Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario
Abstract
1. Introduction
2. Materials and Methods
2.1. Uridine Phosphorylation under ‘Warm-Pool Model’ Theme
2.2. 31P-NMR and Mass Spectrometry (MS) Analyses of Uridine Phosphorylation Reactions
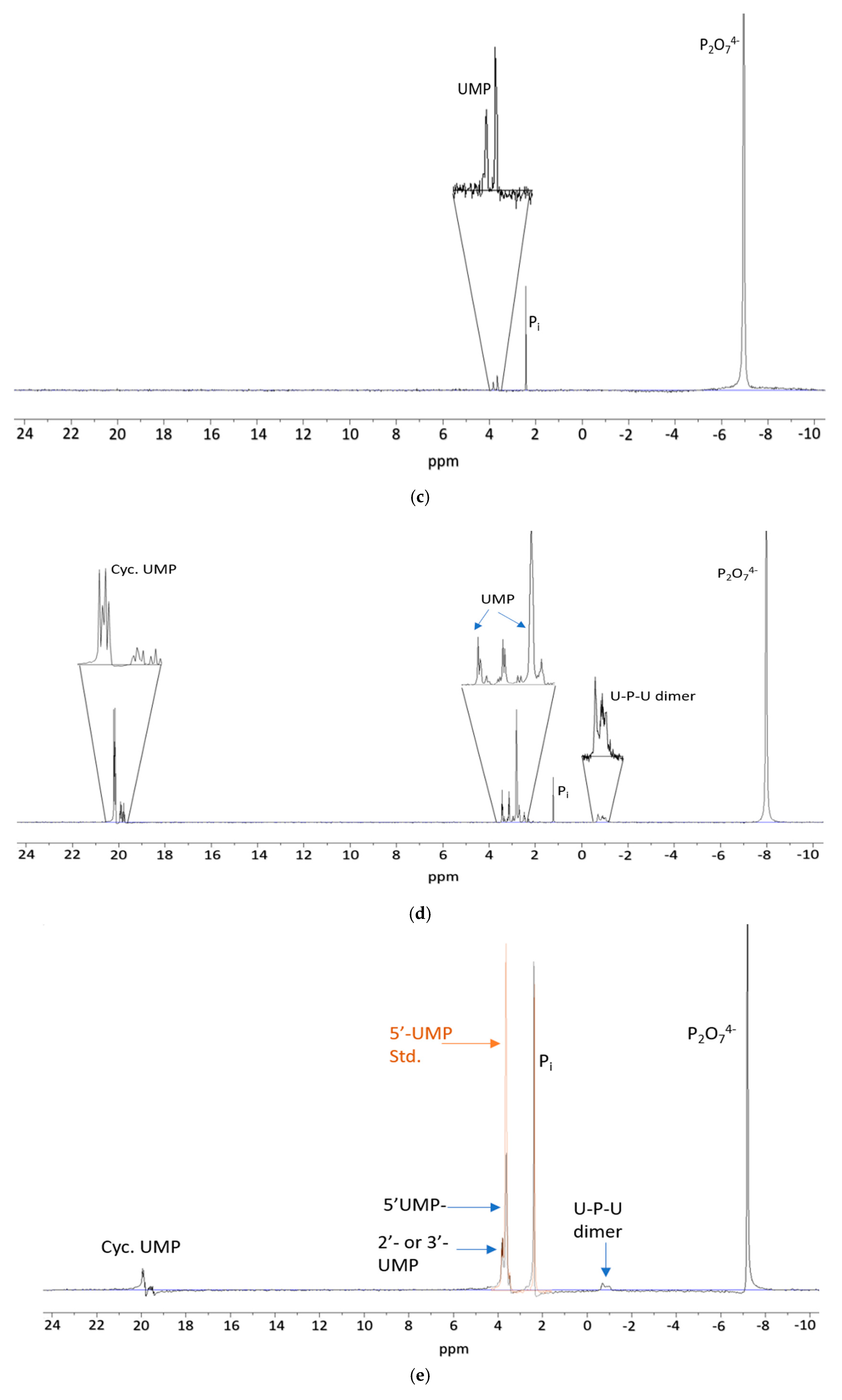
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woese, C. The Evolution of the Genetic Code. The Genetic Code; Harper & Row: New York, NY, USA, 1967; pp. 179–195. [Google Scholar]
- Crick, F.H. The origin of the genetic code. J. Mol. Biol. 1968, 38, 367–379. [Google Scholar] [CrossRef]
- Orgel, L.E. Evolution of the genetic apparatus. J. Mol. Biol. 1968, 38, 381–393. [Google Scholar] [CrossRef]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P. On the origin of phosphorylated biomolecules. In Origins of Life: The Primal Self-Organization; Springer: Berlin/Heidelberg, Germany, 2011; pp. 57–84. [Google Scholar]
- Pasek, M.A.; Gull, M.; Herschy, B. Phosphorylation on the early earth. Chem. Geol. 2017, 475, 149–170. [Google Scholar] [CrossRef]
- Gull, M. Prebiotic phosphorylation reactions on the early Earth. Challenges 2014, 5, 193–212. [Google Scholar] [CrossRef]
- Gajewski, E.; Steckler, D.K.; Goldberg, R.N. Thermodynamics of the Hydrolysis of Adenosine 5′-Triphosphate to Adenosine 5′-Diphosphate. J. Biol. Chem. 1986, 261, 12733–12737. [Google Scholar] [PubMed]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Orig. Life 1976, 7, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Mauro, E.D. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Mauro, E.D. Nucleoside phosphorylation by phosphate minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernández, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef]
- Gull, M.; Cafferty, B.J.; Hud, N.V.; Pasek, M.A. Silicate-Promoted Phosphorylation of Glycerol in Non-Aqueous Solvents: A Prebiotically Plausible Route to Organophosphates. Life 2017, 7, 29. [Google Scholar] [CrossRef]
- Burcar, B.; Pasek, M.; Gull, M.; Cafferty, B.J.; Velasco, F.; Hud, N.V.; Menor-Salván, C. Darwin’s warm little pond: A one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. Engl. 2016, 55, 13249–13253. [Google Scholar] [CrossRef] [PubMed]
- Burcar, B.; Castañeda, A.; Lago, J.; Daniel, M.; Pasek, M.A.; Hud, N.V.; Orlando, T.; Menor-Salván, C. A Stark Contrast to Modern Earth: Phosphate Mineral Transformation and Nucleoside Phosphorylation in an Iron-and Cyanide-Rich Early Earth Scenario. Angew. Chem. Int. Ed. Engl. 2019, 58, 16981–16987. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L.; Chalmers, J.H.; Cleaves, H.J. Is formamide a geochemically plausible prebiotic solvent? Phys. Chem. Chem. Phys. 2016, 18, 20085–20090. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.L.; Burcar, B.T.; Hud, N.V.; Febrian, R.; Mehta, C.; Bracher, P.J.; Atlas, Z.D.; Pasek, M.A. The Prebiotic Provenance of Semi-Aqueous Solvents. Orig. Life Evol. Biosph. 2020, 50, 1–14. [Google Scholar] [CrossRef]
- Pasek, M.A. Thermodynamics of prebiotic phosphorylation. Chem. Rev. 2019, 120, 4690–4706. [Google Scholar] [CrossRef]
- Lohrmann, R.; Orgel, L.E. Prebiotic synthesis: Phosphorylation in aqueous solution. Science 1968, 161, 64–66. [Google Scholar] [CrossRef]
- Schwartz, A.W.; van-der, V.M.; Bisseling, T.; Chittenden, G.J.F. Prebiotic nucleotide synthesis—Demonstration of a geologically plausible pathway. Orig. Life Evol. Biosph. 1975, 6, 163–168. [Google Scholar] [CrossRef]
- Ponnamperuma, C.; Mack, R. Nucleotide synthesis under possible primitive earth conditions. Science 1965, 148, 1221–1223. [Google Scholar] [CrossRef]
- Bishop, M.J.; Lohrman, R.; Orgel, L.E. Prebiotic phosphorylation of thymidine at 65 °C in simulated desert conditions. Nature 1972, 237, 162–164. [Google Scholar] [CrossRef]
- Yamagata, Y.; Matsukawa, T.; Mohri, T.; Inomata, K. Phosphorylation of adenosine in aqueous-solution by electric discharges. Nature 1979, 282, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Mohri, T.; Yamakoshi, M.; Inomata, K. Constant AMP synthesis in aqueous solution by electric discharges. Orig. Life Evol. Biosph. 1981, 11, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Handschuh, G.J.; Orgel, L.E. Struvite and prebiotic phosphorylation. Science 1973, 179, 483–484. [Google Scholar] [CrossRef]
- Österberg, R.; Orgel, L.E.; Lohrmann, R. Further studies of urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.; Chang, S.; Ponnamperuma, C. Phosphorylation by way of inorganic phosphate as a potential prebiotic process. Nature 1968, 218, 442–443. [Google Scholar] [CrossRef]
- Handschuh, G.J.; Lohrmann, R.; Orgel, L.E. The effect of Mg2+ and Ca2+ on urea-catalyzed phosphorylation reactions. J. Mol. Evol. 1973, 2, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, L.; Orgel, L.E. Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science 1971, 171, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Gull, M.; Mojica, M.A.; Fernández, F.M.; Gaul, D.A.; Orlando, T.M.; Liotta, C.L.; Pasek, M.A. Nucleoside phosphorylation by the mineral schreibersite. Sci Rep. 2015, 5, 17198. [Google Scholar] [CrossRef]
- Kaye, K.; Bryant, D.E.; Marriott, K.E.R.; Ohara, S.; Fishwick, C.W.G.; Kee, T.P. Selective Phosphonylation of 5′-Adenosine Monophosphate (5′-AMP) via Pyrophosphite [PPi(III)]. Orig. Life Evol. Biosph. 2016, 46, 425–434. [Google Scholar] [CrossRef]
- Ozawa, K.; Nemoto, A.; Imai, E.-I.; Honda, H.; Hatori, K.; Matsuno, K. Phosphorylation of nucleotide molecules in hydrothermal environments. Orig. Life Evol. Biosph. 2004, 34, 465–471. [Google Scholar] [CrossRef]
- Mullen, L.B.; Sutherland, J.D. Formation of potentially prebiotic amphiphiles by reaction of beta-hydroxy-n-alkylamines with cyclotriphosphate. Angew. Chem. Int. Ed. Engl. 2007, 46, 4166–4168. [Google Scholar] [CrossRef] [PubMed]
- Saffhill, R. Selective phosphorylation of the cis-2′,3′-diol of unprotected ribonucleosides with trimetaphosphate in aqueous solution. J. Org. Chem. 1970, 35, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Fan, C.; Wan, R.; Tong, C.; Miao, Z.; Chen, J.; Zhao, Y. Phosphorylation of adenosine with trimetaphosphate under simulated prebiotic conditions. Orig. Life Evol. Biosph. 2002, 32, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, R. Formation of nucleoside-5′-phosphoramidates under potentially prebiological conditions. J. Mol. Evol. 1977, 10, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W.; Ponnamperuma, C. Phosphorylation on the primitive earth: Phosphorylation of adenosine with linear polyphosphate salts in aqueous solution. Nature 1968, 218, 443. [Google Scholar] [CrossRef] [PubMed]
- Gibard, C.; Bhowmik, S.; Karki, M.; Kim, E.K.; Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 2018, 10, 212. [Google Scholar] [CrossRef]
- Gibard, C.; Gorrell, I.B.; Jiménez, E.I.; Kee, T.P.; Pasek, M.A.; Krishnamurthy, R. Geochemical Sources and Availability of Amidophosphates on the Early Earth. Angew. Chem. Int. Ed Engl. 2019, 58, 8151–8155. [Google Scholar] [CrossRef] [PubMed]
- Baltscheffsky, M.; Schultz, A.; Baltscheffsky, H. H+—Proton—Pumping inorganic pyrophosphatase: A tightly membrane-bound family. FEBS Lett. 1999, 452, 121–127. [Google Scholar] [CrossRef]
- Serrano, A.; Pérez-Castiñeira, J.R.; Baltscheffsky, M.; Baltscheffsky, H. H+-PPases: Yesterday, today and tomorrow. IUBMB Life 2007, 59, 76–83. [Google Scholar] [CrossRef]
- Pross, A. Toward a general theory of evolution: Extending Darwinian theory to inanimate matter. J. Syst. Chem. 2011, 2, 1–14. [Google Scholar] [CrossRef]
- Babich, L.; Hartog, A.F.; van der Horst, M.A.; Wever, R. Continuous-flow reactor-based enzymatic synthesis of phosphorylated compounds on a large scale. Chem. Eur. J. 2012, 18, 6604–6609. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphate under primitive Earth conditions. Nature 1991, 204, 516–519. [Google Scholar] [CrossRef]
- Holm, N.G.; Baltscheffsky, H. Links between hydrothermal environments, pyrophosphate, Na+, and early evolution. Orig. Life Evol. Biosph. 2011, 41, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.A.; Kee, T.P.; Bryant, D.E.; Pavlov, A.A.; Lunine, J.I. Production of potentially prebiotic condensed phosphates by phosphorus redox chemistry. Angew. Chem. Int. Ed. Engl. 2008, 47, 7918–7920. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Miller, S.L. Are polyphosphates or phosphate esters prebiotic reagents? Orig. Life Evol. Biosph. 1995, 41, 693–702. [Google Scholar] [CrossRef]
- Holm, N.G. The significance of Mg in prebiotic geochemistry. Geobiology 2012, 10, 269–279. [Google Scholar] [CrossRef]
- Yamagata, Y.; Inoue, H.; Inomata, K. Specific effect of magnesium ion on 2, 3′-cyclic AMP synthesis from adenosine and trimeta phosphate in aqueous solution. Orig. Life Evol. Biosph. 1995, 25, 47–52. [Google Scholar] [CrossRef]
- Schoonen, M.; Smirnov, A.; Cohn, C. A perspective on the role of minerals in prebiotic synthesis. AMBIO 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Pasek, M.; Dworkin, J.; Lauretta, D. A radical pathway for phosphorylation during schreibersite corrosion with implications for the origin of life. Geochim. Cosmochim. Acta 2007, 71, 1721–1736. [Google Scholar] [CrossRef]
- Bowler, F.R.; Chan, C.K.; Duffy, C.D.; Gerland, B.; Islam, S.; Powner, M.W.; Sutherland, J.D.; Xu, J. Prebiotically plausible oligoribonucleotide ligation facilitated by chemo selective acetylation. Nat. Chem. 2013, 5, 383–389. [Google Scholar] [CrossRef]
- Miller, S. Production of some organic compounds under possible primitive Earth conditions. J. Am. Chem. Soc. 1955, 77, 2351–2361. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral surfaces, geochemical complexities, and the origins of life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef]
- Lambert, J.B.; Gurusamy-Thangavelu, S.A.; Ma, K. The silicate-mediated formose reaction: Bottom-up synthesis of sugar silicates. Science 2010, 327, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Larralde, R.; Robertson, M.P.; Miller, S.L. Rates of decomposition of ribose and other sugars: Implications for chemical evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 8158–8160. [Google Scholar] [CrossRef] [PubMed]


| Reaction Sets | Description |
|---|---|
| Set 1: To each sample, 0.1 g Na4P2O7 and 0.5 g uridine were added to about 7–8 mL DI water and dissolved to form a clear solution. The reaction temperatures were kept at a range of 60–65 °C for about 5 days. The remaining description, regarding the additional materials added to each of the sample in set 1, is as follows: | |
| Sample 1 | 0.1 g urea, 0.1 g magnesium sulphate, and 0.3 g white sand |
| Sample 2 | 0.1 g urea only |
| Sample 3 | 0.1 g magnesium sulphate only |
| Sample 4: | 0.3 g white sand only |
| Sample 5 | No additional material |
| Set 2: To each sample in this set, similar amounts of reactants were added as in set 2. However, the temperature window was 70–75 °C for about 5 days. The remaining description, regarding the additional materials added to each of the sample in set 2, is as follows: | |
| Sample 6 | 0.1 g urea, 0.1 g magnesium sulphate, and 0.3 g white sand |
| Sample 7 | 0.1 g urea only |
| Sample 8 | 0.1 g magnesium sulphate only |
| Sample 9 | 0.3 g white sand only |
| Sample 10 | No additional material |
| Sample No. | P2O74− | Pi | 5′-UMP | 2′- Or 3′-UMP | Cyc. UMP | Dimer (U-P-U) | Net Org. PO4 |
|---|---|---|---|---|---|---|---|
| 1 | 67 | 1 | 10.3 | 5.7 | 11 | 5 | 32 |
| 2 | 90 | 1 | 2.48 | 0.52 | 5 | 1 | 9 |
| 3 | 92.7 | 0.5 | 2.2 | 0.30 | 3.5 | 0.8 | 6.8 |
| 4 | 93.8 | 0.2 | 1 | 0.2 | 4.4 | 0.4 | 6 |
| 5 | 27 | 64 | 7.5 | 1 | 0.5 | ND | 9 |
| 6 | 66 | 1.5 | 10.8 | 4.2 | 14 | 3.5 | 32.5 |
| 7 | 91.2 | 1 | 1 | ND | 6 | 0.8 | 7.8 |
| 8 | 91.3 | 0.3 | 2.72 | 1.28 | 3.4 | 1 | 8.4 |
| 9 | 89.5 | 0.3 | 1.5 | 0.5 | 7 | 1.2 | 10.2 |
| 10 | 87.7 | 6.7 | 3.2 | 1.8 | 0.6 | ND | 5.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gull, M.; Omran, A.; Feng, T.; Pasek, M.A. Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario. Life 2020, 10, 122. https://doi.org/10.3390/life10080122
Gull M, Omran A, Feng T, Pasek MA. Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario. Life. 2020; 10(8):122. https://doi.org/10.3390/life10080122
Chicago/Turabian StyleGull, Maheen, Arthur Omran, Tian Feng, and Matthew A. Pasek. 2020. "Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario" Life 10, no. 8: 122. https://doi.org/10.3390/life10080122
APA StyleGull, M., Omran, A., Feng, T., & Pasek, M. A. (2020). Silicate-, Magnesium Ion-, and Urea-Induced Prebiotic Phosphorylation of Uridine via Pyrophosphate; Revisiting the Hot Drying Water Pool Scenario. Life, 10(8), 122. https://doi.org/10.3390/life10080122








