) [28] | Asian | HB | NA | 169 to 203 | 135/126/39 | 157/171/26 | NA | NA | MALDI-TOF MS (Sequenom) | 0.024 |
| Mostowska et al. (2012) [33] | European descent | HB | NA | NA | 62/105/39 | 120/230/96 | 79/98/29 | 156/196/94 | PCR-HRM | 0.467/0.028 |
| Bagordakis et al. (2013) [31] | Mixed | PB | NA | NA | 74/140/85 | 127/172/85 | NA | NA | Multiplex PCR | 0.067 |
| Zhong-wei et al. (2013) [29] | Asian | PB | NA | NA | 54/91/36 | 36/50/18 | NA | NA | TaqMan | 0.928 |
| Ludwig et al. (2014) [9] | Mixed | PB | NA | 102 to 111 | 37/73/33 | 100/163/66 | NA | NA | MALDI-TOF MS (Sequenom) | 0.977 |
| do Rego Borges et al. (2015) [23] | Mixed | HB | NA | NA | 76/152/65 | 74/187/91 | NA | NA | TaqMan | 0.223 |
| Babu Gurramkonda et al. (2015) [34] | European descent | PB | NA | NA | 46/72/26 | 61/80/35 | 41/68/35 | 57/87/32 | Kompetitive allele specific PCR (KASP) | 0.348/0.905 |
| Mi et al. (2015) [30] | Asian | HB | 4.98 to 5.20 | NA | 88/104/30 | 158/137/29 | 79/107/36 | 113/157/54 | Mini-sequencing (SNAPSHOT) | 0.928/0.965 |
| Velázquez-Aragón et al. (2016) [7] | Mixed | PB | 5.5 to 1.33 | 99 to 132 | 44/56/32 | 54/137/68 | 32/71/27 | 71/131/53 | Kompetitive allele specific PCR (KASP) | 0.326/0.602 |
| Wu et al. (2018) [8] | Asian | PB | NA | NA | 103/116/29 | 111/145/24 | 92/126/30 | 91/154/35 | PCR-RFLP | 0.014/0.015 |
| First Author, (Year) | Selection (Four Points) | Comparability (Two Points) | Exposure (Three Points) | Total Points |
|---|---|---|---|---|
| Pan et al. (2011) [27] | *** | ** | *** | 8 |
| Fontoura et al. (2012) [32] | *** | - | *** | 7 |
| Huang et al. (2012) [28] | *** | * | *** | 7 |
| Mostowska et al. (2012) [33] | *** | ** | *** | 8 |
| Bagordakis et al. (2013) [31] | **** | - | *** | 7 |
| Zhong-wei et al. (2013) [29] | **** | - | *** | 7 |
| Ludwig et al. (2014) [9] | **** | - | *** | 7 |
| do Rego Borges et al. (2015) [23] | *** | - | *** | 7 |
| Babu Gurramkonda et al. (2015) [34] | **** | ** | *** | 9 |
| Mi et al. (2015) [30] | *** | ** | *** | 8 |
| Velázquez-Aragón et al. (2016) [7] | **** | * | *** | 8 |
| Wu et al. (2018) [8] | **** | - | *** | 7 |
| Genetic Model | First Author, Publication Year | NSCL/P | Control | Weight | Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | M-H, Random, 95%CI | |||
| G vs. A | Pan, 2011 | 277 | 742 | 274 | 768 | 9.2% | 1.07 [0.87, 1.32] |
| Mostowska, 2012 | 183 | 412 | 422 | 892 | 8.7% | 0.89 [0.70, 1.13] | |
| Huang, 2012 | 204 | 600 | 223 | 708 | 8.8% | 1.12 [0.89, 1.41] | |
| Fontoura, 2012 | 290 | 640 | 449 | 800 | 9.2% | 0.65 [0.53, 0.80] | |
| Zhong-wei, 2013 | 163 | 362 | 86 | 208 | 6.7% | 1.16 [0.82, 1.64] | |
| Bagordakis, 2013 | 310 | 598 | 342 | 767 | 9.1% | 1.34 [1.08, 1.66] | |
| Ludwig, 2014 | 139 | 286 | 295 | 658 | 7.9% | 1.16 [0.88, 1.54] | |
| Mi, 2015 | 164 | 444 | 195 | 648 | 8.3% | 1.36 [1.05, 1.76] | |
| do Rego Borges, 2015 | 282 | 586 | 369 | 704 | 9.0% | 0.84 [0.68, 1.05] | |
| Babu Gurramkonda, 2015 | 124 | 288 | 150 | 352 | 7.2% | 1.02 [0.74, 1.39] | |
| Velázquez-Aragón, 2016 | 120 | 264 | 273 | 518 | 7.5% | 0.75 [0.56, 1.01] | |
| Wu, 2018 | 174 | 496 | 193 | 560 | 8.3% | 1.03 [0.80, 1.32] | |
| Subtotal (95%CI) | 5718 | 7583 | 100.0% | 1.01 [0.88, 1.15] | |||
| Total Events | 2430 | 3271 | |||||
| Heterogeneity: Tau2 = 0.04; Chi2 = 39.48, df = 11 (p < 0.0001); I2 = 72%; Test for overall effect: Z = 0.10 (p = 0.92) | |||||||
| GG vs. AA | Pan, 2011 | 51 | 226 | 57 | 224 | 9.1% | 0.85 [0.55, 1.32] |
| Mostowska, 2012 | 39 | 101 | 96 | 216 | 8.8% | 0.79 [0.49, 1.27] | |
| Huang, 2012 | 39 | 174 | 26 | 183 | 8.3% | 1.74 [1.01, 3.01] | |
| Fontoura, 2012 | 86 | 202 | 123 | 197 | 9.4% | 0.45 [0.30, 0.67] | |
| Zhong-wei, 2013 | 36 | 60 | 18 | 54 | 6.6% | 3.00 [1.39, 6.45] | |
| Bagordakis, 2013 | 85 | 159 | 85 | 212 | 9.3% | 1.72 [1.13, 2.60] | |
| Ludwig, 2014 | 33 | 70 | 66 | 166 | 8.1% | 1.35 [0.77, 2.37] | |
| Mi, 2015 | 30 | 118 | 29 | 187 | 8.1% | 1.86 [1.05, 3.29] | |
| do Rego Borges, 2015 | 65 | 141 | 91 | 165 | 9.0% | 0.70 [0.44, 1.09] | |
| Babu Gurramkonda, 2015 | 26 | 72 | 35 | 96 | 7.6% | 0.99 [0.52, 1.86] | |
| Velázquez-Aragón, 2016 | 32 | 76 | 68 | 122 | 8.0% | 0.58 [0.32, 1.03] | |
| Wu, 2018 | 29 | 132 | 24 | 135 | 7.8% | 1.30 [0.71, 2.38] | |
| Subtotal (95%CI) | 1531 | 1957 | 100.0% | 1.08 [0.79, 1.47] | |||
| Total EVENTS | 551 | 718 | |||||
| Heterogeneity: Tau2 = 0.23; Chi2 = 47.66, df = 11 (p < 0.00001); I2 = 77%; Test for overall effect: Z = 0.47 (p = 0.64) | |||||||
| AG vs. AA | Pan, 2011 | 175 | 320 | 160 | 327 | 9.3% | 1.26 [0.92, 1.72] |
| Mostowska, 2012 | 105 | 167 | 230 | 350 | 8.6% | 0.88 [0.60, 1.30] | |
| Huang, 2012 | 126 | 261 | 171 | 328 | 9.2% | 0.86 [0.62, 1.19] | |
| Fontoura, 2012 | 118 | 234 | 203 | 277 | 8.7% | 0.37 [0.26, 0.54] | |
| Zhong-wei, 2013 | 91 | 145 | 50 | 86 | 6.9% | 1.21 [0.70, 2.09] | |
| Bagordakis, 2013 | 140 | 214 | 172 | 299 | 8.8% | 1.40 [0.97, 2.01] | |
| Ludwig, 2014 | 73 | 110 | 163 | 263 | 7.7% | 1.21 [0.76, 1.93] | |
| Mi, 2015 | 104 | 192 | 137 | 295 | 8.8% | 1.36 [0.95, 1.96] | |
| Babu Gurramkonda, 2015 | 72 | 118 | 80 | 141 | 7.4% | 1.19 [0.73, 1.96] | |
| do Rego Borges, 2015 | 152 | 228 | 187 | 261 | 8.6% | 0.79 [0.54, 1.16] | |
| Velázquez-Aragón, 2016 | 56 | 100 | 137 | 191 | 7.3% | 0.50 [0.30, 0.83] | |
| Wu, 2018 | 116 | 219 | 145 | 256 | 8.8% | 0.86 [0.60, 1.24] | |
| Subtotal (95% CI) | 2308 | 3074 | 100.0% | 0.93 [0.73, 1.17] | |||
| Total Events | 1328 | 1835 | |||||
| Heterogeneity: Tau2 = 0.13; Chi2 = 46.53, df = 11 (p < 0.00001); I2 = 76%; Test for overall effect: Z = 0.63 (p = 0.53) | |||||||
| AG + GG vs. AA | Pan, 2011 | 226 | 371 | 217 | 384 | 9.1% | 1.20 [0.90, 1.60] |
| Fontoura, 2012 | 204 | 320 | 326 | 400 | 8.7% | 0.40 [0.28, 0.56] | |
| Huang, 2012 | 165 | 300 | 197 | 354 | 8.9% | 0.97 [0.71, 1.33] | |
| Mostowska, 2012 | 144 | 206 | 326 | 446 | 8.5% | 0.85 [0.59, 1.23] | |
| Zhong-wei, 2013 | 127 | 181 | 68 | 104 | 7.2% | 1.25 [0.74, 2.08] | |
| Bagordakis, 2013 | 225 | 299 | 257 | 384 | 8.7% | 1.50 [1.07, 2.11] | |
| Ludwig, 2014 | 106 | 143 | 229 | 329 | 7.8% | 1.25 [0.80, 1.95] | |
| Mi, 2015 | 134 | 222 | 166 | 324 | 8.6% | 1.45 [1.03, 2.05] | |
| do Rego Borges, 2015 | 217 | 293 | 278 | 352 | 8.5% | 0.76 [0.53, 1.10] | |
| Babu Gurramkonda, 2015 | 68 | 144 | 115 | 176 | 7.7% | 0.47 [0.30, 0.75] | |
| Velázquez-Aragón, 2016 | 88 | 132 | 205 | 259 | 7.6% | 0.53 [0.33, 0.84] | |
| Wu, 2018 | 145 | 248 | 169 | 280 | 8.6% | 0.92 [0.65, 1.31] | |
| Subtotal (95%CI) | 2859 | 3792 | 100.0% | 0.89 [0.70, 1.14] | |||
| Total Events | 1849 | 2553 | |||||
| Heterogeneity: Tau2 = 0.15; Chi2 = 59.28, df = 11 (p < 0.00001); I2 = 81%; Test for overall effect: Z = 0.90 (p = 0.37) | |||||||
| GG vs. AA + AG | Pan, 2011 | 51 | 371 | 57 | 384 | 9.6% | 0.91 [0.61, 1.37] |
| Fontoura, 2012 | 86 | 320 | 123 | 400 | 12.2% | 0.83 [0.60, 1.15] | |
| Huang, 2012 | 39 | 300 | 26 | 354 | 6.9% | 1.89 [1.12, 3.18] | |
| Mostowska, 2012 | 39 | 206 | 96 | 446 | 9.4% | 0.85 [0.56, 1.29] | |
| Bagordakis, 2013 | 85 | 299 | 85 | 384 | 11.4% | 1.40 [0.99, 1.98] | |
| Zhong-wei, 2013 | 36 | 181 | 18 | 104 | 5.3% | 1.19 [0.63, 2.22] | |
| Ludwig, 2014 | 33 | 143 | 66 | 329 | 7.9% | 1.20 [0.74, 1.92] | |
| do Rego Borges, 2015 | 65 | 293 | 91 | 352 | 10.9% | 0.82 [0.57, 1.18] | |
| Mi, 2015 | 30 | 222 | 29 | 324 | 6.6% | 1.59 [0.92, 2.73] | |
| Babu Gurramkonda, 2015 | 26 | 144 | 35 | 176 | 6.2% | 0.89 [0.51, 1.56] | |
| Velázquez-Aragón, 2016 | 32 | 132 | 68 | 259 | 7.7% | 0.90 [0.55, 1.46] | |
| Wu, 2018 | 29 | 248 | 24 | 280 | 6.1% | 1.41 [0.80, 2.50] | |
| Subtotal (95%CI) | 2859 | 3792 | 100.0% | 1.08 [0.91, 1.26] | |||
| Total Events | 551 | 718 | |||||
| Heterogeneity: Tau2 = 0.03; Chi2 = 17.14, df = 11 (p = 0.10); I2 = 36%; Test for overall effect: Z = 0.87 (p = 0.38) | |||||||
| Genetic Model | First Author, Publication Year | NSCL/P | Control | Weight | Odds Ratio | ||
|---|---|---|---|---|---|---|---|
| Events | Total | Events | Total | M-H, Fixed, 95%CI | |||
| A vs. C | Fontoura, 2012 | 243 | 766 | 306 | 806 | 26.6% | 0.76 [0.62, 0.94] |
| Mostowska, 2012 | 183 | 412 | 422 | 892 | 19.4% | 0.89 [0.70, 1.13] | |
| Mi, 2015 | 179 | 444 | 265 | 648 | 16.8% | 0.98 [0.76, 1.25] | |
| Babu Gurramkonda, 2015 | 138 | 288 | 151 | 352 | 9.2% | 1.22 [0.90, 1.67] | |
| Velázquez-Aragón, 2016 | 125 | 260 | 237 | 510 | 10.9% | 1.07 [0.79, 1.44] | |
| Wu, 2018 | 186 | 496 | 224 | 560 | 17.2% | 0.90 [0.70, 1.15] | |
| Subtotal (95%CI) | 2666 | 3768 | 100.0% | 0.92 [0.83, 1.02] | |||
| Total Events | 1054 | 1605 | |||||
| Heterogeneity: Chi2 = 7.74, df = 5 (p = 0.17); I2 = 35%; Test for overall effect: Z = 1.57 (p = 0.12) | |||||||
| AA vs. CC | Fontoura, 2012 | 44 | 228 | 57 | 211 | 26.6% | 0.65 [0.41, 1.01] |
| Mostowska, 2012 | 29 | 108 | 94 | 250 | 23.1% | 0.61 [0.37, 1.00] | |
| Babu Gurramkonda, 2015 | 35 | 76 | 32 | 89 | 8.8% | 1.52 [0.81, 2.84] | |
| Mi, 2015 | 36 | 115 | 54 | 167 | 16.8% | 0.95 [0.57, 1.59] | |
| Velázquez-Aragón, 2016 | 27 | 59 | 53 | 124 | 10.3% | 1.13 [0.61, 2.11] | |
| Wu, 2018 | 30 | 122 | 35 | 126 | 14.4% | 0.85 [0.48, 1.50] | |
| Subtotal (95%CI) | 708 | 967 | 100.0% | 0.85 [0.68, 1.05] | |||
| Total Events | 201 | 325 | |||||
| Heterogeneity: Chi2 = 7.49, df = 5 (p = 0.19); I2 = 33%; Test for overall effect: Z = 1.52 (p = 0.13) | |||||||
| CA vs. CC | Fontoura, 2012 | 155 | 339 | 192 | 346 | 31.0% | 0.68 [0.50, 0.91] |
| Mostowska, 2012 | 98 | 177 | 196 | 352 | 17.6% | 0.99 [0.69, 1.42] | |
| Mi, 2015 | 107 | 186 | 157 | 270 | 16.3% | 0.97 [0.67, 1.42] | |
| Babu Gurramkonda, 2015 | 68 | 109 | 87 | 144 | 8.5% | 1.09 [0.65, 1.81] | |
| Velázquez-Aragón, 2016 | 71 | 103 | 131 | 202 | 8.3% | 1.20 [0.72, 2.00] | |
| Wu, 2018 | 126 | 218 | 154 | 245 | 18.4% | 0.81 [0.56, 1.18] | |
| Subtotal (95% CI) | 1132 | 1559 | 100.0% | 0.88 [0.75, 1.03] | |||
| Total Events | 625 | 917 | |||||
| Heterogeneity: Chi2 = 5.93, df = 5 (p = 0.31); I2 = 16%; Test for overall effect: Z = 1.57 (p = 0.12) | |||||||
| CA + AA vs. CC | Fontoura, 2012 | 199 | 383 | 249 | 403 | 31.1% | 0.67 [0.50, 0.89] |
| Mostowska, 2012 | 127 | 206 | 290 | 446 | 18.7% | 0.86 [0.61, 1.22] | |
| Babu Gurramkonda, 2015 | 103 | 144 | 119 | 176 | 8.1% | 1.20 [0.74, 1.95] | |
| Mi, 2015 | 143 | 222 | 211 | 324 | 16.3% | 0.97 [0.68, 1.39] | |
| Velázquez-Aragón, 2016 | 98 | 130 | 184 | 255 | 8.2% | 1.18 [0.73, 1.92] | |
| Wu, 2018 | 156 | 248 | 189 | 280 | 17.6% | 0.82 [0.57, 1.17] | |
| Subtotal (95%CI) | 1333 | 1884 | 100.0% | 0.87 [0.75, 1.00] | |||
| Total Events | 826 | 1242 | |||||
| Heterogeneity: Chi2 = 7.05, df = 5 (p = 0.22); I2 = 29%; Test for overall effect: Z = 1.91 (p = 0.06) | |||||||
| AA vs. CC + CA | Mostowska, 2012 | 29 | 206 | 94 | 446 | 23.6% | 0.61 [0.39, 0.97] |
| Fontoura, 2012 | 44 | 383 | 57 | 403 | 22.8% | 0.79 [0.52, 1.20] | |
| Babu Gurramkonda, 2015 | 35 | 144 | 32 | 176 | 10.1% | 1.44 [0.84, 2.48] | |
| Mi, 2015 | 36 | 222 | 54 | 324 | 17.0% | 0.97 [0.61, 1.53] | |
| Velázquez-Aragón, 2016 | 27 | 130 | 53 | 255 | 13.1% | 1.00 [0.59, 1.68] | |
| Wu, 2018 | 30 | 248 | 35 | 280 | 13.4% | 0.96 [0.57, 1.62] | |
| Subtotal (95%CI) | 1333 | 1884 | 100.0% | 0.89 [0.74, 1.09] | |||
| Total Events | 201 | 325 | |||||
| Heterogeneity: Chi2 = 6.39, df = 5 (p = 0.27); I2 = 22%; Test for overall effect: Z = 1.12 (p = 0.26) | |||||||
| Variable (N) | G vs. A | GG vs. AA | AG vs. AA | AG + GG vs. AA | GG vs. AA + AG |
|---|---|---|---|---|---|
| OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | |
| Overall (12) | 1.01 (0.88, 1.15), 72, <0.0001 | 1.08 (0.79, 1.47), 77, <0.00001 | 0.93 (0.73, 1.16), 76, <0.00001 | 0.89 (0.70, 1.14), 81, <0.00001 | 1.08 (0.91, 1.26), 36, 0.10 |
| Ethnicity | |||||
| Asian (5) | 1.13 (1.01, 1.27), 0, 0.59 | 1.53 (1.01, 2.31), 62, 0.03 | 1.08 (0.92, 1.26), 35, 0.19 | 1.13 (0.97, 1.32), 10, 0.35 | 1.30 (1.03, 1.63), 27, 0.24 |
| European Descent (3) | 0.82 (0.63, 1.08), 71, 0.03 | 0.67 (0.42, 1.09), 64, 0.06 | 0.72 (0.36, 1.45), 88, 0.0002 | 0.55 (0.34, 0.88), 79, 0.009 | 0.85 (0.67, 1.07), 0, 0.98 |
| Mixed (4) | 1.00 (0.76, 1.31), 79, 0.003 | 0.99 (0.59, 1.68), 78, 0.004 | 0.92 (0.60, 1.42), 76, 0.006 | 0.94 (0.60, 1.49), 81, 0.001 | 1.07 (0.87, 1.30), 41, 0.17 |
| Source of Controls | |||||
| Hospital-Based (6) | 0.94 (0.86, 1.03), 80, 0.0001 | 0.83 (0.69, 1.01), 80, 0.0002 | 0.87 (0.76, 1.01), 84, <0.00001 | 0.89 (0.78, 1.02), 85, <0.00001 | 0.98 (0.83, 1.15), 57, 0.04 |
| Population-Based (6) | 1.07 (0.91, 1.26), 52, 0.06 | 1.29 (0.86, 1.94), 66, 0.01 | 1.02 (0.76, 1.36), 60, 0.03 | 0.91 (0.62, 1.33), 80, 0.0002 | 1.18 (0.97, 1.44), 0, 0.63 |
| Variable (N) | A vs. C | AA vs. CC | CA vs. CC | CA + AA vs. CC | AA vs. CC + CA |
|---|---|---|---|---|---|
| OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | OR (95%CI), I2 (%), Ph | |
| Overall (6) | 0.92 (0.83, 1.02), 35, 0.17 | 0.85 (0.68, 1.05), 33, 0.19 | 0.88 (0.75, 1.03), 16, 0.31 | 0.87 (0.75, 1.00), 0.29, 0.22 | 0.89 (0.74, 1.09), 22, 0.27 |
| Ethnicity | |||||
| Asian (2) | 0.94 (0.79, 1.12), 0, 0.65 | 0.90 (0.62, 1.32), 0, 0.76 | 0.89 (0.68, 1.16), 0, 0.49 | 0.89 (0.69, 1.15), 0, 0.51 | 0.97 (0.68, 1.36), 0, 0.99 |
| European Descent (3) | 0.92 (0.71, 1.18), 68, 0.04 | 0.81 (0.48, 1.36), 67, 0.05 | 0.83 (0.67, 1.03), 46, 0.15 | 0.85 (0.62, 1.16), 56, 0.11 | 0.87 (0.55, 1.38), 66, 0.05 |
| Mixed (1) | 1.07 (0.79, 1.44) | 1.13 (0.61, 2.11) | 1.20 (0.72, 2.00) | 1.18 (0.73, 1.92) | 1.00 (0.59, 1.68) |
| Source of Controls | |||||
| Hospital-Based (2) | 0.85 (0.67, 1.09), 57, 0.13 | 0.77 (0.55, 1.07), 21, 0.26 | 0.80 (0.56, 1.14), 55, 0.14 | 0.79 (0.55, 1.14), 61, 0.11 | 0.86 (0.63, 1.18), 0, 0.52 |
| Population-Based (4) | 0.98 (0.86, 1.12), 11, 0.34 | 0.91 (0.68, 1.20), 47, 0.13 | 0.97, (0.79, 1.20), 0, 0.62 | 0.95 (0.78, 1.16), 0, 0.44 | 0.91 (0.71, 1.17), 49, 0.12 |
| Variable | Polymorphism | Allele | Homozygote | Heterozygote | Recessive | Dominant | |
|---|---|---|---|---|---|---|---|
| Publication Year | rs560426 | R | 0.025 | 0.047 | 0.113 | 0.172 | 0.075 |
| Adjusted R2 | −0.099 | −0.098 | −0.086 | −0.068 | −0.094 | ||
| P | 0.937 | 0.884 | 0.726 | 0.593 | 0.816 | ||
| rs481931 | R | 0.406 | 0.456 | 0.243 | 0.388 | 0.495 | |
| Adjusted R2 | −0.044 | 0.010 | −0.176 | −0.062 | 0.057 | ||
| P | 0.424 | 0.364 | 0.643 | 0.447 | 0.318 | ||
| Number of Participants | rs560426 | R | 0.098 | 0.410 | 0.171 | 0.118 | 0.036 |
| Adjusted R2 | −0.089 | 0.085 | −0.068 | −0.085 | −0.099 | ||
| P | 0.761 | 0.185 | 0.594 | 0.714 | 0.912 | ||
| rs481931 | R | 0.953 | 0.913 | 0.810 | 0.650 | 0.814 | |
| Adjusted R2 | 0.886 | 0.793 | 0.570 | 0.279 | 0.579 | ||
| P | 0.003 | 0.011 | 0.051 | 0.162 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imani, M.M.; Sadeghi, M.; Tadakamadla, S.K.; Brühl, A.; Sadeghi Bahmani, D.; Taheri, M.; Brand, S. Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis. Life 2021, 11, 58. https://doi.org/10.3390/life11010058
Imani MM, Sadeghi M, Tadakamadla SK, Brühl A, Sadeghi Bahmani D, Taheri M, Brand S. Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis. Life. 2021; 11(1):58. https://doi.org/10.3390/life11010058
Chicago/Turabian StyleImani, Mohammad Moslem, Masoud Sadeghi, Santosh Kumar Tadakamadla, Annette Brühl, Dena Sadeghi Bahmani, Mohammad Taheri, and Serge Brand. 2021. "Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis" Life 11, no. 1: 58. https://doi.org/10.3390/life11010058
APA StyleImani, M. M., Sadeghi, M., Tadakamadla, S. K., Brühl, A., Sadeghi Bahmani, D., Taheri, M., & Brand, S. (2021). Polymorphisms of ATP-Binding Cassette, Sub-Family A, Member 4 (rs560426 and rs481931) and Non-Syndromic Cleft Lip/Palate: A Meta-Analysis. Life, 11(1), 58. https://doi.org/10.3390/life11010058







