High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Plasmids
2.3. Transfection of HACs
2.4. Ribonucleoprotein Immunoprecipitation, mRNA–miRNA Isolation and miRNA Reverse Tanscription (RT), and Real-Time PCR (qPCR)
2.5. mRNA-Seq Library Construction, Sequencing, and Analysis
2.6. mRNA Reverse Transcription (RT) and Real-Time PCR (qPCR)
2.7. Western Blotting
2.8. Statistical Analysis
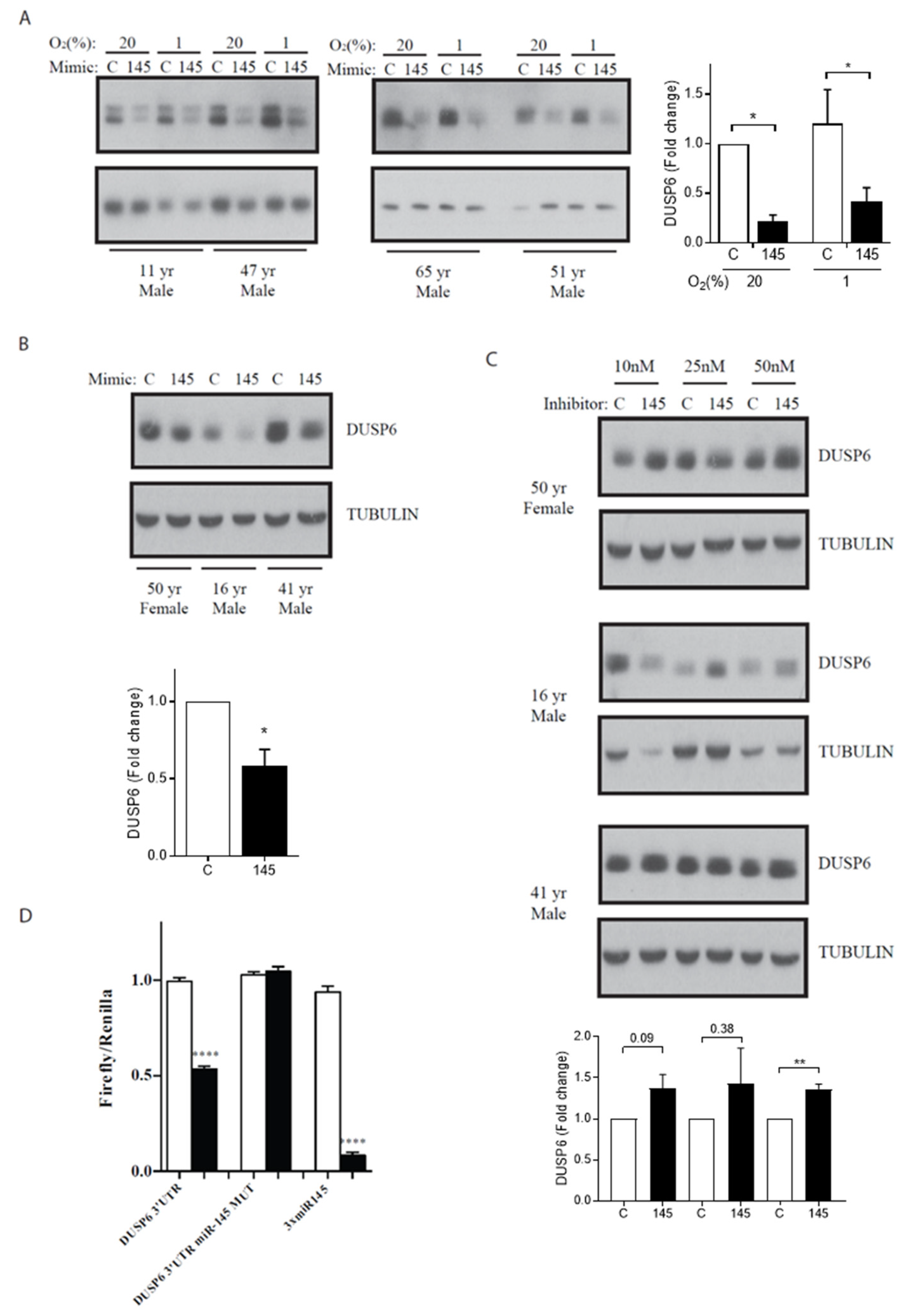
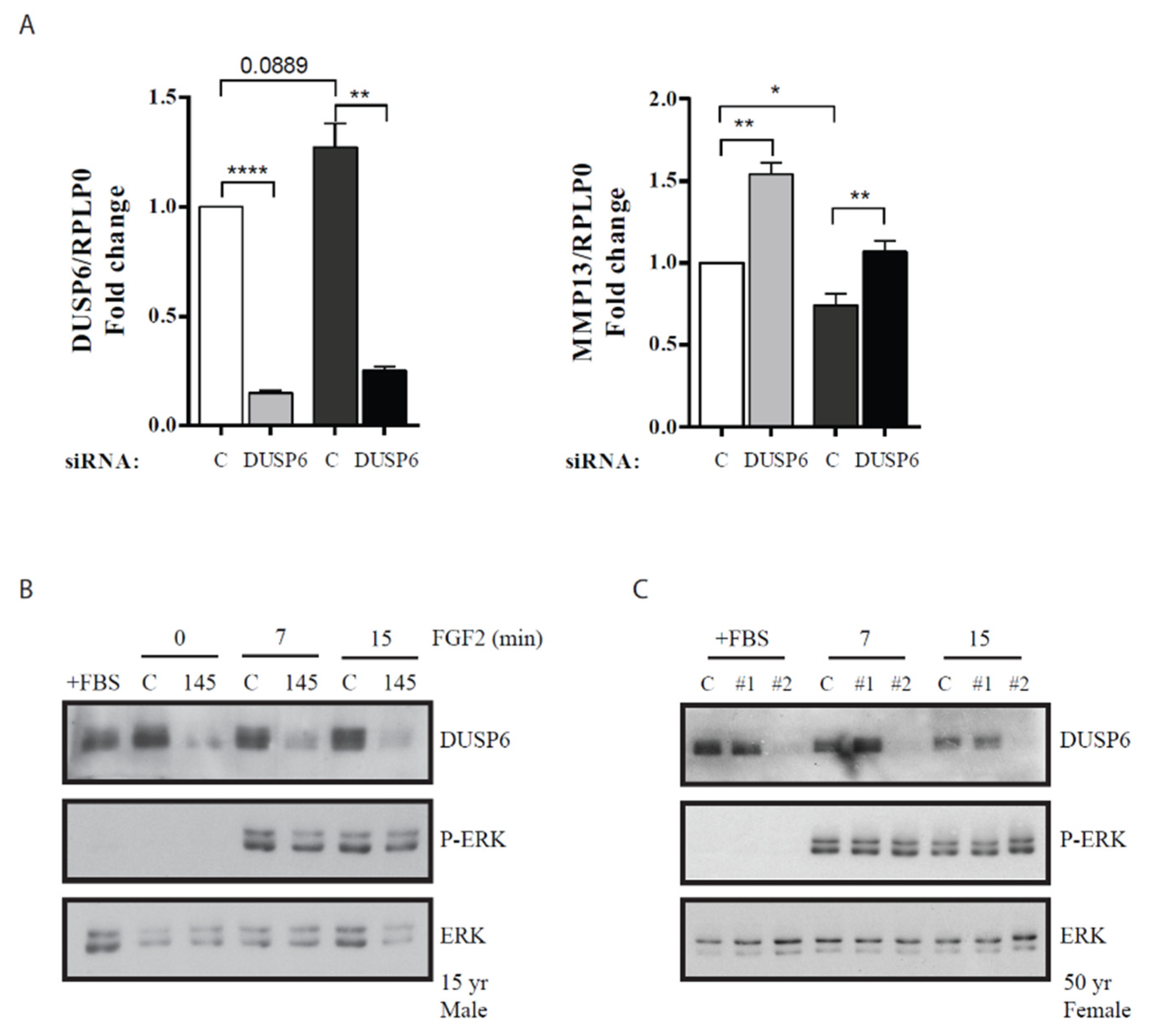
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Billinghurst, R.C.; Dahlberg, L.; Ionescu, M.; Reiner, A.; Bourne, R.; Rorabeck, C.; Mitchell, P.; Hambor, J.; Diekmann, O.; Tschesche, H.; et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Investig. 1997, 99, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of Inflammation, and the Immune System in the Progression of Osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Wang, X.; Manner, P.A.; Horner, A.; Shum, L.; Tuan, R.S.; Nuckolls, G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. 2004, 12, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, K.L.; Wang, J. Cell-based articular cartilage repair: The link between development and regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef]
- Colombini, A.; Perucca Orfei, C.; Kouroupis, D.; Ragni, E.; De Luca, P.; Vigano, M.; Correa, D.; de Girolamo, L. Mesenchymal stem cells in the treatment of articular cartilage degeneration: New biological insights for an old-timer cell. Cytotherapy 2019, 21, 1179–1197. [Google Scholar] [CrossRef]
- Bartel, D.P. microRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, 68–73. [Google Scholar] [CrossRef]
- Sun, K.; Lai, E.C. Adult-specific functions of animal microRNAs. Nat. Rev. Genet. 2013, 14, 535–548. [Google Scholar] [CrossRef]
- Emde, A.; Hornstein, E. miRNAs at the interface of cellular stress and disease. EMBO J. 2014, 33, 1428–1437. [Google Scholar] [CrossRef]
- Kloosterman, W.P.; Plasterk, R.H. The diverse functions of microRNAs in animal development and disease. Dev. Cell 2006, 11, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Miyaki, S.; Sato, T.; Inoue, A.; Otsuki, S.; Ito, Y.; Yokoyama, S.; Kato, Y.; Takemoto, F.; Nakasa, T.; Yamashita, S.; et al. microRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010, 24, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Swingler, T.E.; Wheeler, G.; Carmont, V.; Elliott, H.R.; Barter, M.J.; Abu-Elmagd, M.; Donell, S.T.; Boot-Handford, R.P.; Hajihosseini, M.K.; Münsterberg, A.; et al. The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 2012, 64, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Liu, H.; Zhou, Y. Roles of microRNAs in prenatal chondrogenesis, postnatal chondrogenesis and cartilage-related diseases. J. Cell Mol. Med. 2013, 17, 1515–1524. [Google Scholar] [CrossRef]
- Martinez-Sanchez, A.; Dudek, K.A.; Murphy, C.L. Regulation of human chondrocyte function through direct inhibition of cartilage master regulator SOX9 by microRNA-145 (miRNA-145). J. Biol. Chem. 2012, 287, 916–924. [Google Scholar] [CrossRef]
- Yang, B.; Kang, X.; Xing, Y.; Dou, C.; Kang, F.; Li, J.; Quan, Y.; Dong, S. Effect of microRNA-145 on IL-1β-induced cartilage degradation in human chondrocytes. FEBS Lett. 2014, 588, 2344–2352. [Google Scholar] [CrossRef]
- Hamzeiy, H.; Allmer, J.; Yousef, M. Computational methods for microRNA target prediction. Methods Mol. Biol. 2014, 1107, 207–221. [Google Scholar]
- Martinez-Sanchez, A.; Murphy, C.L. MicroRNA Target Identification-Experimental Approaches. Biology 2013, 2, 189–205. [Google Scholar] [CrossRef]
- Baroni, T.E.; Chittur, S.V.; George, A.D.; Tenenbaum, S.A. Advances in RIP-chip analysis: RNA-binding protein immunoprecipitation-microarray profiling. Methods Mol. Biol. 2008, 419, 93–108. [Google Scholar]
- Tan, L.P.; Seinen, E.; Duns, G.; de Jong, D.; Sibon, O.C.; Poppema, S.; Kroesen, B.J.; Kok, K.; van den Berg, A. A high throughput experimental approach to identify miRNA targets in human cells. Nucleic Acids Res. 2009, 37, e137. [Google Scholar] [CrossRef]
- Keene, J.D.; Komisarow, J.M.; Friedersdorf, M.B. RIP-Chip: The isolation and identification of mRNAs, microRNAs, and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006, 1, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, A.; Murphy, C.L. miR-1247 functions by targeting cartilage transcription factor SOX9. J. Biol. Chem. 2013, 288, 30802–30814. [Google Scholar] [CrossRef]
- Lamble, S.; Batty, E.; Attar, M.; Buck, D.; Bowden, R.; Lunter, G.; Crook, D.; El-Fahmawi, B.; Piazza, P. Improved workflows for high throughput library preparation using the transposome-based Nextera system. BMC Biotechnol. 2013, 13, 104. [Google Scholar] [CrossRef]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq-a Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; McCarthy, D.J.; Chen, Y.; Okoniewski, M.; Smyth, G.K.; Huber, W.; Robinson, M.D. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 2013, 8, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Rasmussen, S.H.; Jacobsen, A.; Krogh, A. cWords-systematic microRNA regulatory motif discovery from mRNA expression data. Silence 2013, 4, 2. [Google Scholar] [CrossRef]
- Lal, A.; Navarro, F.; Maher, C.A.; Maliszewski, L.E.; Yan, N.; O’Day, E.; Chowdhury, D.; Dykxhoorn, D.M.; Tsai, P.; Hofmann, O.; et al. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3’UTR microRNA recognition elements. Mol. Cell 2009, 35, 610–625. [Google Scholar] [CrossRef]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M., Jr.; Jungkamp, A.C.; Munschauer, M.; et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Djuranovic, S.; Nahvi, A.; Green, R. miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 2012, 336, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Uejima, A.; Amano, T.; Nomura, N.; Noro, M.; Yasue, T.; Shiroishi, T.; Ohta, K.; Yokoyama, H.; Tamura, K. Anterior shift in gene expression precedes anteriormost digit formation in amniote limbs. Dev. Growth Differ. 2010, 52, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.W.; Dominguez-Steglich, M.A.; Guioli, S.; Kwok, C.; Weller, P.A.; Stevanović, M.; Weissenbach, J.; Mansour, S.; Young, I.D.; Goodfellow, P.N. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 1994, 372, 525–530. [Google Scholar] [CrossRef]
- Li, C.; Scott, D.A.; Hatch, E.; Tian, X.; Mansour, S.L. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 2007, 134, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Chubinskaya, S.; Pacione, C.; Im, H.J. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum. 2005, 52, 3910–3917. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.G.; Kim, H.J.; Park, H.J.; Kim, Y.J.; Yoon, W.J.; Lee, S.J.; Ryoo, H.M.; Cho, J.Y. Runx2 phosphorylation induced by fibroblast growth factor 2/protein kinase C pathways. Proteomics 2006, 6, 1166–1174. [Google Scholar] [CrossRef]
- Yan, D.; Chen, D.; Im, H.J. Fibroblast growth factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis that coordinates with the PKCδ pathway in human articular chondrocytes. J. Cell Biochem. 2012, 113, 2856–2865. [Google Scholar] [CrossRef]
- Im, H.J.; Muddasani, P.; Natarajan, V.; Schmid, T.M.; Block, J.A.; Davis, F.; van Wijnen, A.J.; Loeser, R.F. Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. J. Biol. Chem. 2007, 282, 11110–11121. [Google Scholar] [CrossRef]
- Otsuki, S.; Hanson, S.R.; Miyaki, S.; Grogan, S.P.; Kinoshita, M.; Asahara, H.; Wong, C.H.; Lotz, M.K. Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 10202–10207. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Halvey, P.J.; Shyr, Y.; Slebos, R.J.; Liebler, D.C.; Zhang, B. Integrative omics analysis reveals the importance and scope of translational repression in microRNA-mediated regulation. Mol. Cell Proteomics 2013, 12, 1900–1911. [Google Scholar] [CrossRef]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N. The mechanics of miRNA-mediated gene silencing: A look under the hood of miRISC. Nat. Struct Mol. Biol. 2012, 19, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wagner, E.J.; Cullen, B.R. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 2002, 9, 1327–1333. [Google Scholar] [CrossRef]
- Kundu, P.; Fabian, M.R.; Sonenberg, N.; Bhattacharyya, S.N.; Filipowicz, W. HuR protein attenuates miRNA-mediated repression by promoting miRISC dissociation from the target RNA. Nucleic Acids Res. 2012, 40, 5088–5100. [Google Scholar] [CrossRef]
- Easow, G.; Teleman, A.A.; Cohen, S.M. Isolation of microRNA targets by miRNP immunopurification. RNA 2007, 13, 1198–1204. [Google Scholar] [CrossRef]
- Chi, S.W.; Hannon, G.J.; Darnell, R.B. An alternative mode of microRNA target recognition. Nat. Struct Mol. Biol. 2012, 19, 321–327. [Google Scholar] [CrossRef]
- Seok, H.; Ham, J.; Jang, E.S.; Chi, S.W. MicroRNA Target Recognition: Insights from Transcriptome-Wide Non-Canonical Interactions. Mol. Cells 2016, 39, 375–381. [Google Scholar]
- Penalva, L.O.; Tenenbaum, S.A.; Keene, J.D. Gene expression analysis of messenger RNP complexes. Methods Mol. Biol. 2004, 257, 125–134. [Google Scholar] [PubMed]
- Penalva, L.O.; Burdick, M.D.; Lin, S.M.; Sutterluety, H.; Keene, J.D. RNA-binding proteins to assess gene expression states of co-cultivated cells in response to tumor cells. Mol. Cancer 2004, 3, 24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lyle, A.N.; Deshpande, N.N.; Taniyama, Y.; Seidel-Rogol, B.; Pounkova, L.; Du, P.; Papaharalambus, C.; Lassègue, B.; Griendling, K.K. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ. Res. 2009, 105, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Grange, L.; Nguyen, M.V.; Lardy, B.; Derouazi, M.; Campion, Y.; Trocme, C.; Paclet, M.H.; Gaudin, P.; Morel, F. NAD(P)H oxidase activity of Nox4 in chondrocytes is both inducible and involved in collagenase expression. Antioxid. Redox Signal. 2006, 8, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.; Shao, P.; Weis, M.A.; Steinmann, B. The kyphoscoliotic type of Ehlers-Danlos syndrome (type VI): Differential effects on the hydroxylation of lysine in collagens I and II revealed by analysis of cross-linked telopeptides from urine. Mol. Genet. Metab. 2002, 76, 211–216. [Google Scholar] [CrossRef]
- Trojanowska, M. Ets factors and regulation of the extracellular matrix. Oncogene 2000, 19, 6464–6471. [Google Scholar] [CrossRef]
- Sgouras, D.N.; Athanasiou, M.A.; Beal, G.J.; Fisher, R.J.; Blair, D.G.; Mavrothalassitis, G.J. ERF: An ETS domain protein with strong transcriptional repressor activity, can suppress ets-associated tumorigenesis and is regulated by phosphorylation during cell cycle and mitogenic stimulation. EMBO J. 1995, 14, 4781–4793. [Google Scholar] [CrossRef]
- Wong, M.; Kireeva, M.L.; Kolesnikova, T.V.; Lau, L.F. Cyr61, product of a growth factor-inducible immediate-early gene, regulates chondrogenesis in mouse limb bud mesenchymal cells. Dev. Biol. 1997, 192, 492–508. [Google Scholar] [CrossRef]
- Elia, L.; Quintavalle, M.; Zhang, J.; Contu, R.; Cossu, L.; Latronico, M.V.; Peterson, K.L.; Indolfi, C.; Catalucci, D.; Chen, J.; et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: Correlates with human disease. Cell Death Differ. 2009, 16, 1590–1598. [Google Scholar] [CrossRef]
- Koutsis, G.; Siasos, G.; Spengos, K. The emerging role of microRNA in stroke. Curr. Top. Med. Chem. 2013, 13, 1573–1588. [Google Scholar] [CrossRef]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A therapeutic triad in atherosclerosis? Arterioscler Thromb Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Bian, C.; Yang, Z.; Bo, Y.; Li, J.; Zeng, L.; Zhou, H.; Zhao, R.C. miR-145 inhibits breast cancer cell growth through RTKN. Int. J. Oncol. 2009, 34, 1461–1466. [Google Scholar] [PubMed]
- Wang, F.; Xia, J.; Wang, N.; Zong, H. miR-145 inhibits proliferation and invasion of esophageal squamous cell carcinoma in part by targeting c-Myc. Onkologie 2013, 36, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, L.H.; Jacobsen, A.B.; Frankel, L.B.; Wen, J.; Krogh, A.; Lund, A.H. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS ONE 2010, 5, e8836. [Google Scholar] [CrossRef] [PubMed]
- Dynoodt, P.; Speeckaert, R.; De Wever, O.; Chevolet, I.; Brochez, L.; Lambert, J.; Van Gele, M. miR-145 overexpression suppresses the migration and invasion of metastatic melanoma cells. Int. J. Oncol. 2013, 42, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Enokida, H.; Yoshino, H.; Itesako, T.; Chiyomaru, T.; Kinoshita, T.; Fuse, M.; Nishikawa, R.; Goto, Y.; Naya, Y.; et al. The tumor-suppressive microRNA-143/145 cluster inhibits cell migration and invasion by targeting GOLM1 in prostate cancer. J. Hum. Genet. 2014, 59, 78–87. [Google Scholar] [CrossRef]
- Endisha, H.; Rockel, J.; Jurisica, I.; Kapoor, M. The complex landscape of microRNAs in articular cartilage: Biology, pathology, and therapeutic targets. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Si, H.B.; Yang, T.M.; Li, L.; Tian, M.; Zhou, L.; Li, D.P.; Huang, Q.; Kang, P.D.; Yang, J.; Zhou, Z.K.; et al. miR-140 Attenuates the Progression of Early-Stage Osteoarthritis by Retarding Chondrocyte Senescence. Mol. Ther. Nucleic Acids 2020, 19, 15–30. [Google Scholar] [CrossRef]
- Seidl, C.I.; Martinez-Sanchez, A.; Murphy, C.L. Derepression of MicroRNA-138 Contributes to Loss of the Human Articular Chondrocyte Phenotype. Arthritis Rheumatol. 2016, 68, 398–409. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Guo, X.Q.; Qi, L.; Yang, J.; Wang, Y.; Wang, C.; Li, Z.M.; Li, L.; Qu, Y.; Wang, D.; Han, Z.M. Salidroside accelerates fracture healing through cell-autonomous and non-autonomous effects on osteoblasts. Cell Tissue Res. 2017, 367, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Liu, W.; Wang, W. Salidroside protects ATDC5 cells against lipopolysaccharide-induced injury through up-regulation of microRNA-145 in osteoarthritis. Int. Immunopharmacol 2019, 67, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, S.; Yamada, N.; Kumazaki, M.; Yasui, Y.; Iwasaki, J.; Naito, S.; Akao, Y. socs7, a target gene of microRNA-145, regulates interferon-β induction through STAT3 nuclear translocation in bladder cancer cells. Cell Death Dis. 2013, 4, e482. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Lu, D.; Peng, W.; Wang, T.; Zhang, Y.; Lin, H. Differential expression profiling of spleen microRNAs in response to two distinct type II interferons in Tetraodon nigroviridis. PLoS ONE 2014, 9, e96336. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, B.; Zhang, Z.; Xu, D.; Ma, G. DUSP6 Inhibitor (E/Z)-BCI Hydrochloride Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Murine Macrophage Cells via Activating the Nrf2 Signaling Axis and Inhibiting the NF-κB Pathway. Inflammation 2019, 42, 672–681. [Google Scholar] [CrossRef]
- Bennett, A.M. DUSPs, twists and turns in the Journey to Vascular Inflammation. FEBS J. 2018, 285, 1589–1592. [Google Scholar] [CrossRef]
- Bonneau, E.; Neveu, B.; Kostantin, E.; Tsongalis, G.J.; De Guire, V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. EJIFCC 2019, 30, 114–127. [Google Scholar]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef]
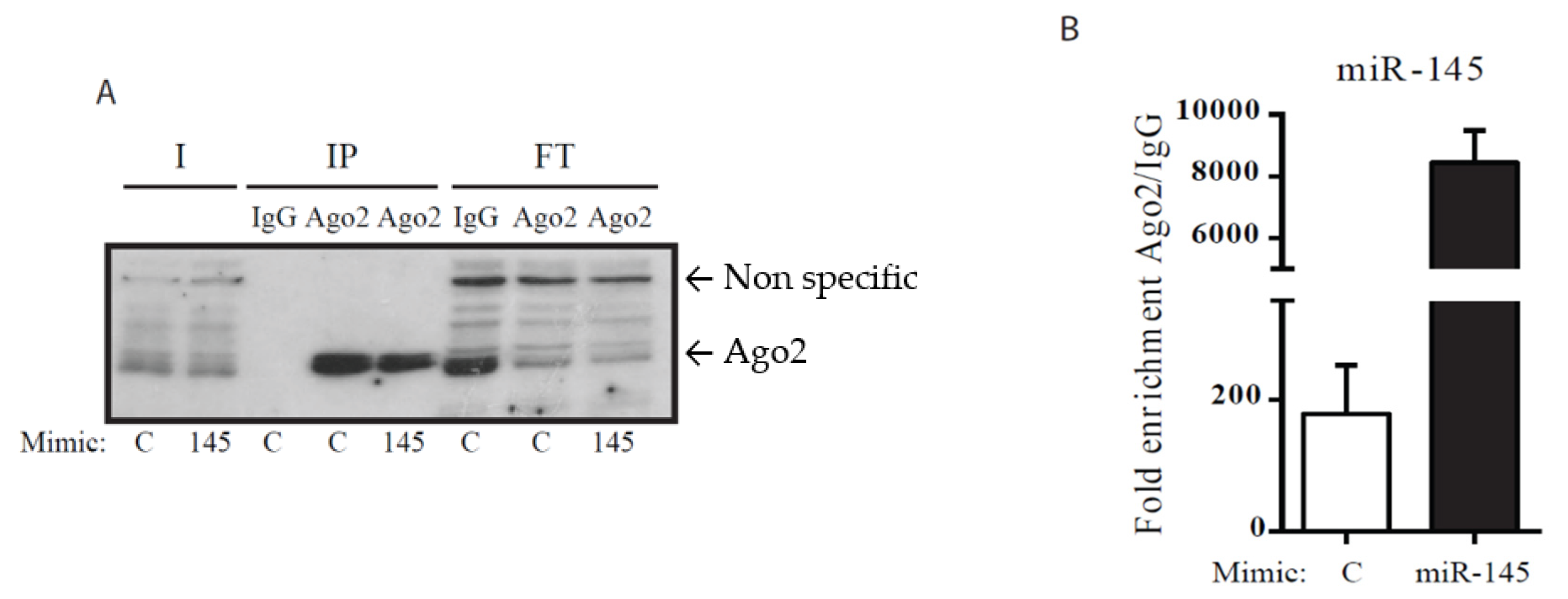
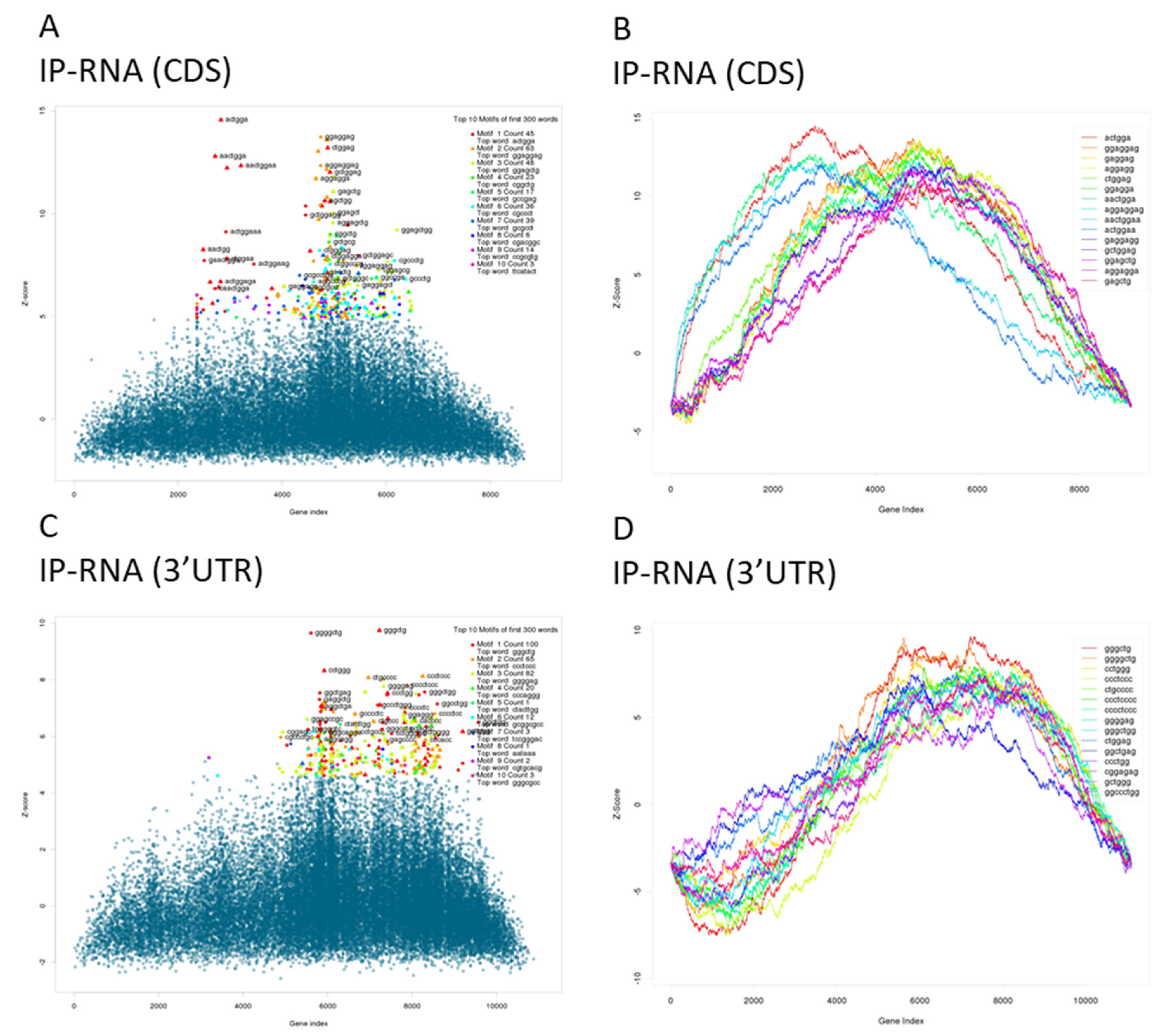
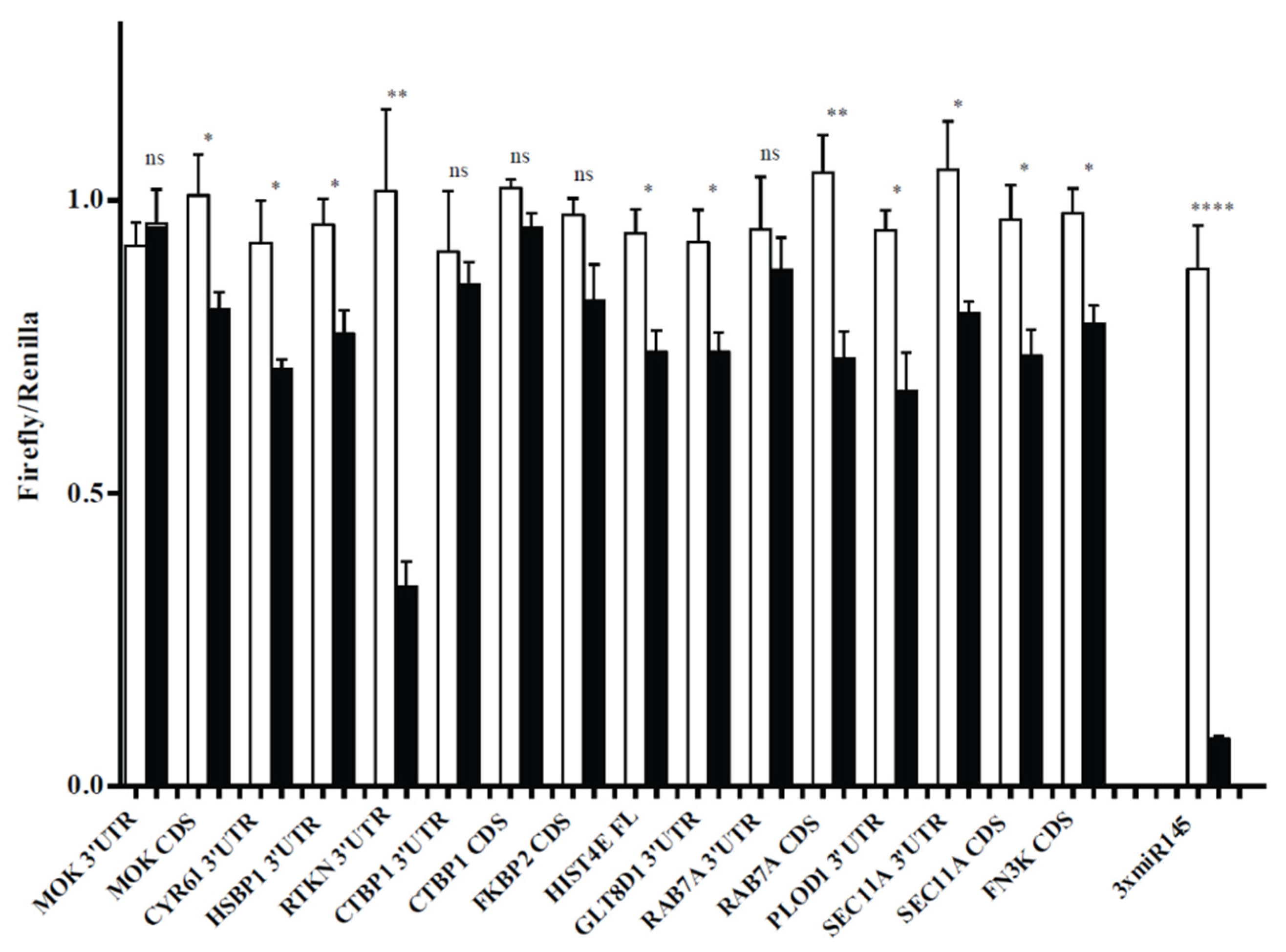
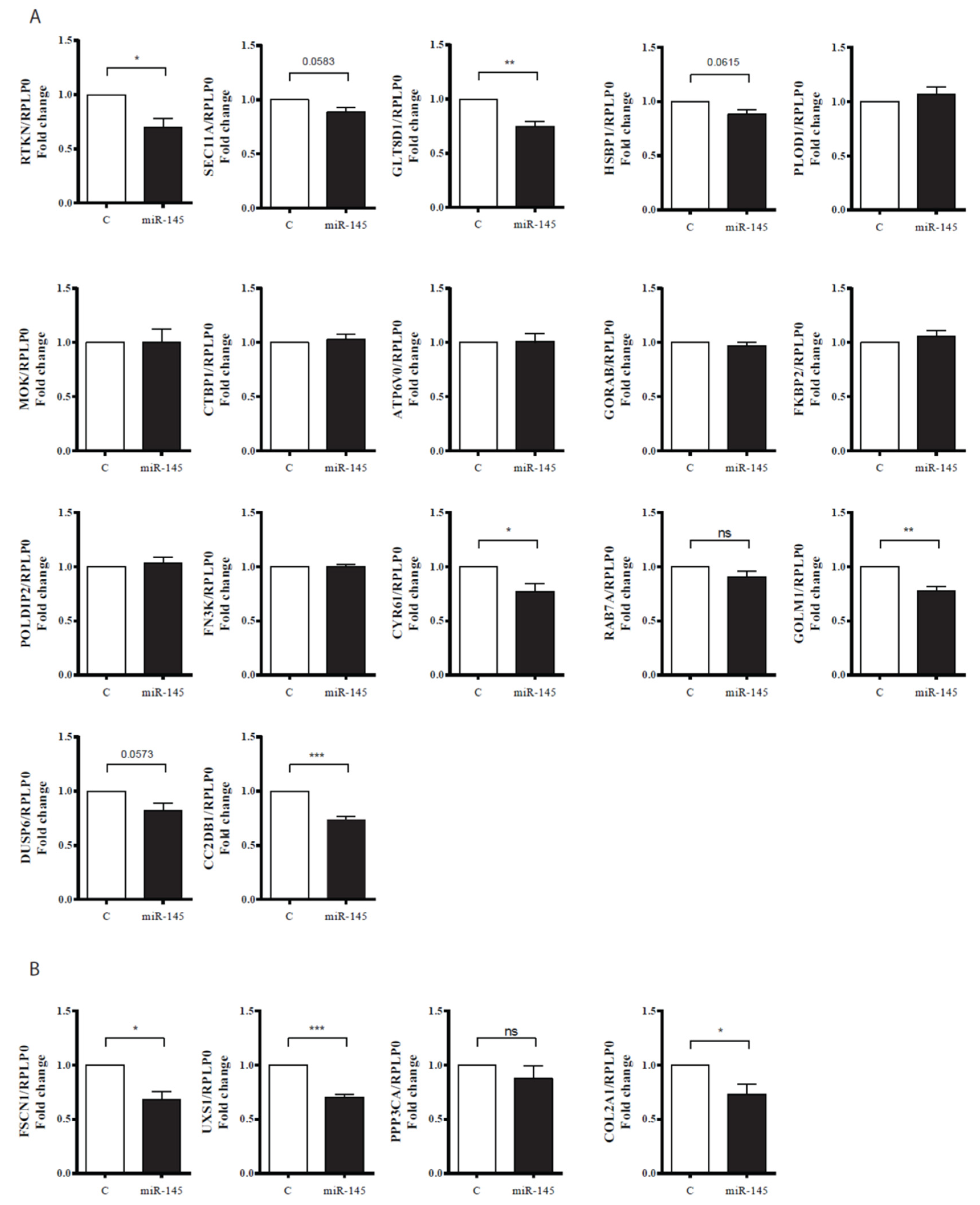
| Gene | LogFC | FDR |
|---|---|---|
| RPL3L | −2.31149 | 0.018536 |
| RP11-365D23.4 | −2.16109 | 0.02382 |
| FSCN1 * | −1.44751 | 1.52 × 10−10 |
| HIST1H4L | −1.39957 | 0.013354 |
| RP11-498C9.15 | −1.39914 | 0.007266 |
| STOX2 | −1.3254 | 0.034993 |
| RP11-649A18.7 | −1.02532 | 0.024884 |
| PKP1 | −1.00064 | 0.032246 |
| ABRACL | −0.98904 | 4.36 × 10−7 |
| CXCL6 | −0.95445 | 0.012477 |
| RP11-166P13.3 | −0.93763 | 0.024801 |
| CTC-448F2.1 | −0.88035 | 0.000899 |
| MALAT1 | −0.85554 | 0.00094 |
| EID2B | −0.84762 | 0.02382 |
| DTD1 | −0.81024 | 0.000533 |
| ADPGK | −0.78387 | 0.002089 |
| CLCN2 | −0.7662 | 0.017386 |
| AC145291.1 | −0.75286 | 0.010585 |
| RP11-620J15.3 | −0.75057 | 0.000152 |
| FAM108C1 * | −0.72829 | 0.011192 |
| CXCL1 | −0.72783 | 0.010556 |
| TMOD2 | −0.72433 | 0.011264 |
| SERPINE1 | −0.72202 | 0.00137 |
| TMEM9B | −0.72166 | 0.000757 |
| USP46 | −0.71885 | 0.004563 |
| GCSH | −0.70966 | 0.001641 |
| NT5DC2 | −0.70882 | 0.001507 |
| GMFB * | −0.68607 | 0.004577 |
| AL132709.8 | −0.68606 | 0.008869 |
| HOMER2 | −0.68387 | 0.001653 |
| DANCR | −0.68207 | 0.015835 |
| CTC-459F4.2 | −0.68075 | 0.034195 |
| ZMYND10 | −0.6622 | 0.039097 |
| MTRNR2L10 | −0.65594 | 0.028528 |
| CCDC43 * | −0.65181 | 0.004142 |
| RPL13AP25 | −0.65105 | 0.039418 |
| AKR1B1 | −0.65088 | 0.001828 |
| SNHG9 | −0.64141 | 0.01704 |
| BAALC | −0.64103 | 0.011259 |
| UXS1 | −0.63656 | 0.002733 |
| AC005035.1 | −0.63071 | 0.025323 |
| PIGF * | −0.6181 | 0.045224 |
| VASN | −0.61722 | 0.000629 |
| RP1-278E11.3 | −0.60875 | 0.004343 |
| AKR1B10 | −0.60848 | 0.049377 |
| ATP13A2 | −0.60351 | 0.002099 |
| SNX24 | −0.5985 | 0.001368 |
| ZNRD1 | −0.59494 | 0.014698 |
| AL132709.5 | −0.5915 | 0.001592 |
| GPRC5C | −0.58401 | 0.048364 |
| C15orf59 | −0.58256 | 0.014698 |
| GCLM | −0.55711 | 0.008631 |
| CC2D1B ** | −0.55688 | 0.012839 |
| PCBP4 | −0.5459 | 0.037638 |
| CTB-63M22.1 | −0.53644 | 0.008388 |
| PPP3CA | −0.53575 | 0.007346 |
| PMEPA1 | −0.5323 | 0.028545 |
| TRIM65 | −0.52934 | 0.04805 |
| FBF1 | −0.52885 | 0.039097 |
| G0S2 | −0.52582 | 0.045454 |
| FAM45A | −0.52388 | 0.016243 |
| RPL22L1 | −0.51543 | 0.02124 |
| MAP3K11 | −0.51431 | 0.017156 |
| RPS3AP5 | −0.49364 | 0.009962 |
| MT1E | −0.48805 | 0.02457 |
| PSAT1 | −0.48716 | 0.01541 |
| SMO | −0.48585 | 0.049462 |
| SWI5 | −0.48225 | 0.028528 |
| H2AFX | −0.47418 | 0.01541 |
| PAFAH1B2 | −0.46644 | 0.037335 |
| DTYMK | −0.46583 | 0.042746 |
| RP11-543P15.1 | −0.4624 | 0.027114 |
| IMP3 * | −0.462 | 0.04281 |
| RPS28 | −0.46182 | 0.013354 |
| RP11-20O24.4 | −0.44976 | 0.034761 |
| RP1-292B18.1 | −0.44362 | 0.041162 |
| RPS2P55 | −0.43723 | 0.037335 |
| RP11-864N7.2 | −0.43695 | 0.040095 |
| RPL9 | −0.43489 | 0.044893 |
| C7orf73 * | −0.42472 | 0.042746 |
| Gene | LogFC | FDR | TargetScan | RNAhybrid (3’UTR) | RNAhybrid (CDS) |
|---|---|---|---|---|---|
| COA6 (C1orf31) | 4.783081 | 4.8 × 10−6 | + | ||
| EXOG | 3.41819 | 9.44 × 10−5 | |||
| HIST1H4E | 3.404645 | 4.49 × 10−19 | |||
| RP11-539I5.1 | 2.924915 | 0.0196 | |||
| HSBP1 | 2.435244 | 3.97 × 10−9 | + | ||
| HIRIP3 | 2.303426 | 0.005801 | + | ||
| DCAF13 | 2.288777 | 2.13 × 10−6 | |||
| BANF1 | 2.250307 | 3.08 × 10−12 | + | ||
| RAB7A | 2.223753 | 5.22 × 10−5 | + | ||
| TMEM223 | 2.20113 | 0.007146 | + | ||
| FN3K | 2.177953 | 0.001289 | + | ||
| SEC11A | 2.040026 | 9.75 × 10−10 | + | + | |
| MOK | 2.005743 | 0.000539 | + | + | |
| TALDO1 | 1.989063 | 1.19 × 10−9 | + | ||
| ULBP3 | 1.963432 | 0.032833 | + | ||
| ARHGEF10L | 1.93225 | 2.92 × 10−8 | + | + | |
| CYR61 | 1.868992 | 1.77 × 10−6 | + | + | |
| TMOD3 | 1.818792 | 0.001511 | + | + | |
| GORAB | 1.803528 | 0.030719 | + | + | |
| C6orf106 | 1.772683 | 1.55 × 10−7 | + | ||
| SUPT16H | 1.764385 | 0.004327 | |||
| DECR1 | 1.743822 | 0.029152 | |||
| CC2D1B* | 1.743593 | 3.69 × 10−6 | + | + | + |
| FARSA | 1.737866 | 5.11 × 10−7 | + | ||
| TPI1P1 | 1.723506 | 5.57 × 10−7 | |||
| GLT8D1 | 1.698869 | 0.000477 | + | ||
| MRPL51 | 1.659022 | 1.95 × 10−5 | + | ||
| ATP6V0B | 1.653234 | 0.000656 | + | + | |
| FAM96A | 1.632677 | 0.023186 | |||
| FKBP2 | 1.60429 | 0.001637 | + | ||
| SCAND1 | 1.593582 | 0.014036 | |||
| IFFO1 | 1.551748 | 0.001511 | + | + | |
| CDC6 | 1.543387 | 0.012934 | + | ||
| PSMD11 | 1.532049 | 0.007204 | + | + | + |
| MCM5 | 1.531026 | 0.013155 | + | ||
| MBD2 | 1.495552 | 0.013169 | + | + | |
| MRPL41 | 1.473089 | 0.029152 | + | ||
| TPI1 | 1.451595 | 0.005256 | + | + | |
| DUSP6 | 1.424208 | 0.019572 | + | + | |
| TIGD5 | 1.419209 | 0.007146 | + | + | |
| RPL39 | 1.411136 | 0.025767 | |||
| OGFR | 1.399202 | 0.002343 | + | ||
| RTKN | 1.364258 | 0.04595 | + | + | + |
| CTBP1 | 1.351367 | 0.00019 | + | ||
| ARL2 | 1.332863 | 0.002363 | + | ||
| TADA3 | 1.239423 | 0.032833 | + | ||
| PTGR1 | 1.236809 | 0.016147 | |||
| KLHDC10 | 1.192891 | 0.048934 | + | + | + |
| GOLM1 | 1.141642 | 0.006373 | + | + | |
| METRNL | 1.100353 | 0.015776 | + | ||
| PLOD1 | 1.098426 | 0.047772 | + | + | |
| ADM | 1.08839 | 0.012934 | |||
| SIX1 | 1.066897 | 0.042474 | + | + | |
| RCN3 | 1.046073 | 0.038849 | + | ||
| POLDIP2 | 1.041776 | 0.015349 | + | ||
| COX6B1 | 1.032669 | 0.030567 | + | ||
| KIAA0930 | 1.010146 | 0.030719 | + | + | + |
| ERF | 1.006507 | 0.047544 | + | + | + |
| ATP5A1 | 0.984198 | 0.029152 | + |
| Term | p-Value |
|---|---|
| Interferon-mediated immunity | 1.10 × 10−13 |
| Immunity and defense | 3.30 × 10−5 |
| Proteolysis | 8.80 × 10−3 |
| Glycolysis | 1.20 × 10−2 |
| T-cell mediated immunity | 1.30 × 10−2 |
| Macrophage-mediated immunity | 2.30 × 10−2 |
| MHCI-mediated immunity | 4.90 × 10−2 |
| Protein metabolism and modification | 6.50 × 10−2 |
| Protein biosynthesis | 9.00 × 10−2 |
| AngiogenesisCOA6 (C1orf31) | 9.60 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Sanchez, A.; Lazzarano, S.; Sharma, E.; Lockstone, H.; Murphy, C.L. High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes. Life 2020, 10, 58. https://doi.org/10.3390/life10050058
Martinez-Sanchez A, Lazzarano S, Sharma E, Lockstone H, Murphy CL. High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes. Life. 2020; 10(5):58. https://doi.org/10.3390/life10050058
Chicago/Turabian StyleMartinez-Sanchez, Aida, Stefano Lazzarano, Eshita Sharma, Helen Lockstone, and Christopher L. Murphy. 2020. "High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes" Life 10, no. 5: 58. https://doi.org/10.3390/life10050058
APA StyleMartinez-Sanchez, A., Lazzarano, S., Sharma, E., Lockstone, H., & Murphy, C. L. (2020). High-Throughput Identification of MiR-145 Targets in Human Articular Chondrocytes. Life, 10(5), 58. https://doi.org/10.3390/life10050058





