Rat Animal Model of Pectus Excavatum
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animals
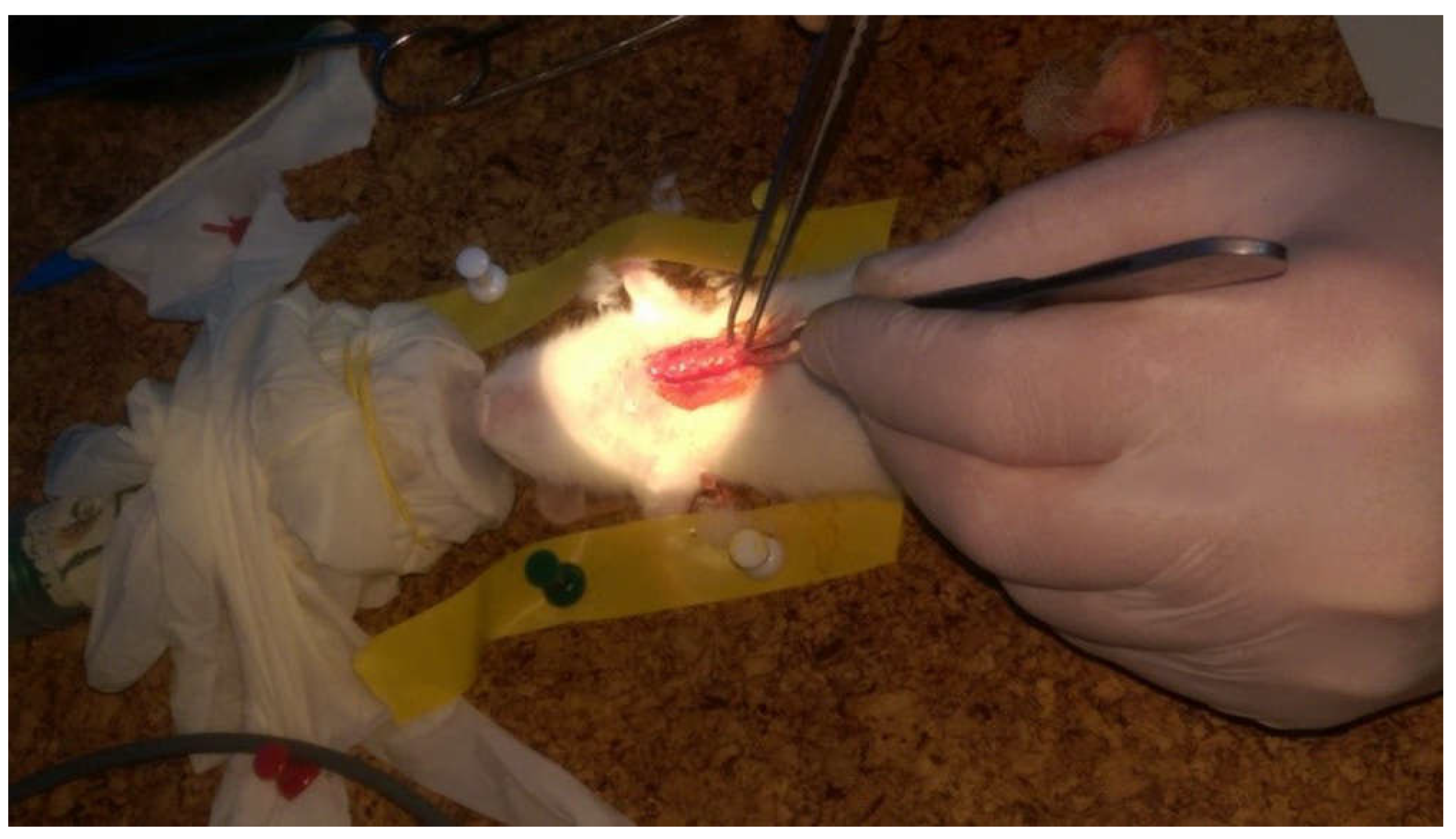
2.2. Surgical Procedure
2.3. CT Scan, Assessment
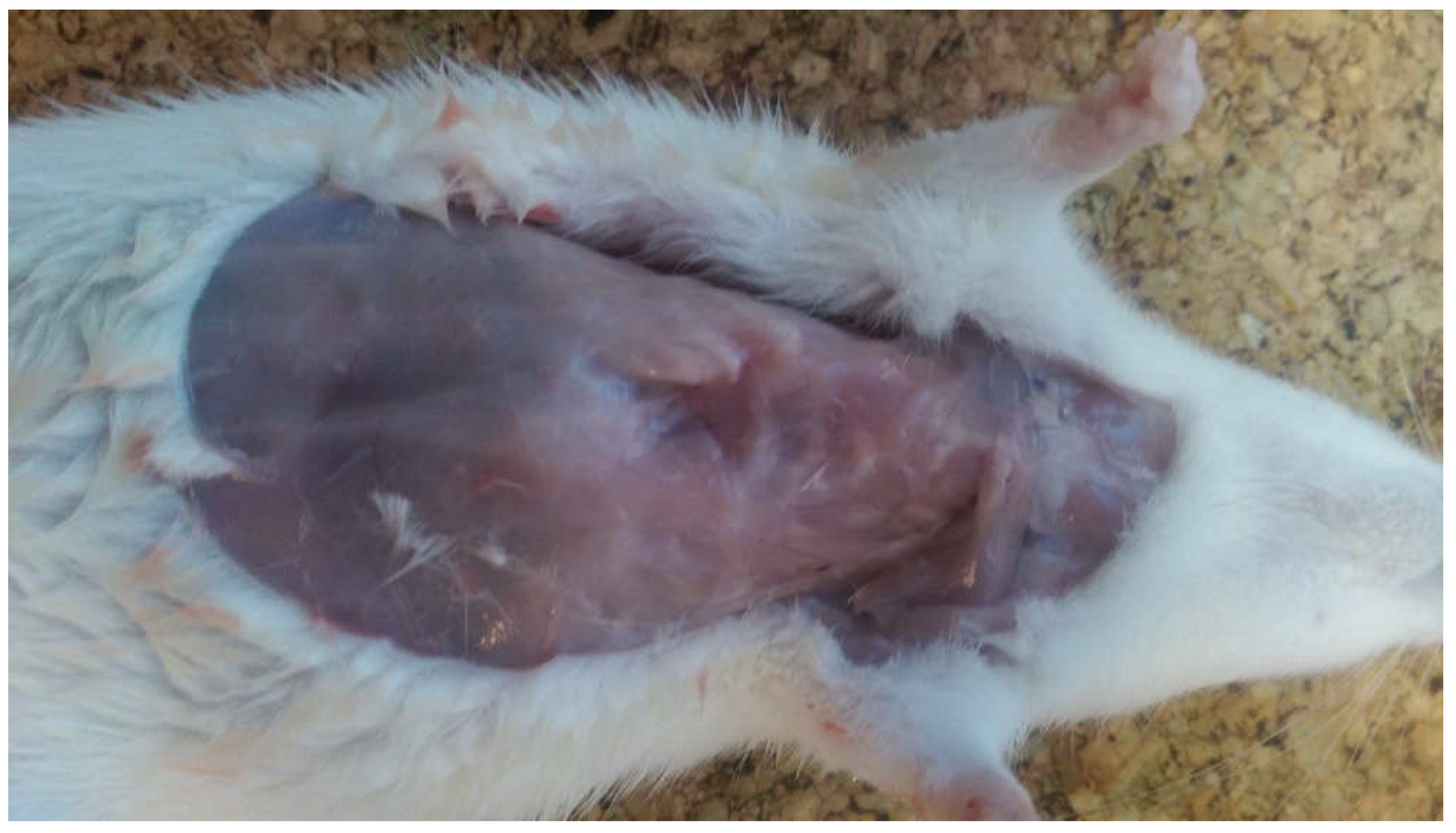
2.4. Direct Assessment and Measurements of the Thoracic Cage
2.5. Statistical Analysis
3. Results
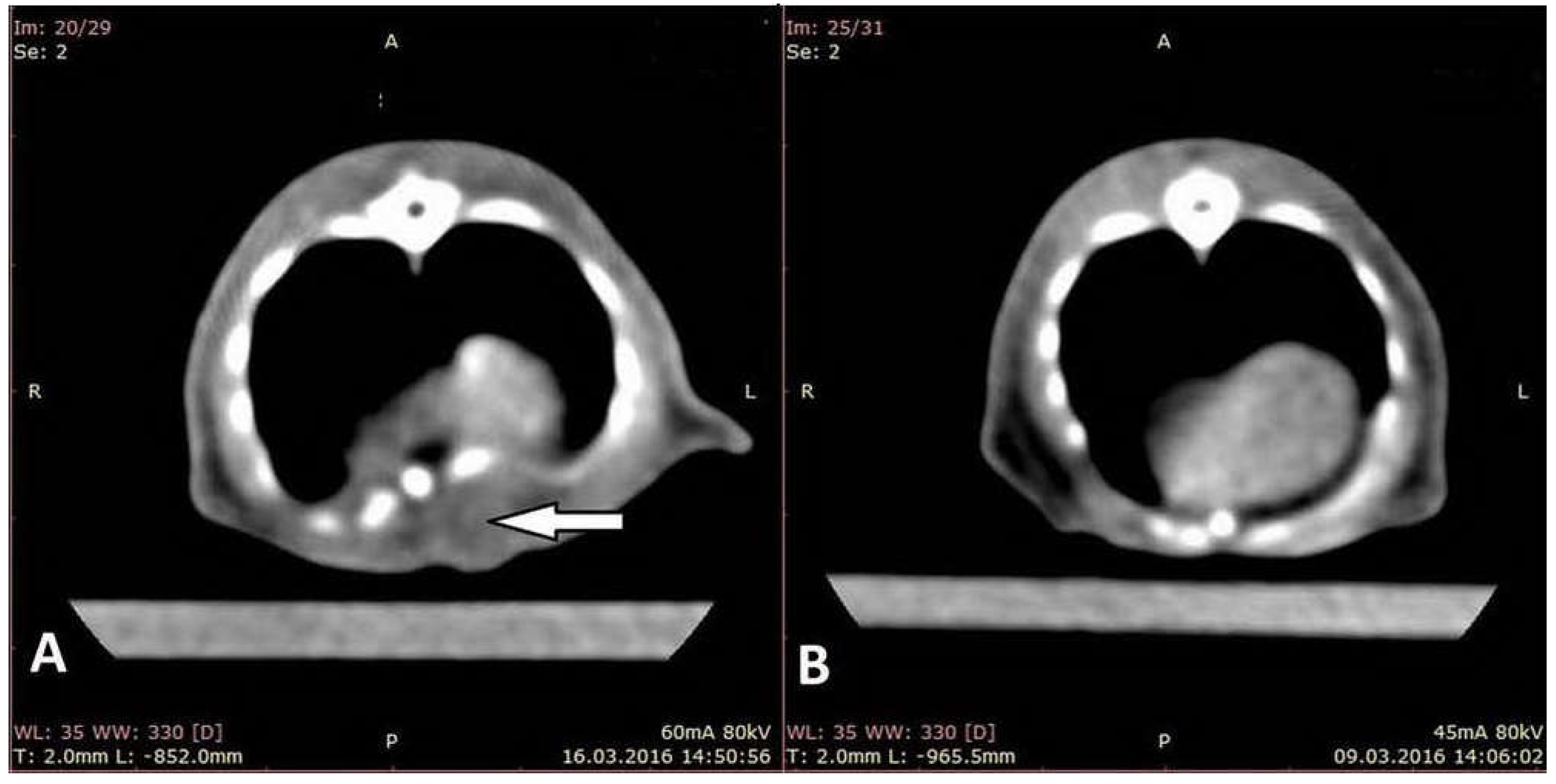
3.1. Assessment on CT Images
3.2. Direct Assessment and Measurements of the Thoracic Cage
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kelly, R.E., Jr. Pectus excavatum: Historical background, clinical picture, preoperative evaluation and criteria for operation. Semin. Pediatr. Surg. 2008, 17, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Nuss, D.; Obermeyer, R.J.; Kelly, R.E., Jr. Pectus excavatum from a pediatric surgeon’s perspective. Ann. Cardiothorac. Surg. 2016, 5, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Nicodin, A.; Boia, E.S.; Popoiu, M.C.; Cozma, G.; Nicodin, G.; Badeti, R.; Trailescu, M.; Adam, O.; David, V.L. Preliminary results after Nuss procedure. Chirurgia 2010, 105, 203–210. [Google Scholar] [PubMed]
- Goretsky, M.J.; Kelly, R.E., Jr.; Croitoru, D.; Nuss, D. Chest wall anomalies: Pectus excavatum and pectus carinatum. Adolesc. Med. Clin. 2004, 15, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, H.; Freiberger, N. Light microscopic studies of the cartilage in funnel chest. A new view of the pathogenesis. Z. Fur Exp. Chir. Transplant. und Kunstl. Organe Organ der Sekt. Exp. Chir. Ges. Chir. DDR 1989, 22, 314–318. [Google Scholar]
- Kurkov, A.V.; Shekhter, A.B.; Paukov, V.S. Costal cartilage structural and functional changes in children with a funnel or keeled chest. Arkhiv. Patol. 2017, 79, 57. [Google Scholar] [CrossRef]
- A Tsvetkova, T.; A Kozlov, E.; Rudakov, S.S.; A Del’Vig, A. Extractability of collagen from the rib cartilage and skin in funnel chest in children. Vopr. Meditsinskoi Khimii 1988, 34, 71–74. [Google Scholar]
- Kuritsyn, V.M.; Shabanov, A.M.; Shekhonin, B.V.; Rukosuev, V.S.; Rudakov, S.S. Pathohistology of costal cartilage and immunomorphologic characteristics of collagen in unnel chest. Arkhiv Patol. 1987, 49, 20. [Google Scholar]
- Feng, J.; Hu, T.; Liu, W.; Zhang, S.; Tang, Y.; Chen, R.; Jiang, X.; Wei, F. The biomechanical, morphologic, and histochemical properties of the costal cartilages in children with pectus excavatum. J. Pediatr. Surg. 2001, 36, 1770–1776. [Google Scholar] [CrossRef]
- David, V.L.; Izvernariu, D.A.; Popoiu, C.M.; Puiu, M.; Boia, E.S. Morphologic, morphometrical and histochemical proprieties of the costal cartilage in children with pectus excavatum. Romanian J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2011, 52, 625–629. [Google Scholar]
- Kurkov, A.V.; Paukov, V.S.; Fayzullin, A.L.; Shekhter, A.B. Costal cartilage changes in children with pectus excavatum and pectus carinatum. Arkhiv Patol. 2018, 80, 8–15. [Google Scholar] [CrossRef]
- Asmar, A.; Semenov, I.; Kelly, R.; Stacey, M. Abnormal response of costal chondrocytes to acidosis in patients with chest wall deformity. Exp. Mol. Pathol. 2019, 106, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Komsta, R.; Osiński, Z.; Dębiak, P.; Twardowski, P.; Lisiak, B. Prevalence of pectus excavatum (PE), pectus carinatum (PC), tracheal hypoplasia, thoracic spine deformities and lateral heart displacement in thoracic radiographs of screw-tailed brachycephalic dogs. PLoS ONE 2019, 14, e0223642. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-Y.; Mann, F.; Jeong, S.-W. Surgical correction of pectus excavatum in two cats. J. Vet. Sci. 2008, 9, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Gifford, A.T.; Flanders, J.A. External splinting for treatment of pectus excavatum in a dog with right ventricular outflow obstruction. J. Vet. Cardiol. 2010, 12, 53–57. [Google Scholar] [CrossRef]
- Risselada, M.; De Rooster, H.; Liuti, T.; Polis, I.; Van Bree, H. Use of internal splinting to realign a noncompliant sternum in a cat with pectus excavatum. J. Am. Vet. Med. Assoc. 2006, 228, 1047–1052. [Google Scholar] [CrossRef]
- Karner, C.M.; Long, F.; Solnica-Kreze, L.; Monk, K.R.; Gray, R.S. Gpr126/Adgrg6 Deletion in Cartilage Models Idiopathic Scoliosis and Pectus Excavatum in Mice. Hum. Mol. Genet. 2015, 24, 4365–4373. [Google Scholar] [CrossRef]
- Geisbe, H.; Buddecke, E.; Flach, A.; Müller, G.; Stein, U. 88. Biochemical, morphological and physical as well as animal experimental studies on the pathogenesis of funnel chest. Langenbeck’s Archiv. Surg. 1967, 319, 536–541. [Google Scholar] [CrossRef]
- Wang, R.; Yang, W.; Cai, L.; Yang, J.; Xiang, X.; Zhang, C.; Jiang, Q.; Yang, Y.; Chen, Z. Establishment of a rabbit model of pectus excavatum. Int. J. Clin. Exp. Med. 2017, 10, 6429–6436. [Google Scholar]
- Hoinoiu, B.; Jiga, L.P.; Nistor, A.; Dornean, V.; Barac, S.; Miclaus, G.; Ionac, M.; Hoinoiu, T. Chronic Hindlimb Ischemia Assessment; Quantitative Evaluation Using Laser Doppler in a Rodent Model of Surgically Induced Peripheral Arterial Occlusion. Diagnosis 2019, 9, 139. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Cook, O. Cardio-Respiratory Studies in Pre and Post Operative Funnel Chest (Pectus Excavatum). Dis. Chest 1951, 20, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Giem, R.N.; Paulsen, G.A.; Dykes, J. Pectus deformities. Calif. Med. 1961, 94, 306–309. [Google Scholar] [PubMed]
- Hubrecht, R.C.; Kirkwood, J. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
| CT1 (Preoperative) | CT2 (7 Days) | CT3 (14 Days) | CT4 (21 Days) | CT5 (28 Days) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CG | EG | CG | EG | CG | EG | CG | EG | CG | EG | |
| AD (mm) | 29.12 ± 1.08 | 28.24 ± 2.03 | 31.76 ± 1.95 | 31.50 ± 1.79 | 35.62 ± 0.56 | 36.27 ± 1.00 | 38.80 ± 0.84 | 38.38 ± 1.24 | 40.56 ± 1.00 | 40.08 ± 1.54 |
| p = 0.39 | p = 0.80 | p = 0.20 | p = 0.51 | p = 0.55 | ||||||
| SSD (mm) | 4.77 ± 0.14 | 4.95 ± 0.29 | 5.09 ± 0.24 | 5.24 ± 0.28 | 5.54 ± 0.18 | 5.65 ± 0.35 | 5.77 ± 0.27 | 6.12 ± 0.24 | 6.00 ± 0.22 | 6.71 ± 0.42 |
| p = 0.24 | p = 0.33 | p = 0.53 | p = 0.35 | p = 0.01 | ||||||
| SD4 (mm) | 8.63 ± 0.44 | 8.57 ± 0.48 | 10.61 ± 0.28 | 10.93 ± 0.90 | 12.04 ± 0.45 | 11.08 ± 0.78 | 12.72 ± 0.64 | 12.72 ± 0.92 | 13.92 ± 0.63 | 13.76 ± 0.65 |
| p = 0.81 | p = 0.47 | p = 0.02 | p = 0.99 | p = 0.67 | ||||||
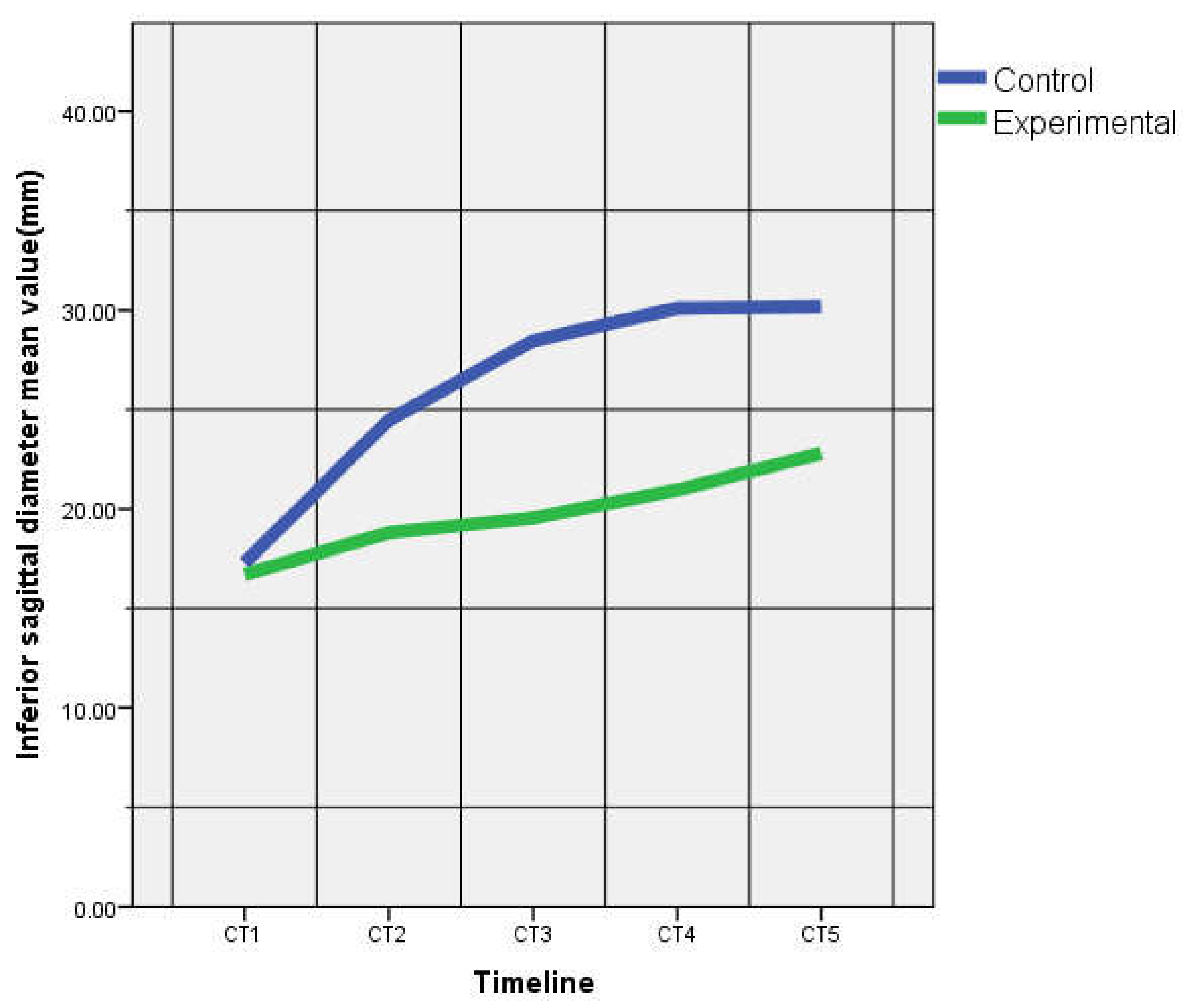
| ISD (mm) | 17.28 ± 0.58 | 16.71 ± 1.16 | 24.48 ± 0.74 | 18.80 ± 3.14 | 28.46 ± 2.54 | 19.54 ± 2.00 | 30.10 ± 0.45 | 20.95 ± 2.25 | 30.18 ± 0.57 | 22.78 ± 2.12 |
| p = 0.33 | p = 0.02 | p = 0.00 | p = 0.00 | p = 0.00 | ||||||
| STD (mm) | 6.92 ± 0.12 | 6.84 ± 0.22 | 7.12 ± 0.09 | 7.52 ± 0.37 | 7.30 ± 0.21 | 7.76 ± 0.56 | 7.92 ± 0.14 | 8.29 ± 0.42 | 8.54 ± 0.37 | 8.45 ± 0.54 |
| p = 0.373 | p = 0.03 | p = 0.10 | p = 0.08 | p = 0.73 | ||||||
| TD4 (mm) | 15.12 ± 0.68 | 14.52 ± 0.90 | 17.56 ± 0.40 | 17.52 ± 0.99 | 19.96 ± 0.38 | 19.30 ± 1.30 | 21.36 ± 0.48 | 20.72 ± 0.87 | 22.64 ± 0.34 | 22.18 ± 0.80 |
| p =0.19 | p = 0.92 | p = 0.29 | p = 0.13 | p = 0.34 | ||||||
| ITD (mm) | 24.82 ± 0.30 | 24.13 ± 1.32 | 28.94 ± 0.70 | 27.65 ± 1.61 | 31.16 ± 0.38 | 29.63 ± 1.20 | 32.66 ± 0.92 | 30.35 ± 1.50 | 32.98 ± 0.32 | 31.95 ± 1.21 |
| p = 0.28 | p = 0.12 | p = 0.19 | p = 0.01 | p = 0.09 | ||||||
| CT1 (Preoperative) | CT2 (7 Days Postop) | CT3 (14 Days Postop) | CT4 (21 Days Postop) | CT5 (28 Days Postop) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CG | EG | CG | EG | CG | EG | CG | EG | CG | EG | |
| SHI | 1.45 ± 0.02 | 1.38 ± 0.03 | 1.40 ± 0.08 | 1.44 ± 0.12 | 1.31 ± 0.08 | 1.38 ± 0.17 | 1.37 ± 0.07 | 1.35 ± 0.07 | 1.42 ± 0.09 | 1.26 ± 0.11 |
| p = 0.03 | p = 0.49 | p = 0.38 | p = 0.68 | p = 0.02 | ||||||
| HI4 | 1.75 ± 0.07 | 1.61 ± 0.13 | 1.65 ± 0.07 | 1.61 ± 0.14 | 1.65 ± 0.07 | 1.74 ± 0.14 | 1.68 ± 0.07 | 1.63 ± 0.12 | 1.62 ± 0.03 | 1.61 ± 0.08 |
| p = 0.22 | p = 0.52 | p = 0.16 | p = 0.41 | p = 0.70 | ||||||
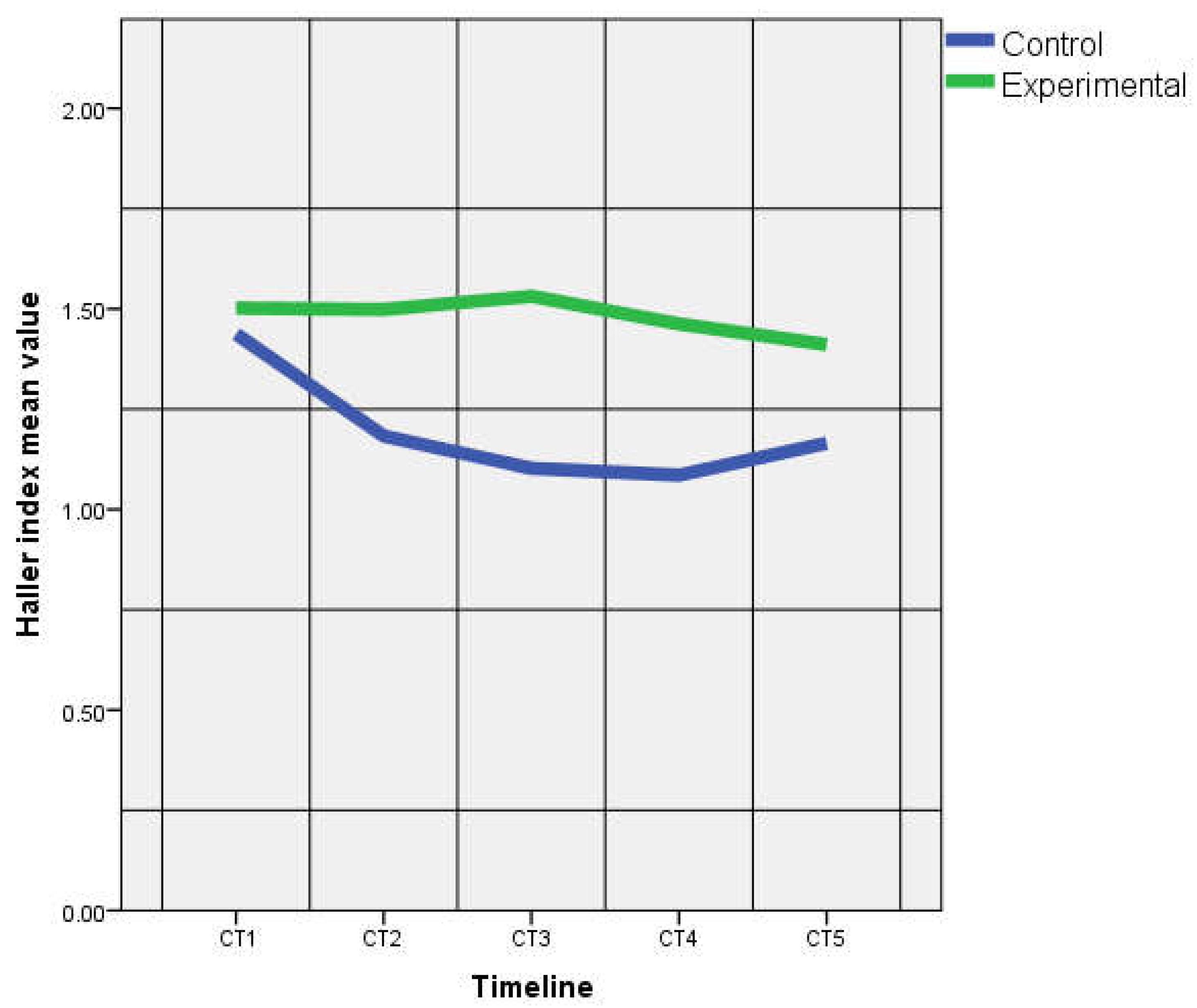
| IHI | 1.43 ± 0.03 | 1.50 ± 0.10 | 1.18 ± 0.04 | 1.49 ± 0.23 | 1.10 ± 0.10 | 1.49 ± 0.23 | 1.08 ± 0.04 | 1.46 ± 0.15 | 1.16 ± 0.10 | 1.41 ± 0.11 |
| p = 0.20 | p = 0.03 | p = 0.00 | p = 0.00 | p = 0.04 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, V.-L.; Ciornei, B.; Horhat, F.-G.; Amaricai, E.; Horhat, I.-D.; Hoinoiu, T.; Boia, E.-S. Rat Animal Model of Pectus Excavatum. Life 2020, 10, 96. https://doi.org/10.3390/life10060096
David V-L, Ciornei B, Horhat F-G, Amaricai E, Horhat I-D, Hoinoiu T, Boia E-S. Rat Animal Model of Pectus Excavatum. Life. 2020; 10(6):96. https://doi.org/10.3390/life10060096
Chicago/Turabian StyleDavid, Vlad-Laurentiu, Bogdan Ciornei, Florin-George Horhat, Elena Amaricai, Ioana-Delia Horhat, Teodora Hoinoiu, and Eugen-Sorin Boia. 2020. "Rat Animal Model of Pectus Excavatum" Life 10, no. 6: 96. https://doi.org/10.3390/life10060096
APA StyleDavid, V.-L., Ciornei, B., Horhat, F.-G., Amaricai, E., Horhat, I.-D., Hoinoiu, T., & Boia, E.-S. (2020). Rat Animal Model of Pectus Excavatum. Life, 10(6), 96. https://doi.org/10.3390/life10060096







