Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation
Abstract
1. Introduction
2. Methods
2.1. Samples and Hydrothermal Experiments
2.2. High-Performance Liquid Chromatograph (HPLC) Analysis
2.3. FTIR Micro-Spectroscopy Analysis
2.4. Gel Filtration Chromatography Analysis
3. Results
3.1. Amino Acid Concentrations
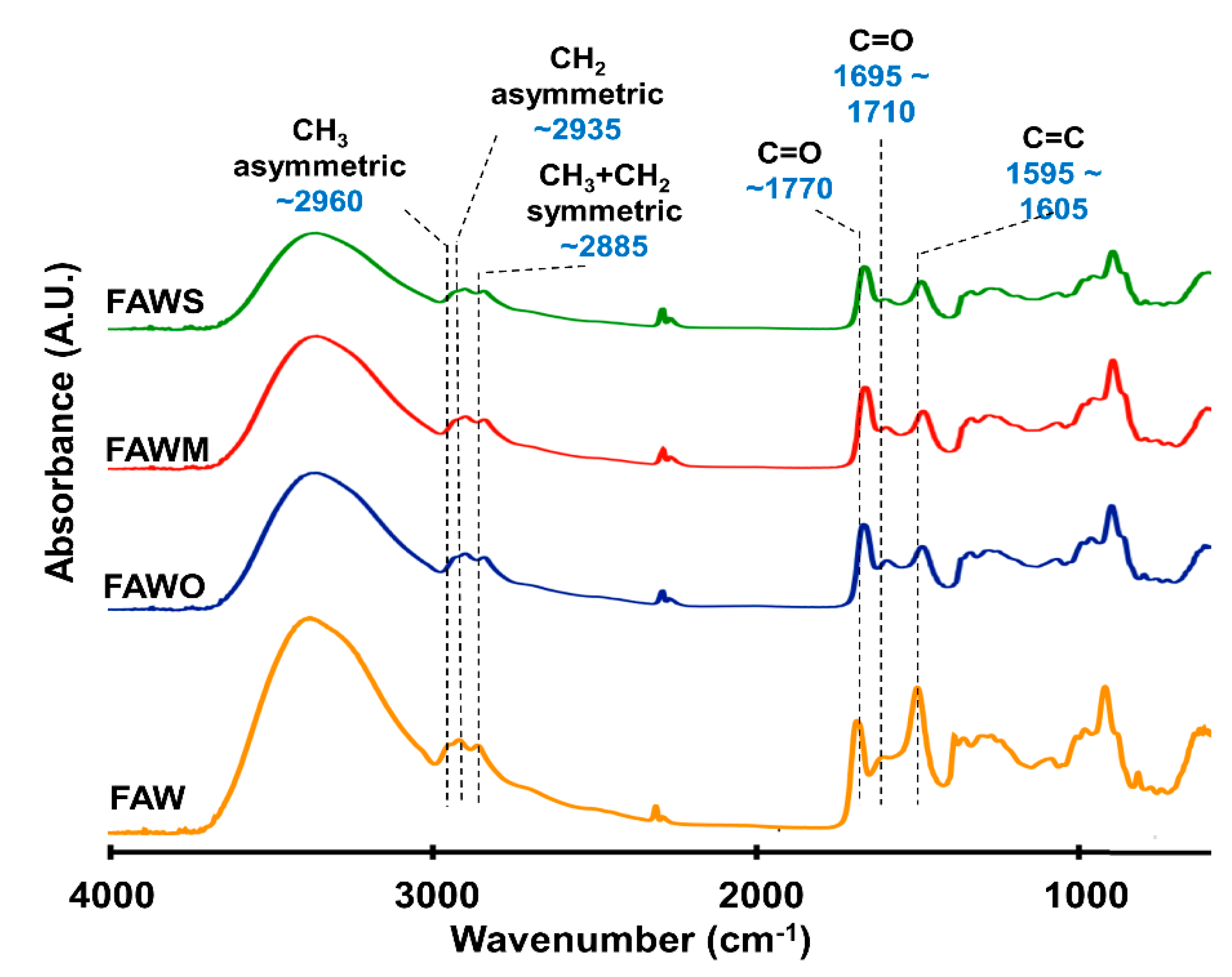
3.2. FTIR Absorption Spectra
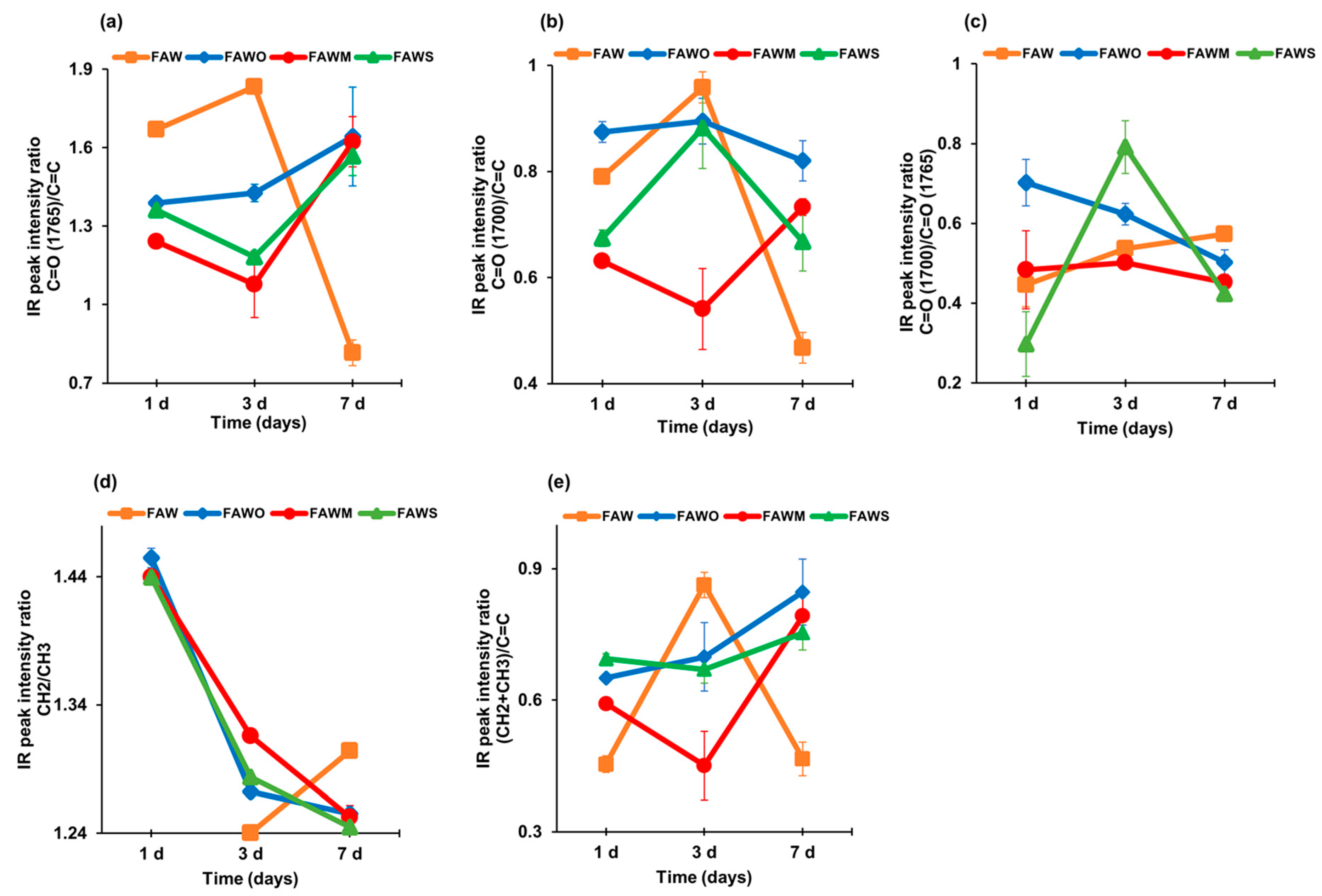
3.2.1. IR Peak Intensity Ratios
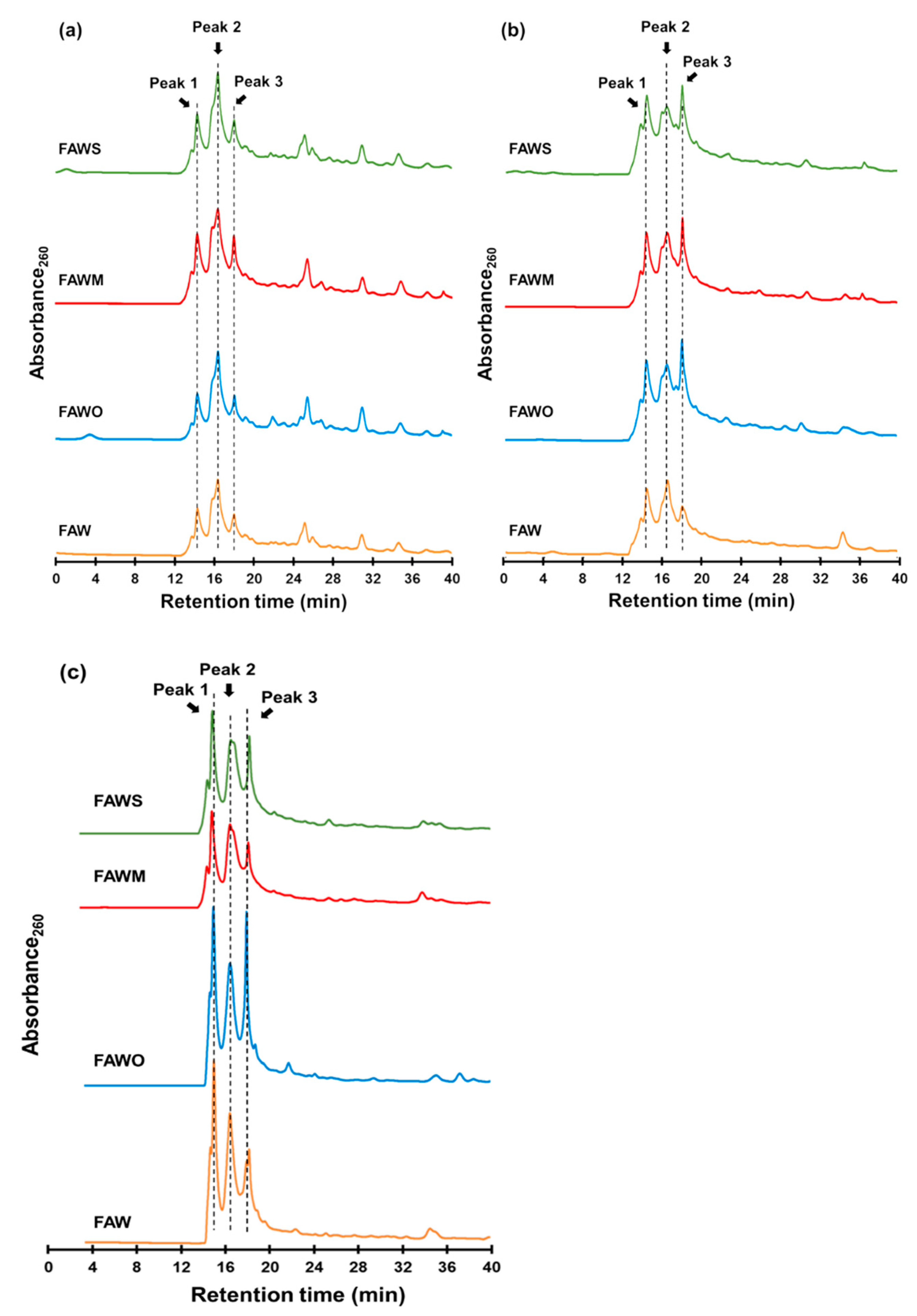
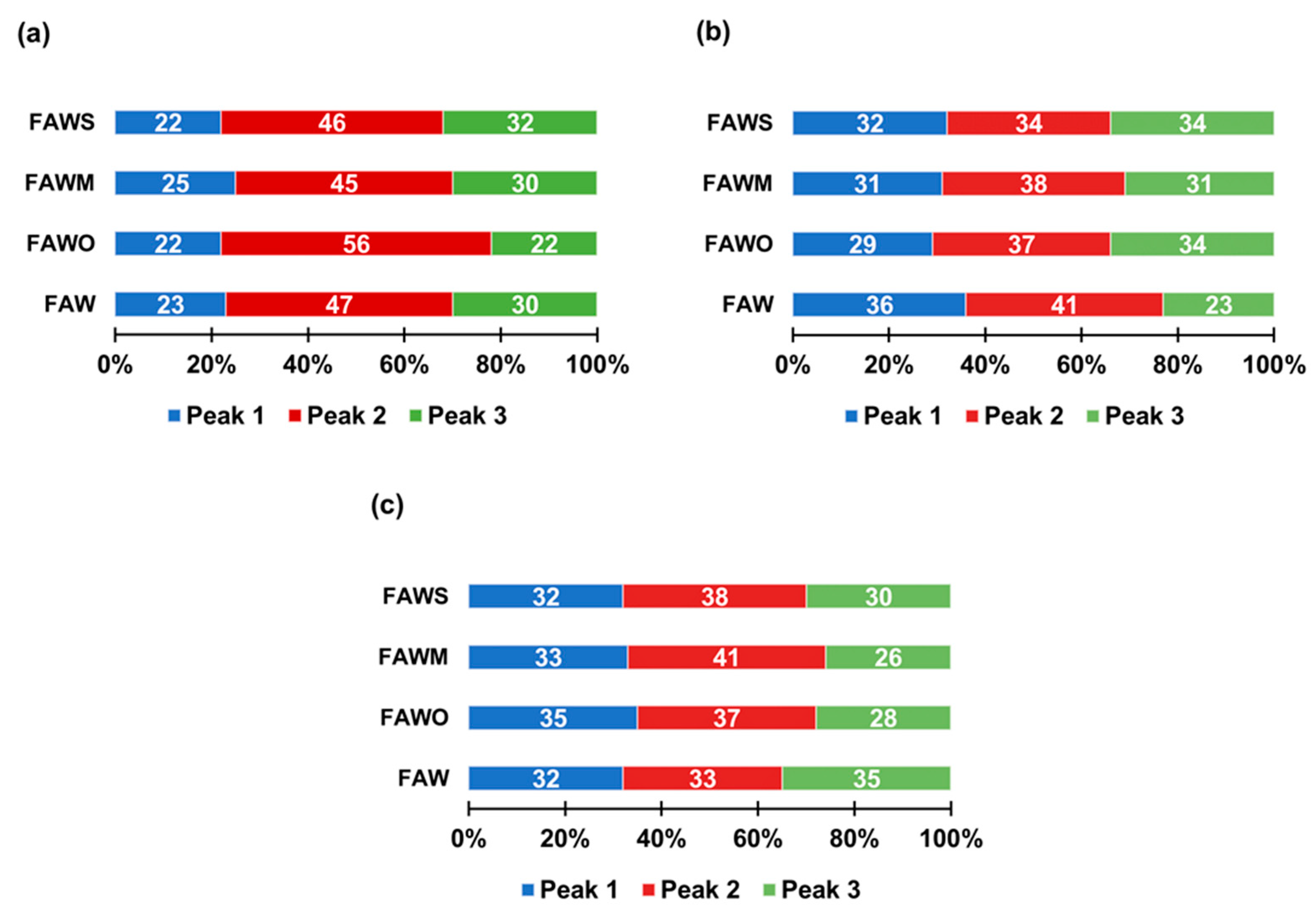
3.3. Molecular Weight Estimation
4. Discussion
4.1. Amino Acid Production during Aqueous Alteration in Small Bodies in the Solar System
4.2. The Effects of Minerals on Amino Acid Formation
4.3. Molecular Structure Variations of FAW with Minerals
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert, F.; Epstein, S. The concentration and isotopic composition of hydrogen, carbon and nitrogen in carbonaceous meteorites. Geochim. Cosmochim. Acta 1982, 46, 81–95. [Google Scholar] [CrossRef]
- Pearson, V.K.; Sephton, M.A.; Franchi, I.A.; Gibson, J.M.; Gilmour, I. Carbon and nitrogen in carbonaceous chondrites: Elemental abundances and stable isotopic compositions. Meteorit. Planet. Sci. 2006, 41, 1899–1918. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Fogel, M.; Yabuta, H.; Cody, G.D. The origin and evolution of chondrites recorded in the elemental and isotopic compositions of their macromolecular organic matter. Geochim. Cosmochim. Acta 2007, 71, 4380–4403. [Google Scholar] [CrossRef]
- Botta, O.; Bada, J.L. Extraterrestrial Organic Compounds in Meteorites. Surv. Geophys. 2002, 23, 411–467. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Alexander, C.M.O.; Aponte, J.C.; Dworkin, J.P.; Elsila, J.E.; Yabuta, H. The Origin and Evolution of Organic Matter in Carbonaceous Chondrites and Links to Their Parent Bodies. In Primitive Meteorites and Asteroids; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–271. ISBN 978-0-12-813325-5. [Google Scholar]
- Kobayashi, K.; Kaneko, T.; Saito, T. Characterization of complex organic compounds formed in simulated planetary atmospheres by the action of high energy particles. Adv. Space Res. 1999, 24, 461–464. [Google Scholar] [CrossRef]
- Takano, Y.; Ohashi, A.; Kaneko, T.; Kobayashi, K. Abiotic synthesis of high-molecular-weight organics from an inorganic gas mixture of carbon monoxide, ammonia, and water by 3 MeV proton irradiation. Appl. Phys. Lett. 2004, 84, 1410–1412. [Google Scholar] [CrossRef]
- Elmasry, W.; Kebukawa, Y.; Kaneko, T.; Obayashi, Y.; Fukuda, H.; Oguri, Y.; Kobayashi, K. Alteration and Stability of Complex Macromolecular Amino Acid Precursors in Hydrothermal Environments. Orig. Life Evol. Biosph. 2020, 50, 15–33. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbier, B.; Arcones Segovia, A.; Rosenbauer, H.; Thiemann, W.H.-P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef]
- Nuevo, M.; Auger, G.; Blanot, D.; D’Hendecourt, L. A Detailed Study of the Amino Acids Produced from the Vacuum UV Irradiation of Interstellar Ice Analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Filippi, J.-J.; De Marcellus, P.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. N-(2-Aminoethyl)glycine and Amino Acids from Interstellar Ice Analogues. ChemPlusChem 2012, 77, 186–191. [Google Scholar] [CrossRef]
- Modica, P.; Martins, Z.; Meinert, C.; Zanda, B.; D’Hendecourt, L.L.S. The Amino Acid Distribution in Laboratory Analogs of Extraterrestrial Organic Matter: A Comparison to CM Chondrites. Astrophys. J. 2018, 865, 41. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Bernard, S.; Le Guillou, C.; Remusat, L. Evolution of interstellar organic compounds under asteroidal hydrothermal conditions. Icarus 2018, 305, 358–370. [Google Scholar] [CrossRef]
- Le Guillou, C.; Bernard, S.; Brearley, A.J.; Remusat, L. Evolution of organic matter in Orgueil, Murchison and Renazzo during parent body aqueous alteration: In situ investigations. Geochim. Cosmochim. Acta 2014, 131, 368–392. [Google Scholar] [CrossRef]
- Alexander, C.M.O.; Cody, G.D.; De Gregorio, B.T.; Nittler, L.R.; Stroud, R.M. The nature, origin and modification of insoluble organic matter in chondrites, the major source of Earth’s C and N. Geochemistry 2017, 77, 227–256. [Google Scholar] [CrossRef]
- Brearley, A.J. The action of water. In Meteorites and the Early Solar System II; Lauretta, D.S., McSween, J.H.Y., Eds.; University of Arizona Press: Tucson, AZ, USA, 2006; pp. 587–624. [Google Scholar]
- Zolensky, M.E. Extraterrestrial Water. Elements 2005, 1, 39–43. [Google Scholar] [CrossRef]
- Urey, H.C. The cosmic abundances of potassium, uranium, and thorium and the heat balances of the earth, the moon, and mars. Proc. Natl. Acad. Sci. USA 1955, 41, 127–144. [Google Scholar] [CrossRef]
- Grimm, R.E.; McSween, H.Y., Jr. Heliocentric zoning of the asteroid belt by aluminum-26 heating. Science 1993, 259, 653–655. [Google Scholar] [CrossRef][Green Version]
- Alexander, C.M.O.; Howard, K.T.; Bowden, R.; Fogel, M.L. The classification of CM and CR chondrites using bulk H, C and N abundances and isotopic compositions. Geochim. Cosmochim. Acta 2013, 123, 244–260. [Google Scholar] [CrossRef]
- Trigo-Rodríguez, J.M.; Rubin, A.E.; Wasson, J.T. Non-nebular origin of dark mantles around chondrules and inclusions in CM chondrites. Geochim. Cosmochim. Acta 2006, 70, 1271–1290. [Google Scholar] [CrossRef]
- Rubin, A.; Trigo-Rodríguez, J.; Huber, H.; Wasson, J. Progressive alteration of CM carbonaceous chondrites. Geochim. Cosmochim. Acta 2007, 71, 2361–2382. [Google Scholar] [CrossRef]
- Trigo-Rodríguez, J.M. Aqueous alteration in chondritic asteroids and comets from the study of carbonaceous chondrites. In Planetary Materials; Lee, M.R., Leroux, H., Eds.; EMU Notes in Mineralogy; European Mineralogical Union and the Mineralogical Society: London, UK, 2015; Volume 15, pp. 67–87. [Google Scholar]
- Zolensky, M.E.; Mittlefehldt, D.W.; Lipschutz, M.E.; Wang, M.-S.; Clayton, R.N.; Mayeda, T.K.; Grady, M.M.; Pillinger, C.; David, B. CM chondrites exhibit the complete petrologic range from type 2 to 1. Geochim. Cosmochim. Acta 1997, 61, 5099–5115. [Google Scholar] [CrossRef]
- Cody, G.D.; Heying, E.; Alexander, C.M.O.; Nittler, L.R.; Kilcoyne, A.L.D.; Sandford, S.A.; Stroud, R.M. Establishing a molecular relationship between chondritic and cometary organic solids. Proc. Natl. Acad. Sci. USA 2011, 108, 19171–19176. [Google Scholar] [CrossRef]
- Kebukawa, Y.; David Kilcoyne, A.L.; Cody, G.D. Exploring the potential formation of organic solids in chondrites and comets through polymerization of interstellar formaldehyde. Astrophys. J. 2013, 771, 19. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Chan, Q.H.S.; Tachibana, S.; Kobayashi, K.; Zolensky, M.E. One-pot synthesis of amino acid precursors with insoluble organic matter in planetesimals with aqueous activity. Sci. Adv. 2017, 3, e1602093. [Google Scholar] [CrossRef]
- Rotelli, L.; Trigo-Rodríguez, J.M.; Moyano-Cambero, C.E.; Carota, E.; Botta, L.; Di Mauro, E.; Saladino, R. The key role of meteorites in the formation of relevant prebiotic molecules in a formamide/water environment. Sci. Rep. 2016, 6, 38888. [Google Scholar] [CrossRef]
- Pearson, V.K.; Sephton, M.A.; Kearsley, A.T.; Bland, P.A.; Franchi, I.A.; Gilmour, I. Clay mineral-organic matter relationships in the early solar system. Meteorit. Planet. Sci. 2002, 37, 1829–1833. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A Perspective on the Role of Minerals in Prebiotic Synthesis. Ambio 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Ganor, J.; Reznik, I.J.; Rosenberg, Y.O. Organics in Water-Rock Interactions. Rev. Miner. Geochem. 2009, 70, 259–369. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Le Guillou, C.; Bernard, S.; Viennet, J.C.; Jaber, M.; Remusat, L. Influence of phyllosilicates on the hydrothermal alteration of organic matter in asteroids: Experimental perspectives. Geochim. Cosmochim. Acta 2020, 269, 150–166. [Google Scholar] [CrossRef]
- Vinogradoff, V.; Remusat, L.; McLain, H.L.; Aponte, J.C.; Bernard, S.; Danger, G.; Dworkin, J.P.; Elsila, J.E.; Jaber, M. Impact of Phyllosilicates on Amino Acid Formation under Asteroidal Conditions. ACS Earth Space Chem. 2020, 4, 1398–1407. [Google Scholar] [CrossRef]
- Yamashita, Y.; Naraoka, H. Two homologous series of alkylpyridines in the Murchison meteorite. Geochem. J. 2014, 48, 519–525. [Google Scholar] [CrossRef]
- Davidson, I. Hydrolysis of Samples for Amino Acid Analysis. Methods Mol. Biol. 2003, 211, 111–122. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Gilani, G.S. Amino Acid Analysis. Curr. Protoc. Protein Sci. 2009, 58, 11.9.1–11.9.37. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Kebukawa, Y.; Cody, G.D. A kinetic study of the formation of organic solids from formaldehyde: Implications for the origin of extraterrestrial organic solids in primitive Solar System objects. Icarus 2015, 248, 412–423. [Google Scholar] [CrossRef]
- Igisu, M.; Ueno, Y.; Shimojima, M.; Nakashima, S.; Awramik, S.M.; Ohta, H.; Maruyama, S. Micro-FTIR spectroscopic signatures of Bacterial lipids in Proterozoic microfossils. Precambrian Res. 2009, 173, 19–26. [Google Scholar] [CrossRef]
- Charnley, S.B.; Rodgers, S.D. Interstellar Reservoirs of Cometary Matter. Space Sci. Rev. 2008, 138, 59–73. [Google Scholar] [CrossRef]
- Rubin, A.E. Petrologic Evidence for Collisional Heating of Chondritic Asteroids. Icarus 1995, 113, 156–167. [Google Scholar] [CrossRef]
- Kopetzki, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Moreno, R.; Crovisier, J.; Colom, P.; Lis, D.C.; Sandqvist, A.; Boissier, J.; Despois, D.; Milam, S.N. Ethyl alcohol and sugar in comet C/2014 Q2 (Lovejoy). Sci. Adv. 2015, 1, e1500863. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Glavin, D.P.; Kminek, G.; Bada, J.L. Relative Amino Acid Concentrations as a Signature for Parent Body Processes of Carbonaceous Chondrites. Orig. Life Evol. Biosph. 2002, 32, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Martins, Z.; Ehrenfreund, P. Amino acids in Antarctic CM1 meteorites and their relationship to other carbonaceous chondrites. Meteorit. Planet. Sci. 2007, 42, 81–92. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P.; Aubrey, A.; Botta, O.; Doty, J.H.; Martins, Z.; Bada, J.L. Amino acid analyses of Antarctic CM2 meteorites using liquid chromatography-time of flight-mass spectrometry. Meteorit. Planet. Sci. 2006, 41, 889–902. [Google Scholar] [CrossRef]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 45, 1948–1972. [Google Scholar] [CrossRef]
- Yanagawa, H.; Kobayashi, K. Chapter 8 An experimental approach to chemical evolution in submarine hydrothermal systems. Orig. Life Evol. Biosph. 1992, 22, 147–159. [Google Scholar] [CrossRef]
- Marshall, W.L. Hydrothermal synthesis of amino acids. Geochim. Cosmochim. Acta 1994, 58, 2099–2106. [Google Scholar] [CrossRef]
- Shimoyama, A.; Blair, N.; Ponnamperuma, C. Synthesis of amino acids under primitive earth conditions in the presence of clay. Orig. Life 1978, 1978, 95–99. [Google Scholar]
- Hashizume, H. Role of clay minerals in chemical evolution and the origins of life. In Clay Minerals in Nature—Their Characterization, Modification and Application; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Dos Santos, R.; Patel, M.; Cuadros, J.; Martins, Z. Influence of mineralogy on the preservation of amino acids under simulated Mars conditions. Icarus 2016, 277, 342–353. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Shimoyama, A.; Friebele, E. Clay and the origin of life. Orig. Life Evol. Biosph. 1982, 12, 9–40. [Google Scholar] [CrossRef]
- Lambert, J.-F. Adsorption and Polymerization of Amino Acids on Mineral Surfaces: A Review. Orig. Life Evol. Biosph. 2008, 38, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Marshall-Bowman, K.; Ohara, S.; Sverjensky, D.A.; Hazen, R.M.; Cleaves, H.J. Catalytic peptide hydrolysis by mineral surface: Implications for prebiotic chemistry. Geochim. Cosmochim. Acta 2010, 74, 5852–5861. [Google Scholar] [CrossRef]
- Stievano, L.; Piao, L.Y.; Lopes, I.; Meng, M.; Costa, D.; Lambert, J.-F. Glycine and lysine adsorption and reactivity on the surface of amorphous silica. Eur. J. Miner. 2007, 19, 321–331. [Google Scholar] [CrossRef]
- Meng, M.; Stievano, L.; Lambert, J.-F. Adsorption and Thermal Condensation Mechanisms of Amino Acids on Oxide Supports. 1. Glycine on Silica. Langmuir 2004, 20, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Parbhakar, A.; Cuadros, J.; Sephton, M.A.; Dubbin, W.; Coles, B.J.; Weiss, D. Adsorption of l-lysine on montmorillonite. Colloids Surf. A Physicochem. Eng. Asp. 2007, 307, 142–149. [Google Scholar] [CrossRef]
- Kitadai, N.; Yokoyama, T.; Nakashima, S. In situ ATR-IR investigation of L-lysine adsorption on montmorillonite. J. Colloid Interface Sci. 2009, 338, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.S. Clay and clay-supported reagents in organic synthesis. Tetrahedron 2002, 58, 1235–1255. [Google Scholar] [CrossRef]
- Wu, L.M.; Zhou, C.H.; Keeling, J.; Tong, D.S.; Yu, W.H. Towards an understanding of the role of clay minerals in crude oil formation, migration and accumulation. Earth Sci. Rev. 2012, 115, 373–386. [Google Scholar] [CrossRef]
- Tian, M.; Liu, B.S.; Hammonds, M.; Wang, N.; Sarre, P.J.; Cheung, A.S.-C. Formation of polycyclic aromatic hydrocarbons from acetylene over nanosized olivine-type silicates. Phys. Chem. Chem. Phys. 2012, 14, 6603–6610. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Alexander, C.M.O.; Cody, G.D. Compositional diversity in insoluble organic matter in type 1, 2 and 3 chondrites as detected by infrared spectroscopy. Geochim. Cosmochim. Acta 2011, 75, 3530–3541. [Google Scholar] [CrossRef]
- Sephton, M.A.; Verchovsky, A.B.; Bland, P.A.; Gilmour, I.; Grady, M.M.; Wright, I.P. Investigating the variations in carbon and nitrogen isotopes in carbonaceous chondrites. Geochim. Cosmochim. Acta 2003, 67, 2093–2108. [Google Scholar] [CrossRef]
- Huang, Y.; Alexandre, M.R.; Wang, Y. Structure and isotopic ratios of aliphatic side chains in the insoluble organic matter of the Murchison carbonaceous chondrite. Earth Planet. Sci. Lett. 2007, 259, 517–525. [Google Scholar] [CrossRef]
- McCabe, R. Clay Chemistry. In Inorganic Materials, 2nd ed.; Bruce, D., O’Hare, D., Eds.; Wiley and Sons: New York, NY, USA, 1996; pp. 313–376. [Google Scholar]
- Houk, K.N.; Strozier, R.W. Lewis acid catalysis of Diels-Alder reactions. J. Am. Chem. Soc. 1973, 95, 4094–4096. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Dahryn Trivedi, A.B.; Khemraj Bairwa, H.S. Fourier Transform Infrared and Ultraviolet-Visible Spectroscopic Characterization of Biofield Treated Salicylic Acid and Sparfloxacin. Nat. Prod. Chem. Res. 2015, 3, 3. [Google Scholar] [CrossRef]
- Guan, X.; Chen, G.; Shang, C. ATR-FTIR and XPS study on the structure of complexes formed upon the adsorption of simple organic acids on aluminum hydroxide. J. Environ. Sci. 2007, 19, 438–443. [Google Scholar] [CrossRef]
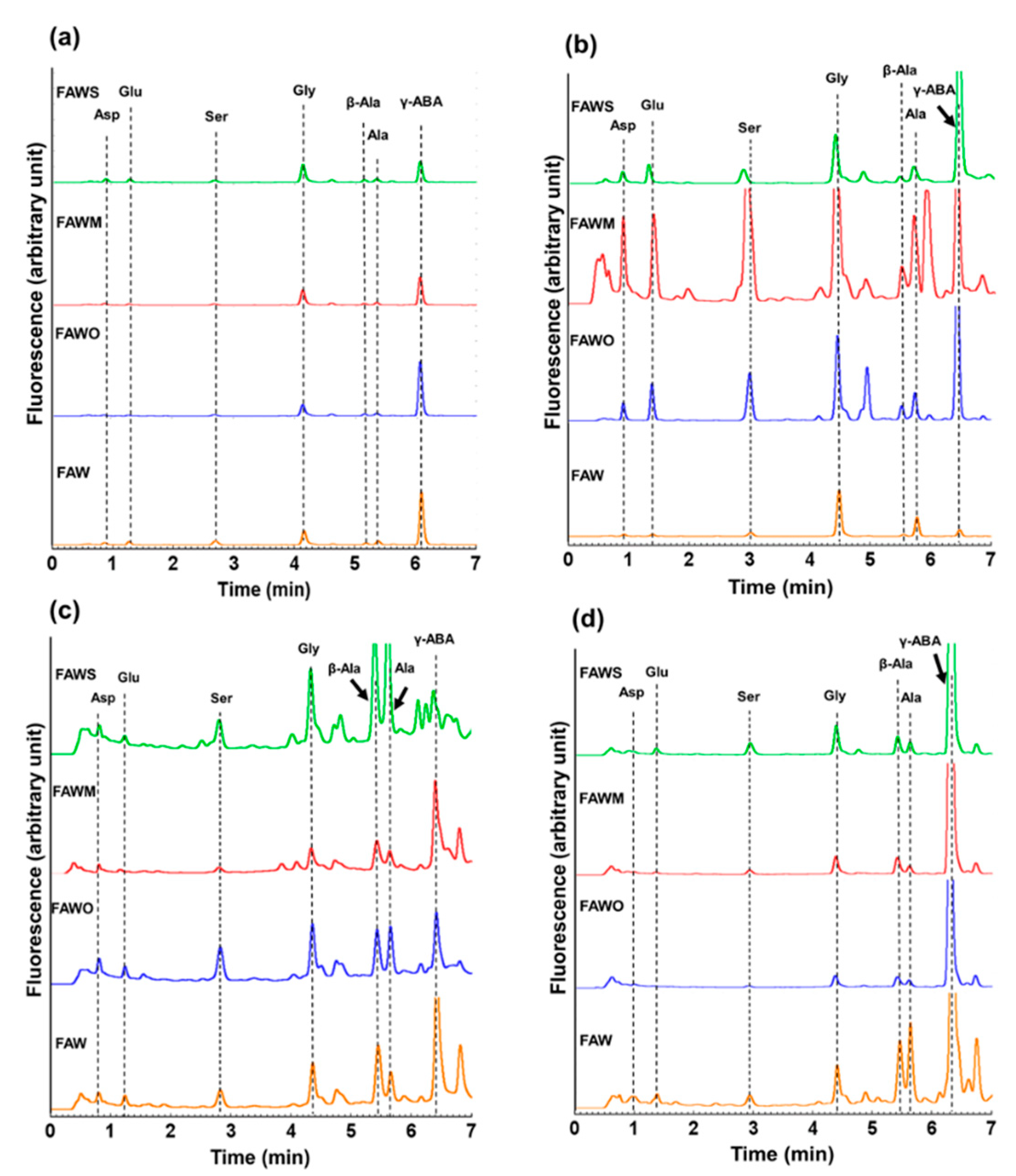
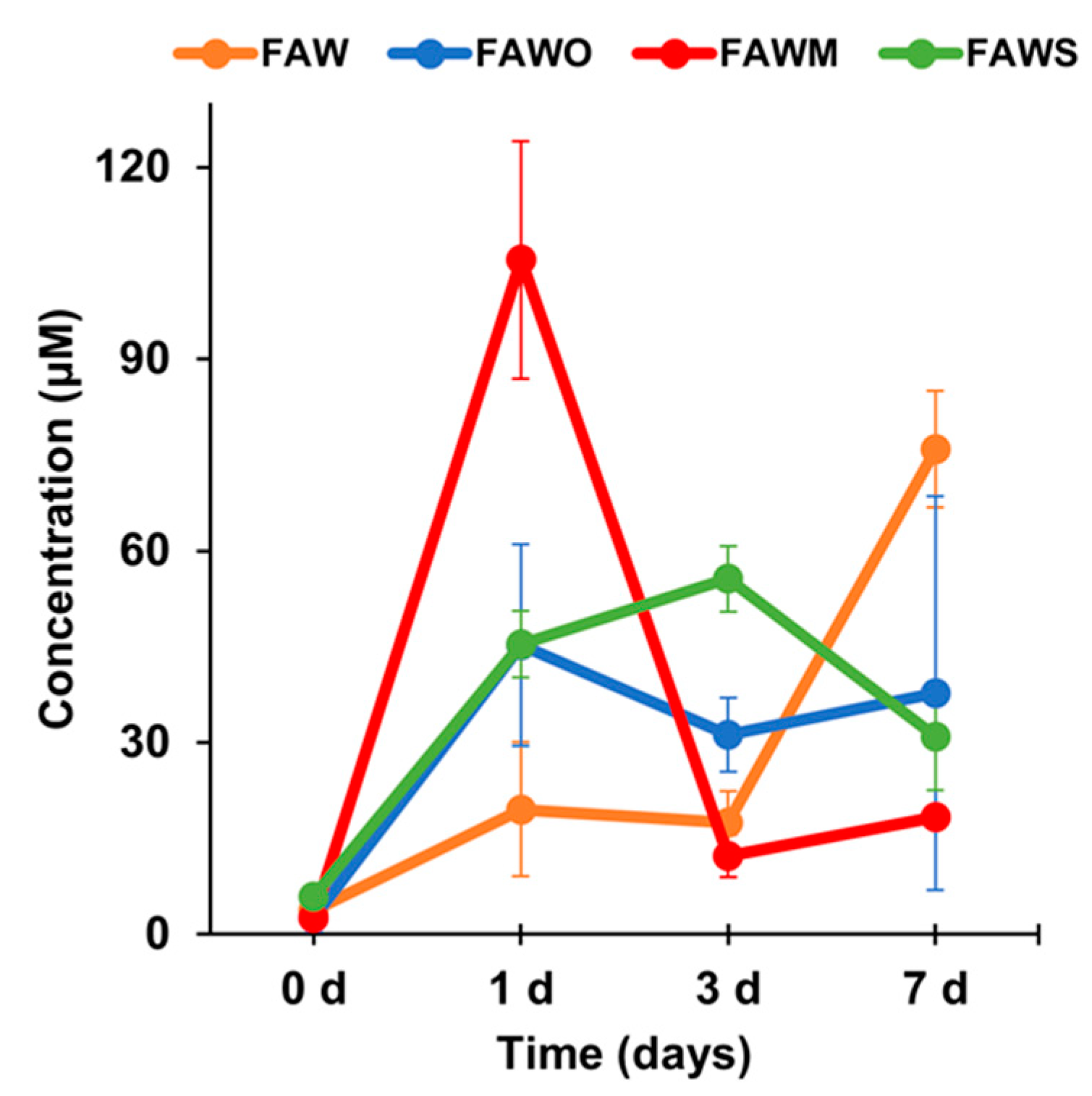
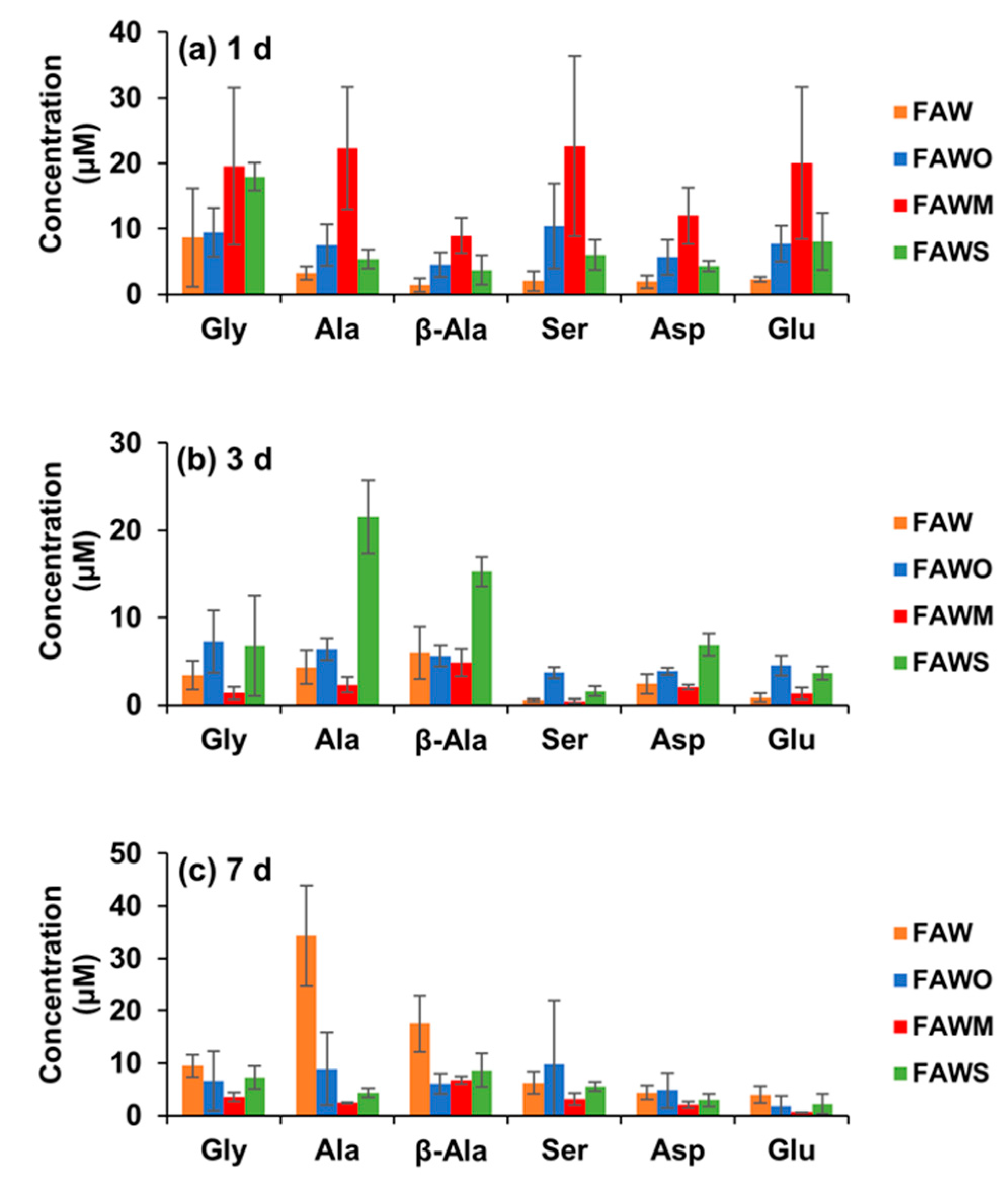
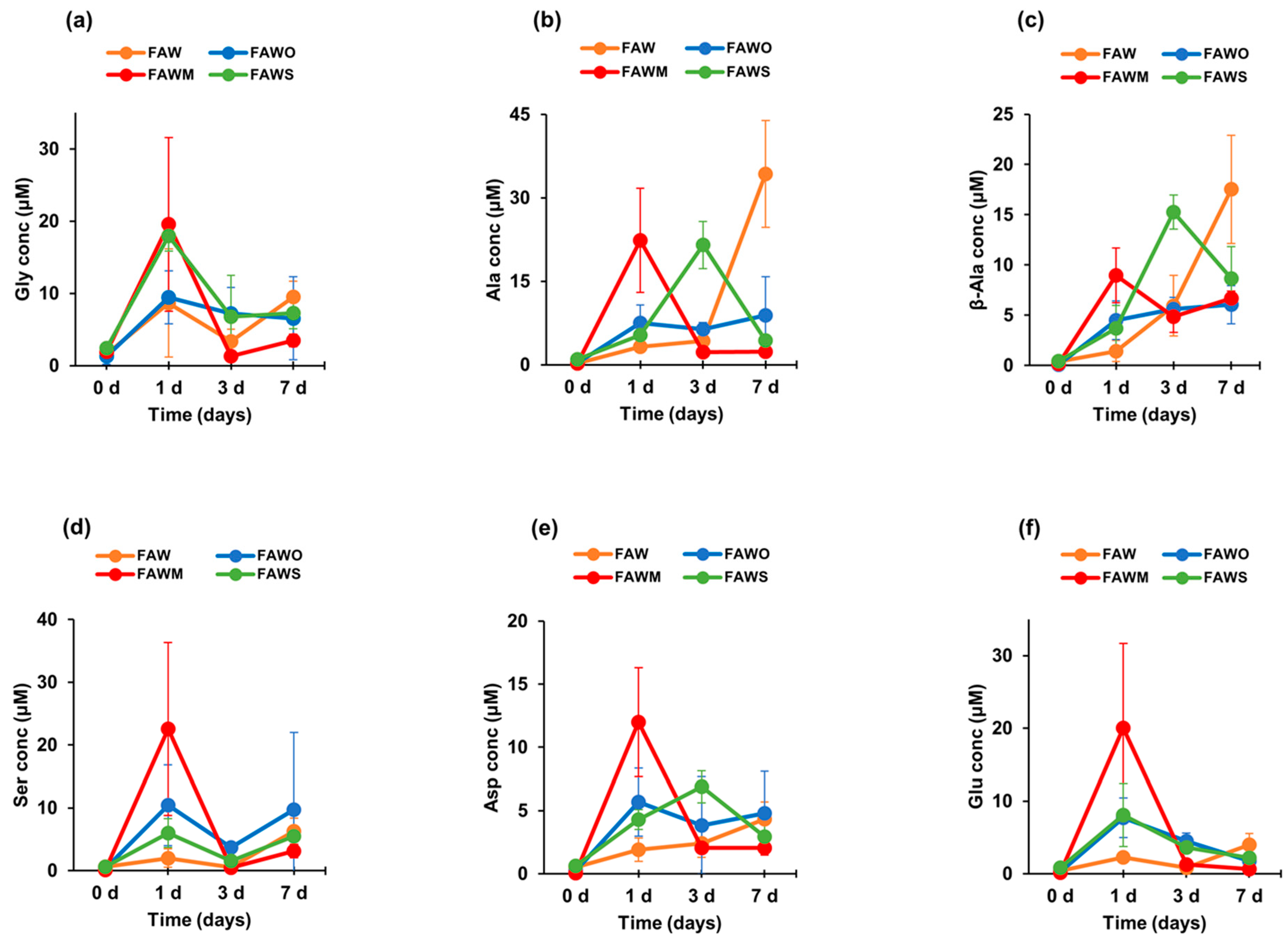



| Amino Acid Concentration (μM) | FAW | FAWO | ||||||
|---|---|---|---|---|---|---|---|---|
| RT | 150 °C | RT | 150 °C | |||||
| 0 d | 1 d | 3 d | 7 d | 0 d | 1 d | 3 d | 7 d | |
| Gly | 1.6 ± 1.0 | 8.7 ± 7.5 | 3.4 ± 1.7 | 9.5 ± 2.2 | 1.3 ± 1.1 | 9.4 ± 3.7 | 7.2 ± 3.6 | 6.6 ± 5.7 |
| Ala | 0.3 ± 0.1 | 3.3 ± 1.0 | 4.3 ± 1.9 | 34.3 ± 9.6 | 0.3 ± 0.2 | 7.5 ± 3.2 | 6.4 ± 1.2 | 8.9 ± 7.0 |
| β-Ala | 0.4 ± 0.3 | 1.4 ± 1.1 | 5.9 ± 3.0 | 17.5 ± 5.4 | 0.1 ± 0.08 | 4.5 ± 1.9 | 5.6 ± 1.2 | 6.0 ± 1.9 |
| Ser | 0.6 ± 0.2 | 2.0 ± 1.5 | 0.6 ± 0.2 | 6.2 ± 2.1 | 0.4 ± 0.3 | 10.4 ± 6.5 | 3.7 ± 0.6 | 9.7 ±12.2 |
| Asp | 0.5 ± 0.3 | 1.9 ± 0.9 | 2.4 ± 1.1 | 4.3 ± 1.3 | 0.24 ± 0.2 | 5.7 ± 2.7 | 3.8 ± 0.4 | 4.8 ± 3.3 |
| Glu | 0.4 ± 0.2 | 2.2 ± 0.4 | 0.9 ± 0.5 | 4.0 ± 1.6 | 0.3 ± 0.2 | 7.7 ± 2.7 | 4.5 ± 1.1 | 1.7 ± 1.9 |
| Total | 3.8 ± 2.0 | 19.5 ± 10.5 | 17.5 ± 4.9 | 75.8 ± 9.1 | 2.6 ± 2.2 | 45.2 ± 15.8 | 31.2 ± 5.8 | 37.7 ± 30.8 |
| Amino Acid Concentration (μM) | FAWM | FAWS | ||||||
| RT | 150 °C | RT | 150 °C | |||||
| 0 d | 1 d | 3 d | 7 d | 0 d | 1 d | 3 d | 7 d | |
| Gly | 2.0 ± 0.4 | 19.6 ± 12.0 | 1.3 ± 0.7 | 3.5 ± 0.9 | 2.4 ± 0.2 | 18.0 ± 2.1 | 6.8 ± 5.8 | 7.3 ± 2.2 |
| Ala | 0.2 ± 0.1 | 22.4 ± 9.4 | 2.3 ± 0.9 | 2.4 ± 0.2 | 1.0 ± 0.4 | 5.4 ± 1.4 | 21.5 ± 4.2 | 4.3 ± 0.9 |
| β-Ala | 0.2 ± 0.1 | 9.0 ± 2.7 | 4.8 ± 1.5 | 6.7 ± 0.7 | 0.4 ± 0.02 | 3.7 ± 2.3 | 15.3 ± 1.7 | 8.7 ± 3.2 |
| Ser | 0.1 ± 0.06 | 22.6 ± 13.8 | 0.4 ± 0.3 | 3.1 ± 1.1 | 0.6 ± 0.4 | 6.0 ± 2.3 | 1.6 ± 0.6 | 5.5 ± 0.8 |
| Asp | 0.1 ± 0.04 | 12.0 ± 4.3 | 2.0 ± 0.3 | 2.0 ± 0.6 | 0.6 ± 0.04 | 4.3 ± 0.8 | 6.9 ± 1.3 | 3.0 ± 1.2 |
| Glu | 0.2 ± 0.1 | 20.0 ± 11.6 | 1.3 ± 0.7 | 0.6 ± 0.03 | 0.8 ± 0.1 | 8.1 ± 4.3 | 3.6 ± 0.8 | 2.2 ± 1.9 |
| Total | 2.8 ± 0.8 | 105.6 ± 18.6 | 12.1 ± 3.3 | 18.3 ± 1.6 | 5.8 ± 1.1 | 45.5 ± 5.2 | 55.7 ± 5.1 | 31.0 ± 8.5 |
| Peak Position (Wavenumber/cm−1) | Assignments | Species |
|---|---|---|
| 3370 | OH | Carbonyl, alcohol, associated water |
| 2960–2970 | C–H asymmetric stretch | Aliphatic CH3 |
| 2935–2940 | C–H asymmetric stretch | Aliphatic CH2 |
| 2885 | C–H symmetric stretch | Aliphatic CH3 + CH2 |
| 1760–1770 | C=O stretch | Ester |
| 1695–1710 | C=O stretch | Carboxyl, aldehyde, amide |
| 1595–1605 | C=C stretch | Olefinic, aromatic |
| 1050 | C–O stretch |
| Peak Position (Wavenumber/cm−1) | FAW | FAWO | FAWM | FAWS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 d | 3 d | 7 d | 1 d | 3 d | 7 d | 1 d | 3 d | 7 d | 1 d | 3 d | 7 d | |
| CH3 | - * | 2960 | 2960 | 2970 | 2960 | 2960 | 2970 | 2960 | 2960 | 2970 | 2960 | 2960 |
| CH2 | 2945 | 2938 | 2935 | 2940 | 2935 | 2935 | 2940 | 2940 | 2935 | 2935 | 2935 | 2935 |
| C=O (ester) | 1760 | 1765 | 1770 | 1760 | 1765 | 1770 | 1765 | 1768 | 1770 | 1770 | 1665 | 1770 |
| C=O (Carboxyl, aldehyde, amide) | 1700 | 1705 | 1695 | 1698 | 1705 | 1705 | 1695 | 1700 | 1710 | 1710 | 1695 | 1710 |
| C=C | 1645 | 1603 | 1595 | 1620 | 1603 | 1600 | 1600 | 1595 | 1600 | 1600 | 1605 | 1605 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmasry, W.; Kebukawa, Y.; Kobayashi, K. Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation. Life 2021, 11, 32. https://doi.org/10.3390/life11010032
Elmasry W, Kebukawa Y, Kobayashi K. Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation. Life. 2021; 11(1):32. https://doi.org/10.3390/life11010032
Chicago/Turabian StyleElmasry, Walaa, Yoko Kebukawa, and Kensei Kobayashi. 2021. "Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation" Life 11, no. 1: 32. https://doi.org/10.3390/life11010032
APA StyleElmasry, W., Kebukawa, Y., & Kobayashi, K. (2021). Synthesis of Organic Matter in Aqueous Environments Simulating Small Bodies in the Solar System and the Effects of Minerals on Amino Acid Formation. Life, 11(1), 32. https://doi.org/10.3390/life11010032







