Abstract
Despite more than one hundred years of work on organosilicon chemistry, the basis for the plausibility of silicon-based life has never been systematically addressed nor objectively reviewed. We provide a comprehensive assessment of the possibility of silicon-based biochemistry, based on a review of what is known and what has been modeled, even including speculative work. We assess whether or not silicon chemistry meets the requirements for chemical diversity and reactivity as compared to carbon. To expand the possibility of plausible silicon biochemistry, we explore silicon’s chemical complexity in diverse solvents found in planetary environments, including water, cryosolvents, and sulfuric acid. In no environment is a life based primarily around silicon chemistry a plausible option. We find that in a water-rich environment silicon’s chemical capacity is highly limited due to ubiquitous silica formation; silicon can likely only be used as a rare and specialized heteroatom. Cryosolvents (e.g., liquid N2) provide extremely low solubility of all molecules, including organosilicons. Sulfuric acid, surprisingly, appears to be able to support a much larger diversity of organosilicon chemistry than water.
1. Introduction
Life in environments very different from Earth’s could have a biochemistry that is very distinct from Earth’s life [1,2,3,4,5,6]. A persistent contender as an alternative elemental basis for life is silicon, mainly due to the chemical analogies between silicon and carbon. The idea that life could be based on silicon rather than carbon was first proposed in 1891 by the German astrophysicist Julius Scheiner [7]. Silicon shares many similarities with carbon and is, in the form of various silicates in rocks, the second most abundant element (after oxygen) in Earth’s crust [8]. However, despite its abundance, terrestrial life only uses silicon as silicic acid and silica (see Appendix A). There are no known examples of life using any other type of silicon chemistry. When people speak of “silicon-based life”, they are usually referring to the diverse organosilicon chemistry, and specifically chemistry in which carbon in organic molecules is replaced by silicon. So why is life’s use of silicon so restricted, and could a wider silicon chemistry be used in other biochemistries?
The possibility of silicon-based life is usually either accepted as plausible (e.g., [1]) or dismissed out of hand (e.g., Carl Sagan famously called himself a “carbon chauvinist” [9]). However, despite a century of speculation and experimental work on organosilicon chemistry, the plausibility of silicon-based life has never been systematically addressed nor objectively reviewed. This paper seeks to fill this gap. We review what is known, modeled, or speculated about silicon as a basis of life, and provide a comprehensive assessment of the opportunities for silicon in a non-terrestrial biochemistry, with a detailed discussion of the arguments for and against silicon as a building block of biochemistry.
We start with the discussion of the general requirements for the chemistry of life, no matter its chemical basis (Section 2). We next provide the overview of silicon chemistry (Section 3.1), including discussion of the diversity of silicon chemistry (Section 3.2) and thermodynamics of formation of silicon compounds (Section 3.3). We then discuss how silicon chemistry meets the requirements for the chemistry of life, focusing on the classes of environments in which life using silicon as a component of biochemistry could occur (Section 4). We conclude first that in water or ammonia silicon cannot be the main building block for life but might be used as a rare heteroatom, second that cryosolvents are poor environments for any biochemistry, and third that in sulfuric acid silicon could be used much more widely by life (Section 5). We relegate discussion of the details of how terrestrial life uses silicon to Appendix A, and more detailed background on silicon chemistry and speculations on silicon biochemistry to Appendix B, Appendix C and Appendix D.
2. General Requirements for the Chemistry of Life
We begin by describing the general requirements that any biochemistry must fulfil. The general characteristics that have to be met by any chemical basis for life can be condensed to three essential requirements: sufficient chemical diversity (Section 2.1); stability and reactivity (Section 2.2); and the presence of a solvent (Section 2.3) (reviewed in [10]). Although chemical diversity, reactivity, and solvent requirements are linked, we discuss them separately below. If silicon chemistry is to be a component of any biochemistry, then it must fulfil these requirements.
2.1. Chemical Diversity
Life needs a diverse set of chemicals with different functions. On Earth, the diverse set of chemicals are amino acids (to make proteins), sugars, and nitrogenous bases (to make nucleic acids), hydroxyl and keto acids (as core metabolic intermediates), lipids (to make membranes), and more. To build a diverse set of chemicals, life needs a set of elements capable of building molecules composed of many atoms that will provide sufficient biological functionality.
Sufficient chemical diversity needed to build a molecular repertoire suitable for life can only be achieved by a scaffolding element bonded with heteroatom elements. The scaffold atom is one that can join in chains and clusters to construct the skeleton or shape of a molecule, and the heteroatoms provide chemical activity in a molecule. Scaffolds provide the ability to make large molecules, and hence a large number of different molecules (compare the number of stable molecules of the type XnHm that can be formed with X=Nitrogen (three–NH3, N2H4, N2H2) and with X=Carbon (an essentially infinite number of hydrocarbons)). Scaffold elements and heteroatoms tend to be different atoms. The scaffold needs to be relatively stable and unreactive, while at the same time bonding to functional atoms (heteroatoms) that provide chemical functionality and distinctiveness to each molecule (Figure 1).
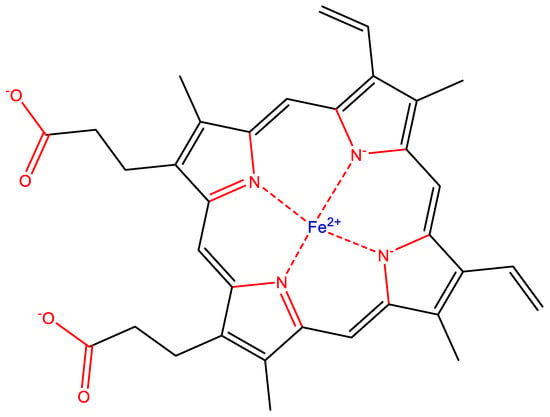
Figure 1.
Scaffolding elements and heteroatoms build biochemicals. Scaffolding elements are responsible for creating the overall structure and shape of molecules, while heteroatoms enable necessary chemical diversity, reactivity and directional bonding. For example, in heme (a crucial molecule used by virtually all life on Earth), carbon is a scaffolding element and O and N elements are heteroatoms that allow for the necessary reactivity and directional bonding required for coordination of catalytically important iron ion (Fe2+). Heteroatoms and bonds to them are colored red, scaffold elements (carbon) and bonds are colored black.
On Earth, carbon forms the major scaffold element (hence the term “carbon-based life”). Several other non-metal elements could be viable scaffold alternatives to carbon in that regard [1,2,3,4]. Sulfur, boron and, in particular, silicon, are capable of forming covalent compounds in which many atoms of the same type are bound together to form large molecules, and therefore could, in principle, be considered as “scaffolding elements” [10] (Figure 2). Sulfur-based polymers, e.g., amphiphilic polythionates [11], are, however, limited to linear chains, which severely restricts the diversity of possible shapes in sulfur-based systems. Boron, on the other hand, forms polymeric structures that are clusters of atoms rather than smaller, isolated molecules, e.g., decaborane (14), B10H14, which, while stoichiometrically a boron analogue of the hydrocarbon decane, is in structure more similar to a diamond nanoparticle [12]. Among these alternatives, silicon seems to be the most promising choice as a substituent for carbon in biochemistry (Figure 2).
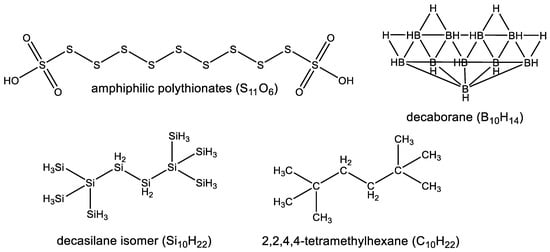
Figure 2.
Comparison of four (sulfur, boron, silicon and carbon) scaffolding elements and their pros and cons as main building blocks of biochemistry. Sulfur (e.g., amphiphilic polythionates [11]) forms chains with itself, and in an alternation with carbon, nitrogen, or oxygen (heteroatoms), but has a very limited branched structure, which severely limits the diversity of possible shapes in sulfur-based molecules. Boron (e.g., decaborane [12]) forms chains with itself, and in an alternation with carbon, nitrogen, or oxygen (heteroatoms), but forms clusters of atoms rather than smaller isolated molecules (a problem which is opposite to sulfur). Carbon (e.g., decane isomer) and silicon (e.g., decasilane isomer [15]), on the other hand, form chains with themselves, form chains in alternation with various heteroatoms, and can form diverse linear or branched structures. Out of the possible alternative scaffolding elements, silicon appears to be the most promising choice as a substituent for carbon in biochemistry.
Because the scaffolding of carbon (and hydrogen) alone provides only the very limited chemical function required for metabolism, heteroatoms are needed to provide chemical reactivity. Heteroatoms can form covalent bonds to carbon (or other scaffolding elements) but are more electropositive or electronegative. This difference in electronegativity provides the reactivity of molecules (see Section 2.2). Heteroatom reactivity is responsible for the great majority of the metabolic reactions occurring in Earth’s organisms [13]. Life on Earth uses several heteroatoms (e.g., O, N, S and P) that form covalent bonds with the carbon scaffold of biomolecules and with each other.
Heteroatoms can conveniently be divided into common heteroatoms and rare heteroatoms (although this is for convenience and does not represent any absolute class of abundance, which in any case will vary from organism to organism). Common heteroatoms (e.g., nitrogen or oxygen in terrestrial life) are used in many different molecules in biochemistry. On Earth, oxygen dominates among the heteroatoms used by life. In fact, the oxygen heteroatom is so ubiquitous that Earth biochemistry has been described as the “chemistry of carbonyl groups” [4]. By contrast, rare heteroatoms are used in a small number of specific contexts. Examples in terrestrial life include selenium and fluorine. It is plausible that these elements are difficult for life to use widely but confer specific advantage in the rare molecules in which they are found. For example, the thermodynamics of fluorine make it hard to incorporate into organic molecules, but the toxicity of the resulting molecules makes fluorine valuable in plant defense chemicals [14].
We note that the chemical diversity in small molecules implies a potential chemical diversity in polymers. Proteins are enormously diverse because they can be made from a chemically diverse set of amino acid monomers.
Together, scaffolds and heteroatoms provide the chemical diversity and reactivity required for life.
2.2. Chemical Stability and Reactivity
Chemical stability and reactivity are the second general requirement of the chemistry of life. There must be a balance between biochemicals’ stability and reactivity in their solvent. Biochemicals must be stable to reaction both with the solvent and with each other, over whatever timescale is required for their biological function. Thus, for example, sugars cannot be a component of a biochemistry that uses concentrated sulfuric acid as a solvent, because sugars are dehydrated to amorphous carbon in under a minute in sulfuric acid conditions [16]. However, biochemicals have to be reactive in their solvent to some extent, in order to be able to perform their required biological functions.
Aprotic solvents (those that cannot donate protons) tend to be less reactive than water, ammonia, and sulfuric acid. Furthermore, reactivity is highly reduced at low temperatures so almost any chemistry is stable in liquid methane or liquid nitrogen. We would therefore expect very stable chemicals not to be components of life in cold, aprotic solvents, as they would not be reactive enough.
Stability and reactivity are therefore a function of the solvent as well as the chemistry itself. We discuss the solvent requirement next.
2.3. Solvent
Chemical life has to operate in a medium that allows molecules to move (as do liquids and gases) but is dense enough to stop large molecules from simply falling out as aggregates (as are solids and liquids). This means that the chemistry of life has to operate in a dense fluid solvent, most plausibly a liquid. Water is widely considered the ideal, possibly the only, solvent for life [17]. Water actively assists in the self-organization of membranes and polymers of life, therefore assisting in compartmentalization, a phenomenon that is likely to be a general requirement for life [18]. On Earth, liquid water also plays an active role in life’s metabolic processes.
Although water is a good solvent for Earth’s carbon-based life, many other solvents have been proposed as well suited for alternative biochemistries (e.g., sulfuric acid, carbon dioxide, hydrogen cyanide, propane, ammonia, hydrogen sulfide, ethane and methane, nitrogen, and even neon and argon) [1,3,4,19,20,21,22]. These liquids broadly fall into the protic solvents (solvents that, like water, can donate or accept protons from chemicals dissolved in them, such as H2SO4, H2S, NH3), and aprotic solvents (such as liquid hydrocarbons or nitrogen). In general, protic solvents are chemically aggressive, and their aggressiveness limits the chemistry that can stably dissolve in them. In contrast to protic solvents, aprotic solvents are generally less reactive and stably dissolve a wider range of chemicals than protic solvents.
Solubility of solids in any solvent generally increases with temperature, so cosmically common aprotic solvents such as liquid methane and nitrogen are poor solvents because they are liquid only at very low temperatures. Thus, the nature and temperature of the solvent in which life operates affects both what scaffolds are viable and what heteroatom chemistry is stable in that solvent.
3. Overview of Silicon Chemistry
We start the assessment of silicon as a building block of biochemistry by presenting an overview of the basic physico-chemical properties of silicon chemistry compared to carbon (Section 3.1), followed by the discussion of the chemical diversity of silicon chemistry as compared to carbon (Section 3.2). We then address the thermodynamics of formation of silicon chemicals, with a comparison to carbon equivalents, and discuss the thermodynamic barriers in the synthesis of complex silicon molecules. We present new findings on the possible diversity of silicon chemistry based on thermodynamic limitations (Section 3.3).
3.1. Silicon Chemistry Overview in Comparison to Carbon
Silicon is the closest analogue of carbon. Both silicon and carbon are tetravalent atoms that form primarily covalent (non-ionic) compounds. However, there is surprisingly little similarity between them that goes beyond the statement that both elements “can form four covalent bonds”. In this section, we summarize the similarities and differences of silicon and carbon and discuss the consequences of their different reactivities and chemical properties on the formation of complex chemistry.
The covalent radius of a silicon atom is larger than that of carbon which results in generally longer bond lengths and different bond angles. Different bond lengths and angles have especially large effects in ring structures containing silicon atoms, resulting in distinct ring conformations and ring reactivity as compared to their carbon-containing counterparts (see for e.g., sila-venlafaxine (9) in Section 3.2.2 below).
The bonding preferences of silicon are also different than carbon, mainly due to the availability of low-lying 3d orbitals that allow silicon to form compounds that have five- or six-coordinated silicon atoms. Especially, penta-coordinated silicon compounds are readily formed, which allows for many more reaction paths between tetra-coordinate silicon compounds than are available to carbon [23,24]. Stable penta- and hexa-coordinate silicon compounds are “super-chiral”.
Silicon is more electropositive as compared to C, N, O, and H. The higher electropositivity of Si creates an electron-deficient center in silicon and results, e.g., in a stronger bond polarization as compared to analogous carbon bonds, or in a reversed bond polarization of the C–H and Si–H bonds (Table 1). As a result of those differences, most bonds that silicon forms with non-metals are more strongly polarized than their carbon counterparts and thus more susceptible to electrophilic and nucleophilic attack. Even bonds that are considered to be very stable, like Si−C, have higher reactivity as compared to their carbon analogues. For example, silicon tetrachloride (containing Si–Cl bonds) is hydrolyzed almost instantly in water, whereas carbon tetrachloride (containing analogous C–Cl bonds), which is also thermodynamically unstable to hydrolysis, is stable for years in the presence of water. Silanes (SiH4, Si2H6 etc.) are stable as pure chemicals for many years but are very sensitive to water in the presence of trace alkali, unlike alkanes. Reactions of both disilane (Si2H6) and ethane (C2H6) with oxygen are clearly exothermic, but ethane may be mixed with oxygen at 200 °C without reacting, whereas disilane spontaneously combusts in air at 0 °C [25]. We discuss the implications of such differences in reactivity in Section 3.2.2.

Table 1.
An overview of the basic physico-chemical properties of carbon and silicon, including their respective bond and common functional group reactivity. Data based on values collected in [26,28,29]. *SMILES description of multiple bonds is used throughout the paper [30]. For example, C=C is a double carbon–carbon bond; C:C is an aromatic carbon–carbon system; C#C is a triple carbon–carbon bond. For more details on the chemistry of organosilicon compounds and examples of some exceptionally exotic chemistry that silicon atoms can participate in, see Appendix C.
The differences between bond energies for carbon- and silicon-containing chemical bonds are also reflected by the disparities in the reactivity of silicon and carbon-containing compounds (Table 1). The strength of a C–C bond is a little bit greater than of a C–O bond, while the analogous Si–O bond is much stronger than the Si–Si bond [26], although the exact bond energy depends on the substituents on the C or Si. As a result of these differences in individual bond strengths, the chemistry of organic carbon molecules is dominated by C–C polymerization (catenation, the formation of long chains of covalently linked carbon atoms, e.g., in hydrocarbons). Even though silicon is capable of formation of Si–Si catenated structures (e.g., in silanes), they are much more reactive than their C–C counterparts (especially in water). As a consequence of the greater reactivity of the Si–Si bond, the most common stable polymers of silicon are built from Si–O chains, as the Si–O bond is disproportionately stronger than any other Si-containing bond (Table 1). Moreover, the polymerization of silicon often leads to a meshwork of Si–O chains and not linear polymers like for carbon—recall that the formation of long linear polymers is often cited as an absolute, general characteristic of any biochemistry [27]. As a result, Si chemistry in oxygen-rich environments (e.g., water) ultimately leads to silica (SiO2) (which is a refractory solid rather than a gas, with no double bonds to oxygen as in its carbon equivalent CO2) (Figure 3).
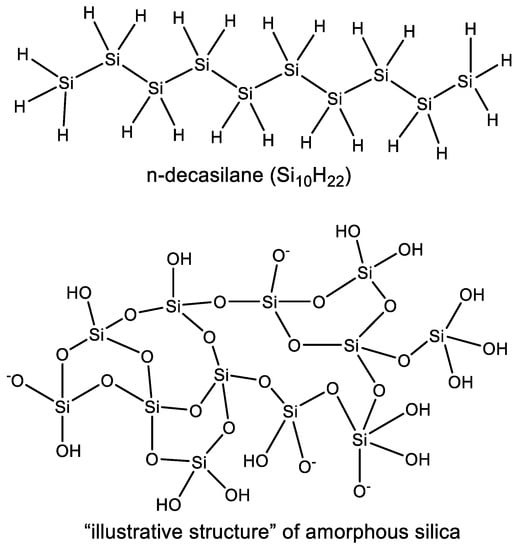
Figure 3.
Polymerization of silicon in oxygen-rich aqueous environments leads to a meshwork of Si–O chains (e.g., “illustrative structure” of amorphous silica) and not linear polymers like silanes (e.g., n-decasilane (Si10H22)). As a result, Si chemistry in oxygen-rich environments (e.g., water) ultimately leads to silica (SiO2), a refractory solid.
3.2. Diversity of Silicon Chemistry
In this section, we explore the potential diversity of silicon chemistry from three viewpoints: the theoretical diversity of organosilicon chemistry; the observed functional diversity of silicon molecules; and the potential diversity of silicon-based polymers. We conclude with a new assessment that there is, in theory, sufficient potential diversity in silicon chemistry to build biochemistry.
3.2.1. Theoretical Diversity of Silicon Chemistry
Silicon can form stable covalent bonds with the same crucial elemental building blocks as carbon. It can covalently bind itself, carbon, nitrogen, oxygen, sulfur, phosphorus, and the halogens, as well as semi-metals like germanium. Silicon can also form covalent bonds with many metals [31,32]. On top of the versatile binding to many other elements in its most common tetra-coordinate state, Si can also form stable penta- and hexa-coordinate compounds with crucial biogenic elements like nitrogen, carbon or oxygen, with or without overall charge on the molecule (see Table 1 for detailed description of the physico-chemical properties of Si). Moreover, the coordination numbers of silicon (3, 4, 5, 6) are different than that of carbon (1, 2, 3, 4). Such differences often result in the formation of silicon chemicals that do not have direct carbon analogues, emphasizing that the potential chemical flexibility of silicon (although achieved through different means) could be as great as that of carbon.
There are two types of measure of chemical diversity: structural and functional. Hydrocarbons show a staggering structural diversity but are chemically monotonous. As noted in Section 2.1, there are only three stable NmHn compounds, but they show more divergent chemical behavior than all the hydrocarbons. In this section, we address the structural diversity of organosilicons; in the following section (Section 3.2.2), we discuss the functional diversity of silicon.
To illustrate the scale of possible structural diversity in organosilicon chemistry as compared to carbon, we generated a set of possible chemical structures that are composed of up to seven atoms selected from S, P, O, N, F and Cl as heteroatoms, (with as many additional H atoms as was required to satisfy valence rules), using an algorithm derived from that described in the work of [33,34]. In short, the algorithm takes a set of topological structures defined as alkanes and substitutes heteroatoms into them according to bonding rules. Starting structures and bonding rules are defined to reflect stability and reactivity in different solvents (see Appendix E.1 for more details on the calculations of the silicon chemical space).
We generated sets of structures likely to be stable in water, sulfuric acid or in a hypothetical aprotic cryosolvent (we did not consider solubility in cryosolvents for the chemical diversity estimation; see Section 4.4 for discussion of the solubility limitation of cryosolvents), generated with or without silicon atoms. These calculations do not include penta- and hexacoordinate systems, which are under-represented in small molecules (by definition, there are no 5-atom molecules in which a silicon atom is bonded to five other atoms).
From our combinatorics study, we find that adding silicon to the repertoire of the chemistry possible in water adds little additional structural chemical diversity (Figure 4). Such a very modest increase in chemical diversity is due to the fact that almost all Si–X bonds, including Si–Si bonds, are readily attacked and hydrolyzed in water (Table 1). In contrast, we find that adding silicon to the repertoire of structures stable in sulfuric acid has a greater positive impact on the available chemical diversity. In aprotic solvents, where neither attack by H+ or OH- is possible, the increase in chemical diversity is greater still. However, our calculations suggest that, overall, silicon does not have a major impact on the structural chemical diversity available to life (as compared to carbon). This is because carbon can readily form double and triple bonds with C, N, O and S, whereas silicon double bonds are strongly disfavored (although see Appendix C for an overview of exceptions to this generalization about silicon chemistry). Such limitations of silicon chemistry are further exemplified by the efforts to make silicon analogues of terrestrial biochemicals, which have only revealed how different silicon and carbon really are [35]. We discuss the observed functional diversity of silicon chemistry next.
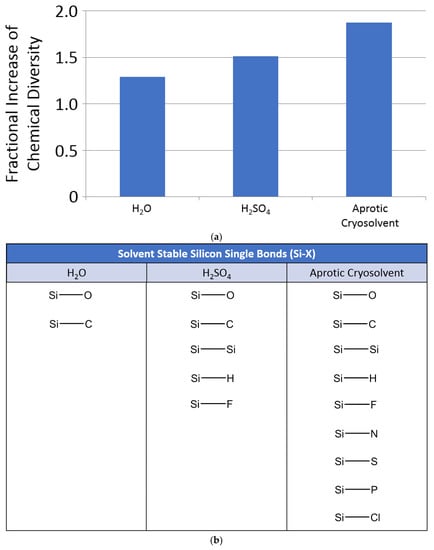
Figure 4.
Theoretical chemical diversity of silicon chemistry. (a) Relative size of the chemical space with silicon included in SPONCH + F + Cl chemistry, compared to the chemical space without silicon. Y axis: size of the chemical space of chemical structures with up to six non-hydrogen atoms predicted to be stable in respective solvents generated by including silicon chemistry. The relative size of the chemical space is calculated as S/N, where S is the number of molecules in the chemical space with silicon and N is the number without silicon. See main text and Appendix E.1 for details. (b) Silicon single bonds generally stable in H2O, H2SO4 and aprotic cryosolvent (a qualitative assessment, based on the chemical reactivity of silicon compounds discussed in detail in Section 4). Very few Si-containing chemical bonds are stable in water. Note that, while some Si–O bonds are generally stable to hydrolysis in water (e.g., in the context of C–Si bonds, like C–Si–OH), many Si–O-bond-containing functional groups are not (e.g., O–Si–O–C), further limiting the scope of Si chemistry available for life in water. Sulfuric acid can support larger number of Si-containing bonds, and, as a consequence, more diverse silicon chemistry. Virtually all chemistry is stable in aprotic cryosolvents.
We conclude with a few points. First, the vast potential theoretical space of silicon chemistry is almost entirely unstable in water, and hence not available to a biochemistry based on water as a solvent (see also Section 4.1). This major point has been implied from several examples (e.g., [1,3]) but never fully emphasized. Second, the presence of Si adds a greater diversity of molecules—perhaps enough to contribute as a heteroatom component of biochemistry—to the available chemistry of hypothetical life based in sulfuric acid (see also Section 4.3). Third, the majority of the chemical space of silicon chemistry is available in aprotic solvents such as dry liquid hydrocarbons or liquid nitrogen.
3.2.2. Observed Functional Diversity of Silicon Chemistry
The functional diversity of silicon chemistry is demonstrated by the wide range of compounds that have been created in the lab, and by their very varied chemical properties, including even some biological uses. We can only provide a brief overview of the diversity of molecules containing silicon (either as a scaffolding element or as a major heteroatom) that are available to silicon chemistry. Such an overview will serve as an illustration of a proof of principle for the chemical diversity of synthetic organosilicon molecules. We discuss several such examples below.
Silanes (silicon analogues of hydrocarbons), both branched and unbranched [15], and diverse cyclosilanes (e.g., cyclohexasilane) are commonly known silicon analogues of hydrocarbons [36]. Polymeric siloxenes (cyclosilane rings with attached –OH groups) have no direct carbon analogue, but are reminiscent of functionalized graphenes [37]. The linked siloxene ring structures have structural and electronic similarities to the porphyrin units of heme and chlorophyll [37] (see Appendix C for the discussion of hypothetical, analogous solutions of silicon to the common biological functions). Many complex cage systems, composed of multiple connected ringed systems, are also known. One example is silsesquioxanes, containing Si–O-bond-rich “core” structures that can be modified with other groups that allow for a precise spatial orientation of the entire molecule, therefore opening the possibility for the complex regulation of the chemical accessibility of the core structure [38,39,40,41].
Mere structural diversity in general is insufficient to support a biochemistry (Section 3.2.1). The chemistry of life must support chemical functional diversity as well. Silicon provides the basis for diverse chemical function when combined with other atoms (other than carbon). Several examples include Si–S-bond-containing silicon analogues of thiols (thiosilanes) [42,43,44] and Si–N-bond-containing α-sila-amino acids [45]. Dynamic, highly polarized silicon systems, where rapid, reversible chemistry occurs, are also known [46]. Silicon can also provide electron conduction down an Si–Si silane chain [47,48].
Diverse silicon chemicals that have organic carbon scaffolds around the silicon atom (i.e., where silicon is acting as a heteroatom, not a scaffold element) are also known, although many react very rapidly with water. Examples of such chemicals include zwitterionic silicon compounds [49]; a range of organosilicon molecules with negatively charged silicon centers [50,51,52]; positively charged silicium (tricoordinate silicon [53]); and pentacoordinate silicons [54,55], some of which have silicon bonded to five different atoms at once [23], that can have useful catalytic properties in carbon–carbon bond formation [56].
Silicon can form stable structures inaccessible to carbon, such as stable gem diols, which have been shown to both be stable in water and to be pharmacologically promising [57] (see also Section 4.1). Silyl fluorides have also shown specific biological effects [58], as have other, more exotic, molecules like hexa-coordinate silicon (10) [59].
When silicon is incorporated into an organic compound, the chemical and physical differences contributed by the silyl group can provide such compounds with unique chemical properties, which can lead to subtle biological function different from carbon. These new unique properties lead to useful pharmacological and medicinal applications of organosilicon molecules and open new avenues for unique specific interactions between organosilicon molecules and biological macromolecules. Good examples of such new biologically and pharmacologically relevant properties were shown for sila-venlafaxine (9), where the introduction of silicon into the ring structure provides a conformation that is not accessible with the corresponding carbon analogue [58].
One of the persistent myths about silicon chemistry is that it is “monotonous”, and this is in part because many of the key analogous functional groups in carbon-based biochemistry cannot exist in silicon chemistry. Thus, despite decades of effort, no-one has succeeded in making a compound with a –H and an –OH group attached to the same silicon atom (Reinhold Tacke, per comm). Thus, direct silicon analogues of primary alcohols or sugars are impossible. Similarly, silicon doubly bonded to oxygen is not stable under Earth surface conditions even in the absence of water or molecular oxygen, so silicon analogues of aldehydes, ketones and carboxylic acids are impossible. However, this is not the point at issue. The question is whether silicon can provide diverse function, and, as we argue on the examples presented above, it can. Silicon does so, however, through different chemistry than carbon. We discuss the capability of silicon for complex, diverse chemical functionality (on the example of polymer chemistry) next.
3.2.3. Observed Structural Diversity of Complex Silicon Polymer Chemistry
As a subset of observed silicon chemical diversity, we turn to silicon polymer chemistry. Functional chemical diversity provided by silicon is only useful to life if silicon can form polymers. Virtually every biological process uses polymers. While the specific long polymers used by life on Earth, like proteins, DNA or RNA, do not have to be universal for all life, it is likely that the utilization of some form of polymers is one of the required general characteristics of all life, no matter its chemical composition [2,13,27]. It is therefore prudent to ask if silicon can provide sufficient chemical diversity, beyond small molecules, as a building block of complex polymers for biochemistry.
The capability of silicon polymer chemistry to form sufficiently diverse molecules to support complex biochemistry is often called into question. Silicon chemistry is often called “monotonous” in comparison to possible carbon equivalents [60]. The myth of the chemical monotony of organo-silicon polymers and small molecules likely stems from the comparison of the enormous diversity of carbon-based molecules produced by life on Earth with the known organo-silicon polymers used in industry. Clearly, most industrial organo-silicon polymers are monotonous, but so are most industrial carbon polymers, especially those that are not inspired by natural products. Chemical monotony is often required by the intended function of the industrial polymer. Chemical monotony is especially prevalent in plastics, but it is not an inherent feature of organosilicon chemistry. There is no inherent chemical reason why diverse high-molecular-weight silanes or siloxanes could not have a highly diverse and highly structured set of side chains.
In fact, the modern copolymer industry has many examples of very diverse organosilicon polymers, containing many different silicon-bearing monomers in single- or cross-linked polymeric chains [61,62,63,64]. (Many industrial polymeric structures are produced on a very large scale, e.g., popular silicones are produced in an impressive amount of two million metric tonnes per year [65])). Silicon is known to form stable polysilane polymers with an –Si–Si– scaffolding, containing as many as 500 consecutive Si–Si bonds [66]. Shorter chiral oligosilanes, with up to 26 consecutive Si–Si bonds, were also synthetized [2]. Such oligosilanes are capable of supporting many different side-chain groups of variable chemical functionality, including carboxylic acid groups (1) that are soluble in water and can self-aggregate into amphiphilic vesicles and micelle like structures or alkyl side chains that are soluble in non-polar solvents (2) [2]. Note that carbon often plays the role of a heteroatom in polymers that are scaffolded by silicon.
There is, therefore, little doubt that the true chemical diversity of silicon-bearing polymers is sufficient to build a complex scaffolding of biochemicals, analogous in their biological functionality to proteins, nucleic acids or carbohydrates in Earth’s biochemistry.
The capability of a scaffolding element to build diverse molecules, especially polymeric structures, is likely one of the universal requirements for life, no matter its underlying chemical composition. Silicon clearly meets that requirement. Furthermore, silicon chemistry can also lead to the formation of molecular barriers necessary to achieve compartmentalization, another likely universal requirement for life. For example, formation of complex macromolecular assemblies that can potentially serve as molecular barriers akin to lipid bilayers in Earth’s carbon biochemistry is also known. Examples of such large flexible systems include sheets, tubes, strings and many other shapes that can be formed by various derivatives of silane polyols [67,68,69]. Even on Earth, life recognizes a unique advantage of silicon as a useful structural element (see Appendix A).
The substitution of Si for C in the context of the Si–C bond has been successfully demonstrated for a series of pharmaceutically and biologically important compounds, including amino acids (e.g., (3–6)) [70]. In some cases, the silicon-containing group is incorporated as a side chain substituent, in peptide-like backbone-containing molecules (e.g., in silanediols). Only very recently, the silicon atom been incorporated at the central α-position of an amino acid (7, 8) in a peptide chain forming short silicon-based peptides such as (7) [45]. Synthesis of α-sila-peptides (7) further exemplifies the potential for silicon as a heteroatom in the context of aqueous biochemistry (see Section 4.1).
We conclude that silicon chemistry can provide equivalent diversity of function to carbon chemistry, both from the point of view of theoretical as well as synthetic functional chemical space.
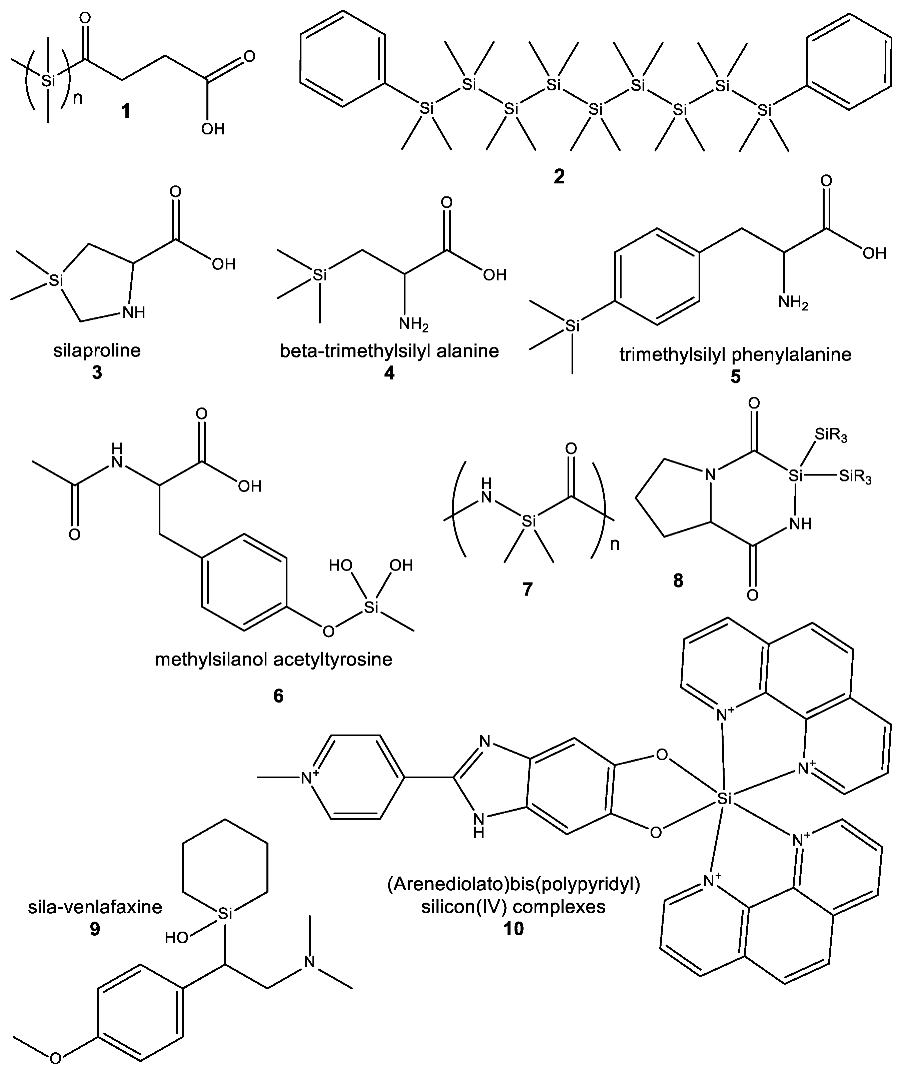
3.3. Thermodynamic Aspects of Formation of Silicon Compounds
We now address the thermodynamics of formation of silicon chemicals, providing a new analysis of energies of formation of silicon chemicals (see Appendix E.2 and Table 2). While thermodynamics is never an absolute barrier to life, chemicals that are energetically costly to make might be underrepresented in metabolism if less energetically costly functional equivalents are available.

Table 2.
Energy of formation of silicon-containing compounds. The energies of formation of Si-containing compounds are generally much higher, and therefore less favorable, than their carbon counterparts. Thermodynamic values were collected from the literature [72,73], including NIST-JANAFF thermochemical tables [74] and calculated by the GAMESS model for entropy (v1.0) [75,76,77]. All calculations are done for molecules in their standard state. XO2 corresponds to carbon- or silicon-containing substrates, carbon dioxide and silica, respectively (see Appendix E.2 for details on the modeled chemical reactions).
In this section, we discuss whether there is a thermodynamic barrier to the formation of a diverse space of silicon-containing molecules and explore if basic thermodynamics could be the reason for the improbability of silicon-based biochemistry.
All compounds containing silicon bonded to atoms of similar electronegativity (Si, P) require substantially more energy to make than equivalent carbon compounds (Table 2). However, silicon bonded to atoms of greater electronegativity (including C) is relatively more thermodynamically favorable (negative ΔG of formation from SiO2) (Table 2). Note that SiF4 is actually more thermodynamically stable than CF4. This observation is consistent with the fact that silicon tetrafluoride is stable and produced as a trace gas by volcanoes on Earth [71].
Our thermodynamic calculations show that the energies of formation of Si-containing compounds are generally much higher, and therefore less favorable, than their carbon counterparts (Table 2). Such a high thermodynamic cost of the synthesis of silicon compounds could contribute to the scarcity of silicon in biochemistry; it is not, however, an absolute limitation.
4. Potential for Silicon Biochemistry in Different Solvents
A solvent provides the immediate chemical environment for life. In this section, we discuss the general requirements for Si biochemistry through the lens of the solvent, by assessing the degree to which several of the cosmically abundant liquids can support complex silicon chemistry. In this paper, we focus on the examples of the most plausible and cosmically abundant solvents; we note, however, that some speculations have suggested the possibility of liquid rock as a solvent for life [6,78]. Feinberg and Shapiro postulate a planet “Thermia” with a surface temperature above the melting point of rock and silicate-based life “swimming” in it [78]. Although such a liquid would be composed of silicates, it is not a plausible host for silicon-using biochemistry. Covalent bonds in silicate rocks are Si–O bonds, the strongest bonds silicon forms (Table 1). All the covalent bonds in solid rock are mobilized on melting, becoming no more stable than the hydrogen bonds in liquid water. Of necessity, no complex silicon-based compounds could be stable in an environment where the silicon–oxygen bonds are being broken on a millisecond timescale by heat (although we note that planet Corot-7b could be a real-Universe model for Feinberg’s and Shapiro’s planet “Thermia” [79]).
Throughout, when we discuss the abundance or rarity of silicon in biochemicals, we refer to the number of chemical structures containing silicon, not the total mass of silicon compounds in the biosphere.
4.1. Silicon Biochemistry in Water
Only a tiny fraction of the theoretical chemical space of silicon chemistry can be stable in water (Section 3.2.1). In fact, some of the commonly held views about the low diversity of silicon chemistry come directly from the instability of silicon chemistry in water. Silicon chemistry in water also requires substantially more energy to access than equivalent carbon chemistry (Section 3.3). For all of the above reasons, we argue in this subsection that silicon is unlikely to be a scaffold element or a common heteroatom element in water. Silicon may be a rare heteroatom, found in a small number of chemicals where the stability and/or thermodynamic barriers are sufficiently minor.
The observation that the biochemistry of silicon in terrestrial organisms is extremely chemically limited is consistent with the limitations of silicon chemistry in water. All biological silicon-containing structures are derivatives of only one molecule (silicic acid, H4SiO4) (11) and its dehydration product, silica. In all of Earth’s life, the silicon atom is bonded exclusively to oxygen, forming a Si–O single bond. No naturally occurring lifeforms synthesize bonds between silicon and any other atom (see Appendix A for a detailed overview of silicon biochemistry in life on Earth).
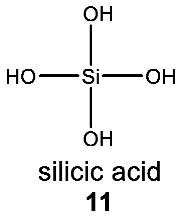
We now turn to discuss two scenarios in which Si chemistry can potentially be used in water-rich environments.
Silicon cannot be used as scaffold or major heteroatom element in water
Biochemistry based purely on Si–Si scaffolding is almost certainly impossible in water. Many Si–Si compounds hydrolyze almost instantaneously in water. The hydrolytic instability of the majority of silicon compounds is likely an unavoidable barrier to exclusive Si–Si scaffolding of life in aqueous environments [60] (Figure 3). Similarly, silicon as a major scaffold bonded to H, N, S or P atoms is implausible for its hydrolytic instability.
It is, however, possible to envision a scenario in which both carbon and silicon together play a role in scaffolding of biochemistry. The possible scaffolding scenarios for such Si–C “hybrid biochemistry” could include silanolate –Si–O–C– functional groups (not unlike the proposed cross-links in plant cell wall or vertebrate extracellular matrix, see Appendix A), although these are also hydrolytically labile. A hybrid backbone could also be built of silicon–carbon single bonds. The silicon–carbon single bond is a strong, water- and other-solvent-stable covalent bond that in principle could be utilized in biochemistry as a scaffolding bond (Table 1). Such use of silicon would be analogous to the role nitrogen plays in the backbone of proteins, or that phosphorus and oxygen play in the backbone of nucleic acids. However, including silicon as a major scaffolding element would have to give a very significant evolutionary advantage that would offset the tremendous energetic cost of mobilizing large amounts of Si.
In a water-rich environment, on a typical rocky planet where the C/O ratio is heavily skewed towards O, the main form of silicon will be sequestered in highly unreactive and insoluble silica-rich rocks (see Appendix B for the discussion of rare exceptions from this rule). The excess cosmic abundance of elemental oxygen, as compared to other elements that silicon could be stably bonded with, ensures that the great majority of the available silicon is almost exclusively bonded to oxygen. Similarly, a very high affinity of oxygen to silicon makes it unlikely that bonds between silicon and other elements (like Si–Si or Si–C) would be anything but a rare oddity in environments where oxygen is plentiful. It is, therefore, very likely that silicon chemistry on the planetary bodies in the Galaxy is dominated by the chemistry of silicon and oxygen in the silica rocks.
Utilization of silicon for building a rich and chemically diverse biochemistry in water necessities prior breaking of the Si–O bond, a feat that, as of yet, life on Earth appears to be incapable of doing. The incredible stability and strength of the Si–O bond makes hybrid Si–C scaffolding using Si as a major heteroatom element, tremendously energetically costly and therefore very unlikely. It does not mean, however, that such Si–C scaffolds for complex biochemistry are completely impossible. In environments on planets which have C/O ratios favoring C over O, or with much less overall O content (the hypothetical carbon planets [80]), the main building blocks for Si–C hybrid biochemistry might be more readily available (see Appendix D). It is important to stress, however, that such environments might be very rare.
Silicon can be used as a rare heteroatom element in water
We now present several examples of how Si could in principle be used as a rare heteroatom in water-rich terrestrial biochemistry.
The fact that life on Earth does not use silicon in any other capacity than silicic acid and silica is not in itself an evidence for an inherent limitation of life’s biochemical machinery. For example, life cannot make Si–Cl bonds in water. Similarly, Si–H bonds hydrolyze to Si–OH in a matter of a few hours under mammalian physiological conditions (e.g., [81]). But Si–C bonds that are stable in water could in principle be used by life (Section 3.1). No natural enzyme system can break Si–O bonds and synthesize Si–C bonds. Naturally occurring terrestrial enzymes process silicon-containing drugs and synthetic molecules via the carbon moieties, with silicon-containing functional groups left intact or readily hydrolyzed [58,82]. However, the possibility for silicon’s incorporation as a rare heteroatom in organic molecules by water-based life appears much more likely than previously thought, thanks to a series of elegant experiments in directed evolution by Frances Arnold’s laboratory. The experiments suggest that, at least in some capacity, life is capable of evolving means to create Si–C bonds previously not known to biology [83,84]. A chiral Si–C bond formation is catalyzed by an artificially evolved variant of Rhodothermus marinus cytochrome c. However, the formation of the Si–C bond happens in contrived conditions, in a thermodynamically favorable conversion of the Si–H bond in phenyldimethylsilane substrate to an additional fourth Si–C bond (Figure 5). Such experiments show that terrestrial biochemistry could generate Si–C bonds, but not how it could reduce silica to a silane as the feedstock for such a reaction.
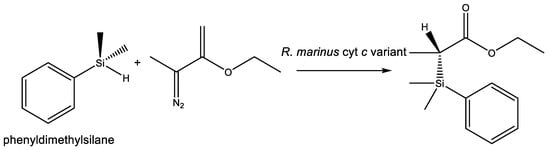
Figure 5.
Formation of chiral Si–C bond catalyzed by a laboratory-evolved variant of Rhodothermus marinus cytochrome c [83,84].
The concept that terrestrial life could use silicon bonded to carbon as a rare heteroatom is supported by the example of boron in biochemistry. Boron (B) is present in Earth’s crust as borate, which, like silicate, requires substantial energy to reduce. Boron, like silicon, is used widely by life as an oxyacid. Compounds containing B–C and B–N bonds, like silicon compounds, are known to organic chemists and utilized in pharmacology, so there is clear evidence that organoboron compounds have useful biological functions [85,86,87]. However, unlike the case of silicon, there is a reported example of boron as a heteroatom in a natural compound containing a B–C bond (12) [88], suggesting that there is an evolutionary benefit to breaking the difficult B–O bond for inclusion of boron in biochemicals.
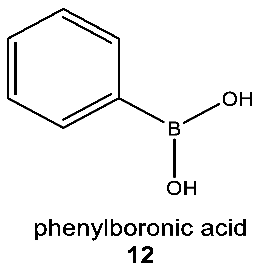
The example of a phenylboronic acid (12), a natural B–C-bond-containing compound isolated from cranberry fruit (Vaccinium sp.), shows that life is capable of overcoming the very high energetic cost of breaking and chemical transformation of the very stable B–O bonds if there is a useful function for such chemistry that cannot be achieved through other means (it is not known what that advantage is). It is very much possible that, similarly to organoboron, some small number of organosilicon natural products with silicon heteroatoms await discovery. Therefore, looking for analogous natural products containing a Si–C bond is not without merit, especially since, as we discuss below, organosilicon chemicals could provide life with a unique and useful biological function. Such an evolutionary advantage might be enough to offset the energetic costs of breaking the very stable Si–O bonds.
What might that selective advantage be? Clearly, it must derive from the unique chemistry of silicon, and one such chemistry is the unique chemical functionality of silanols, silanediols and silatriols (13, 14, 15).
Silanols, silanediols, and silatriols (13, 14, 15) are silicon-containing analogues of alcohols that are characterized by unusual solubility properties, often being similarly soluble both in water and other solvents, like hexane [89,90,91,92]. Such solubility behavior is likely the result of the formation of strong hydrogen-bonded molecular complexes in solution [69,93,94]. Such enhanced hydrogen-bonding abilities and increased acidity of silanols, relative to carbon analogues, have potentially useful biological applications that their carbon analogues cannot provide (Figure 6). For example, silanediols are present almost exclusively as the geminal diol tautomer over silanone (i.e., Si=O). Silanediols mimic the geminal diol form of carbonyl as a transition state analogue in the catalytic cycle of proteases; they are therefore considered potent protease inhibitors [57,95,96,97,98,99]. This characteristic of silicon organic chemistry could be potentially explored for a useful biological function by water-based terrestrial life.
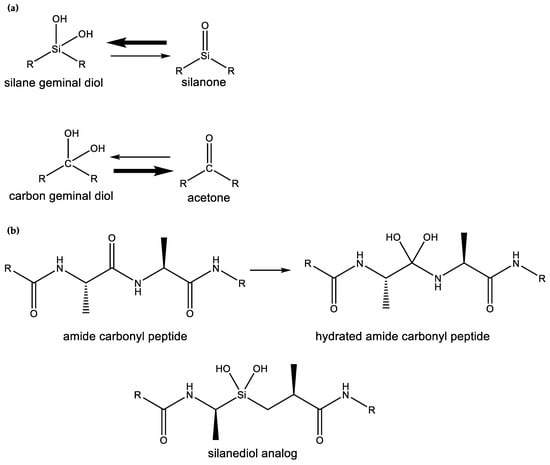
Figure 6.
Different bonding preferences between carbon and silicon. (a) Silanones (double-bonded Si=O functional groups), are not favored as compared to geminal silicon diols, which, in contrast to carbon germinal diols, are stable. (b) Geminal silicon diols mimic the unstable hydrated carbonyl. Silanediol analogues do not have stable carbon analogues and have potent biological functions, e.g., as protease inhibitors, which could be utilized by water-based life. Figure modified from [99].
In conclusion, the usage of Si in the capacity of a rare heteroatom for water-based biochemistry can, in principle, provide life with unique biological functionality of silicon chemistry that cannot be provided by other heteroatoms. In theory, this advantage might counterbalance the significant energetic expense to an organism to mobilize silicon from inorganic silica. The water stability of Si–C and Si–O bonds makes them potentially attractive as carriers of useful biological functions and opens up the possibility for specialized utilization of Si as a rare heteroatom.
4.2. Silicon Biochemistry in Non-Aqueous Acid/Base Solvents
A number of protic solvents have some chemical similarity to water and could in principle be solvents for life. However, bearing chemical similarities to water, protic solvents pose challenges for silicon biochemistry.
The principle protic solvent similar to water is ammonia. Both water and ammonia are acid/base solvents that self-ionize to form a significant concentration of conjugate acid and conjugate base (H3O+/OH− in water, NH4+/NH2− in ammonia). The conjugate acid can act as an electrophile or Lewis acid, the conjugate base as a nucleophile or Lewis base.
Ammonia on planetary bodies is unlikely to exist as a pure solvent, for two reasons. First, ammonia vapor is easily photolyzed to produce molecular nitrogen, a process that is effectively irreversible outside of the deep atmospheres of giant planets. A substantial planetary ammonia ocean could therefore only be maintained if the planet atmosphere and surface is protected from UV radiation. Secondly, oxygen is cosmically more abundant than nitrogen, such that any environment with condensed ammonia would also have condensed water. Because ammonia and water are fully miscible in each other, the result would be a mixed water–ammonia ocean.
In any event, an ammonia solution is quite basic, and as a result very aggressive to silicon chemistry. As a strongly protonating solvent, we would expect ammonolysis (an analogous process to hydrolysis in water) to pose a serious limitation to any complex silicon chemistry in NH3 solvent. As far as we are aware, however, Si compound chemistry has not been studied in liquid ammonia.
Other protic solvents could include H2S and HCN, but they are not cosmically abundant and not expected to be commonly present on planetary bodies.
4.3. Silicon Biochemistry in Sulfuric Acid
Sulfuric acid is considered an even more chemically aggressive solvent than water, and, as a consequence, an implausible solvent for biochemistry. Terrestrial biochemistry is rapidly destroyed by concentrated sulfuric acid. However, we have found, unexpectedly, that a significant fraction of silicon chemistry is stable in the harsh conditions of concentrated H2SO4 (see Section 3.2.1; (Figure 4), despite sulfuric acid’s highly aggressive, polar, and chaotropic character. Below, we expand on the chemistry of silicon in sulfuric acid and assess the viability of silicon biochemistry in this unforgiving solvent.
Silicon chemistry is more stable in sulfuric acid than in water
Perhaps surprisingly, a larger fraction of silicon chemical space is stable in concentrated sulfuric acid than in water. This is because much of the instability of silicon compounds in water arises from nucleophilic attack by OH- ions on the positive silicon atom (Section 3.2.1) and the stability of the resulting pentacoordinate structure. In contrast, in concentrated sulfuric acid, electrophilic attack dominates and silicon atoms, being electron-poor in almost all compounds, are not efficient targets of electrophilic chemistry.
Such a difference in reactivity means that a range of chemical groups are stable in sulfuric acid but relatively unstable in water. For example, trifluoralkyl groups (alkyl-SiF3) are almost instantly hydrolyzed in water, whereas they are stable in concentrated sulfuric acid [100]. Chlorosilanes take hours to days to hydrolyze in 100% sulfuric acid at 20 °C [101], whereas in water they hydrolyze effectively instantly. Alkyl silanes are resistant to cleavage of the Si–C bond under sulfuric acid conditions that will sulfonate an aromatic group [102]. Si–Si bonds are more stable in 100% sulfuric acid than Si–C bonds (that are, themselves, also stable in sulfuric acid if the carbon is aliphatic, not aromatic) [103]. Si–OH groups can be sulfated to form sulfate esters, depending on conditions [102]. Silane moieties (Si–H bonds) are stable to reaction with sulfuric acid at room temperature in some contexts—in others, where ring strain is present (e.g., silacyclopentane), they are hydrolyzed [104]. Note that Si–H groups are stable in pure water, but the slightest trace of alkali compounds, including the presence of ordinary glass, catalyzes their rapid hydrolysis [25]. There is no data available on the stability of Si-S and Si-N bonds in sulfuric acid.
There are very few exceptions to the above, Si chemicals that are more stable in water than in sulfuric acid. One notable example is that Si-phenyl bonds are readily broken in sulfuric acid, but not in water [105,106]. In addition, low-molecular-weight silicones are generally more readily rearranged into silanols or sulfate esters in sulfuric acid [107] than in water.
Possible advantages of silicon chemistry for hypothetical sulfuric-acid-based life
The fact that a larger number of silicon functional groups appear to be stable in concentrated sulfuric acid than in water opens the possibility for hypothetical life in sulfuric acid to use silicon to a considerable extent (Figure 4). We therefore turn to specific examples of chemical functionalities of silicon chemistry in sulfuric acid.
The first functionality comes from the very stable hydrogen bonds that silicon compounds can form in general. For example, Si–OH and Si–F bonds are highly polarized and would be expected to form extremely strong hydrogen bond donors and acceptors, respectively. The silicon–hydrogen bond strength could be valuable to overcome the chaotropic effects of sulfuric acid in forming stable macromolecular structures. The hydrogen bond energies between Si–F and H–X are not known, but the energy of the similar, very stable, H–F:H–OH dimer hydrogen bond is ~45 kJ/mol, compared to the HO–H:H–OH dimer of 21 kJ/mol [108]. With silicon being slightly more electropositive than hydrogen, H-bonds involving Si–F are expected to be even more stable than H–F ones.
Silanes (silicon molecules containing Si–Si bonds) can exclusively provide another potentially useful biological functionality for hypothetical sulfuric-acid-based life. The Si–Si chains, many of which are known to be stable in concentrated sulfuric acid, have a degree of σ orbital overlap that allows electron conduction down the scaffold of the molecule [47]. Such electron conduction is analogous to conjugated alkene systems in Earth life’s biochemistry. Conjugated alkenes such as isoprene are very rapidly attacked in concentrated H2SO4 [109,110], and so, in principle, long-chain silanes in sulfuric acid could substitute for biochemical functions carried out by conjugated dienes in terrestrial chemistry.
We also note that some silicon-containing polymers are highly resistant to sulfuric acid (e.g., polymers where silicon and carbon atoms alternate in the backbone, rather than the silicon–oxygen alternation of silicones, are stable to 98% H2SO4 at 90 °C [111]. Such unique silicon chemistry might also provide necessary biological functionality that is otherwise difficult to attain in sulfuric acid through exclusively carbon-based chemistry.
We emphasize that these examples of potential biological uses of silicon chemistry are speculations, not predictions. We use them here solely to illustrate that silicon has specific, potential advantages as a heteroatom for compounds in a sulfuric acid solvent—advantages that either do not apply, or apply less, in water. Adding silicon to the repertoire of structures stable in sulfuric acid has a greater positive impact on the available structural and functional chemical diversity than in water (Section 3.2.1 and Figure 4). This greater scope of stable silicon functional groups could result in greater evolutionary advantage for sulfuric-acid-based life (as compared to Earth’s water-based life) to use silicon chemistry. Not only is the size of available silicon chemical space in sulfuric acid greater than that in water, but the overrepresentation of stable silicon functional groups could offset the smaller number of carbon-based functional groups that are stable in the aggressive conditions of concentrated sulfuric acid.
Therefore, the evolutionary pressure for any sulfuric-acid-based life to explore silicon does not come solely from the advantages of the larger scope of available silicon chemistry but also from the potential necessity to explore silicon chemical space in sulfuric acid to perform biological functions.
Planetary environments with sulfuric acid
Sulfuric acid has been suggested to be an abundant solvent on the surface of planets [21]. However, unlike other speculated high-temperature solvents such as HCN and NH3, there is a precedent in the Solar System for the planetary-scale existence of liquid with concentrated sulfuric acid, and that is the Venusian clouds.
Venus has a temperate cloud layer (a region spanning from 48 to 60 km altitude with temperatures < 100 °C and pressures < 2 bar) believed to be composed of liquid sulfuric acid droplets. A permanent Venusian aerial biosphere has been a topic of scientific speculation for many decades (see, e.g., [112,113,114]). It is unknown what biochemistry could exist in such a highly reactive and aggressive protic solvent as sulfuric acid, but, as our discussion above indicates, a biochemistry that makes wider use of silicon is a possibility.
4.4. Silicon Biochemistry in Cold Aprotic Solvents
Silicon chemistry—really, any chemistry—is much more stable in aprotic solvents than in protic solvents. Aprotic solvents are non-ionizing solvents, which, unlike protic solvents (like water or ammonia), do not contain labile H+. In planetary terms, common aprotic solvents such as methane, ethane and nitrogen, that only form liquid phases at very low temperatures, are commonly called cryosolvents. Here, we use the term cryosolvent (short for cryogenic solvent, a solvent that is liquid at temperatures below −100 °C) for cold aprotic solvents.
Planetary environments with cryosolvents
Surface seas of aprotic cryosolvents might be a common occurrence on planets. In fact, aprotic cryosolvents like methane, ethane (C2H6) or liquid nitrogen may be the most abundant liquids on planetary surfaces, based on an exhaustive analysis of the propensity of stable surface oceans composed of liquids different than water [21]. N2 itself is a very common chemical on the cosmic scale, with abundances rivalling that of water.
Surface non-protonating solvents like liquid nitrogen (N2) oceans could be especially common on planets (or moons) orbiting M-dwarf stars [21]. The very low melting (−210 °C) and boiling points (−196 °C) of N2 necessitate that planets and moons hosting liquid N2 oceans have to receive far less incident stellar energy than planets hosting water oceans. The corresponding large planet–star separation (e.g., >1 a.u. for an M5 star [21] could mitigate the detrimental effects of high stellar activity of M-dwarf stars—planets orbiting close to the stars may be subjected to the catastrophic loss of an atmosphere from stellar flares. Such advantages could result in stable, “clement” conditions that could potentially allow for liquid N2 oceans to persist on a planetary surface for billions of years despite the relatively narrow temperature range for liquid N2 (−210 °C to −196 °C, at 1 bar). Such conditions could also exist in our own Solar System, on Neptune’s moon Triton, which orbits in an “N2 habitable zone” [115].
The low temperatures of aprotic cryosolvent seas pose at least two serious limitations as solvents for life (regardless of if such life is silicon- or alternative-carbon-based). The first problem is the low rate of any chemistry at such low temperatures. The second problem stems from the low solubility of molecules at cryogenic temperatures. Of those two limitations, the first, i.e., slow rates of chemical reactions, is easier to overcome.
Low chemical reactivity in cryosolvents
Slow reaction rates could be prohibitive for the formation of, or reactivity between, complex molecules. Chemical reactions occurring in cryosolvents would proceed very slowly, much more slowly than in Earth’s surface environment. The speed of chemical reactions generally drops by a factor of 2–3 for every 10 °C temperature decrease [116]. This drop of chemical reaction rate, however, is not an absolute limitation; it could actually be an advantage specific to silicon chemistry. The key factor in the formation of complex chemicals at any temperature is the selection of chemical reactions that are specifically tailored to a given temperature range [3]. Many silicon chemicals that are too reactive at Earth surface temperatures may have chemical reactivities “just right” at the temperature ranges of cryosolvents (including very low temperatures of liquid N2). (Silicon can do very fast chemical reactions at extremely low temperatures of liquid O2, as exemplified by experiments on the reactivity of amorphous silicon and oxygen [117].)
Specifically, two features of silicon chemistry support the notion that the reactivity of complex silicon chemistry could be uniquely suited for cryosolvent temperatures.
First, the increased reactivity of silicon—a disadvantage in water—could be an advantage in cryosolvents. Silicon is more electropositive than carbon, most Si bonds with non-metals are more polarized than the equivalent C bonds. As a result, such bonds are more liable to electrophilic and nucleophilic attack (see Section 3), allowing chemistry using weaker nucleophiles or electrophiles. Such reactivity is predominant in solvents like water (and ammonia). Such differences in reactivity are important because strong nucleophiles or electrophiles are themselves likely to be polarized and hence insoluble in cryosolvents (see below for further discussion on solubility). Additionally, common Si–X bonds are generally weaker than equivalent C–X bonds, and, as such, require less thermal energy to break for any given reaction mechanism. Again, weaker bonds might be considered an advantage over “classical” carbon chemistry in very-low-temperature environments.
The inherently greater reactivity of organosilicon-based chemicals could also be an advantage through enabling greater control and regulation of silicon-based biochemical processes. For example, chemical reactions involved in the formation and breakage of hydrogen bonds are much slower at cryotemperatures, which could allow for complex regulation of their formation by catalysts [4]. The much stronger nature of hydrogen bonds in cryosolvents could stabilize molecules to a much greater degree than in liquid water at Earth’s surface temperatures. While, sometimes, such stabilization effects might be viewed as a detriment (e.g., much stronger Si–OH H bonding), they could be beneficial for easy catalytic control of reactivity and stabilization of the genetic polymer molecules of hypothetical silicon-based life forms.
Secondly, in non-polar cryosolvents, many silicon-bearing functional groups that are completely unstable in water (including exotic unsaturated silanes) are stable and could in principle be utilized for useful biological function. Such higher structural and functional diversity of silicon chemistry in cryosolvents could make utilization of silicon chemistry much more attractive for life and could potentially offset the high energy requirements needed for cleavage of the Si–O bond and the mobilization of silicon from silica rocks. For example, functional groups containing multiple bonds between silicon atoms (e.g., Si=Si, Si=C, and Si#Si) are well known (see Appendix C, for detailed discussion of this unusual silicon chemistry), but so far are only known to be stable in sterically constrained compounds (i.e., compounds where the other silicon valences are occupied by very bulky groups) and in the absence of water, ammonia and a range of other groups, like carbonyls and alkynes [118,119]. In colder environments, such systems, though still reactive, are stable enough that their reactivity is much easier to regulate and control and, hence, much more useful.
Thus, the generally lower reactivity of chemicals at cryogenic temperatures is likely not a major barrier for silicon chemistry in cryosolvents.
Low solubility of chemicals in cryosolvents
The second, much more serious barrier for the possibility of complex organic chemistry of any kind in cryosolvents is the very low solubility of molecules (especially large complex polymers) under low-temperature conditions. The low solubility of molecules likely means that no cryosolvents are suitable for life. (There does not appear to be a solubility barrier in warmer solvents like water or sulfuric acid.)
Cryosolvents can in principle dissolve non-polar solutes. The solubility of non-polar molecules that do not form strong hydrogen bonds depends on their molecular weight as well as weak electrostatic interactions. However, due to the very low temperatures, even small non-polar molecules such as butane have very low solubility in liquid methane or liquid nitrogen. Polar molecules such as water, which form strong hydrogen bonds, are effectively completely insoluble in cryosolvents (e.g., [120]).
To assess the degree of the solubility limitation on the possibility of silicon biochemistry we estimate the solubility of silicon molecules at cryogenic temperatures. For our calculations, we use the example of liquid nitrogen (N2). We find that even the simplest silicon compounds have very low solubility in liquid nitrogen, confirming that the low solubility is likely the main limitation for life at cryogenic temperatures (Figure 7). We discuss the details of our calculations below.
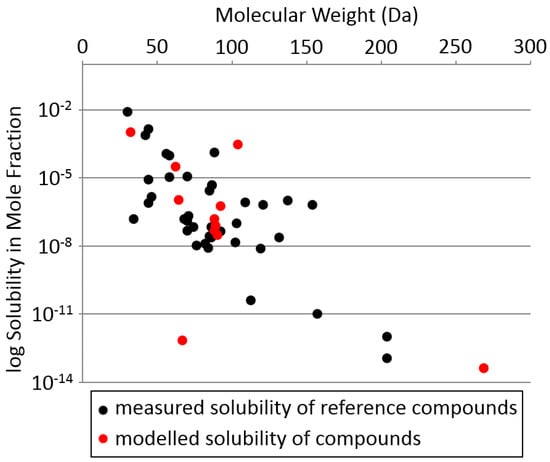
Figure 7.
Solubility of compounds vs. molecular weight in liquid nitrogen. Y axis: solubility in mole fraction plotted on log scale. X axis: molecular weight in daltons (Da). Black circles: solubility of measured reference compounds. Red circles: modeled solubility of molecules in Table 2. The results are an “order-of-magnitude” indication of solubility, not a quantitative prediction. In all cases (measured and modeled), the solubility of molecules in liquid N2 is very low and decreases gradually with increasing molecular weight. Solubility data for the reference compounds: [120,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142].
We estimate the solubility of the silicon compounds listed in Table 2 in liquid nitrogen at −196 °C (its boiling point at 1 bar), using the modified linear free-energy relationship method of Abraham [121] (see Appendix E.3 for more details).
Our results illustrate that even the simplest silicon compounds—SiH4 and Si2H6—are expected to only have parts-per-thousand solubility in liquid nitrogen, and more complex molecules of complexity equivalent to amino acid glycine will have sub-parts-per-million solubility. The exception is silicon tetrafluoride, which is estimated to be anomalously soluble. For context, of the few thousand chemicals in Earth’s life core metabolism [143], probably only a dozen or so are soluble in liquid N2 at >ppm level; none of these have an –OH group or a molecular weight over 100. For comparison, the solubility of biochemicals in water is much higher, with solubilities reaching molar concentrations. Sugars alone, with the general formula CH2O, and any number of carbons from 3 (trioses such as glyceraldehyde) through 6 (hexoses such as glucose) up to 9 (such as sialic acid) are all soluble in water at molar concentrations, and can build thousands of possible water-soluble structures.
Life requires a diverse set of chemicals and a solvent, as summarized at the start of this paper. If a solvent cannot, even in principle, dissolve a diverse set of chemicals, then that solvent cannot support life.
We conclude that, despite the favorable conditions for stability and reactivity, the solubility barrier is detrimental for Si and any other chemistry in cryosolvents.
We summarize the interconnected nature of solubility and chemical stability in Figure 8. Water does indeed occupy an optimal position in this diagram, balancing reactivity with solubility.
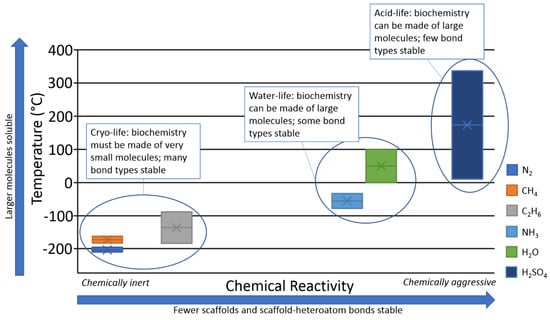
Figure 8.
Schematic representation of the viability of different solvents for complex biochemistry, as a function of chemical reactivity. Y axis: Temperature in °C. X axis: Qualitative estimation of chemical reactivity of a solvent. The height of each bar represents the temperature range of a liquid phase of a solvent (at 1 bar).
5. Synthesis and Conclusions
We have reviewed silicon chemistry in comparison to carbon chemistry and highlighted key points related to the possibility of Si as a building block of life.
- Silicon and carbon are “false twins”. Their similarities are superficial and insufficient to mitigate their crucial differences. Chemistry that is stable and normal for carbon is unstable and exotic for silicon, and, similarly, chemistry that is unstable and impossible for carbon is stable and routine for silicon. Silicon’s distinct chemical characteristics and reactivity make it a challenging choice for life (Section 3.1).
- Silicon-based life that uses Si exclusively as a scaffold element is often portrayed in science fiction. An iconic example of a fictional silicon-based life form is Horta from the twenty-fifth episode—“The Devil in the Dark”—of the first season of the popular science fiction television series Star Trek TOS, itself possibly based on the “Siliconey” in Asimov’s short story “The Talking Stone” (1955). However, silicon-based life that uses Si exclusively as a scaffold element is almost certainly impossible.
- −
- Despite the potentially rich chemistry of silicon, direct substitution of C for Si in organic molecules is often impossible (Section 3.1; Section 3.2).
- −
- Formation of many biologically crucial functional groups is much less favorable for silicon than for their carbon counterparts (e.g., unsaturated silicon structures are generally only stable at cryogenic temperatures, if at all) (Section 3.2.1).
- −
- Due to the very high affinity of silicon to oxygen the most common stable polymers of silicon are built from a meshwork of Si–O chains instead of linear Si–Si silane polymers (Section 3.1).
- −
- The excess cosmic abundance of elemental oxygen ensures that the great majority of the available silicon is almost exclusively, and stably, bonded to oxygen (in the form of unreactive silica). Therefore, while “carbon chemistry is the chemistry of life, silicon chemistry is the chemistry of rocks” (Section 4.1). The substantial energy needed to turn rocks into life, compared to that needed to turn CO2 into life, argues against silicon.
- The vast potential theoretical space of silicon chemistry is almost entirely unstable in water, and hence not available to a biochemistry based on water as a solvent (Section 3.2.1; Section 4.1).
- Earth’s life silicon biochemistry is extremely chemically limited. In all of Earth’s life, the silicon atom is bonded exclusively to oxygen, forming a Si–O single bond. No naturally occurring life forms synthesize bonds between silicon and any other atom (Section 4.1).
- The usage of Si in the capacity of a rare heteroatom for water-based biochemistry can, in principle, be possible. The water stability of Si–C and Si–O bonds makes silicon attractive as a carrier of specialized biological functions. We postulate that some small number of organosilicon natural products with rare silicon heteroatoms await discovery (Section 4.1).
- The energies of formation of Si-containing compounds are generally much higher, and, therefore, less favorable, than their carbon counterparts. The thermodynamics puts additional, although not absolute, constraints on the potential of silicon-based life (Section 3.3).
- Any sort of biochemistry is implausible in cryogenic solvents, because of solubility limits (Section 4.4).
- Going forward, we should think about silicon as a contributor to biochemistry (as a common heteroatom in sulfuric acid and a rare heteroatom in water solvent) rather than a main building block of life.
During our review, we have synthesized one new key finding.
- A larger fraction of the silicon chemical space is stable in concentrated sulfuric acid than in water. Such greater diversity of possible stable silicon molecules could be exploited by hypothetical sulfuric-acid-based life (e.g., a hypothetical, strictly aerial biosphere living in the sulfuric acid clouds of Venus). Even though carbon would still dominate, silicon could be widely used as a heteroatom component in sulfuric acid biochemistry (Section 4.3).
Even if the most serious objections to the biological use of silicon as a heteroatom can in principle be circumvented, the question of sufficient evolutionary pressure for the use of silicon over carbon as a main component for life still remains.
Author Contributions
Conceptualization, J.J.P.; methodology, W.B. and J.J.P.; chemical calculations, W.B.; writing—original draft preparation, J.J.P.; writing—review and editing, J.J.P., W.B. and S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MIT Professor Amar G. Bose Research Grant Program.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A. Biochemistry of Silicon in Life on Earth
Silicon has been suggested as an “accessory element” of all life on Earth, even with crucial roles in life’s origin. One of the theories on the origin of life on Earth (OOL), called the “clay world” [144] hypothesis, argues for the active involvement of silicates in the origin and evolution of metabolism. The clay world hypothesis suggests that crucial OOL reactions happened on the surfaces of clay minerals. Such reactions include, e.g., CO2 and N2 fixation that could have led to carboxylic acids, amino acids, sugars and nitrogenous bases, etc. In the clay world scenario, metabolism originated first and precedes the emergence of the RNA world. We note, however, that even if silicon was crucial in OOL, Si compounds that were involved in OOL were always simple silicates, and they only had a facilitating role in the formation of more complex carbon-based organics. None of the proposed clay world scenarios involve or result in the formation of organosilicon compounds.
Silicon has been found in virtually all major branches of the tree of life, including fungi and bacteria. The role of Si in bacterial and fungal metabolism and pathogenicity might be widely underappreciated [145,146]. For example, several isolated early studies suggest that bacterial silicon metabolism could be related to pathogenicity in at least several strains of bacteria (e.g., [147]). However, the biochemical mechanisms that govern silicon metabolism in living organisms on Earth are still elusive.
The biochemistry of silicon, and silicic acid in particular, was extensively studied in three broad groups of Eukaryotes—plants, diatoms and animals. Below, we briefly summarize the known biochemistry of silicon in those groups of organisms, with a special focus on the chemical bonding and interactions of the silicic acid (H4SiO4) (11), the only known source of silicon for biochemistry, with the other components of cellular metabolism.
Appendix A.1. Silicon Biochemistry in Plants
For years, the status of Si as an essential element for plants was debatable. By the early 1980s, the role of Si in a multitude of diverse physiological processes in plants was proven beyond doubt, and it is now clear that Si has a central role in plant growth and development [148]. Integration of Si within plant cell wall increases cell wall rigidity (which, in turn, boosts the overall mechanical strength of the entire plant) [149], protects plants against excessive loss of water [150], protects from diseases [151], and protects from stresses such as heat and drought, heavy metals and radiation [152,153,154,155].
All Si-containing structures and compounds in plants use silicic acid (H4SiO4) as a substrate and the simplest building block [156]. Silicic acid is a weak acid (pKa 9.8) composed of a Si atom tetrahedrally bound to four hydroxyl groups (11). Dissolved H4SiO4 is absorbed by plant roots from the soil in its uncharged form [148,157,158,159,160] with the help of specialized silicic acid transporters (e.g., Lsi6) [161,162,163,164,165,166]. Saturation of dissolved silicic acid occurs at a 1.67-mM concentration, above which silicic acid undergoes rapid polymerization, which results in deposition of solid amorphous silica (16) [167,168].
Plant amorphous silica can be deposited in any plant organ, even within or between plant cells, as well as being an integral part of the cell wall, e.g., as phytoliths [169,170,171,172]. Once amorphous silica deposits are formed, they are immobile and are not redistributed within the plant [173]. The composition and localization of amorphous silica varies significantly between plant species and highly depends on environmental conditions [174,175]).
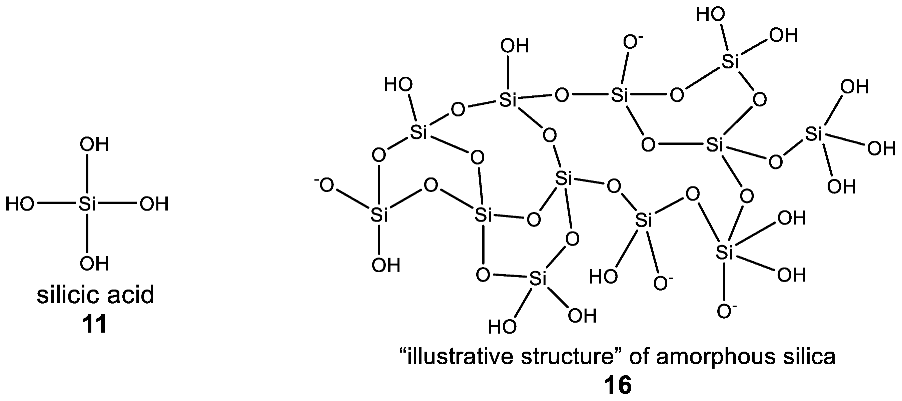
The chemical and molecular basis of the association of silicic acid, and amorphous silica in general, with various organic polymers of the plant cell wall (cellulose, hemicellulose, pectins, lignins, various phenolic compounds and proteins) is not fully understood. The most commonly hypothesized compounds to bind silicic acid in the plant cell wall are phenol–carbohydrate and lignin–carbohydrate complexes.
Over the years, on few separate occasions, it was suggested that, apart from phytoliths and other amorphous silica polymers, silicic acid is bound to sugar polymers in the cell wall in the form of silanolate-derivative -Si–O–C– covalent cross-links. However, the nature and even existence of the –Si–O–C– cross-link itself also awaits more rigorous experimental confirmation [176,177]. Nevertheless, early studies on the covalent nature of potential silanolate cross-links in the plant cell wall postulated the involvement of polyphenol compounds. Such statements were supported by early reports suggesting the association of Si with phenolic compounds in the root cell walls of rice (Oryza sativa), which could lead to the formation of Si-facilitated cross-links between lignin and carbohydrates [178]. Indeed, compounds like lignin or callose are heavily involved in the deposition of silica in a variety of plant species (e.g., horsetail (Equisetum arvense) [177,179,180,181]. Recent studies focusing on the distribution of the Si content of the cell wall in single isolated cells of Oryza sativa further confirmed a pool of Si that is firmly bound, likely via –Si–O–C– bonds, with cell wall hemicellulose macromolecular components [182,183]. Such –Si–O–C– cross-links in the context of the single plant cell likely play a structural role (maintaining of the overall shape of the cell), and provide additional stability of the cell wall, especially during its expansion and subsequent cell division (including facilitating of cell wall regeneration) [182,183]. We note that it is not inconceivable that the proposed covalent –Si–O–C– cross-links (17) in the cell walls of various plants are structurally analogous (and likely, to a certain degree, functionally analogous as well) to borate –B–O–C– cross-linking (18) identified in rhamnogalacturonan II carbohydrate polymers within the primary cell wall of Arabidopsis thaliana [184]. The borate moiety forms covalent cross-links with two apiose sugar residues belonging to two separate rhamnogalacturonan II chains. Such –B–O–C– covalent cross-links were shown to be crucial for normal plant growth and development [184].
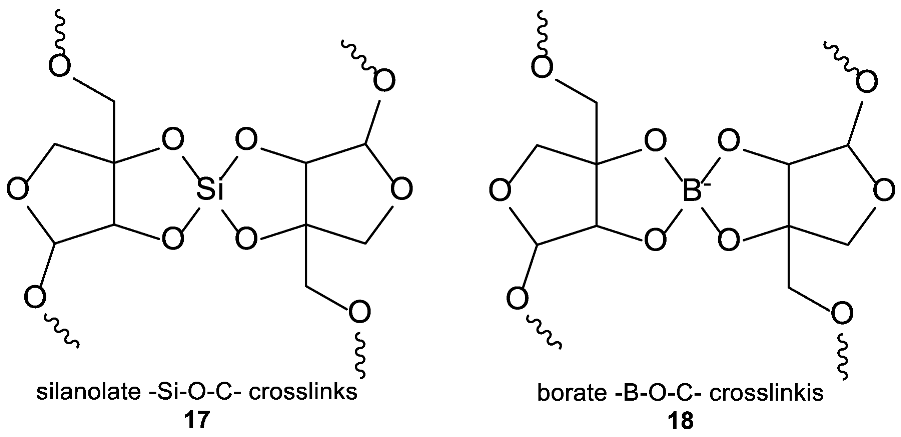
Appendix A.2. Silicon Biochemistry in Diatoms
Unlike in plants, where silica is a minor, albeit important, component of the cell wall, in some planktonic organisms it forms the bulk of the cell wall. Diatoms, in particular, make a dazzling variety of cell wall structures almost entirely from silica. Studies on the chemistry and physiology of silicification in various planktonic organisms (e.g., in the model organism Thalassiosira pseudonana) are mostly focused on understanding silicic acid transport into the diatom cell, formation of silica deposition vesicles, and identification of proteins facilitating and regulating the polymerization of silicic acid and subsequent formation of amorphous silica deposits [185].
Several protein families (e.g., silaffins, silacidins and cingulins) facilitate and regulate the polymerization of silicic acid in diatoms. All proteins involved in silicification in diatoms, especially silaffins, are heavily post-translationally (PTM) modified (e.g., N-methylated, O-phosphorylated, glycosylated, sulfated and N-polyaminated). PTM-modified silaffin peptides (15–22 amino acids long) are the main protein components responsible for the polymerization of silicic acid to amorphous silica [186]. The polymerization appears to be facilitated through general electrostatic interactions between silicic acid and free and silaffin-bound long-chain polyamines.
In contrast to the formation of amorphous silica in plants, it is not clear if carbohydrates have a specific role in facilitation of the polymerization of silicic acid in diatoms. Callose- and mannan-rich carbohydrates were identified in the cell walls of all studied species, and it is likely that they are involved in some capacity in regulation of morphology of the amorphous silica deposits [185]. To date, there are no reports on the possibility of utilization of covalent –Si–O–C– cross-links in the cell wall of diatoms.
Appendix A.3. Silicon Biochemistry in Animals
Si is an essential micronutrient in animals. In vertebrates, Si is crucial for proper development of the extracellular matrix (e.g., collagen formation) and, as a consequence, healthy bone development and wound healing [187]. Unfortunately, apart from the very recent discovery of a mammalian efflux H4SiO4 transporter Slc34a2 (also known as NaPiIIb, a known sodium–phosphate co-transporter) [188], virtually nothing is known on the biochemical pathways regulating vertebrate silicon metabolism, or what the silicon is doing in tissues. Early studies suggested that silicic acid is covalently bound, in the form of silanolate –Si–O–C cross-links, to glycosaminoglycans (GAG) found in the extracellular matrix of the connective tissues [189], and it is speculated that –Si–O–C– links maintain the overall architecture of the connective tissue in vertebrates and ensure its resilience [190,191,192].
Much more is known on the metabolism of silicic acid in sponges. Sponges form skeletal structures (spicules) made of polymerized amorphous silica, with the help of the specialized cells called sclerocytes. The process of polymerization of the precursor, silicic acid, is strictly dependent on the protein silicatein, an enzyme that shares the classical serine, histidine, and asparagine catalytic triad with serine proteases.
It is assumed, as it is in the case of all other amorphous silica-utilizing organisms, that silicic acid is a direct precursor of silicatein-mediated polymerization of amorphous silica in sponges. However, the direct precursor role of silicic acid was not proven in vitro or in vivo. On the contrary, careful in-vitro experiments suggest that the “best” substrates for silicateins are silicon alkoxides, for example tetraethoxysilane (19). It is postulated that, during the reaction, a transient enzyme–substrate –Si–O–C– covalent intermediate is formed (similar to reaction intermediates formed in the related hydrolases). The subsequent hydrolysis of the transient intermediate releases silanol, which polymerizes by dehydration. The relevance of this is unclear, as silicon alkoxides have never been demonstrated in vivo. However, they are very easily hydrolyzed. If silicon alkoxides are the preferred endogenous substrates for silicateins in vivo, it would demonstrate wider use of silicon chemistry by life than previously thought, albeit still within the realm of the chemistry of silicic acid and its derivatives.
Another potential unique feature of the silicon metabolism in sponges, as compared to vertebrates, diatoms and plants, is the capability to depolymerize the deposited amorphous silica deposits. The depolymerization of deposited amorphous silica is facilitated by a silicase (an enzyme related to carbonic anhydrase) [193]. Together, sponge silicateins and silicases maintain the dynamic equilibrium between condensed, polymerized amorphous silica and the soluble fraction of the silicic acid [194].
Appendix B. Chemical Diversity of Silicon Compounds Observed in the Natural Environment
The theoretical calculations on the size of the possible chemical space of organosilicon chemistry are in stark contrast to the diversity of silicon compounds observed and identified in the natural environment (either in the interstellar medium or on planetary bodies and meteorites). In this section, we compare the diversity of silicon- and carbon-containing molecules identified in the variety of cosmic environments and discuss the reasons for the discrepancy in the observed chemical diversity between the two elements.
There are several reports available that suggest that silicon species could be detected in interstellar medium, protostellar discs or circumstellar shells. However, while polymeric carbon compounds are ubiquitous from the astrochemical point of view, the analogous silicon compounds seem to be much less abundant. For example, a multitude of different carbon-containing molecules were detected in the interstellar medium, within nebulae, in the heads of comets or inside meteorites [195,196,197,198,199]. There are approx. 490~ different chemical species detected in astrochemical observations, ~420 of them containing carbon. Only relatively few of them (~55) are silicon-containing compounds [200]. The majority of silicon-containing molecules have been detected in circumstellar shells (in the gas streaming outwards from evolved stars) or in laboratory conditions that were designed to mimic circumstellar shell conditions [201,202]. The Si-bearing species detected include SiO, SiNC and SiCN, SiS, SiN, SiH4 and silicon carbides (SiC) with variable-length (n = 1–4) carbon atom chains, and even the first Si-bearing species with two Si atoms—SiCSi [203]. Silanes (e.g., SiH4 and other molecules containing a Si–H group) may also be common in some stellar environments, like protostellar discs (e.g., W33A) or comets, where the characteristic 4.6-µm IR absorbance ascribed to silane groups has been detected [204]. Silanes could be formed in these environments by the effects of stellar wind on reduced grains, or the condensation of shock-vaporized silicon atoms in hydrogen-rich domains.
The large amounts of silicon–carbon species in some special stellar environments (e.g., in the inner zones of the envelope of the carbon star IRC +10216 (CW Leonis) [203,205,206] could play an important role in the formation of silicon carbide dust grains in carbon-rich stellar envelopes [203]. Recently, other Si-containing species (e.g., SiS) have been identified in the carbon star IRC +10216 with ALMA and at mid-IR wavelengths as well [206,207]. Together with SiC2, Si2C, SiO and SiS, they dominate the fraction of available silicon [206,208]. SiCN and SiNC have been also detected in the external layers of the stellar envelope [209,210], but their abundances are much lower than of the silicon carbide species SiC3 and SiC4. More recent studies have expanded the known Si chemical repertoire even further, with the detection of methyl silane, CH3SiH3, and silyl cyanide, SiH3CN, in the envelope of the carbon star IRC +10216 (CW Leonis) [211].
Stellar environments are not the only conditions where non-Si–O silicon-containing molecules were detected. Despite the generally dominant silica environments, Si–C species were also identified in a variety of planetary geological contexts on Earth and beyond. Silicon carbide mineral can be found even in the highly oxidized environment of Earth’s silica-rich crust [212], where it may be the result of metamorphic reprocessing of silicate under highly reducing conditions [213]. Silicon carbide and silicon nitride are also widely distributed components of carbon-rich grains in carbonaceous chondrites [214,215]. Both silicon carbide and silicon nitride are postulated to be abundant minerals on hypothetical carbon planets (see Appendix C for the discussion of carbon planets as possible abodes for silicon-based life). The discovery of silicon carbide and silicon nitride is surprising in this context. The isotope ratios in the grains suggest that they were formed in oxygen-rich regions of supernova remnants [216]. SiC is thermodynamically disfavored over CO2 and SiO2 in the presence of abundant oxygen, although it is suggested that grains themselves are oxygen-poor [217]. How the oxygen and the silicon became separated is still unknown [218].
Compounds containing Si–C bonds can also be synthesized naturally under rare terrestrial surface conditions. The authors of [219] have demonstrated the presence of organosilicon compounds in impact glasses called irghizites in the Zhamanshin meteorite impact crater. Mass spectrometry of the contents of bubbles on the surface of impact glasses shows ions characteristic of polyorganosiloxanes (C3H9Si, C5H15OSi2, C7H15O2Si3 etc.). Organosilane signature ions were less abundant at the mouth of the crater than at the base [219]. Similar ions are not found in Australian strewn field tektites (which are mostly ~0.7 million years old [111]). The authors of [219] postulate that organosilanes are formed during condensation of the hot vapor created by the impact, which condenses without hydrolysis in the dry environment of the glass particles. Over geological time, organosilanes are hydrolyzed by water diffusing through the glass. The authors of [1] have suggested an equivalent process by which silanes and silanols could be deposited in cryogenic environments (see Section 4.4 for the discussion of silicon chemistry in cryosolvents).
Appendix C. Formation of Unsaturated Organosilicon Compounds
The formation of double-bonded, triple-bonded or aromatic compounds with silicon is generally highly disfavored, and requires special conditions, e.g., sterically constrained systems where bulky flanking groups kinetically stabilize the structure. The complete absence of water and, often, low temperatures are also required. The difficulty of formation and the overall instability of unsaturated silicon systems is in stark contrast to carbon, where double-bonded, triple-bonded and aromatic systems are formed easily. Therefore, even if theoretically possible, the reactivity of silicon-unsaturated systems results in a lot of the chemical space that is normally available to carbon having no direct silicon analogues.
The diminished structural chemical diversity of silicon chemistry due to the lack of unsaturated functional groups is not compensated by the fact that silicon forms some unique compounds that do not have stable carbon analogues. For example, penta- and hexa-coordinate Si compounds are stable [23,59,220]. ((Arenediolato)bis(polypyridyl)silicon(IV) complexes (10) contain hexa-coordinate silicon and are surprisingly stable against hydrolysis in water and are investigated as potential DNA binders [59].) Silanediols have been developed as protease inhibitors and are water-stable as well [99]. Even if life could exploit such unique chemical functionalities of organosilicon chemistry, it does not provide full functional compensation for the lack of stable unsaturated systems. Thus, at first glance, carbon has more “chemical options” than silicon.
The key differences in the multiple bond formation capabilities of both elements are also illustrated by silicon and carbon elemental forms. Both elements form a “diamond”-like structure, but only carbon is known to form graphene–double/aromatic bond conjugated systems [221,222].
The apparent lack of formation of aromatic π-conjugated systems and double or triple bond connections between atoms is often cited as a severe limitation of silicon chemistry and the main reason why silicon is not a good candidate as a scaffolding element for life [2,3]. The formation of such unsaturated organosilicon systems is unlikely at Earth’s conditions (0–100 °C temperature regime; abundance of liquid water; O2-rich atmosphere). However, recent developments in organosilicon synthetic chemistry show that silanes (silicon analogues of hydrocarbons) can undergo a versatile chemistry under conditions that are very different from Earth’s environment (cryogenic conditions; lack of liquid water; no O2 [223]). We discuss some such exceptional silicon chemistry briefly below.
Even if silanes are generally not known to readily form classical aromatic π-conjugated systems, a structural analogue of benzene was successfully made from silicon atoms [224]. The resulting silicon benzene analogue (20) is not flat like a carbon-based ring, but rather adopts a chair conformation where only the electrons in the central part of the ring are delocalized. This new type of aromatic stabilization, unknown for organic carbon molecules, is called “dismutational aromaticity”. The same authors suggest that the existence of a fully planar hexasilabenze might be possible if different substituents or synthetic precursors are used [224].
Recent modern synthetic chemistry approaches were able to uncover synthetic routes to many different unsaturated silicon compounds, not only containing Si#Si triple bonds (21, 22) [225], silicon analogues of alkynes, but also diverse structural motifs containing Si=Si double bonds. Some more interesting examples include 1,2-disilabenzenes (23) [226,227,228], silicon analogues of allenes (24), or even silicon conjugated systems (25) structurally resembling 2-methyl-1,3-butadiene (isoprene), a chemical motif widely used by life on Earth (see, e.g., [229]). For a very detailed, comprehensive review of recent breakthroughs in organosilicon chemistry, please see [230].
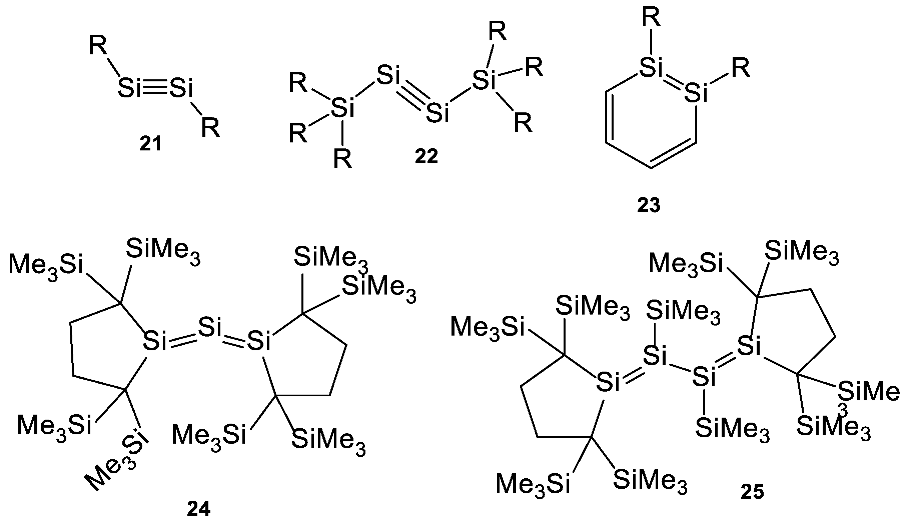
Unsaturated silicon chemistry as a crucial component of silicon biochemistry in cryosolvents?
Under the unlikely assumption that the solubility barriers to silicon chemistry in cryosolvents discussed in Section 4.4 are overcome (Figure 7; Figure 8), the unsaturated silicon systems discussed above could be crucial components of silicon biochemistry in the cold. Below, we discuss the potential for silicon unsaturated systems in the context of the crucial biological functionality that they can provide.
The existence of unsaturated silicon systems that are direct silicon structural analogues greatly increases the possible diversity of silicon chemistry and the scope of potential biological functionality of silicon-containing molecules. Direct structural similarity, however, is not the only way to achieve analogous biological function. Carbon chemistry achieves specific functional goals through compounds with double and triple bonds, such as carbonyl groups, whose silicon analogues both have different properties and are highly unstable. However, in looking for the potential for a silicon-based biochemistry, we need to explore the existence of functional rather than direct structural analogues of terrestrial carbon compounds. The silicon analogue of a carboxylic acid may be a silane triol rather than a “sila-carboxylate”. Below, we present some speculations on how silicon chemistry could provide fundamental biological functions.
The biological function potentially most affected by the intrinsic limitations of silicon chemistry is capture and delocalization of electronic energy, a biochemical function that unsaturated carbon-based molecules perform quite readily. Therefore, functional biological silicon equivalents of such biochemical processes as photosynthesis and synthesis of a silicon functional analogue of ATP are required. If there are no silicon-based functional alternatives, with or without stable unsaturated silicon systems, for such crucial biological functions, then the viability of silicon chemistry as a building block of life looks rather bleak. The solutions to this limitation of silicon were discussed before on several occasions [1,2,3]; we summarize and expand on them further.
Such complex electronic effects could be performed by unsaturated silicon systems. While they are spectacularly unstable and reactive under water-rich, room-temperature conditions, their reactivity is much lower in cryogenic conditions; in fact, their reactivity might be “just right” in cryosolvents. Silicon can also form σ-conjugated polysilanes [231] that can have similar electronic properties to carbon-based molecules containing aromatic π-conjugated systems [2]. The ability of polysilanes and other silicon-containing compounds to delocalize electrons and have light-activated electronic effects, likely crucial for the development of any kind of photosynthetic system, has been discussed before on several occasions as well [1,3]. It is clear that organosilicon chemistry is not in any way deficient when it comes to the potential for supporting complex electronic effects, including semiconduction, light activation and electroluminescence [47,48,232,233,234].
Another crucial aspect of terrestrial bioenergetics is charge separation chemistry. Earth life uses light energy to separate charge in water, generating oxygen and “reducing equivalents” (hydrogen associated with a carrier molecule) in the process. For example, on Earth, charge is separated in the process of oxidative phosphorylation, which results in proton gradients much larger than single molecules. How can such a charge redistribution occur in very cold, cryogenic, non-polar liquids? One possibility is that such charge separation could occur intramolecularly, analogously to the initial photon capture events in terrestrial photosynthesis. Polysilanes are photoconductive due to the ability of light energy to separate charge within the polysilane molecule [232]. Therefore, charge separation chemistry in a protonating solvent is not the only way to capture photon energy in chemical form.
Double-bonded silicon systems (e.g., silicon structural analogues of ethene) could be utilized as a functional analogue of ATP in cryosolvents. Note that proton gradients, utilized for ATP synthesis in Earth’s biochemistry, are uniquely suited to polar solvents, like water or possibly ammonia, and are not stable in cryosolvents and, therefore, cannot be coupled to the synthesis of silicon analogues of ATP. Si=Si systems, in turn, can be utilized in such conditions as an energy source for the synthesis of countless other molecules. One can speculate that the synthesis of silicon-unsaturated systems can be achieved with the help of UV light. For example, one of the approaches used to synthesize silicon–carbon double bonds is by UV photolysis of silanes on ultra-cold matrices [118,235], conditions that are not unlike the surface of Triton or Pluto. Similar processes could in principle happen on the surfaces of planets and moons containing liquid N2 seas, providing that the UV output of the parent star at such distances is sufficient (recall that M-dwarfs have less UV output than G-dwarfs [236]). In an analogous way to the highly reactive biochemical systems in Earth’s biochemistry, the Si=Si double bond can be protected from unwanted reactivity within the cores of complex macromolecular assemblies, allowing for the utilization of the reactivity of the Si=Si highly energetic bond in a strictly regulated manner.
Appendix D. Silicon Carbide and Carbon Planets as Potentially Suitable Environments for Silicon Biochemistry?
The likely conditions where complex silicon biochemistry could potentially prevail can be summarized with the following main points [3]: (1) scarcity of water, to limit the efficiency of transformation of various organosilicon species to the insoluble and unreactive silica; (2) low temperatures (below the freezing point of water) to increase the stability of reactive silicon functional groups, like silanes and complex unsaturated silicon systems; (3) the presence of a liquid solvent that is compatible with complex silicon chemistry; (4) the presence of a reducing atmosphere, with no O2, to prevent oxidation of reactive silicon functional groups to chemically unreactive Si–O bonds in silica; (5) the presence of available silicon precursor compounds (simple silicon species that are sufficiently reactive to be mobilized and included in the complex chemistry, likely with no dominance of SiO2).
It is clear from the list of requirements above that the majority of “planetary real estate” does not favor silicon-based life. The predominance of silica on the majority of planetary bodies might even make the use of silicon as a heteroatom for carbon-based life unlikely. There is, however, one type of environment, namely, “carbon planets” [80], that may be more favorable to the use of silicon in biochemistry, a concept that has not previously been discussed.
Appendix D.1. The Introduction to Carbon Planets
A carbon planet (or carbide planet) is a theoretical type of a planet that contains significant amounts of carbon, much more than a typical rocky (silica-rich) planet. The existence of carbon planets was theorized, and the propensity for such exotic worlds is a topic of a heated debate. Formation of a carbide planet is controversial, but models suggest that it is more probable with increasing stellar C/O ratio and increasing distance from the host star [80,237,238,239]. A number of stars with C/O ratios of greater than 0.8 are known [240]. The abundance of such worlds in the galaxy has been questioned on many occasions [241,242,243,244], but they have not been ruled out, so carbon planets remain an exciting possibility for an environment in which life could, in principle, arise.
The composition of the interiors of carbon planets has been modeled extensively (e.g., [245,246,247,248]). In contrast to Earth, where the great majority of oxygen is bound with silicon to form silicates, the predominant chemical species in Earth’s crust, carbon planets are predicted to contain large amounts of carbon monoxide (CO). CO is going to be one of the main sinks responsible for depleting oxygen [249]. The rest of the available carbon (depending on the amount of oxygen) is likely going to be bound with silicon to form siliconoxycarbide (SiOC), silicon carbide (SiC) and titanium carbide (TiC), or will form graphite and diamond layers [249,250,251,252]. The core is built from mostly Fe carbides. Depending on the C/O ratio, the surfaces of carbon planets can be rich in graphite, other C-rich minerals or hydrocarbons (like methane or tar). The atmospheres (if they exist at all) could contain CO or methane. Water is likely to be rare, as the reaction of water with carbon (graphite or diamond) to form CO2 and H2 is exothermic, and so would remove water from volcanic gases.
Appendix D.2. Can Carbon Planets Have Favourable Conditions for Complex Silicon Chemistry?
There are a few characteristics that might make carbon planets a better place for a complex chemistry containing silicon to arise than silicate planets. As we mentioned in the section above, carbon planets likely form further away from their host stars [238], meaning that they could contain a more diverse collection of solvents on their surface [21]. In particular, the high probability of formation of HCN on the surface or in the atmosphere of carbon planets might make liquid HCN the preferred solvent for complex organosilicon chemistry [253]. HCN, with a liquid range of relatively high temperatures (−13–26 °C), might provide sufficiently high solubility for biologically active macromolecules. The surfaces of carbon planets are also likely rich in various heavier hydrocarbons, like propane or butane. Propane and butane, with their higher boiling points, might also be preferred solvents for the formation of complex Si–C hybrid chemistry.
Moreover, carbon planets will probably be devoid of water, one of the most common reactants for silicon chemistry. Any amount of water or other oxygen-containing species that is, e.g., delivered via comets or meteors will readily react with carbides on the surface, limiting the sequestration of Si in the form of unreactive silica.
There are, however, some serious limitations to complex silicon chemistry on carbon planets as well. One such limitation is analogous to the sequestration of Si in silica on water or oxygen-rich rocky planets. As we have mentioned above, one of the main components of carbon planets is silicon carbide, which can be considered an analogue of silica rocks for carbon planets. Silicon carbide is a very strong and hard mineral, composed of a tetrahedral assembly of carbon and silicon atoms with very strong bonds in the crystal lattice. It is very chemically resistant, to both acids and alkali and even molten salts, up to temperatures of 800 °C. Thus, mobilizing Si and C from SiC will be very energetically costly (maybe even more so than breaking Si–O bonds in silica!) and might be prohibitive for life. The global geological aspects of the durability of silicon carbide rocks can also have detrimental consequences for such potentially crucial phenomena as plate tectonics or rock weathering. Silicates are easily weathered by water, as silica is sparingly soluble in water. Silicon carbide is not soluble in any solvent, so weathering and, hence, cycling of elements could be much more difficult on carbon planets. This might affect their long-term climate and habitability [253]. On the other hand, silicon carbide has unique and potentially beneficial properties as an exceptional support for various metal catalysts. Silicon carbide is often used in industry for catalysis of chemical reactions like oxidation of hydrocarbons, such as n-butane, to maleic anhydride [254,255]. Involvement of silicon carbide in the catalysis of various chemical reactions might help in facilitation of the formation of complex chemistry on carbon planets. It also is likely that not all Si and not all C are going to be locked in silicon carbide rocks. For example, silanes will be stable in the majority of conditions on carbon planets. If the abundance and diversity of Si–C compounds in the interstellar medium is any indication, then we could expect, in principle, a diverse collection of Si–C building blocks for biochemistry even if they exist on a largely chemically inert (but catalytically active) silicon carbide surface. For perspective, the vast majority of iron on Earth is locked in the Earth’s core, but that does not mean that trace iron cannot be a key part of terrestrial metabolism.
Appendix E. Chemical Diversity, Thermodynamics and Solubility Calculations
Appendix E.1. Chemical Diversity Calculations with Combimol-B
Theoretical chemical structures were generated by the program Combimol-B, which is based on the concepts implemented in the original Combimol as described in [33]. Combimol-B operates as follows.
As input, Combimol-B takes an alkane structure, which defines the topology of atoms. A new alkane is therefore needed for each topology, and an increasing number are needed for each size class of molecule. For convenience, the notation N = is used to describe molecules with N non-hydrogen atoms in them. Thus, for N = 2, the input is ethane, for N = 3, the input is propane and cyclopropane, for N = 4 the input is butane, isobutane, methylcyclopropane and cyclobutane, and so on. The program is provided with a list of atoms, a list of bonding rules for those atoms, and a list of rules for which bonds can form between those atoms. Thus, for phosphorus (III), the program might state that is must be bonded to between one and three atoms, and that it can be bonded to carbon but not to chlorine, thus implicitly stating that P–Cl bonds are unstable (as they are in water). The bonding rules and atom lists are user-defined. Using these rules, the program recursively replaces all the atoms in the molecule with atoms from its input list as follows. First, the single bonds between input carbons are replaced systematically with double or triple bonds. Specific rules prevent triple bonds in rings. A user-defined switch in the program can select whether to accept cumulene structures (C=C=C); in the execution used for this work, cumulenes were accepted. Cumulenes are not allowed in rings (the exclusion of alkynes and cumulenes from rings is pragmatic, based on the small size of the molecules being generated, to avoid structures such as cycloprop-1,2-diene-C1=C=C1, which are implausible). For each of these hydrocarbon “cores”, the program recursively substitutes carbons for other atoms in the list, according to the rules of bonding provided in the two files, which specify which atom can be joined to which, and which atom can support double or triple bonds. Thus if the input structure was ethane and the atom list was N and O, the program would generate structures whose SMILES description would be CC, C=C, C#C, CO, C=O, CN, C=N, C#N, NO, N=O, OO, O=O, N=N and N#N. If bonds between N and O were not allowed, then NO and N=O would not be generated. If N was specified not to be able to support double bonds, then N=O, N=N, C=N, C#N and N#N would not be generated. The program also filters out chains of non-carbon atoms. While nitrogen can be singly bonded to nitrogen (as in hydrazine), the structure N-N-N-N-N is never found in any stable molecule. Several specific rules, mostly user-defined, implement these filters. Duplicate molecules are identified and removed via their InChI code.
Some molecules cannot be generated this way, such as nitric oxide (which violates classical bonding rules). However, these are a relatively small fraction of all possible molecules. Some which are plausible on classical bonding terms, such as N=O, are actually not realistic.
Pentavalent atoms cannot be accommodated in this process, and so, to accommodate pentavalent phosphorus and arsenic and hexavalent sulfur, specific atom types are pre-defined in the program. There are listed below (Table A1) separately from S and P, which are assumed to be S(II) and P(III) respectively.
For this exercise, two sets of staring alkanes were used. For the aprotic solvent, an exhaustive set of alkanes of N = 2 through N = 7 was used, including rings and fused ring systems. For the water and sulfuric acid solvent set, only five-, six- and seven-membered rings were used. Three-and four-membered hydrocarbon rings in which one (or more) carbon has been substituted with a silicon are highly reactive, reacting explosively with acid for example. The aprotic solvent was assumed to be a “cryogenic solvent” like liquid nitrogen, which allows for the formation of many bonds. The only phosphorus species allowed in sulfuric acid are phosphonium groups, as phosphate and thiophosphate esters are rapidly hydrolyzed and phosphines are oxidized by sulfuric acid. Silicon is defined in water as requiring four bonds (i.e., Si–H bonds, the default if no other element is specified, are not allowed). In sulfuric acid, Si–H bonds are allowed.
Bonds defined for the three sets of solvents are shown in Table A1. Bonds not allowed in each solvent are ones which are broadly liable to hydrolysis in those solvents. The filtering of structures for stability is only partly effective, but, as the over-estimation of the size of the chemical space is likely to be the same for all sets of molecules generated, the filtering approach can be used to compare the extent of the subsets of chemical space that reflect the stability and reactivity of chemicals in different solvents.
The Combimol-B PC executable, source code and documentation, and some example input and configuration files, are available from W.B. on request. The program is written in QB64 BASIC.

Table A1.
Silicon bonds stable in cryosolvents (top, (a)); silicon bonds stable in water (middle, (b)); silicon bonds stable in sulfuric acid (bottom, (c)). Bonding matrices for atoms in three different solvents as used in this analysis. For the sets of atoms used, Combimol-B only allows bonds between atoms marked with a “+” in this matrix. Bonds to carbon are implicitly included for all elements, so the table showing bonds stable in water (middle, (b)) shows that only silicon bonded to carbon is stable against hydrolysis. Note that, while some Si–O bonds are stable to hydrolysis in water, many structures are not (e.g., O–Si–O–C-). Therefore, an Si–O bond is not listed as a stable output of COMBIMOL-B combinatorics for water and sulfuric acid.
Table A1.
Silicon bonds stable in cryosolvents (top, (a)); silicon bonds stable in water (middle, (b)); silicon bonds stable in sulfuric acid (bottom, (c)). Bonding matrices for atoms in three different solvents as used in this analysis. For the sets of atoms used, Combimol-B only allows bonds between atoms marked with a “+” in this matrix. Bonds to carbon are implicitly included for all elements, so the table showing bonds stable in water (middle, (b)) shows that only silicon bonded to carbon is stable against hydrolysis. Note that, while some Si–O bonds are stable to hydrolysis in water, many structures are not (e.g., O–Si–O–C-). Therefore, an Si–O bond is not listed as a stable output of COMBIMOL-B combinatorics for water and sulfuric acid.
| (a) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | P | S | F | Cl | S=O | S=S | S(=O)=O | S(=S)=O | P=O | P=S | Si | |
| N | + | + | + | + | + | + | + | + | + | + | + | + | |
| O | + | + | + | + | + | + | + | + | + | ||||
| P | + | + | + | + | + | + | + | ||||||
| S | + | + | + | + | + | + | + | + | + | + | + | + | |
| F | + | + | + | + | + | + | + | + | + | + | |||
| Cl | + | + | + | + | + | + | + | + | + | ||||
| S=O | + | + | + | + | + | + | + | + | + | ||||
| S=S | + | + | + | + | + | + | + | + | |||||
| S(=O)=O | + | + | + | + | + | + | + | + | + | ||||
| S(=S)=O | + | + | + | + | + | + | + | + | + | ||||
| P=O | + | + | + | + | + | + | + | + | |||||
| P=S | + | + | + | + | + | + | + | + | |||||
| Si | + | + | + | + | + | + | + | + | + | + | + | + | + |
| (b) | |||||||||||||
| N | O | P | S | F | Cl | S=O | S=S | S(=O)=O | S(=S)=O | P=O | P=S | Si | |
| N | + | + | + | + | + | + | + | + | + | ||||
| O | + | + | + | + | + | + | + | + | |||||
| P | + | + | |||||||||||
| S | + | + | + | + | + | + | + | + | |||||
| F | + | ||||||||||||
| Cl | |||||||||||||
| S=O | + | + | + | + | + | ||||||||
| S=S | + | + | + | + | |||||||||
| S(=O)=O | + | + | + | + | + | + | |||||||
| S(=S)=O | + | + | + | + | + | + | |||||||
| P=O | + | + | + | ||||||||||
| P=S | + | + | + | ||||||||||
| Si | |||||||||||||
| (c) | |||||||||||||
| N | O | P+ | S | F | Cl | S=O | S=S | S(=O)=O | S(=S)=O | Si | |||
| N | + | + | |||||||||||
| O | + | + | + | + | + | ||||||||
| P+ | |||||||||||||
| S | + | + | + | + | |||||||||
| F | + | ||||||||||||
| Cl | + | ||||||||||||
| S=O | + | + | + | ||||||||||
| S=S | + | ||||||||||||
| S(=O)=O | + | + | + | + | + | ||||||||
| S(=S)=O | + | + | + | + | + | ||||||||
| Si | + | + | |||||||||||
Appendix E.2. Thermodynamics of Formation of Silicon Compounds
List of chemical reactions considered in calculations of the thermodynamics of formation of silicon compounds (see Section 3.3 of the main text for details):
- (1)
- XO2 + 4H2 -> XH4 + 2H2O
- (2)
- 2XO2 +7H2 -> X2H6 + 4H2O
- (3)
- 3XO2 + 10H2 -> X3H8 + 6H2O
- (4)
- XO2 + 4CO2 + 16H2 -> XC4H12 + 10H2O
- (5)
- XO2 + 4CO2 + 16H2 -> XC4H12 + 10H2O
- (6)
- XO2 + 3CO2 + 12H2 -> XC3H10O + 7H2O
- (7)
- XO2 + HCl + 3H2 -> XH3Cl + 2H2O
- (8)
- 2XO2 + 6HCl + H2 -> X2Cl6 + 4H2O
- (9)
- XO2 + 4HF -> XF4 + 2H2O
- (10)
- XO2 + 3CO2 + ½ N2 + 13½H2 -> XC3H11N + 8H2O
- (11)
- H3PO4 + XO2 + 7H2 -> XPH5 + 6H2O
Appendix E.3. Estimation of the Solubility of Silicon Chemicals in Liquid Nitrogen
We used the Abraham model to predict the solubility of silicon chemicals in liquid nitrogen. The Abraham model predicts the log of solubility as the weighted sum of the excess molar refraction (E), induced dipole polarizability (S) [256], McGowen characteristic volume (V) [257] and two hydrogen bonding factors [258]. As strong hydrogen bonds are not relevant to cryogenic solvents and weak hydrogen bonds are not parameterized for this method, we replaced the hydrogen bonding terms with D and H.
D is the dipole in Debye, as calculated for gas-phase molecules using DFT at B3LYP 6–311/G level of theory to optimize structure and calculate dipole, as implemented in GAMESS [259,260].
H is the number of hydrogen atoms in the molecule (as, to a first approximation, any hydrogen atom can form a weak hydrogen bond under liquid N2 conditions).
Note that it is expected that weak hydrogen bonds (for example, between C–H groups and π electron systems) will have significant effects on solubility in cryogenic solvents [261,262]).
Therefore, the overall equation for solubility is:
Log10(solubility) = 1.01533H − 0.6613E − 0.6498S − 1.0357D − 0.02431V − 2.68817
The model is meant to give an order-of-magnitude estimate of solubility only, based on values that can be computed for any molecule and which do not rely on experimental measurements that are not available for many silicon-containing compounds. For 39 substances for which measured solubility in liquid nitrogen is available in the literature, the model gives an RMS error of prediction vs. measured log10(solubility) = 0.99 and an r2 of predicted vs. measured value of 0.8071.
References
- Bains, W. Many chemistries could be used to build living systems. Astrobiology 2004, 4, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Ricardo, A.; Carrigan, M.A. Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 2004, 8, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Irwin, L.N. The prospect of alien life in exotic forms on other worlds. Naturwissenschaften 2006, 93, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Baross, J.; Benner, S.A.; Cody, G.D.; Copley, S.D.; Pace, N.R.; Scott, J.H.; Shapiro, R.; Sogin, M.L.; Stein, J.L.; Summons, R.; et al. The Limits of Organic Life in Planetary Systems; National Academies Press: Washington, DC, USA, 2007; ISBN 0309179564. [Google Scholar]
- Peng, S. Silicon-Based Life in the Solar System. Silicon 2015, 7, 1–3. [Google Scholar] [CrossRef]
- Schulze-Makuch, D.; Irwin, L.N. Life in the Universe: Expectations and Constraints; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-97657-0. [Google Scholar]
- Darling, D.; Schulze-Makuch, D. The Extraterrestrial Encyclopedia; First Edition Design eBook Publishing: Sarasota, FL, USA, 2016; ISBN 9781506901442. [Google Scholar]
- Asimov, I. The elementary composition of the earth’s crust. J. Chem. Educ. 1954, 31, 70. [Google Scholar] [CrossRef]
- Ferris, T. RollingStone; Penske Media Corporation: New York City, NY, USA, June 1973. [Google Scholar]
- Hoehler, T.; Bains, W.; Davila, A.; Parenteau, M.; Pohorille, A. Life’s requirements, habitability, and biological potential. In Planetary Astrobiology; Meadows, V., Des Marais, D.J., Arney, G., Schmidt, B., Eds.; University of Arizona Press: Tucson, AZ, USA, 2020; in press. [Google Scholar]
- Griebel, J.J.; Glass, R.S.; Char, K.; Pyun, J. Polymerizations with elemental sulfur: A novel route to high sulfur content polymers for sustainability, energy and defense. Prog. Polym. Sci. 2016, 58, 90–125. [Google Scholar] [CrossRef]
- Adams, R.M. Boron, Metallo-Boron Compounds, and Boranes; Interscience Publishers: New York City, NY, USA, 1964. [Google Scholar]
- Benner, S.A. Defining life. Astrobiology 2010, 10, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Naturally occurring organofluorines. In Organofluorines; Springer: Berlin/Heidelberg, Germany, 2002; pp. 121–136. [Google Scholar]
- Christopoulos, V.; Rotzinger, M.; Gerwig, M.; Seidel, J.; Kroke, E.; Holthausen, M.; Wunnicke, O.; Torvisco, A.; Fischer, R.; Haas, M. Synthesis and Properties of Branched Hydrogenated Nonasilanes and Decasilanes. Inorg. Chem. 2019, 58, 8820–8828. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Kubinec, M.; Martin, L.; Schainker, J.; Vento, S. Sugar and Sulfuric Acid. Available online: https://www.youtube.com/watch?v=ZOedJgqTT9E (accessed on 1 May 2020).
- Pohorille, A.; Pratt, L.R. Is water the universal solvent for life? Orig. Life Evol. Biosph. 2012, 42, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- McKay, P.C. Titan as the Abode of Life. Life 2016, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- McLendon, C.; Opalko, F.J.; Illangkoon, H.I.; Benner, S.A. Solubility of polyethers in hydrocarbons at low temperatures. A model for potential genetic backbones on warm titans. Astrobiology 2015, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, F.J.; Fernandez-Soto, A.; Martínez, V.J. Diving into Exoplanets: Are Water Seas the Most Common? Astrobiology 2019, 19, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N.; Schulze-Makuch, D. Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 2014, 4, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Metz, S.; Burschka, C.; Platte, D.; Tacke, R. Pentacoordination of Silicon by Five Different Ligand Atoms: Neutral Silicon (IV) Complexes with SiClSONC and SiISONC Skeletons. Angew. Chem. 2007, 119, 7136–7139. [Google Scholar] [CrossRef]
- Junold, K.; Baus, J.A.; Burschka, C.; Vent-Schmidt, T.; Riedel, S.; Tacke, R. Five-coordinate silicon (II) compounds with Si–M bonds (M = Cr, Mo, W, Fe): Bis [N, N′-diisopropylbenzamidinato (−)] silicon (II) as a ligand in transition-metal complexes. Inorg. Chem. 2013, 52, 11593–11599. [Google Scholar] [CrossRef] [PubMed]
- Stock, A. Hydrides of Boron and Silicon; Cornell University Press: Tucson, AZ, USA, 1933. [Google Scholar]
- Barron, A.R. Chemistry of the Main Group Elements; Rice University Press: Houston, TX, USA, 2014. [Google Scholar]
- Benner, S.A. Detecting Darwinism from Molecules in the Enceladus Plumes, Jupiter’s Moons, and Other Planetary Water Lagoons. Astrobiology 2017, 17, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Spivack, J.L.; Pohl, E.R.; Kochs, P. Organoalkoxysilanes, organosilanols, and organosiloxanols. In Organosilicon Materials; Springer: Berlin/Heidelberg, Germany, 1997; pp. 105–135. [Google Scholar]
- Patai, S.; Rappoport, Z. The Chemistry of Organic Silicon Compounds; Wiley: Hoboken, NJ, USA, 1989. [Google Scholar]
- Weininger, D. SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 1988, 28, 31–36. [Google Scholar] [CrossRef]
- Walsh, R. Bond dissociation energy values in silicon-containing compounds and some of their implications. Acc. Chem. Res. 1981, 14, 246–252. [Google Scholar] [CrossRef]
- Muller, T.; Zilche, W.; Auner, N. Recent advances in the chemistry of Si-heteroatom multiple bonds. Chem. Org. silicon Compd. 1998, 2, 857–1062. [Google Scholar]
- Bains, W.; Seager, S. A combinatorial approach to biochemical space: Description and application to the redox distribution of metabolism. Astrobiology 2012, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Seager, S.; Bains, W.; Petkowski, J.J. Toward a List of Molecules as Potential Biosignature Gases for the Search for Life on Exoplanets and Applications to Terrestrial Biochemistry. Astrobiology 2016, 16, 465–485. [Google Scholar] [CrossRef] [PubMed]
- Kipping, F.S. The bakerian lecture organic derivatives of silicon. Proc. R. Soc. London. Ser. A Math. Phys. Sci. 1937, 159, 139–148. [Google Scholar]
- Hengge, E.; Janoschek, R. Homocyclic silanes. Chem. Rev. 1995, 95, 1495–1526. [Google Scholar] [CrossRef]
- Dettlaff-Weglikowska, U.; Hönle, W.; Molassioti-Dohms, A.; Finkbeiner, S.; Weber, J. Structure and optical properties of the planar silicon compounds polysilane and Wöhler siloxene. Phys. Rev. B 1997, 56, 13132. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Harrison, P.G. Silicate cages: Precursors to new materials. J. Organomet. Chem. 1997, 542, 141–183. [Google Scholar] [CrossRef]
- Jennings, A.R.; Iacono, S.T.; Mabry, J.M. Polyhedral Silsesquioxanes. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–24. ISBN 978-3-319-19454-7. [Google Scholar]
- Feher, F.J. Polyhedral oligosilsesquioxanes and heterosilsesquioxanes. Silicon Ger. Tin Compd. Met. Alkoxides Met. Diketons Silicones. Gelest Tullytown, PA 2000, 43–59. [Google Scholar]
- Haas, A. The chemistry of silicon-sulfur compounds. Angew. Chem. Int. Ed. English 1965, 4, 1014–1023. [Google Scholar] [CrossRef]
- Chung, M.-K.; Schlaf, M. A catalytic synthesis of thiosilanes and silthianes: Palladium nanoparticle-mediated cross-coupling of silanes with thio phenyl and thio vinyl ethers through selective carbon− sulfur bond activation. J. Am. Chem. Soc. 2004, 126, 7386–7392. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tokitoh, N.; Nagase, S.; Okazaki, R. The First Genuine Silicon-Sulfur Double-Bond Compound: Synthesis and Crystal Structure of a Kinetically Stabilized Silanethione. J. Am. Chem. Soc. 1994, 116, 11578–11579. [Google Scholar] [CrossRef]
- Minkovich, B.; Ruderfer, I.; Kaushansky, A.; Bravo-Zhivotovskii, D.; Apeloig, Y. α-Sila-Dipeptides: Synthesis and Characterization. Angew. Chem. 2018, 130, 13445–13449. [Google Scholar] [CrossRef]
- Sharma, H.K.; Pannell, K.H. Activation of the Si–Si bond by transition metal complexes. Chem. Rev. 1995, 95, 1351–1374. [Google Scholar] [CrossRef]
- Tokito, N.; Okazaki, R. Polysilanes: Conformation, chromotropism and conductivity. Chem. Org. silicon 1998, 2, 1063–1104. [Google Scholar]
- West, R. Polysilanes: Conformations, chromotropism and conductivity. PATAI’S Chem. Funct. Groups 2009. [Google Scholar] [CrossRef]
- Tacke, R.; Puelm, M.; Wagner, B. Zwitterionic pentacoordinate silicon compounds. Adv. Organomet. Chem. 1999, 44, 221–275. [Google Scholar]
- Laine, R.M.; Blohowiak, K.Y.; Robinson, T.R.; Hoppe, M.L.; Nardi, P.; Kampf, J.; Uhm, J. Synthesis of pentacoordinate silicon complexes from SiO2. Nature 1991, 353, 642–644. [Google Scholar] [CrossRef]
- Tacke, R.; Burschka, C.; Richter, I.; Wagner, B.; Willeke, R. Pentacoordinate Silicon Compounds with Si O5 Skeletons Containing SiOH or SiOSi Groups: Derivatives of the Pentahydroxosilicate (1−) Anion [Si (OH) 5]-and Its Anhydride [(HO) 4Si− O− Si (OH) 4] 2. J. Am. Chem. Soc. 2000, 122, 8480–8485. [Google Scholar] [CrossRef]
- Stevenson III, W.H.; Wilson, S.; Martin, J.C.; Farnham, W.B. Pseudorotational mechanism for the inversion of 10-Si-5 siliconates: Ligand structure and reactivity. J. Am. Chem. Soc. 1985, 107, 6340–6352. [Google Scholar] [CrossRef]
- Muller, T.; West, R. Cations of group 14 organometallics. Adv. Organomet. Chem. 2005, 53, 155–216. [Google Scholar]
- Kost, D.; Kingston, V.; Gostevskii, B.; Ellern, A.; Stalke, D.; Walfort, B.; Kalikhman, I. Donor-Stabilized Silyl Cations. 3. Ionic Dissociation of Hexacoordinate Silicon Complexes to Pentacoordinate Siliconium Salts Driven by Ion Solvation1. Organometallics 2002, 21, 2293–2305. [Google Scholar] [CrossRef]
- Kalikhman, I.; Gostevskii, B.; Girshberg, O.; Krivonos, S.; Kost, D. Donor-Stabilized Silyl Cations 4: N-Isopropylidene Hydrazides, Novel Bidentate Ligands for Penta-and Hexacoordinate Silicon Chelates1. Organometallics 2002, 21, 2551–2554. [Google Scholar] [CrossRef]
- Gostevskii, B.; Pestunovich, V.; Kalikhman, I.; Sivaramakrishna, A.; Kocher, N.; Deuerlein, S.; Leusser, D.; Stalke, D.; Kost, D. Donor-Stabilized Silyl Cations. 8. Carbon− Carbon Bond Formation through a Novel Interchelate Molecular Rearrangement in Pentacoordinate Siliconium-Ion Salts1. Organometallics 2004, 23, 4346–4348. [Google Scholar] [CrossRef]
- Sieburth, S.M.; Nittoli, T.; Mutahi, A.M.; Guo, L. Silanediols: A new class of potent protease inhibitors. Angew. Chem. Int. Ed. 1998, 37, 812–814. [Google Scholar] [CrossRef]
- Franz, A.K.; Wilson, S.O. Organosilicon molecules with medicinal applications. J. Med. Chem. 2013, 56, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Henker, J.; Wirmer-Bartoschek, J.; Bendel, L.E.; Xiang, Y.; Fu, C.; Harms, K.; Schwalbe, H.; Meggers, E. Progress in the synthesis and bioactivity of hexacoordinate silicon (IV) complexes. Eur. J. Inorg. Chem. 2016, 2016, 5161–5170. [Google Scholar] [CrossRef]
- Pace, N.R. The universal nature of biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Ito, H.; Taenaka, A.; Nagasaki, Y.; Kataoka, K.; Kato, M.; Tsuruta, T. Silicon-containing block copolymer membranes. Polymer (Guildford) 1996, 37, 633–637. [Google Scholar] [CrossRef]
- Czarnecki, S.; Bertin, A. Hybrid Silicon-Based Organic/Inorganic Block Copolymers with Sol–Gel Active Moieties: Synthetic Advances, Self-Assembly and Applications in Biomedicine and Materials Science. Chem. Eur. J. 2018, 24, 3354–3373. [Google Scholar] [CrossRef] [PubMed]
- Indulekha, K.; Roy, R.E.; Vishnu, A.G.; Rajeev, R.S.; Ninan, K.N.; Gouri, C. Silicone copolymers bearing reactive vinyl and hydride functionalities: Synthesis, characterisation and particulate composite thereof for specialty applications. Mater. Chem. Phys. 2018, 206, 213–223. [Google Scholar] [CrossRef]
- Corden, C.; Tyrer, D.; Menadue, H.; Calreo, J.; Dade, J.; Leferink, R. Socio-economic evaluation of the global silicones industry. Final Rep. 2016, 1, 1–115. [Google Scholar]
- Kamata, N.; Terunuma, D.; Ishii, R.; Satoh, H.; Aihara, S.; Yaoita, Y.; Tonsyo, S. Efficient energy transfer from polysilane molecules and its application to electroluminescence. J. Organomet. Chem. 2003, 685, 235–242. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Litster, S.A.; Redhouse, A.D.; Wisener, C.J. Isolation of a tetrahydroxydisiloxane formed during hydrolysis of an alkyltrichlorosilane: Crystal and molecular structure of [But (OH) 2Si] 2O. J. Chem. Soc. Chem. Commun. 1991, 173–174. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Redhouse, A.D.; Thompson, R.J.; Stańczyk, W.A.; Rózga, K. The crystal structure of (HOMe2Si) 2O. J. Organomet. Chem. 1993, 453, 13–16. [Google Scholar] [CrossRef]
- Unno, M.; Takada, K.; Matsumoto, H. Formation of supermolecule by assembling of two different silanols. Chem. Lett. 2000, 29, 242–243. [Google Scholar] [CrossRef]
- Bährle-Rapp, M. Methylsilanol Acetyltyrosine. In Springer Lexikon Kosmetik und Körperpflege; Springer: Berlin/Heidelberg, Germany, 2007; p. 354. [Google Scholar]
- Mori, T.; Sato, M.; Shimoike, Y.; Notsu, K. High SiF 4/HF ratio detected in Satsuma-Iwojima volcano’s plume by remote FT-IR observation. Earth Planets Sp. 2002, 54, 249–256. [Google Scholar] [CrossRef]
- Gurvich, L.V.; Veyts, I. Thermodynamic Properties of Individual Substances: Elements and Compounds; CRC Press: Boca Raton, FL, USA, 1990; Volume 2, ISBN 0891165339. [Google Scholar]
- Becerra, R.; Walsh, R. Thermochemistry of organosilicon compounds. In Organosilicon Compounds; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–113. [Google Scholar]
- Chase, M.W., Jr. NIST-JANAF thermochemical tables. J. Phys. Chem. Ref. data. 1998. Monograph. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Semiempirical Molecular Orbital Methods. In Reviews in Computational Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 45–81. ISBN 9780470125786. [Google Scholar]
- Winget, P.; Clark, T. Enthalpies of formation from B3LYP calculations. J. Comput. Chem. 2004, 25, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, G.; Shapiro, R. Life beyond Earth: The Intelligent Earthling’s Guide to Life in the Universe; William Morrow &Company: New York City, NY, USA, 1980; ISBN 0688036422. [Google Scholar]
- Léger, A.; Rouan, D.; Schneider, J.; Barge, P.; Fridlund, M.; Samuel, B.; Ollivier, M.; Guenther, E.; Deleuil, M.; Deeg, H.J. Transiting exoplanets from the CoRoT space mission-VIII. CoRoT-7b: The first super-Earth with measured radius. Astron. Astrophys. 2009, 506, 287–302. [Google Scholar] [CrossRef]
- Kuchner, M.J.; Seager, S. Extrasolar carbon planets. arXiv Prepr. 2005, arXiv:astro-ph/0504214, 1–17. Available online: https://arxiv.org/abs/astro-ph/0504214 (accessed on 1 May 2020).
- Tacke, R.; Linoh, H. Bioorganosilicon chemistry. Org. Silicon Compd. 1989, 1, 1143–1206. [Google Scholar]
- Zani, P. Biotransformations of organosilicon compounds: Enantioselective reduction of acyl silanes by means of baker’s yeast. J. Mol. Catal. B Enzym. 2001, 11, 279–285. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Lewis, R.D.; Chen, K.; Arnold, F.H. Directed evolution of cytochrome c for carbon–silicon bond formation: Bringing silicon to life. Science (80-) 2016, 354, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.H. Directed evolution: Bringing new chemistry to life. Angew. Chem. Int. Ed. 2018, 57, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Srebnik, M. Synthesis and biological activity of α-aminoboronic acids, amine-carboxyboranes and their derivatives. Tetrahedron 2003, 59, 579–593. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Quntar, A.A.A.; Srebnik, M. Recent advances in the medicinal chemistry of α-Aminoboronic acids, amine-carboxyboranes and their derivatives. Mini Rev. Med. Chem. 2004, 4, 1001–1018. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of biologically active boron-containing compounds. Medchemcomm 2018, 9, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.T.; MacKinnon, S.L.; Yan, X.; Hammond, G.B.; Vaisberg, A.J.; Neto, C.C. Identification of triterpene hydroxycinnamates with in vitro antitumor activity from whole cranberry fruit (Vaccinium macrocarpon). J. Agric. Food Chem. 2003, 51, 3541–3545. [Google Scholar] [CrossRef] [PubMed]
- Murugavel, R.; Chandrasekhar, V.; Voigt, A.; Roesky, H.W.; Schmidt, H.-G.; Noltemeyer, M. New lipophilic air-stable silanetriols: First example of an x-ray crystal structure of a silanetriol with Si-N bonds. Organometallics 1995, 14, 5298–5301. [Google Scholar] [CrossRef]
- Pietschnig, R.; Spirk, S. The chemistry of organo silanetriols. Coord. Chem. Rev. 2016, 323, 87–106. [Google Scholar] [CrossRef]
- Lickiss, P.D. The synthesis and structure of organosilanols. Adv. Inorg. Chem. 1995, 42, 147–262. [Google Scholar]
- Lickiss, P.D. Polysilanols. Chem. Org. Silicon Compd. 2001, 3, 695–744. [Google Scholar]
- Chandrasekhar, V.; Nagendran, S.; Kingsley, S.; Krishnan, V.; Boomishankar, R. Si–O and P–O motifs in inorganic rings and clusters. J. Chem. Sci. 2000, 112, 171–178. [Google Scholar] [CrossRef]
- Bunning, J.D.; Lydon, J.E.; Eaborn, C.; Jackson, P.M.; Goodby, J.W.; Gray, G.W. Classification of the mesophase of di-isobutylsilanediol. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1982, 78, 713–724. [Google Scholar] [CrossRef]
- Mutahi, M.W.; Nittoli, T.; Guo, L.; Sieburth, S.M. Silicon-Based Metalloprotease Inhibitors: Synthesis and Evaluation of Silanol and Silanediol Peptide Analogues as Inhibitors of Angiotensin-Converting Enzyme1. J. Am. Chem. Soc. 2002, 124, 7363–7375. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Glekas, A.; Sieburth, S.M. Silanediol-based inhibitor of thermolysin. Bioorg. Med. Chem. Lett. 2002, 12, 3625–3627. [Google Scholar] [CrossRef]
- Chen, C.-A.; Sieburth, S.M.; Glekas, A.; Hewitt, G.W.; Trainor, G.L.; Erickson-Viitanen, S.; Garber, S.S.; Cordova, B.; Jeffry, S.; Klabe, R.M. Drug design with a new transition state analog of the hydrated carbonyl: Silicon-based inhibitors of the HIV protease. Chem. Biol. 2001, 8, 1161–1166. [Google Scholar] [CrossRef]
- Singh, S.; Sieburth, S.M. Serine protease inhibition by a silanediol peptidomimetic. Org. Lett. 2012, 14, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Sieburth, S.M.; Chen, C. Silanediol protease inhibitors: From conception to validation. Eur. J. Org. Chem. 2006, 2006, 311–322. [Google Scholar] [CrossRef]
- Emeleus, H.J.; Wilkins, C.J. 122. Some new ethyl and phenyl silicon fluorides. J. Chem. Soc. 1944, 454–456. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidbaur, H. Über Silanolester anorganischer Säuren, III. Schwefelsäureester von Methylsilanolen. Chem. Ber. 1960, 93, 878–882. [Google Scholar] [CrossRef]
- Kipping, F.S. LXIX.—Organic derivatives of silicon. Part XXIV. dl-Derivatives of silicoethane. J. Chem. Soc. Trans. 1921, 119, 647–653. [Google Scholar] [CrossRef][Green Version]
- Kumada, M.; Yamaguchi, M.; Yamamoto, Y.; Nakajima, J.-I.; Shiina, K. Synthesis of some methyldisilanes containing functional groups. J. Org. Chem. 1956, 21, 1264–1268. [Google Scholar] [CrossRef]
- Plate, A.F.; Belikova, N.A.; Egorov, Y.P. Reaction of silacyclopentanes and silacyclohexanes with concentrated sulfuric acid. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1956, 5, 1101–1105. [Google Scholar] [CrossRef]
- Kipping, F.S.; Martin, G. LXII.—The action of fuming sulphuric acid on triphenylsilicol. J. Chem. Soc. Trans. 1909, 95, 489–494. [Google Scholar] [CrossRef]
- Sommer, L.H.; Bailey, D.L.; Goldberg, G.M.; Buck, C.E.; Bye, T.S.; Evans, F.J.; Whitmore, F.C. Vinylsilanes, chlorovinylsilanes and β-styryltrimethylsilane. Further studies on the α-silicon effect and β-eliminations involving silicon1. J. Am. Chem. Soc. 1954, 76, 1613–1618. [Google Scholar] [CrossRef]
- Patnode, W.; Wilcock, D.F. Methylpolysiloxanes1. J. Am. Chem. Soc. 1946, 68, 358–363. [Google Scholar] [CrossRef]
- Kollman, P.A.; Allen, L.C. Hydrogen bonded dimers and polymers involving hydrogen fluoride, water, and ammonia. J. Am. Chem. Soc. 1970, 92, 753–759. [Google Scholar] [CrossRef]
- Jensen, J.L.; Uaprasert, V. Acid-catalyzed hydration of dienes. III. Effects of ring strain on rate, enthalpy, and entropy for hydration of 1, 3-cycloalkadienes. J. Org. Chem. 1976, 41, 649–654. [Google Scholar] [CrossRef]
- Connelly, B.M.; Tolbert, M.A. Reaction of isoprene on thin sulfuric acid films: Kinetics, uptake, and product analysis. Environ. Sci. Technol. 2010, 44, 4603–4608. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, J.J.T. Organo Siloxanes and Their Production. U.S. Patent 2,592,682, 15 April 1952. [Google Scholar]
- Limaye, S.S.; Mogul, R.; Smith, D.J.; Ansari, A.H.; Słowik, G.P.; Vaishampayan, P. Venus’ Spectral Signatures and the Potential for Life in the Clouds. Astrobiology 2018, 18, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Makuch, D.; Grinspoon, D.H.; Abbas, O.; Irwin, L.N.; Bullock, M.A. A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Seager, S.; Petkowski, J.J.; Gao, P.; Bains, W.; Bryan, N.C.; Ranjan, S.; Greaves, J. The Venusian Lower Atmosphere Haze as a Depot for Desiccated Microbial Life: A Proposed Life Cycle for Persistence of the Venusian Aerial Biosphere. Astrobiology 2020. accepted. [Google Scholar]
- Soderblom, L.A.; Kieffer, S.W.; Becker, T.L.; Brown, R.H.; Cook, A.F.; Hansen, C.J.; Johnson, T.V.; Kirk, R.L.; Shoemaker, E.M. Triton’s geyser-like plumes: Discovery and basic characterization. Science (80-) 1990, 250, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A. Chemical Kinetics: The Study of Reaction Rates in Solution; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1990; ISBN 156081053X. [Google Scholar]
- Kovalev, D.; Timoshenko, V.Y.; Künzner, N.; Gross, E.; Koch, F. Strong explosive interaction of hydrogenated porous silicon with oxygen at cryogenic temperatures. Phys. Rev. Lett. 2001, 87, 68301. [Google Scholar] [CrossRef] [PubMed]
- West, R. Multiple bonds to silicon: 20 years later. Polyhedron 2002, 21, 467–472. [Google Scholar] [CrossRef]
- Milnes, K.K.; Pavelka, L.C.; Baines, K.M. Cycloaddition of carbonyl compounds and alkynes to (di) silenes and (di) germenes: Reactivity and mechanism. Chem. Soc. Rev. 2016, 45, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Rest, A.J.; Scurlock, R.G.; Wu, M.F. The solubilities of nitrous oxide, carbon dioxide, aliphatic ethers and alcohols, and water in cryogenic liquids. Chem. Eng. J. 1990, 43, 25–31. [Google Scholar] [CrossRef]
- Abraham, M.H.; Smith, R.E.; Luchtefeld, R.; Boorem, A.J.; Luo, R.; Acree Jr, W.E. Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 2010, 99, 1500–1515. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; De Vries, T. The The Solubility of Solid Ethane, Ethylene, and Propylene in Liquid Nitrogen and Oxygen. J. Phys. Chem. 1950, 54, 665–670. [Google Scholar] [CrossRef]
- Preston, G.T.; Funk, E.W.; Prausnitz, J.M. Solubilities of hydrocarbons and carbon dioxide in liquid methane and in liquid argon. J. Phys. Chem. 1971, 75, 2345–2352. [Google Scholar] [CrossRef]
- Wojtaszek, Z.; Szczepaniec-Cieciak, E. The solubility of solid chlorine, hydrogen sulphide, sulphur dioxide, and ammonia in liquid nitrogen in the temperature range 77.4–63.5 K. Cryogenics (Guildford) 1975, 15, 257–260. [Google Scholar] [CrossRef]
- Wojtaszek, Z.; Szczepaniec-Cieciak, E.; Morzyniec, A. The solubility of solid nitrous oxide in liquid nitrogen in the 77.4–63.5 K temperature range. Cryogenics (Guildford) 1975, 15, 351–353. [Google Scholar] [CrossRef]
- Szczepaniec-Cieciak, E.; Nagraba, K. The solubility of solidified toluene in liquid nitrogen. Cryogenics (Guildford) 1978, 18, 601–603. [Google Scholar] [CrossRef]
- Szczepaniec-Cieciak, E.; Kondaurov, V.A.; Melikova, S.M. The solubility of light olefins in liquid nitrogen: Part 2. Cryogenics (Guildford) 1979, 19, 649–651. [Google Scholar] [CrossRef]
- Wojtaszek, Z.; Dabrowska, B.; Skwarczyńska, M. The solubility of solidified dichloromethane in liquid nitrogen at 77.4 K. Cryogenics (Guildford) 1979, 19, 399–400. [Google Scholar] [CrossRef]
- Chen, R.J.J.; Liaw, V.W.; Elliot, D.G. Prediction of CO2 Solubility in Light Hydrocarbon Mixtures at Low Temperatures. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1980; pp. 620–628. [Google Scholar]
- Kuebler, G.P.; McKinley, C. Solubility of Solid tert-Butyl Mercaptan in Liquid Methane and an LNG Mixture. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1980; pp. 616–619. [Google Scholar]
- Szczepaniec-Cieciak, E.; Kondaurov, V.A.; Melikova, S.M. Study on the solubility light alkanes in liquid nitrogen. Cryogenics (Guildford) 1980, 20, 48–51. [Google Scholar] [CrossRef]
- Zelikina, G.Y.; Meister, T.G.; Mamchenko, T.B. Spectroscopic determination of the solubility of several substances in liquefied gases according to electronic absorption bands. J. Appl. Spectrosc. 1980, 32, 348–352. [Google Scholar] [CrossRef]
- Dabrowska, B. The solubility of selected halogen hydrocarbons in liquid nitrogen at 77.4 K. Cryogenics (Guildford) 1984, 24, 276–277. [Google Scholar] [CrossRef]
- Dabrowska, B. Solubility of CFCl3, CHCl3, CCl4 and C2HCl3 in liquid nitrogen at 77.4 K. Cryogenics (Guildford) 1991, 31, 896–899. [Google Scholar] [CrossRef]
- Kuebler, G.P.; McKinley, C. Solubility of solid benzene, toluene, n-hexane, and n-heptane in liquid methane. In Advances in Cryogenic Engineering; Springer: Boston, MA, USA, 1995; pp. 320–326. [Google Scholar]
- Dabrowska, B. The solubility of solidified bromoethane C2H5Br in liquid nitrogen at 77.4 K. Cryogenics (Guildford) 1996, 36, 985–988. [Google Scholar] [CrossRef]
- Szczepaniec-Cieciak, E.; Krzeczkowska, M. Solubility of 1-pentene ice in liquid nitrogen and argon at the standard boiling points of the solvents. J. Solut. Chem. 1998, 27, 485–494. [Google Scholar] [CrossRef]
- Kurdziel, M.; Szczepaniec-Cięciak, E.; Żarnowska, E.; Stach, J.; Nitek, W.; Dąbrowska, B. Solubility of solid 1-hexene and 2-methylpentane in liquid argon and nitrogen at the standard boiling points of the solvents. J. Solut. Chem. 2001, 30, 781–794. [Google Scholar] [CrossRef]
- Kurdziel, M.; Szczepaniec-Cięciak, E.; Golonka, M.; Dąbrowska, B.; Nitek, W. Solubility of solid 1-hexyne in liquid argon and nitrogen at the standard boiling points of the solvents. J. Solut. Chem. 2002, 31, 253–260. [Google Scholar] [CrossRef]
- Kurdziel, M.; Szczepaniec-Cięciak, E.; Dąbrowska, B.; Nitek, W.; Paliś, K.; Ślusarska, E. Solubility of solid 2, 3-dimethylbutane and cyclopentane in liquid argon and nitrogen at the standard boiling points of the solvents. J. Solut. Chem. 2003, 32, 601–615. [Google Scholar] [CrossRef]
- Kurdziel, M.; Szczepaniec-Cieciak, E.; Watorczyk, M.; Dabrowska, B. Solubility of solid 2-methyl-1, 3-butadiene (isoprene) in liquid argon and nitrogen at the standard boiling points of the solvents. J. Solut. Chem. 2004, 33, 453–464. [Google Scholar] [CrossRef]
- Singh, S.; Combe, J.-P.; Cordier, D.; Wagner, A.; Chevrier, V.F.; McMahon, Z. Experimental determination of acetylene and ethylene solubility in liquid methane and ethane: Implications to Titan’s surface. Geochim. Cosmochim. Acta 2017, 208, 86–101. [Google Scholar] [CrossRef]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; De Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef] [PubMed]
- Cairns-Smith, G.A.; Hartman, H. Clay Minerals and the Origin of Life; Cambridge University Press: Cambridge, UK, 1986; Volume 204. [Google Scholar]
- Wainwright, M. The neglected microbiology of silicon-from the origin of life to an explanation for what Henry Charlton Bastian saw. Soc. Gen. Microbiol. Q. 1997, 24, 83–85. [Google Scholar]
- Wainwright, M.; Al-Wajeeh, K.; Grayston, S.J. Effect of silicic acid and other silicon compounds on fungal growth in oligotrophic and nutrient-rich media. Mycol. Res. 1997, 101, 933–938. [Google Scholar] [CrossRef]
- Das, S.; Mandal, S.; Chakrabarty, A.N.; Dastidar, S.G. Metabolism of silicon as a probable pathogenicity factor for Mycobacterium & Nocardia spp. Indian J. Med. Res. 1992, 95, 59–65. [Google Scholar] [PubMed]
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. The Transport and Function of Silicon in Plants. Biol. Rev. 1983, 58, 179–207. [Google Scholar] [CrossRef]
- Emadian, S.F.; Newton, R.J. Growth Enhancement of Loblolly Pine (Pinus taeda L.) Seedlings by Silicon. J. Plant Physiol. 1989, 134, 98–103. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, R.R.; Benhamou, N.; Menzies, J.G. Cytological Evidence of an Active Role of Silicon in Wheat Resistance to Powdery Mildew (Blumeria graminis f. sp. tritici). Phytopathology 2003, 93, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Côté-Beaulieu, C.; Chain, F.; Menzies, J.G.; Kinrade, S.D.; Bélanger, R.R. Absorption of aqueous inorganic and organic silicon compounds by wheat and their effect on growth and powdery mildew control. Environ. Exp. Bot. 2009, 65, 155–161. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Qayyum, M.F.; Irshad, M.K. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2015, 119, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. Silicon in life: A bioinorganic solution to bioorganic essentiality. J. Inorg. Biochem. 1998, 69, 139–144. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.P.; Ma, G.R.; Shui, M.X.; Wan, D.F.; Li, R.H. Silicon isotope study on rice plants from the Zhejiang province, China. Chem. Geol. 2005, 218, 41–50. [Google Scholar] [CrossRef]
- Casey, W.H.; Kinrade, S.D.; Knight, C.T.G.; Rains, D.W.; Epstein, E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant. Cell Environ. 2004, 27, 51–54. [Google Scholar] [CrossRef]
- Ding, T.P.; Zhou, J.X.; Wan, D.F.; Chen, Z.Y.; Wang, C.Y.; Zhang, F. Silicon isotope fractionation in bamboo and its significance to the biogeochemical cycle of silicon. Geochim. Cosmochim. Acta 2008, 72, 1381–1395. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2011, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Mitani, N.; Yamaji, N.; Ma, J.F. HvLsi1 is a silicon influx transporter in barley. Plant J. 2009, 57, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ma, J.F. Identification of Maize Silicon Influx Transporters. Plant Cell Physiol. 2008, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A Transporter Regulating Silicon Distribution in Rice Shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Chiba, Y.; Mitani-Ueno, N.; Feng Ma, J. Functional Characterization of a Silicon Transporter Gene Implicated in Silicon Distribution in Barley. Plant Physiol. 2012, 160, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Currie, H.A.; Perry, C.C. Silica in plants: Biological, biochemical and chemical studies. Ann. Bot. 2007, 100, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Leishman, M.R. Is plant ecology more siliceous than we realise? Trends Plant Sci. 2011, 16, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Luxová, M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 437–441. [Google Scholar] [CrossRef]
- Keller, C.; Rizwan, M.; Davidian, J.-C.; Pokrovsky, O.S.; Bovet, N.; Chaurand, P.; Meunier, J.-D. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta 2015, 241, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Shakoor, S.A.; Bhat, M.A.; Mir, S.H. Phytoliths in plants: A review. Res. Rev. J. Bot. Sci. 2014, 3, 10–24. [Google Scholar]
- Hodson, M.J.; Sangster, A.G. Techniques for the microanalysis of higher plants with particular reference to silicon in cryofixed wheat tissues. Scanning Microsc. 1990, 4, 407–418. [Google Scholar]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Li, Z.; Song, Z.; Cornelis, J.-T. Impact of rice cultivar and organ on elemental composition of phytoliths and the release of bio-available silicon. Front. Plant Sci. 2014, 5, 529. [Google Scholar] [CrossRef] [PubMed]
- Balec, R.; Belanger, R.; Chapman, D.M.; Epstein, E.; Guevel, M.H.; Kinrade, S.D.; Knight, C.T.G.; Rains, D.W.; Terill, M.; Wang, J. Organosilicate chemistry: Evidence for a crosslinking role in plants. In III Silicon in Agriculture Conference. Ed Uberlândia; Federal University of Uberlandia: Uberlandia, Brazil, 2005; Volume 76. [Google Scholar]
- Currie, H.A.; Perry, C.C. Chemical evidence for intrinsic “Si” within Equisetum cell walls. Phytochemistry 2009, 70, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Inanaga, S.; Okasaka, A. Calcium and silicon binding compounds in cell walls of rice shoots. Soil Sci. Plant Nutr. 1995, 41, 103–110. [Google Scholar] [CrossRef]
- Fang, J.; Ma, X. In vitro simulation studies of silica deposition induced by lignin from rice. J. Zhejiang Univ. Sci. B 2006, 7, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Law, C.; Exley, C. New insight into silica deposition in horsetail (Equisetum arvense). BMC Plant Biol. 2011, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Fleck, A.T.; Schulze, S.; Hinrichs, M.; Specht, A.; Waßmann, F.; Schreiber, L.; Schenk, M.K. Silicon promotes exodermal Casparian band formation in Si-accumulating and Si-excluding species by forming phenol complexes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, L.; Liu, J.; Liu, X.; Li, X.; Ma, J.; Lin, Y.; Xu, F. Evidence for ‘silicon’within the cell walls of suspension-cultured rice cells. New Phytol. 2013, 200, 700–709. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Ma, J.; Wang, L. A hemicellulose-bound form of silicon with potential to improve the mechanical properties and regeneration of the cell wall of rice. New Phytol. 2015, 206, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Funakawa, H.; Miwa, K. Synthesis of borate cross-linked rhamnogalacturonan II. Front. Plant Sci. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding diatom cell wall silicification—Moving forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Otzen, D. The role of proteins in biosilicification. Scientifica (Cairo) 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, C.D.; Nielsen, F.H. Silicon deprivation decreases collagen formation in wounds and bone, and ornithine transaminase enzyme activity in liver. Biol. Trace Elem. Res. 2002, 89, 251–261. [Google Scholar] [CrossRef]
- Ratcliffe, S.; Jugdaohsingh, R.; Vivancos, J.; Marron, A.; Deshmukh, R.; Ma, J.F.; Mitani-Ueno, N.; Robertson, J.; Wills, J.; Boekschoten, M. V Identification of a mammalian silicon transporter. Am. J. Physiol. Physiol. 2017, 312, C550–C561. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc. Natl. Acad. Sci. USA 1973, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. Proceedings: Silicon as an essential element. In Federation Proceedings; Federation of American Societies for Experimental Biology: Bethesda, MA, USA, 1974; Volume 33, p. 1758. [Google Scholar]
- Carlisle, E.M. In vivo requirement for silicon in articular cartilage and connective tissue formation in the chick. J. Nutr. 1976, 106, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, E.M. A silicon requirement for normal skull formation in chicks. J. Nutr. 1980, 110, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Schröer, H.C.; Krasko, A.; Le Pennec, G.; Adell, T.; Wiens, M.; Hassanein, H.; Müller, I.M.; Müller, W.E.G. Silicase, an enzyme which degrades biogenous amorphous silica: Contribution to the metabolism of silica deposition in the demosponge Suberites domuncula. In Silicon Biomineralization; Springer: Berlin/Heidelberg, Germany, 2003; pp. 249–268. [Google Scholar]
- Shimizu, K.; Morse, D.E. Silicatein: A unique silica-synthesizing catalytic triad hydrolase from marine sponge skeletons and its multiple applications. In Methods in Enzymology; Elsevier: Cambridge, MA, USA, 2018; Volume 605, pp. 429–455. ISBN 0076-6879. [Google Scholar]
- Fegley, B. Carbon chemistry and organic compound synthesis in the solar nebula. Meteoritics 1987, 22, 378. [Google Scholar]
- Gladstone, G.R.; Towe, K.M.; Kasting, J. Photochemistry in the primitive solar nebula. Science (80-) 1993, 261, 1058–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanon, P.; Chaussidon, M.; Robert, F. The redox state of the solar nebula: C and H concentrations in chondrules. Meteorit. Planet. Sci. Suppl. 1996, 31, A57. [Google Scholar]
- Llorca, J. Gas-grain chemistry of carbon in interplanetary dust particles- Kinetics and mechanism of hydrocarbon formation. Lunar Planet. Sci. XXIX 1998, 29, 1119. [Google Scholar]
- Varela, M.E.; Metrich, N. Carbon in olivines of chondritic meteorites. Geochim. Cosmochim. Acta 2000, 64, 3433–3438. [Google Scholar] [CrossRef]
- McElroy, D.; Walsh, C.; Markwick, A.J.; Cordiner, M.A.; Smith, K.; Millar, T.J. The UMIST database for astrochemistry 2012. Astron. Astrophys. 2013, 550, A36. [Google Scholar] [CrossRef]
- Umeki, H.; Nakajima, M.; Endo, Y. Laboratory detections of SiC2N and SiC3N by Fourier transform microwave spectroscopy. J. Chem. Phys. 2014, 141, 184303. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; Gottlieb, C.A.; Thaddeus, P. Silicon molecules in space and in the laboratory. Mol. Phys. 2003, 101, 697–704. [Google Scholar] [CrossRef][Green Version]
- Cernicharo, J.; McCarthy, M.C.; Gottlieb, C.A.; Agúndez, M.; Prieto, L.V.; Baraban, J.H.; Changala, P.B.; Guélin, M.; Kahane, C.; Martin-Drumel, M.A. Discovery of SiCSi in IRC+ 10216: A missing link between gas and dust carriers of Si–C bonds. Astrophys. J. Lett. 2015, 806, L3. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Fonti, S.; Orofino, V. The 4.6 micron feature of–SiH groups in silicate dust grains and infrared cometary spectra. Planet. Space Sci. 1999, 47, 781–785. [Google Scholar] [CrossRef]
- Cernicharo, J.; Waters, L.; Decin, L.; Encrenaz, P.; Tielens, A.; Agúndez, M.; De Beck, E.; Müller, H.S.P.; Goicoechea, J.R.; Barlow, M.J. A high-resolution line survey of IRC+ 10216 with Herschel/HIFI-First results: Detection of warm silicon dicarbide (SiC). Astron. Astrophys. 2010, 521, L8. [Google Scholar] [CrossRef]
- Velilla Prieto, L.; Cernicharo, J.; Quintana-Lacaci, G.; Agúndez, M.; Castro-Carrizo, A.; Fonfría, J.P.; Marcelino, N.; Zúñiga, J.; Requena, A.; Bastida, A. Si-bearing Molecules Toward IRC+ 10216: ALMA Unveils the Molecular Envelope of CWLeo. Astrophys. J. 2015, 805. [Google Scholar] [CrossRef]
- Fonfría, J.P.; Cernicharo, J.; Richter, M.J.; Fernández-López, M.; Velilla Prieto, L.; Lacy, J.H. The abundance of 28Si32S, 29Si32S, 28Si34S, and 30Si32S in the inner layers of the envelope of IRC+ 10216. Mon. Not. R. Astron. Soc. 2015, 453, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Agúndez, M.; Fonfría, J.P.; Cernicharo, J.; Kahane, C.; Daniel, F.; Guélin, M. Molecular abundances in the inner layers of IRC+ 10216. Astron. Astrophys. 2012, 543, A48. [Google Scholar] [CrossRef]
- Guélin, M.; Muller, S.; Cernicharo, J.; McCarthy, M.C.; Thaddeus, P. Detection of the SiNC radical in IRC+ 10216. Astron. Astrophys. 2004, 426, L49–L52. [Google Scholar] [CrossRef]
- Guélin, M.; Muller, S.; Cernicharo, J.; Apponi, A.J.; McCarthy, M.C.; Gottlieb, C.A.; Thaddeus, P. Astronomical detection of the free radical SiCN. Astron. Astrophys. 2000, 363, L9–L12. [Google Scholar]
- Cernicharo, J.; Agúndez, M.; Prieto, L.V.; Guélin, M.; Pardo, J.R.; Kahane, C.; Marka, C.; Kramer, C.; Navarro, S.; Quintana-Lacaci, G. Discovery of methyl silane and confirmation of silyl cyanide in IRC+ 10216. Astron. Astrophys. 2017, 606, L5. [Google Scholar] [CrossRef] [PubMed]
- Leung, I.; Guo, W.; Friedman, I.; Gleason, J. Natural occurrence of silicon carbide in a diamondiferous kimberlite from Fuxian. Nature 1990, 346, 352. [Google Scholar] [CrossRef]
- Mathez, E.A.; Fogel, R.A.; Hutcheon, I.D.; Marshintsev, V.K. Carbon isotopic composition and origin of SiC from kimberlites of Yakutia, Russia. Geochim. Cosmochim. Acta 1995, 59, 781–791. [Google Scholar] [CrossRef]
- Bernatowicz, T.J.; Amari, S.; Lewis, R.S. Refractory carbides in interstellar graphite. In Proceedings of the Lunar and Planetary Science Conference, Houston, TX, USA, 14–18 March 1994; Lunar and Planetary Institute: Houston, TX, USA, 1994; Volume 25, p. 103. [Google Scholar]
- Lee, M.R.; Russell, S.S.; Arden, J.W.; Pillinger, C.T. The isotopic composition and mineralogy of silicon nitride (Si3N4) within ordinary and enstatite chondrites. Meteoritics 1992, 27, 248–249. [Google Scholar]
- Travaglio, C.; Gallino, R.; Amari, S.; Zinner, E.; Woosley, S.; Lewis, R.S. Low-density graphite grains and mixing in type II supernovae. Astrophys. J. 1999, 510, 325. [Google Scholar] [CrossRef]
- Hoppe, P.; Strebel, R.; Eberhardt, P.; Amari, S.; Lewis, R.S. Small SiC grains and a nitride grain of circumstellar origin from the Murchison meteorite: Implications for stellar evolution and nucleosynthesis. Geochim. Cosmochim. Acta 1996, 60, 883–907. [Google Scholar] [CrossRef]
- Ebel, D.S.; Grossman, L. Condensation from supernova gas made of free atoms1. Geochim. Cosmochim. Acta 2001, 65, 469–477. [Google Scholar] [CrossRef]
- Zbik, M.; Jasieniak, M.; SMART, R.S.C. Organosilane occurrence in irghizite samples from the Zhamanshin impact crater, Kazakhstan. Meteorit. Planet. Sci. 2000, 35, 943–947. [Google Scholar] [CrossRef]
- Baus, J.A.; Burschka, C.; Bertermann, R.; Guerra, C.F.; Bickelhaupt, F.M.; Tacke, R. Neutral six-coordinate and cationic five-coordinate silicon (iv) complexes with two bidentate monoanionic n, s-pyridine-2-thiolato (−) ligands. Inorg. Chem. 2013, 52, 10664–10676. [Google Scholar] [CrossRef] [PubMed]
- Kittel, C.; McEuen, P.; McEuen, P. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1996; Volume 8. [Google Scholar]
- Ashcroft, N.W.; Mermin, N.D. Solid state physics. Saunders Coll. Phila. 1976, 120, 1–848. [Google Scholar]
- Muthukumaran, N.; Velappan, K.; Gour, K.; Prabusankar, G. N-heterocyclic carbene supported halosilylenes: New frontiers in an emerging field. Coord. Chem. Rev. 2018, 377, 1–43. [Google Scholar] [CrossRef]
- Abersfelder, K.; White, A.J.P.; Rzepa, H.S.; Scheschkewitz, D. A tricyclic aromatic isomer of hexasilabenzene. Science (80-) 2010, 327, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, A.; Kinjo, R.; Ichinohe, M. A stable compound containing a silicon-silicon triple bond. Science (80-) 2004, 305, 1755–1757. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Guo, J.-D.; Hashizume, D.; Sasamori, T.; Nagase, S.; Tokitoh, N. The selective formation of a 1, 2-disilabenzene from the reaction of a disilyne with phenylacetylene. Dalt. Trans. 2018, 47, 13318–13322. [Google Scholar] [CrossRef] [PubMed]
- Han, J.S.; Sasamori, T.; Mizuhata, Y.; Tokitoh, N. Reactivity of an aryl-substituted silicon–silicon triple bond: 1, 2-disilabenzenes from the reactions of a 1, 2-diaryldisilyne with alkynes. Dalt. Trans. 2010, 39, 9238–9240. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, R.; Ichinohe, M.; Sekiguchi, A.; Takagi, N.; Sumimoto, M.; Nagase, S. Reactivity of a Disilyne RSi≍ SiR (R=Si i Pr [CH (SiMe3) 2] 2) toward π-Bonds: Stereospecific Addition and a New Route to an Isolable 1, 2-Disilabenzene. J. Am. Chem. Soc. 2007, 129, 7766–7767. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Seager, S.; Petkowski, J.J.; Sousa-Silva, C.; Ranjan, S.; Huang, J.; Bains, W. Assessment of Isoprene as a Possible Biosignature Gas in Exoplanets with Anoxic Atmospheres. Astrobiology 2020. in review. [Google Scholar]
- Blom, B.; Driess, M. Functional Molecular Silicon Compounds II; Springer: Berlin, Germany, 2014. [Google Scholar]
- Maxka, J.; Huang, L.M.; West, R. Synthesis and NMR spectroscopy of permethylpolysilane oligomers Me (SiMe2) 10Me, Me (SiMe2) 16Me, and Me (Me2Si) 22Me. Organometallics 1991, 10, 656–659. [Google Scholar] [CrossRef]
- Fujino, M. Photoconductivity in organopolysilanes. Chem. Phys. Lett. 1987, 136, 451–453. [Google Scholar] [CrossRef]
- Koe, J.; Fujiki, M. Chapter 6—Polysilanes. In Organosilicon Compounds; Lee, V.Y., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 219–300. ISBN 978-0-12-814213-4. [Google Scholar]
- Dahn, J.R.; Way, B.M.; Fuller, E.; Tse, J.S. Structure of siloxene and layered polysilane (Si 6 H 6). Phys. Rev. B 1993, 48, 17872. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.G.; Ando, W.; Chojnowski, J. Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 9401139393. [Google Scholar]
- Ranjan, S.; Wordsworth, R.; Sasselov, D.D. The surface UV environment on planets orbiting M dwarfs: Implications for prebiotic chemistry and the need for experimental follow-up. Astrophys. J. 2017, 843, 110. [Google Scholar] [CrossRef]
- Bond, J.C.; O’Brien, D.P.; Lauretta, D.S. The compositional diversity of extrasolar terrestrial planets. I. In situ simulations. Astrophys. J. 2010, 715, 1050. [Google Scholar] [CrossRef]
- Öberg, K.I.; Murray-Clay, R.; Bergin, E.A. The effects of snowlines on C/O in planetary atmospheres. Astrophys. J. Lett. 2011, 743, L16. [Google Scholar] [CrossRef]
- Booth, R.A.; Clarke, C.J.; Madhusudhan, N.; Ilee, J.D. Chemical enrichment of giant planets and discs due to pebble drift. Mon. Not. R. Astron. Soc. 2017, 469, 3994–4011. [Google Scholar] [CrossRef]
- Fortney, J.J. On the carbon-to-oxygen ratio measurement in nearby Sun-like stars: Implications for planet formation and the determination of stellar abundances. Astrophys. J. Lett. 2012, 747, L27. [Google Scholar] [CrossRef]
- Whitehouse, L.J.; Farihi, J.; Green, P.J.; Wilson, T.G.; Subasavage, J.P. Dwarf carbon stars are likely metal-poor binaries and unlikely hosts to carbon planets. Mon. Not. R. Astron. Soc. 2018, 479, 3873–3878. [Google Scholar] [CrossRef]
- Nissen, P.E. The carbon-to-oxygen ratio in stars with planets. Astron. Astrophys. 2013, 552, A73. [Google Scholar] [CrossRef]
- Bergfors, C.; Farihi, J. Do C/O> 1 main-sequence stars build carbon planets. In AAS/Division for Extreme Solar Systems Abstracts; American Astronomical Society: Washington, DC, USA, 2015; Volume 3. [Google Scholar]
- Wilson, D.J.; Gänsicke, B.T.; Farihi, J.; Koester, D. Carbon to oxygen ratios in extrasolar planetesimals. Mon. Not. R. Astron. Soc. 2016, 459, 3282–3286. [Google Scholar] [CrossRef]
- Madhusudhan, N.; Mousis, O.; Johnson, T.V.; Lunine, J.I. Carbon-rich giant planets: Atmospheric chemistry, thermal inversions, spectra, and formation conditions. Astrophys. J. 2011, 743, 191. [Google Scholar] [CrossRef]
- Kidokoro, Y.; Umemoto, K.; Hirose, K.; Ohishi, Y. Phase transition in SiC from zinc-blende to rock-salt structure and implications for carbon-rich extrasolar planets. Am. Mineral. 2017, 102, 2230–2234. [Google Scholar] [CrossRef]
- Miozzi, F.; Morard, G.; Antonangeli, D.; Clark, A.N.; Dorn, C.; Antoine, R.; Mezouar, M.; Baron, M.A.; Pakhomova, A.; Fiquet, G. An experimental approach to investigate carbon rich exoplanets interior. In Proceedings of the European Planetary Science Congress, Berlin, Germany, 16–21 September 2018; Volume 12. [Google Scholar]
- Miozzi, F.; Morard, G.; Antonangeli, D.; Clark, A.N.; Mezouar, M.; Dorn, C.; Rozel, A.; Fiquet, G. Equation of State of SiC at Extreme Conditions: New Insight Into the Interior of Carbon-Rich Exoplanets. J. Geophys. Res. Planets 2018, 123, 2295–2309. [Google Scholar] [CrossRef]
- Lodders, K.; Fegley Jr, B. Condensation chemistry of carbon stars. In AIP Conference Proceedings; American Institute of Physics: Melville, NY, USA, 1997; Volume 402, pp. 391–423. [Google Scholar]
- Wilson, H.F.; Militzer, B. Interior phase transformations and mass-radius relationships of silicon-carbon planets. Astrophys. J. 2014, 793, 34. [Google Scholar] [CrossRef]
- Futó, P.; Gucsik, A. Basic Mineralogical Models for Silicate-and Carbon-Rich Mega-Earths Considering Compositional and Geophysical Constraints. In Proceedings of the Lunar and Planetary Science Conference, The Woodlands, TX, USA, 9–23 March 2018; Lunar and Planetary Institute: Houston, TX, USA, 2018; Volume 49. [Google Scholar]
- Hakim, K.; Spaargaren, R.; Grewal, D.S.; Rohrbach, A.; Berndt, J.; Dominik, C.; Van Westrenen, W. Mineralogy, Structure, and Habitability of Carbon-Enriched Rocky Exoplanets: A Laboratory Approach. Astrobiology 2019, 19, 867–884. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, P.B.; Rugheimer, S. Hydrogen cyanide in nitrogen-rich atmospheres of rocky exoplanets. Icarus 2019, 329, 124–131. [Google Scholar] [CrossRef]
- Rase, H.F. Handbook of Commercial Catalysts: Heterogeneous Catalysts; CRC Press: Boca Raton, FL, USA, 2000; ISBN 1420036548. [Google Scholar]
- Singh, S.K.; Parida, K.M.; Mohanty, B.C.; Rao, S.B. High surface area silicon carbide from rice husk: A support material for catalysts. React. Kinet. Catal. Lett. 1995, 54, 29–34. [Google Scholar] [CrossRef]
- Bosque, R.; Sales, J. Polarizabilities of solvents from the chemical composition. J. Chem. Inf. Comput. Sci. 2002, 42, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.C. The estimation of solubility parameters and related properties of liquids. J. Chem. Technol. Biotechnol. Chem. Technol. 1984, 34, 38–42. [Google Scholar] [CrossRef]
- Abraham, M.H.; Platts, J.A. Hydrogen bond structural group constants. J. Org. Chem. 2001, 66, 3484–3491. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Gordon, M.S.; Schmidt, M.W. Advances in electronic structure theory: GAMESS a decade later. In Theory and Applications of Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1167–1189. [Google Scholar]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; International Union of Crystal: Oxford, UK, 2001; Volume 9, ISBN 0198509707. [Google Scholar]
- Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 2009, 11, 1757–1788. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).