4. Systematic Paleontology
Cetacea Brisson, 1762
Neoceti Fordyce and Muizon, 2001
Odontoceti Flower, 1867
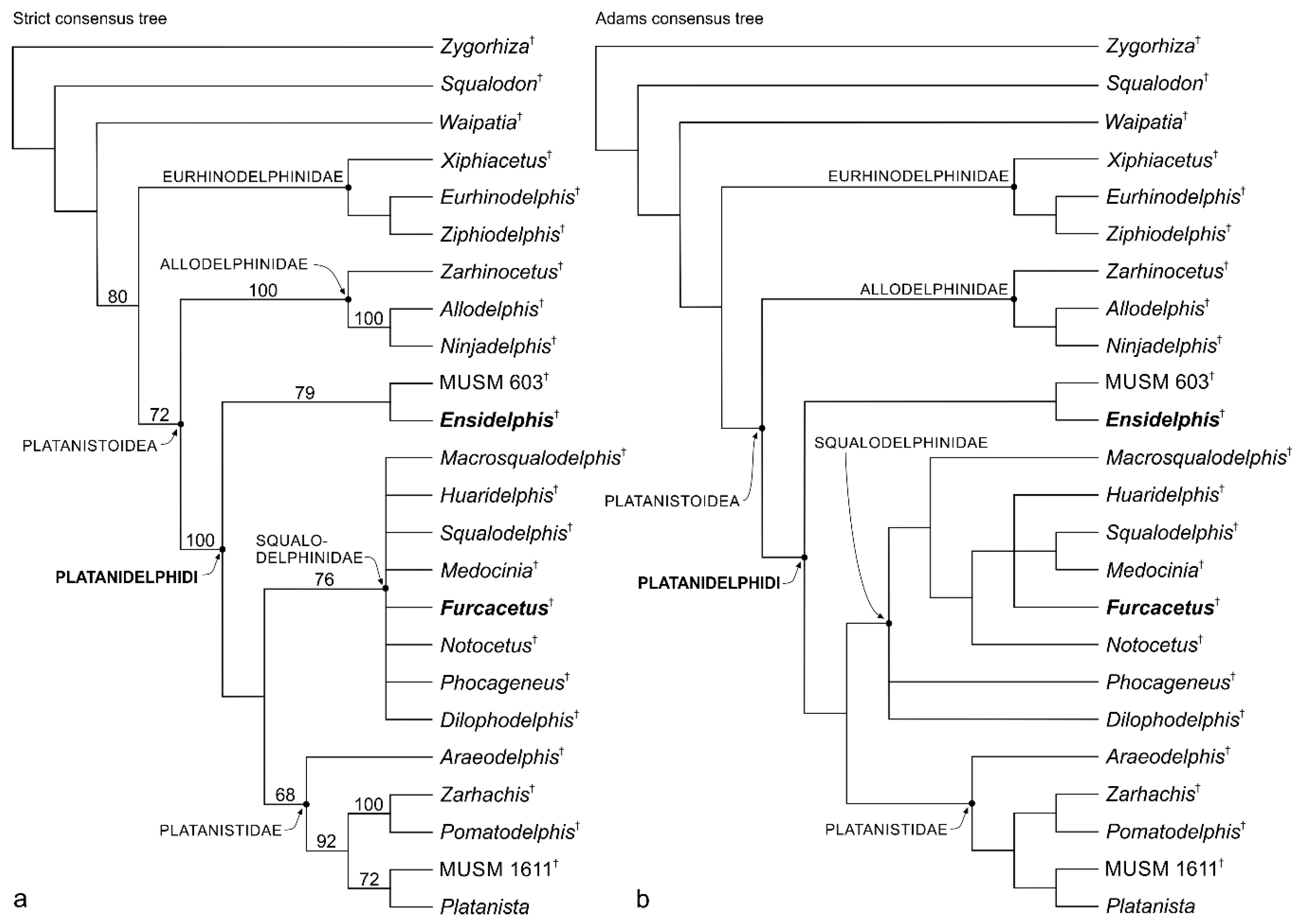
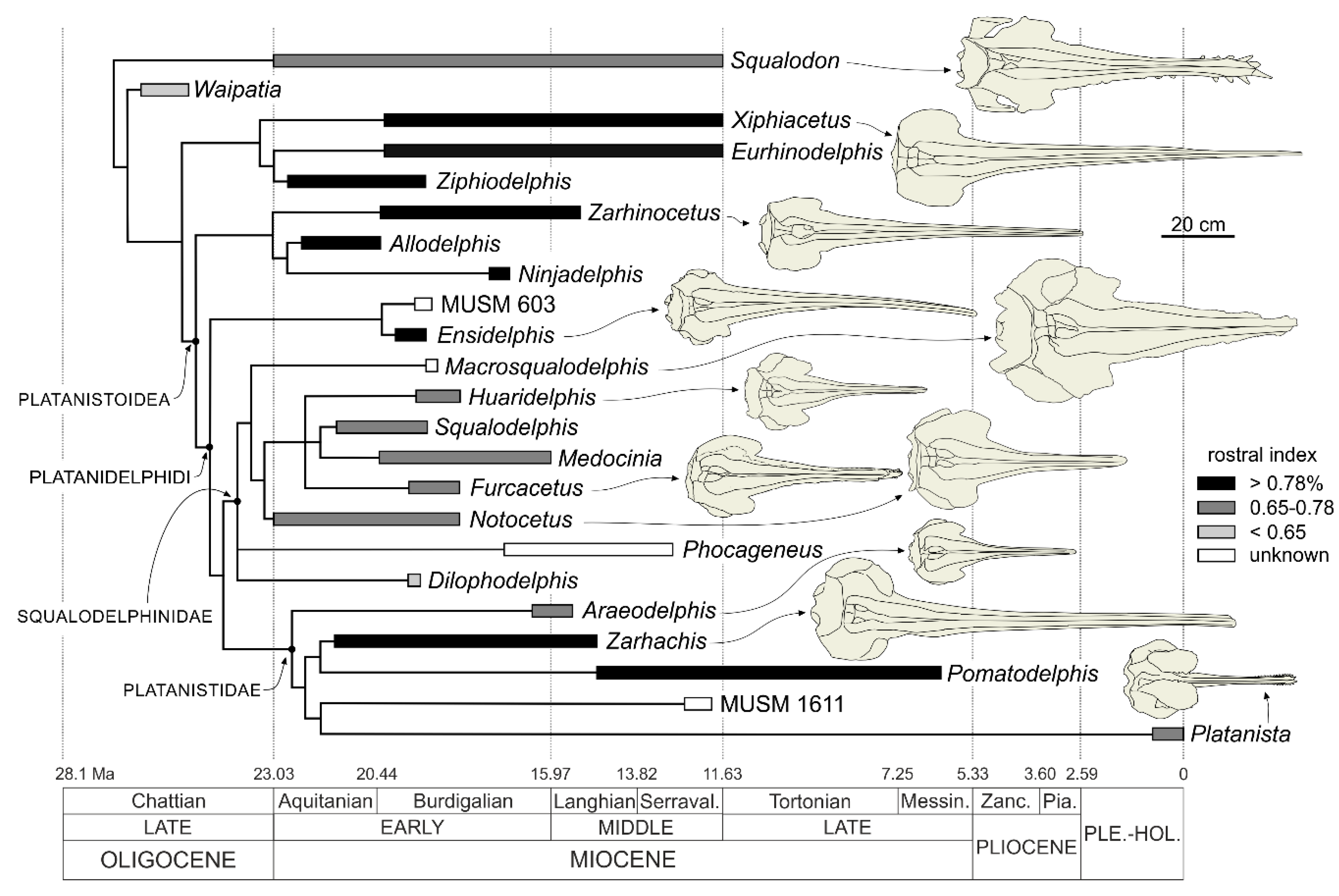
Platanistoidea Gray, 1863
Remarks on the superfamily Platanistoidea and its content. In its first definition proposed by Simpson [
91] the superfamily Platanistoidea included
Platanista, all other extant "river dolphins" (
Lipotes,
Inia, and
Pontoporia, the latter being listed there as
Stenodelphis), and their closest fossil relatives. Currently, the only extant genus recognized as belonging to this clade is
Platanista, whereas the other "river dolphins" are placed within the Delphinida clade [
92,
93,
94,
95,
96,
97]. However, in the past decades a number of fossil taxa have been included in the Platanistoidea, radically changing the concept of this superfamily. Besides the Platanistidae, Muizon [
92] first included in the Platanistoidea the extinct families Squalodelphinidae and Squalodontidae, and, a few years later [
98], also the Dalpiazinidae and
Prosqualodon. Later, Fordyce [
99] added the Waipatiidae and Barnes [
100] the Allodelphinidae. This broad concept of the Platanistoidea has been questioned in several recent phylogenies. For example, the heterodont Squalodontidae and
Prosqualodon were recovered in a more basal position in several analyses [
26,
95,
101,
102], whereas in other analyses the position of these taxa inside or outside Platanistoidea depends on the settings of the phylogenetic analyses (e.g., homoplastic characters being down weighted or not [
103,
104,
105,
106,
107]). The family Waipatidae was also removed from Platanistoidea in some phylogenies (e.g., [
26,
95,
101,
102]), whereas the poorly known Dalpiazinidae were never included in a software-assisted phylogenetic analysis. By contrast, the families Allodelphinidae and Squalodelphinidae appear as two distinct clades closely related to the Platanistidae in part of the recent phylogenies (e.g., [
24,
26,
101,
108]), with allodelphinids being recovered in the basalmost position, sister group of the clade formed by the platanistids and squalodelphinids. In several other recent papers (e.g., [
103,
104,
105,
106,
107]) allodelphinids were not included in the phylogenetic analyses and squalodelphinids were paraphyletic.
Since the phylogenetic analysis presented below confirms again the close relationships between the Platanistidae, Squalodelphinidae, and Allodelphinidae, a new definition of the Platanistoidea sensu stricto is proposed below, only including the above mentioned three families and thus excluding the Dalpiazinidae, Squalodontidae, Waipatidae, and Prosqualodon.
Proposing such a less inclusive, but more stable definition of this superfamily, we do not a priori exclude that in future analyses the Platanistoidea clade falls near to one or more of the above excluded families. In fact, the aim of this restrictive choice is essentially to put order in the controversial systematics of the large odontocete group including extinct platanistoid-like taxa.
Finally, the New Clade Name (NCN) Platanidelphidi is below defined following the rules reported in the International Code of Phylogenetic Nomenclature (PhyloCode [
109]). The Platanidelphidi clade includes the new genus
Ensidelphis described below and the platanistoid MUSM 603 previously referred to aff.
Huaridelphis raimondii [
24], together with the clade formed by Platanistidae + Squalodelphinidae, the latter being repeatedly recovered in morphological phylogenies since the first analyses by Muizon [
92,
98].
Emended diagnosis of Platanistoidea. The members of the Platanistoidea are nearly homodont odontocetes having single-rooted teeth and sharing the following characters: (1) Vertex distinctly shifted to the left compared to the sagittal plane of the skull (absent in Allodelphis and Ninjadelphis); (2) long hamular fossa of the pterygoid sinus extending anteriorly on the palatal surface of the rostrum (also present in Ziphiidae); (3) presence of an articular rim on the lateral surface of the periotic; (4) elongated anterior spine on the tympanic bulla, associated with a marked anterolateral convexity; (5) great reduction of the coracoid process of the scapula and the acromion located on the anterior edge of the scapula (also present in Squalodon and Prosqualodon); (6) neurocranium distinctly shorter than wide, with ratio between neurocranium length (longitudinal, from occipital condyles to level of antorbital notches) and postorbital width < 0.90 (also present in Eurhinodelphinidae); and (7) anterior portion of the zygomatic process of the squamosal in contact with the postorbital process of the frontal (also present in Eurhinodelphinidae).
Platanidelphidi (NCN)
Definition. The branch-based Platanidelphidi consists of the extant Platanista and all species that share a more recent common ancestor with Platanista than with Allodelphis.
Etymology. From Platanista, the type genus of the Platanistidae; and from delphinus, dolphin in Latin. The name is also a combination of Platanistidae and Squalodelphinidae, the two families currently included in the Platanidelphidi.
Diagnosis. The members of the Platanidelphidi clade are platanistoids sharing the following synapomorphies: (1) Asymmetry of the premaxillae on the rostrum at some distance anterior to the premaxillary foramina, with the right premaxilla being distinctly narrower than the left in dorsal view; (2) posterior dorsal infraorbital foramen(ina) along the vertex more medial than the lateralmost margin of the premaxilla on the cranium; (3) deep fossa in the frontal on orbit roof, at the level of the frontal groove (presumably for orbital lobe of pterygoid sinus); (4) palatines not contacting each other on the sagittal plane and displaced dorsolaterally; (5) thick zygomatic process of the squamosal (ratio between the maximum distance from the anteroventral margin of the zygomatic process to the posterodorsal margin, in lateral view, and the vertical distance from the lower margin of the occipital condyles to the vertex of the skull > 0.35); (6) dorsal outline of the zygomatic process of the squamosal in lateral view being dorsally convex (also present in Squalodon and in some specimens of the eurhinodelphinid Xiphiacetus); and (7) ventral edge of the zygomatic process of squamosal in lateral view being almost straight or convex.
Platanidelphidi incertae sedis
Ensidelphis, gen. nov.
LSID: zoobank.org:act: C0D8D0CE-1769-4BA1-85D9-054889976B18
Type and only known species. Ensidelphis riveroi, sp. nov.
Diagnosis. As for the type and only referred species.
Etymology. From ‘ensis’, Latin name of a Roman sword similar to the gladius but longer and narrower; for the very elongated and narrow rostrum; and from ‘delphis’, dolphin in Latin. Gender masculine.
Ensidelphis riveroi, sp. nov.
LSID: zoobank.org:act: act:1DEC0DD9-FEFA-4CC8-A668-11934B4256AA
Holotype and only referred specimen. MUSM 3898 consists of an almost complete and well-preserved cranium (only small portions of both antorbital processes and both the jugals are missing) with articulated, complete mandibles. Both tympanic bullae are exposed on the ventral surface, probably hiding the in situ periotics and accessory ossicles. Only six incomplete teeth are visible, embedded in their alveoli on the right mandible. The atlas, axis, and two additional cervical vertebrae from the same animal are also preserved.
Type locality. Zamaca locality, Western Ica Valley, Ica Region, Southern Peru (
Figure 1a,b). Geographic coordinates: 14°37'1.65" S, 75°37'31.25" W; 307 m above sea level. This specimen was reported in the Zamaca fossil map of Di Celma et al. [
22] with the field number ZM 97 and provisionally referred to “aff. Platanistidae indet.”
Type horizon. The holotype of
Ensidelphis riveroi MUSM 3898 was collected in the Chilcatay Fm exposed at Zamaca locality, and more precisely at 10.7 m above the contact with the underlying Otuma Formation, near the top of the Ct1c facies association of the Ct1 allomember [
21,
22] (
Figure 1d). The age of the Ct1c facies association is constricted between 19.25 ± 0.08 Ma and 19.00 ± 0.28 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
Diagnosis. Ensidelphis is a small odontocete having a narrow and very elongated rostrum (about 80% of the CBL) bearing about 64 small single-rooted teeth in each quadrant. It differs from all other odontocetes in having a protuberance (‘temporal swelling’, new term) on the lateral surface of the temporal fossa dorsal to the squamosal-parietal suture. It is referred to the Platanistoidea as defined above by having: Elongated hamular fossa of the pterygoid sinus extending anteriorly on the palatal surface of the rostrum; neurocranium shorter than wide (ratio < 0.90); and elongated anterior spine on the tympanic bulla associated with a marked anterolateral convexity. It differs from all other Platanistoidea in having a lesser posterior extension of both right and left ascending processes of the premaxillae, ending at the contact with the anterolateral angles of the nasals, and in having an even more elongated anterior spine of the tympanic bulla. It differs from all other Platanistoidea, with the exception of Platanista, for the lesser minimal distance between the temporal crests (see quantification below; character possibly related to the temporal swelling mentioned above). It differs from the similarly hyper-longirostrine Allodelphinidae in having the dorsal opening of the mesorostral groove anterior to the rostrum base narrower than the premaxilla, the lateral rostral suture between premaxilla and maxilla not deeply grooved, proportionally wider premaxillae at rostrum base (>60% of the width of the rostrum), and vertex not strongly transversely pinched. Ensidelphis belongs to the Platanidelphidi clade in having: Asymmetry of the premaxillae on the rostrum at some distance anterior to the premaxillary foramina, with the right premaxilla distinctly narrower than the left in dorsal view; posterior dorsal infraorbital foramen located along the vertex, more medial than the lateralmost margin of the premaxilla in the cranium; vertex distinctly shifted to the left compared to the sagittal plane of the skull; thick zygomatic process of the squamosal (ratio between the maximum distance from the anteroventral margin of the zygomatic process to the posterodorsal margin, in lateral view, and the vertical distance from the lower margin of the occipital condyles to the vertex of the skull > 0.35); and ventral edge of zygomatic process of squamosal in lateral view almost straight. Within the Platanidelphidi, Ensidelphis differs from Platanista, Pomatodelphis, and Zarhachis in the lateral rostral suture between premaxilla and maxilla being not deeply grooved; from Huaridelphis, Notocetus, and Squalodelphis in the presence of a deep lateral groove on the mandible; from Phocageneus, Notocetus, and Squalodelphis in the ventral groove of the tympanic not affecting the whole length of the bone, including the anterior spine; from Araeodelphis, Dilophodelphis, Furcacetus, Huaridelphis, Platanista, and Squalodelphis in the very elongated rostrum (>70% of the CBL); from Furcacetus, Huaridelphis, Macrosqualodelphis, and Notocetus in the smaller size of teeth at rostrum mid-length (diameter <2% of BZW); from Platanista, Pomatodelphis, and Zarhachis in the less elongated mandibular symphysis (61% of the total mandibular length contra >65%) and a consequent smaller angle between the mandibular rami (25° contra roughly 60°).
Etymology. riveroi, honoring Mariano Eduardo de Rivero y Ustariz (1798–1857), prominent Peruvian geologist and archaeologist.
Description and Comparison
Ontogeny. We consider the holotype of Ensidelphis riveroi as an adult animal, having: Nasals and frontals fused together at the vertex, fusion of the premaxillae and maxillae at the anterior end of the rostrum, well individualized upper and lower alveoli, and all epiphyses of preserved post-atlas cervical vertebrae completely fused.
Total body length estimate. The TBL of
Ensidelphis was estimated to 1.95 m, using a BZW value of 196 mm in the equation proposed by Pyenson and Sponberg [
110] for the stem platanistoids. However, considering the extreme elongation of its rostrum, we suspect that the TBL of
Ensidelphis was greater than the one calculated with this equation. Therefore, we tried another way to get a better estimate using the extant
Platanista gangetica and the Miocene
Zarhachis flagellator for comparison. We chose
Zarhachis because it is a relative of
Ensidelphis having the same hyper-longirostrine cranium (see [
111,
112]; ratio between BZW and CBL equals 0.23 and about 0.22 for the holotype of
Ensidelphis riveroi and
Z. flagellator USNM 10485, respectively), and
Platanista because it is the closest extant relative of
Ensidelphis.
The length of the subcomplete thoracic portion (10 vertebrae) of
Z. flagellator USNM 10485 is about 64.05 cm (measurement after Kellogg [
111]; note that the last thoracic of USNM 10485 lacks the centrum, so we estimated its length as a mean value between the ninth thoracic and the first lumbar). The vertebral column of three measured skeletons of
Platanista gangetica (LDUCZ Z2282, MHNP A7945, MSNUP M272) is between 4.12 and 4.76 times the length of its thoracic portion. Using the same proportions, the length of the postcranial skeleton of
Z. flagellator can be estimated between 264 and 305 cm and, adding the skull length (119.5 cm according to Kellogg [
111]), we obtain a skeletal length between 383 to 424 cm. Based on these estimations, the skeletal length of
Z. flagellator is between 3.21 to 3.55 times the length of its skull. Considering that the skull length of
Ensidelphis is 865 cm and applying the same proportions as for
Zarhachis, we obtain an estimate of the skeletal length of
Ensidelphis between 277 (= 865 × 3.21) and 307 cm (865 × 3.55). However, the actual body length is slightly greater than the skeletal length due to soft tissues, including intervertebral disks. Consequently a few more centimeters should be added. Therefore, the body length of
Ensidelphis could have been around 3 m, a length significantly larger that the estimation obtained with the Pyenson and Sponberg [
110] equation.
Cranium
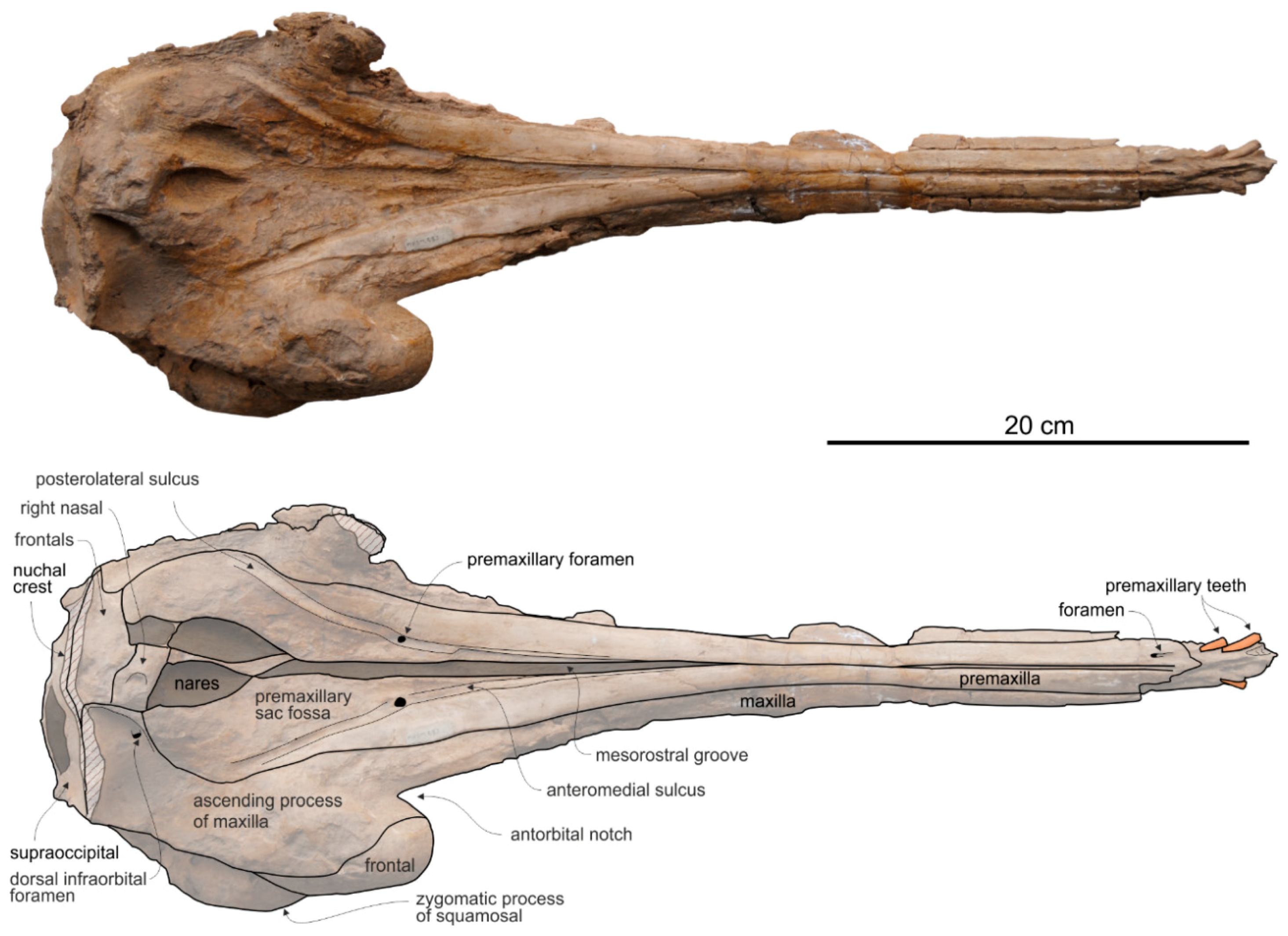
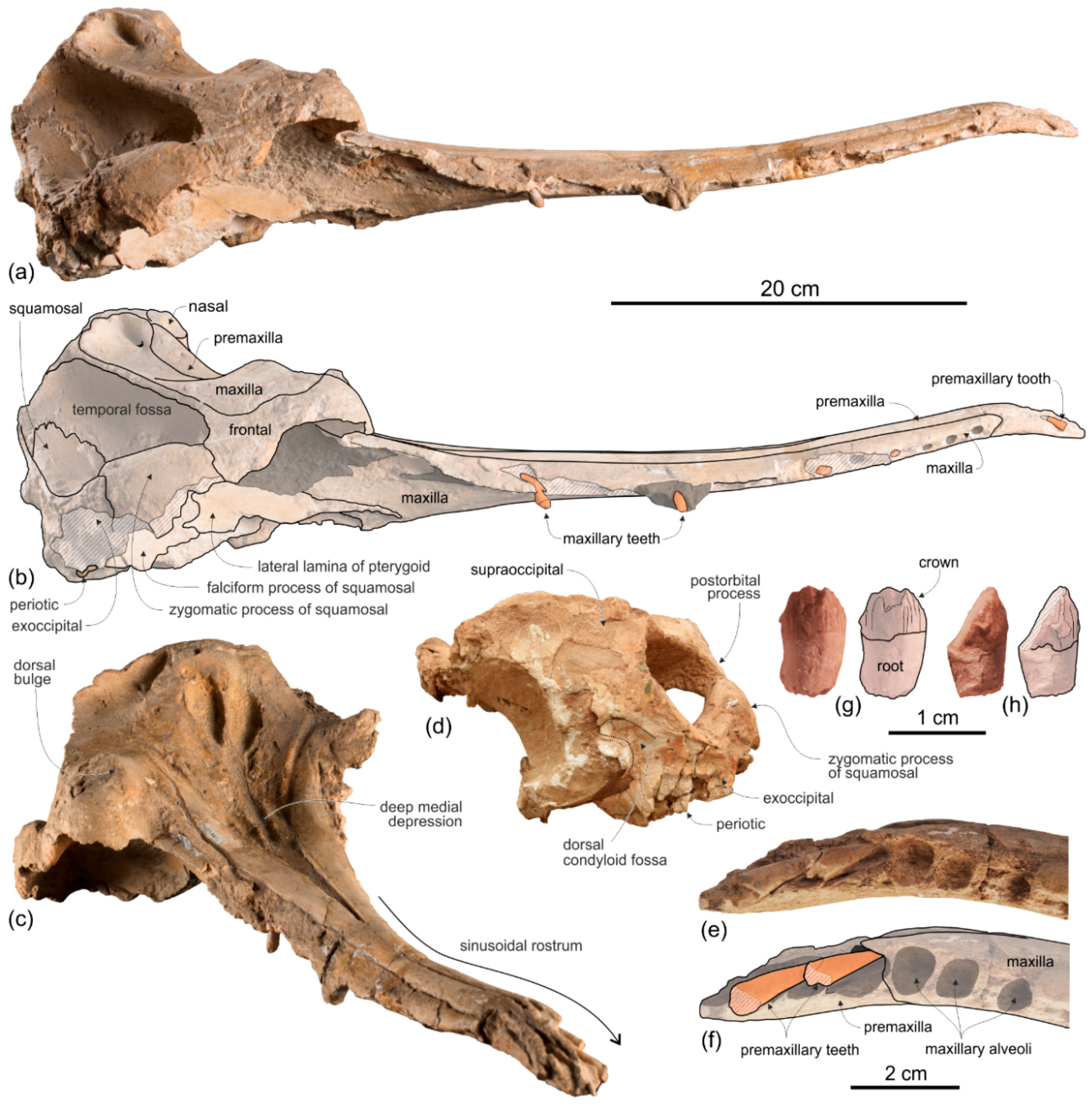
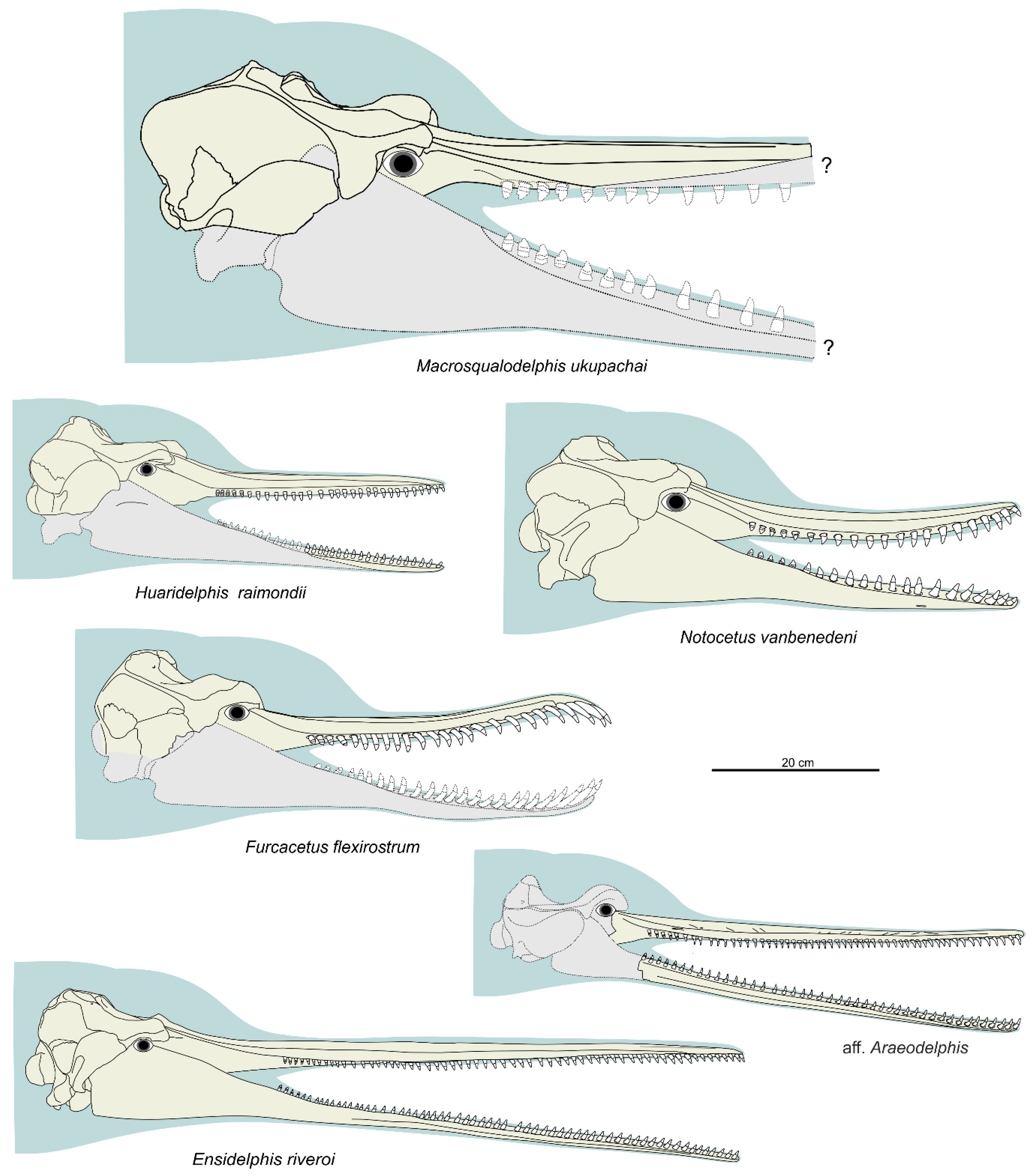
General morphology. The most conspicuous character of the cranium of
Ensidelphis is the extreme elongation of its rostrum (81% of the CBL) (
Figure 2a,b;
Table 1). Among odontocetes, such an elongated rostrum, a state defined as hyper-longirostry [
17], has only been observed in all the allodelphinids (
Allodelphis,
Goedertius,
Ninjadelphis, and
Zarhinocetus), pomatodelphinine platanistids (
Pomatodelphis and
Zarhachis), eurhinodelphinids (i.e.,
Eurhinodelphis,
Schizodelphis,
Xiphiacetus, and probably
Ziphiodelphis), the eoplatanistid
Eoplatanista, and the lipotid
Parapontoporia. Compared with the other hyper-longirostral platanistoids, the rostrum of
Ensidelphis displays a dorsoventral compression intermediate between the less compressed rostra of allodelphinids and the more compressed rostra in pomatodelphinids. As in
Pomatodelphis and
Zarhachis, the dorsoventral compression in
Ensidelphis is more pronounced near the apex of the rostrum. The rostrum of
Ensidelphis clearly differs from the rostrum of the extant
Platanista by not displaying a marked transverse compression, a character also observed in a fragmentary early Miocene fossil rostrum referred to Platanistinae [
100]. Moreover, the rostrum of the
E. riveroi holotype is markedly bent towards the right, a possibly natural condition also observed in some adult females of extant
Platanista gangetica [
113] and in some specimens of
Pontoporia blanivillei [
114] (see below for a possible interpretation of this peculiar asymmetry).
As in all platanistoids and in eurhinodelphinids the neurocranium of Ensidelphis is anteroposteriorly shortened, its length being about 90% of the postorbital width.
As in all Platanidelphidi and the allodelphinid Zarhinocetus, the vertex and the bony nares of Ensidelphis are shifted on the left side and the main transverse axis of the nasals is obliquely oriented, even if this feature is less marked than in some other platanistoids (e.g., Notocetus and Huaridelphis). As a consequence of this shifting, the left frontal is markedly anteroposteriorly shorter than the right on the vertex.
The vertex is low, flat, and weakly sloping anteroventrally; moreover, in lateral view it does not form a pointed crest as observed in several other platanistoids. The transverse compression of the vertex in
Ensidelphis is lesser than observed in all Platanidelphidi with the exception of
Huaridelphis. In fact, in
Ensidelphis and
Huaridelphis the minimum transverse width of the vertex is only slightly greater than the transverse width of bony nares, whereas in all other members of the Platanidelphidi the minimum transverse width of the vertex is the same or slightly lower than the width of bony nares. An exception is represented by
Platanista, displaying a strongly transversely pinched vertex, as observed in the allodelphinids
Allodelphis,
Goedertius,
Ninjadelphis, and
Zarhinocetus, but not in
Arktocara, a putative allodelphinid lacking transverse compression of the vertex [
115].
The temporal fossa displays a remarkable height (ratio between vertical height of the fossa, in lateral view, and vertical distance from the lower margin of the occipital condyles to the vertex of the skull estimated to 0.70) (
Figure 4 and
Figure 5). Among platanistoids, a similar height of the temporal fossa (ratio > 0.60) is only observed in
Furcacetus,
Macrosqualodelphis, Notocetus, and
Platanista. Nevertheless, the temporal fossa of
Ensidelphis differs from the temporal fossa of the aforementioned platanistoids in being anteroposteriorly compressed (height > anteroposterior length).
A clear autapomorphy of
Ensidelphis is the presence of a peculiar protuberance, here named temporal swelling, on the medial wall of the temporal fossa, just above the squamosal–parietal suture. Clearly visible in lateral (
Figure 4c,d,
Figure 5c,d) and posterior (
Figure 6c,f) views of the cranium, this swelling is interpreted here as an original character, neither due to a pathology, or a trauma, or even post-mortem deformation, since it is present with the same shape and exactly in the same position in both the right and the left fossae. Further supporting the non-artificial nature of this character is the low minimum transverse distance between the temporal crests at the very same level of these swellings (best observed in posterior view of the cranium). The ratio between this distance and BZW is 0.55 for
Ensidelphis; among other platanistoids, only
Platanista has a lower value. It is possible that the strong transverse posterior compression of the skull has been compensated (possibly for biomechanical reasons, in relation to the origin of the temporal muscles) by the appearance of the temporal swellings.
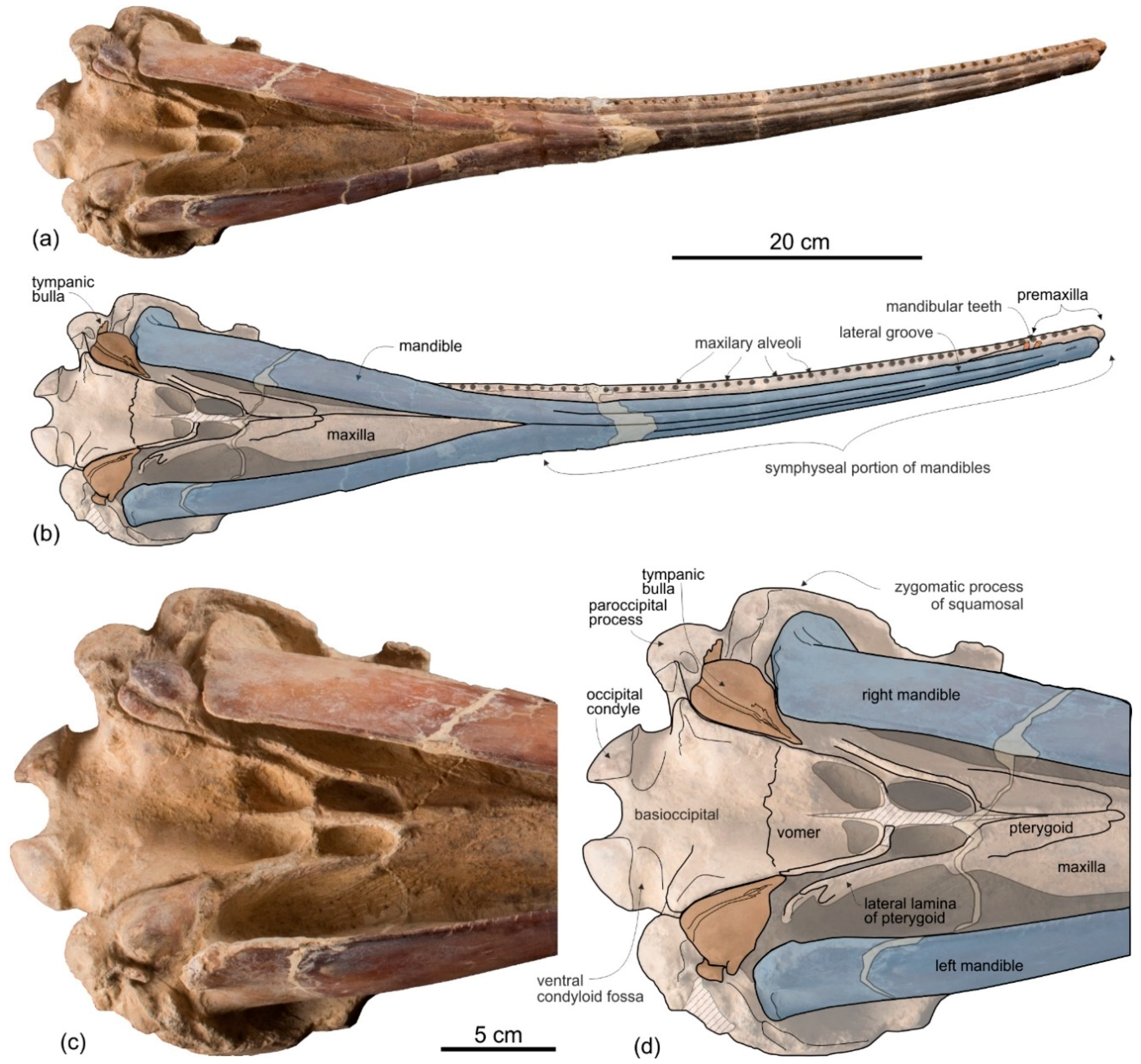
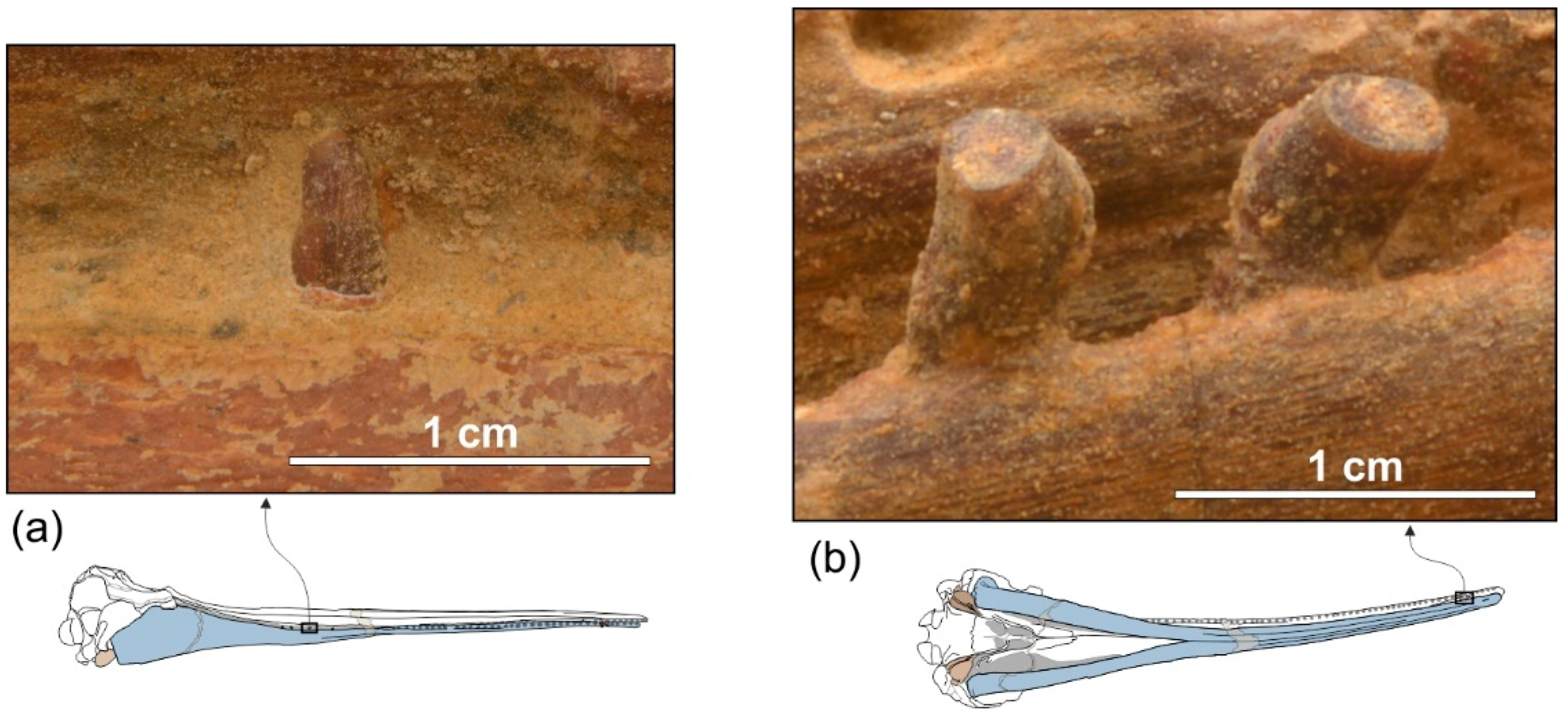
Premaxilla. The apex of the rostrum is formed by the premaxillae only, as clearly showed in ventral view by the oblique maxillary/premaxillary suture that runs anterolaterally, reaching the lateral margin of the rostrum about 60 mm from the end (
Figure 3a,b). Among other platanistoids, the anterior portion of the rostrum being formed by the premaxillae only is also observed in
Dilophodelphis,
Furcacetus,
Huaridelphis,
Notocetus, and
Squalodelphis, whereas in all the allodelphinids and platanistids both premaxillae and maxillae are proposed to reach the apex of the rostrum [
108,
116]. Outside platanistoids, eurhinodelphinids display an even longer anterior premaxillary part of the rostrum (e.g., [
117]).
The premaxillae are joined together dorsomedially, closing the mesorostral groove from the apex of the rostrum to 150 mm anterior to the base of the rostrum (
Figure 2a,b). However, the two premaxillae remain distinct from one another by a narrow but clear medial sulcus. Roughly at the level of the right antorbital notch, the opening of the mesorostral groove reaches its maximum transverse width (11 mm). Among platanistoids a wider dorsal opening of the mesorostral groove near the rostrum base is observed in
Medocinia, Squalodelphis, and in all the allodelphinids.
In dorsal view each premaxilla is laterally fused to the maxilla for about the anterior 100 mm of the rostrum, then the premaxilla–maxilla suture is marked by a thin sulcus becoming deeper towards the posterior portion of the rostrum, but remaining narrow for all the anteroposterior extension of the rostrum, without forming a deep and wide lateral groove as observed in the platanistids Platanista, Pomatodelphis, and Zarhachis.
In the anterior portion of the rostrum the premaxillae are dorsoventrally flattened, then, proceeding posteriorly, their cross section becomes hemicylindrical at rostrum mid-length, before flattening again towards the posterior portion of the rostrum. At the base of the rostrum the dorsal surface of the premaxillae is flat but markedly medioventrally sloping to form a prenarial depression, also observed in most other platanistoids and some other archaic odontocetes (e.g., squalodontids).
About 150 mm anterior to the base of the rostrum, at the level where the premaxillae begin to diverge, the right premaxilla is distinctly transversely narrower than the left premaxilla. This character is observed in all platanistoids, with the exception of Araeodelphis. At the base of the rostrum the premaxillae are moderately wide (transverse width of the premaxillae equals 63% of the width of the rostrum), an intermediate condition between allodelphinids (<60%) on the one side, and Medocinia and Squalodelphis (>75%) on the other side.
A single large premaxillary foramen is present on each premaxilla 35 mm anterior to the rostrum base. The premaxillary foramen is also anterior to the antorbital notch in other platanistoids, with the exception of
Dilophodelphis,
Macrosqualodelphis,
Platanista, and the
Notocetus skulls from the Chilcatay Fm, all having the premaxillary foramen roughly level with the antorbital notch. The anteromedial and posterolateral sulci are wide and clearly discernible, especially on the right side, whereas the posteromedial sulcus is weakly excavated. The premaxillary sac fossae are moderately transversely concave, they slope medioventrally, and they have the same transverse width. The right ascending process of the premaxilla ends with a posterior point incised by a notch followed anteriorly by a longitudinal wide groove, similar to the premaxillary cleft described in
Waipatia [
99], also observed in most other platanistoids (e.g., [
24,
26,
112]), and in several other archaic odontocetes (e.g., [
105,
118]). The left ascending process of the premaxilla has a rounded posterior margin without incision or groove. The posterior end of both the right and left ascending processes of the premaxillae contacts the anterolateral angle of the corresponding nasal. Such a limited posterior extension of the premaxillae distinguishes
Ensidelphis from the other platanistoids, all having the posteromedial margin of the ascending process of both premaxillae in contact with the lateral margin of the nasal. A partial exception is observed in
Furcacetus, whose right premaxilla only contacts the anterolateral angle of the right nasal. Nevertheless, in
Furcacetus the left premaxilla displays a significant posterior extension, as in all other platanistoids except
Ensidelphis. A greater posterior extension of the premaxillae is present in allodelphinids, all having the premaxillae extending posteriorly beyond the nasals.
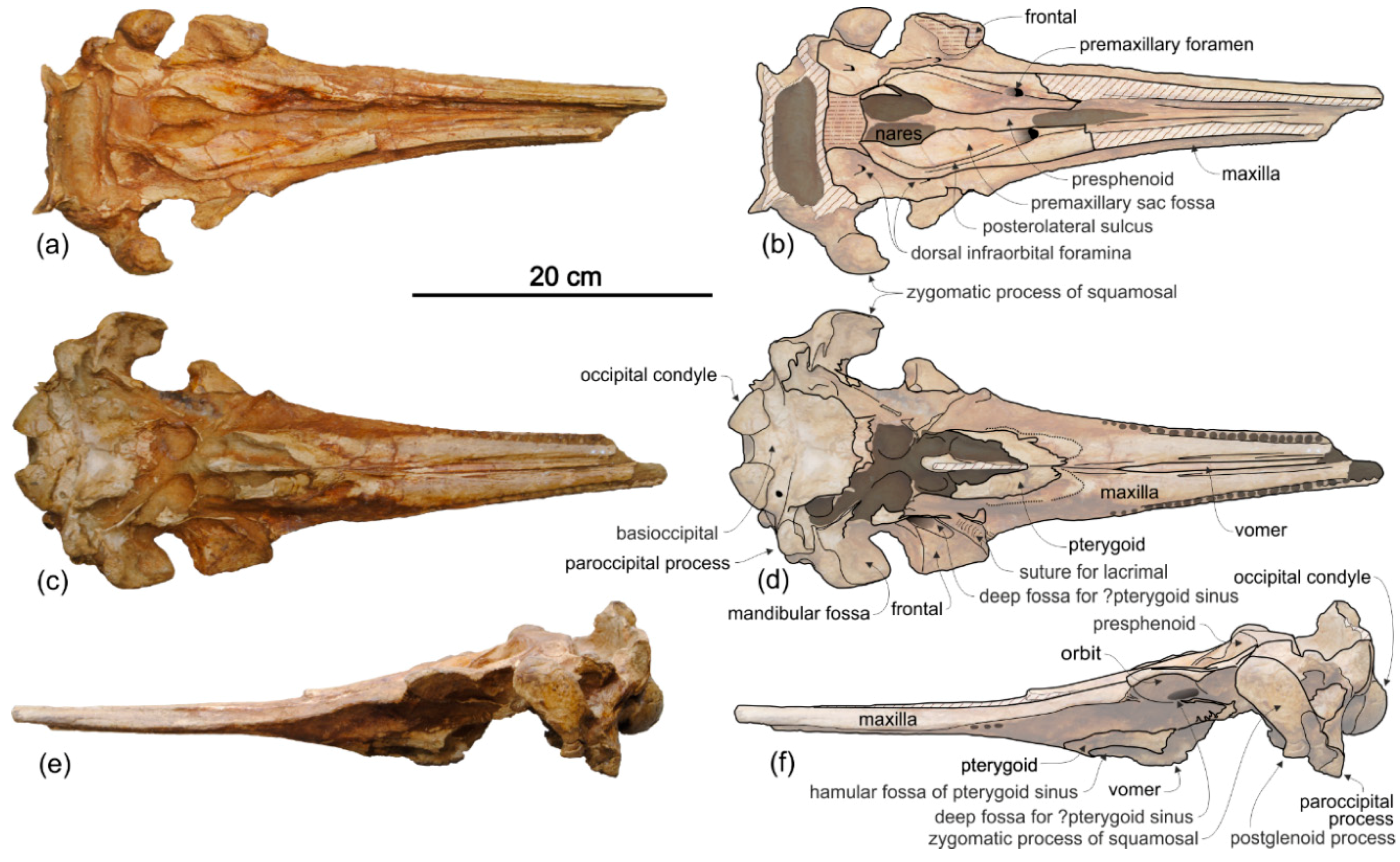
Maxilla. In dorsal view (
Figure 2) the maxilla remains transversely narrow along the entire length of the rostrum, showing a flat dorsal surface roughly parallel to the horizontal plane, only weakly ventrolaterally sloping in the mid portion of the rostrum.
Formed by the maxilla, the lateral margin of the posterior portion of the rostrum is dorsoventrally thin and blade-like as in all platanistoids of the Platanidelphidi clade, with the exception of Huaridelphis, Medocinia, and Notocetus, having a markedly thicker margin.
Both ascending processes of the maxillae are partly broken; consequently, their original dorsal elevation cannot be assessed, although their preserved parts are already higher than the dorsal margin of the rostrum base, a condition shared with all other platanistoids. Due to the incompleteness of the antorbital processes, the shape of the two antorbital notches is unknown.
No dorsal infraorbital foramina are visible around the base of the rostrum, whereas a posterior dorsal infraorbital foramen is located more medial than the lateralmost margin of the premaxilla. The same position of the posterior infraorbital foramina is observed in all known skulls of Platanidelphidi where these foramina are visible.
The posteromedial portions of the ascending processes of the maxillae lateral to the vertex are weakly asymmetrical with the right more anteroposteriorly elongated than the left. Furthermore, the right maxilla descends more abruptly ventrolaterally from the vertex than the left (a condition opposite to that observed in squalodelphinids, all having the left maxilla sloping more abruptly ventrolaterally), to form a deep fossa posterolateral to the right nasal.
The palatal surface of the maxillae is partly covered by the articulated mandibles (
Figure 3); however, the latter are partly shifted to the left, making the ventral surface of the right maxilla largely visible along most of the rostrum. This surface is flat and horizontal; near the lateral margin of the rostrum, it is pierced by well-defined alveoli.
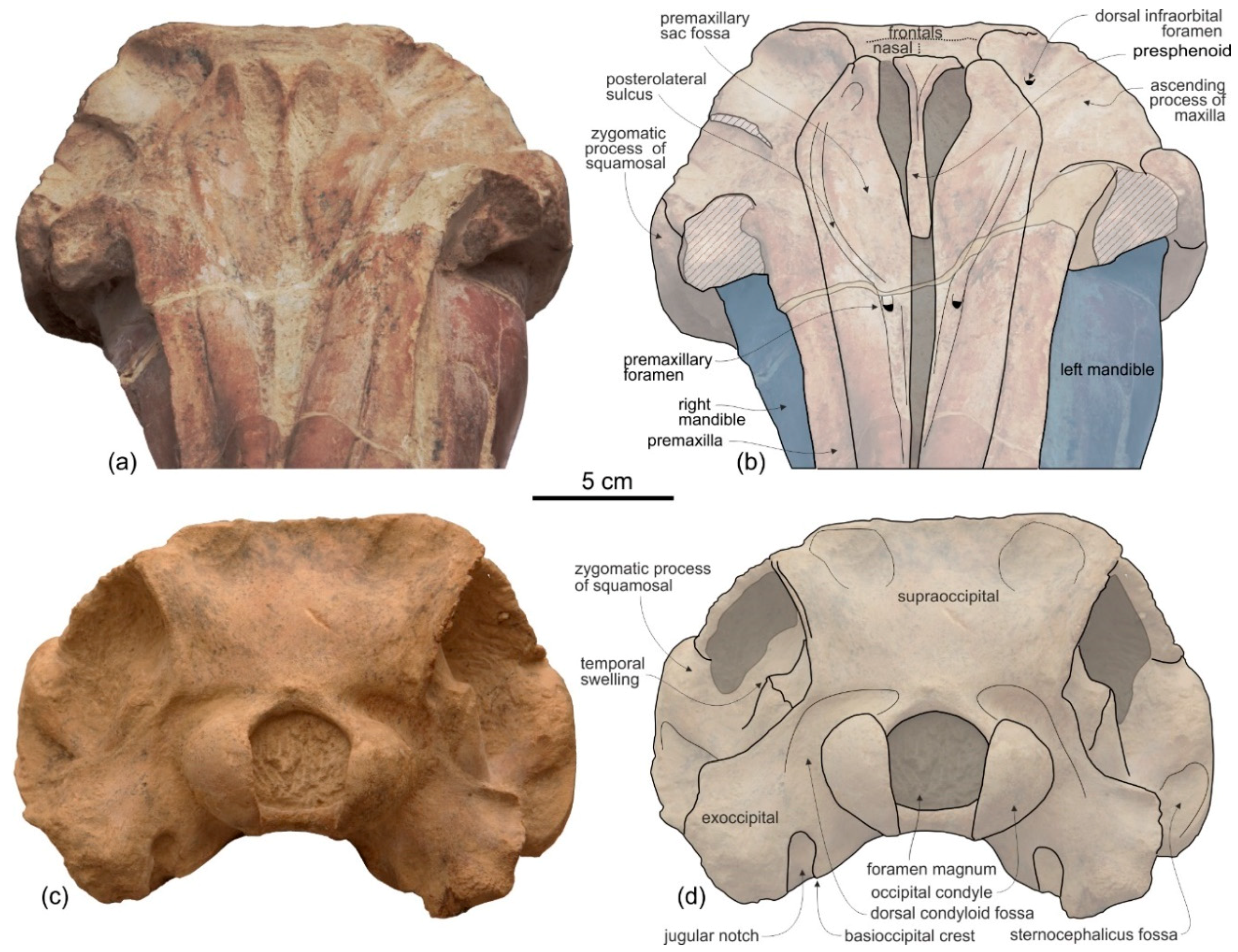
Presphenoid and cribriform plate. The well-preserved nasal septum runs anteroposteriorly along the sagittal plane from the base of the rostrum to the cribriform plate, separating the bony nares (
Figure 2c,d). The cribriform plate borders anteriorly the nasals, reaching dorsally the anterodorsal margin of these bones.
Nasal. The vertex is a flat, trapezoidal area where the sutures between bones are not clearly visible (
Figure 2c,d). This could be due to some post-mortem abrasion of the skull or, more likely, it could be a genuine anatomical feature. In fact, bone fusion at the vertex is observed in other odontocetes, as for example in the beaked whale
Tusciziphius [
119]. If abrasion can be excluded, the nasals of
Ensidelphis have a flat dorsal surface. They are probably anteroposteriorly compressed, being shorter than the frontals at the vertex, and, together in dorsal view, form a rectangle with an angle of about 7° between its anterior side and a coronal plane of the skull. A similar oblique orientation of the longitudinal axis of the nasals is present in most Platanidelphidi and also in the archaic odontocete
Waipatia. Although the nasal-frontal suture is not clearly discernible, being only tentatively reconstructed in
Figure 2b,d, it seems that the maximum transverse compression across the vertex occurs at the level of the nasals, with the frontals progressively widening posteriorly. In lateral view the dorsal margin of the nasals slopes anteroventrally, forming an angle of 12° with the dorsal margin of the rostrum. An anterior slope of the nasals is also observed in
Furcacetus and
Huaridelphis, being more pronounced in the latter (22–35°), whereas it is absent in other platanistoids such as
Macrosqualodelphis and
Notocetus, both displaying an inflated and subhorizontal dorsal surface of the nasals.
Frontal. The frontals at the vertex are trapezoidal, flat and slope anteroventrally with the same inclination as the nasals (
Figure 2c,d). The suture between the right and left frontals is not discernible.
Both preorbital processes of the frontals are broken and only a posteromedial portion is preserved. In lateral view the best-preserved right process (
Figure 5c,d) displays a thickening (ratio between the height of this process measured in lateral view perpendicular to the maxilla-frontal suture and the vertical distance from the lower margin of the occipital condyles to the vertex of the skull = 0.10) that is lesser than in
Dilophodelphis, Furcacetus, Medocinia, Pomatodelphis, and
Zarhachis, all having ratios >0.30. However, this value in
Ensidelphis was measured along the broken lateral surface and a thickening of the preorbital process in its missing lateral portion cannot be excluded. Along the same longitudinal break of the supraorbital process, the section visible in lateral view suggests that the orbit was short and that the postorbital process was robust and triangular.
The articulated mandibles cover most of the ventral surface of the two orbit roofs (
Figure 3); consequently, it is not possible to check for the presence of a deep fossa in the medial portion of the orbit roof, as observed in all other Platanidelphidi.
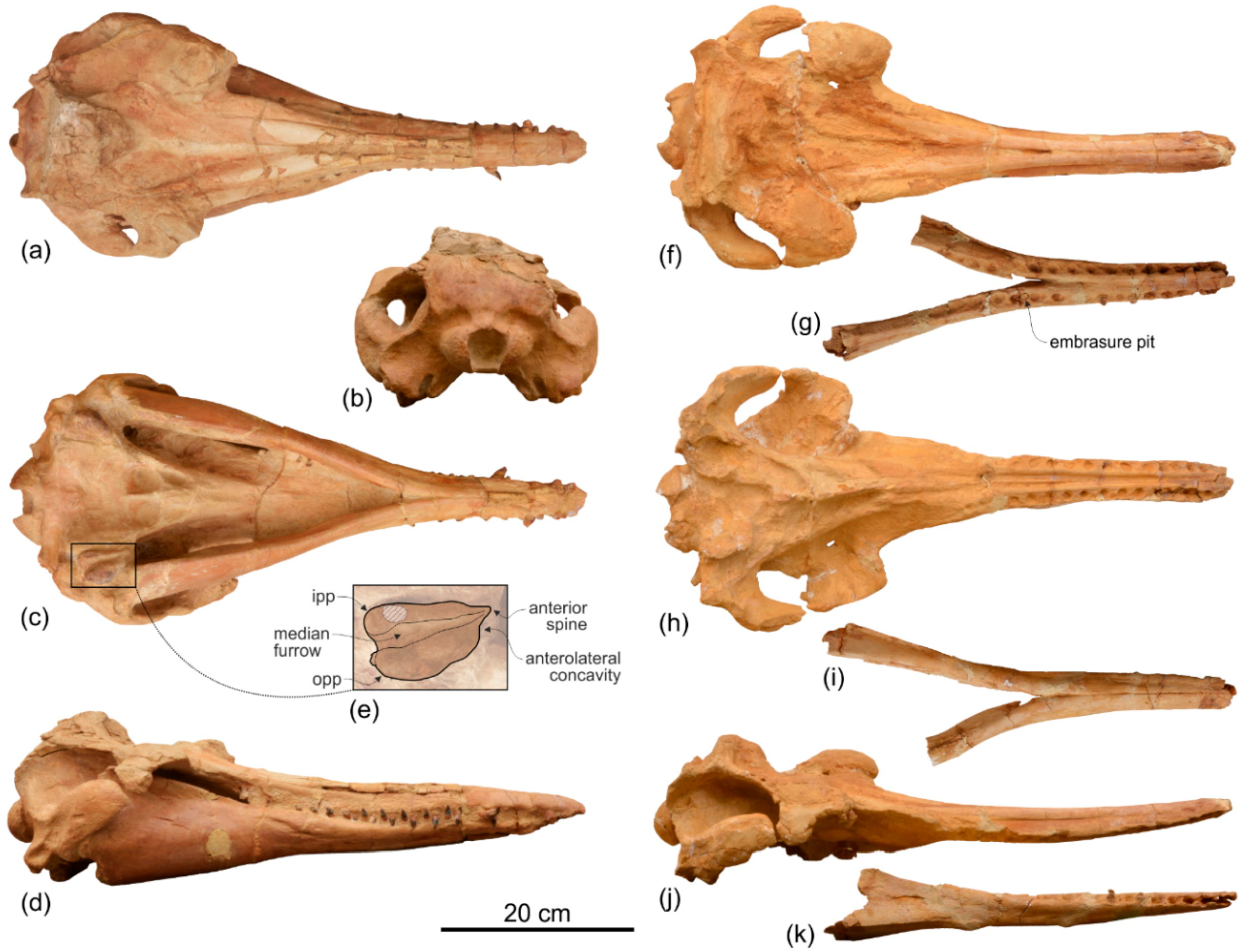
Supraoccipital. In lateral view the nuchal crest is not prominent, even if it represents the highest part of the skull (
Figure 4 and
Figure 5). In anterior (
Figure 6a,b) and posterior (
Figure 6c,d) views of the cranium this crest draws a straight horizontal line, whereas in dorsal view it displays a low posterior concavity.
The lateral margin of the supraoccipital forms, together with the parietal, the temporal crest that delimits posterodorsally the temporal fossa. In posterior view the temporal crest is obliquely oriented due to the gradual transverse narrowing of the occipital shield (supraoccipital + exoccipitals) ventrally, as mentioned above. A maximum transverse constriction of the occipital shield is similarly located ventrally, although less marked, in Macrosqualodelphis. All the other platanistoids have lateral margins of the occipital shield less laterally concave in posterior view; in several cases these margins are almost straight (i.e., pomatodelphinines and allodelphinids). In all these cases the maximum transverse constriction of the shield is not located ventrally as in Ensidelphis and Macrosqualodelphinus. A peculiar condition is observed in Platanista, displaying a remarkable transverse narrowing of the shield related to the transverse widening of the temporal fossae. However, in Platanista the narrowest portion of the occipital is located more dorsally, suggesting a non-homologous origin of this feature in Ensidelphis and in the extant South Asian river dolphin.
The posterodorsal surface of the occipital shield is weakly transversely concave and exhibits two wide, roughly circular depressions with a diameter of about 30 mm, one for each side of the supraoccipital. These depressions might indicate the origin of neck muscles, such as m. semispinalis capitis or m. rhomboideus capitis (see [
120]). There is no external occipital crest (sensu [
27]).
Palatine. In ventral view, palatines are not visible in the well exposed posteromedial portion of the rostrum (
Figure 3c,d), possibly because they are fully covered by the pterygoids. However, the lateral portion of the neurocranium is still covered by sediment and by the two mandibular rami; consequently, it is not possible to check for the presence of a lateral exposure of the palatine.
Pterygoid. The right and left pterygoids, joined together medially, form a narrow point that extends 55 mm beyond the level of the right antorbital notch (
Figure 3c,d). Partially covered by the pterygoid plates, the pterygoid sinus fossae also reach beyond the base of the rostrum, as in all platanistoids. The well-preserved left lateral lamina of the pterygoid is a rectilinear plate that contacts posterolaterally the falciform process of the squamosal.
Jugal-Lacrimal. The lacrimal and the jugal are lost, on both sides of the skull, due to the breakage of the antorbital processes (
Figure 4 and
Figure 5).
Squamosal. In lateral view (
Figure 4 and
Figure 5), the zygomatic process of the squamosal is short and robust, with a maximum thickness making 35% of the vertical distance from the lower margin of the occipital condyles to the vertex. A value similar or greater than in
Ensidelphis was observed in all Platanidelphidi. The zygomatic process of
Ensidelphis also shares with other Platanidelphidi the globose shape in lateral view, due to the dorsal margin being convex and the ventral margin being not concave (in this case it is straight and obliquely oriented). In particular the dorsal margin of the zygomatic process of
Ensidelphis draws a regular arch, as in most Platanidelphidi with the exception of
Pomatodelphis and
Zarhachis, having the posterior portion of the dorsal margin that slopes more abruptly posteriorly. In
Ensidelphis, the anterodorsal surface of the zygomatic process is tightly appressed to the postorbital process of the frontal, a feature due to the anterodorsal development of the zygomatic process and shared with all platanistoids and eurhinodelphinids. In lateral view the sternocephalicus fossa extends on the posteroventral portion of the zygomatic process as a narrow and elongate groove that forms an angle of about 65° with the horizontal plane. The postglenoid process is small and anteroventrally direct as in other Platanidelphidi. The squamosal plate is visible in lateral view, forming the ventral portion of the medial wall of the temporal fossa. This wall is locally laterally inflated, forming the peculiar temporal swelling mentioned above, with the maximum lateral expansion (better seen in posterior view) in correspondence to the squamosal-parietal suture. In
Platanista this suture line is only slightly swollen.
The ventral surface of the zygomatic processes is almost entirely covered by the articulated mandibular condyles (
Figure 3). The thin plate of the left falciform process can be observed, contacting anteriorly the posterolaterally elongated, plate-like lateral lamina of the pterygoid.
Parietal. Visible on the medial wall of the temporal fossa the parietal forms most of the lateral wall of the neurocranium (
Figure 4 and
Figure 5). The aforementioned temporal swelling involves also the ventral portion of the parietal exposed in the temporal fossa.
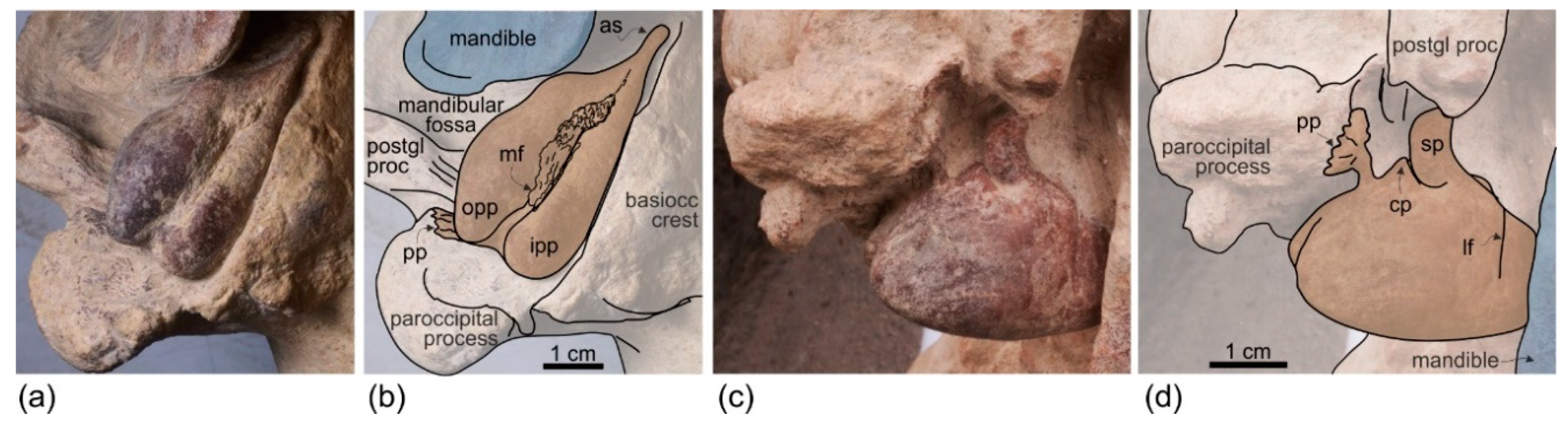
Exoccipital. The occipital condyles are large and posteriorly prominent: they are bordered dorsolaterally by well-excavated dorsal condyloid fossae (visible in posterior view:
Figure 6c,d) and ventrally by ventral condyloid fossae (visible in ventral view:
Figure 3). The foramen magnum is circular and the jugular notches are deeply incised. The paroccipital process is robust; in lateral view it is thicker and significantly more ventrally extended than the postglenoid process of the squamosal, a condition observed in all Platanidelphidi, with the exception of
Platanista, which has an atrophied paroccipital process and a large, ventrally extended postglenoid process.
Basioccipital. The basioccipital basin is transversely narrow, delimited laterally by robust basioccipital crests that are posterolaterally bent, drawing together a small angle (about 30°) (
Figure 3).
Vomer. The vomer is visible in ventral view and delimits posteriorly and medially each choana (
Figure 3).
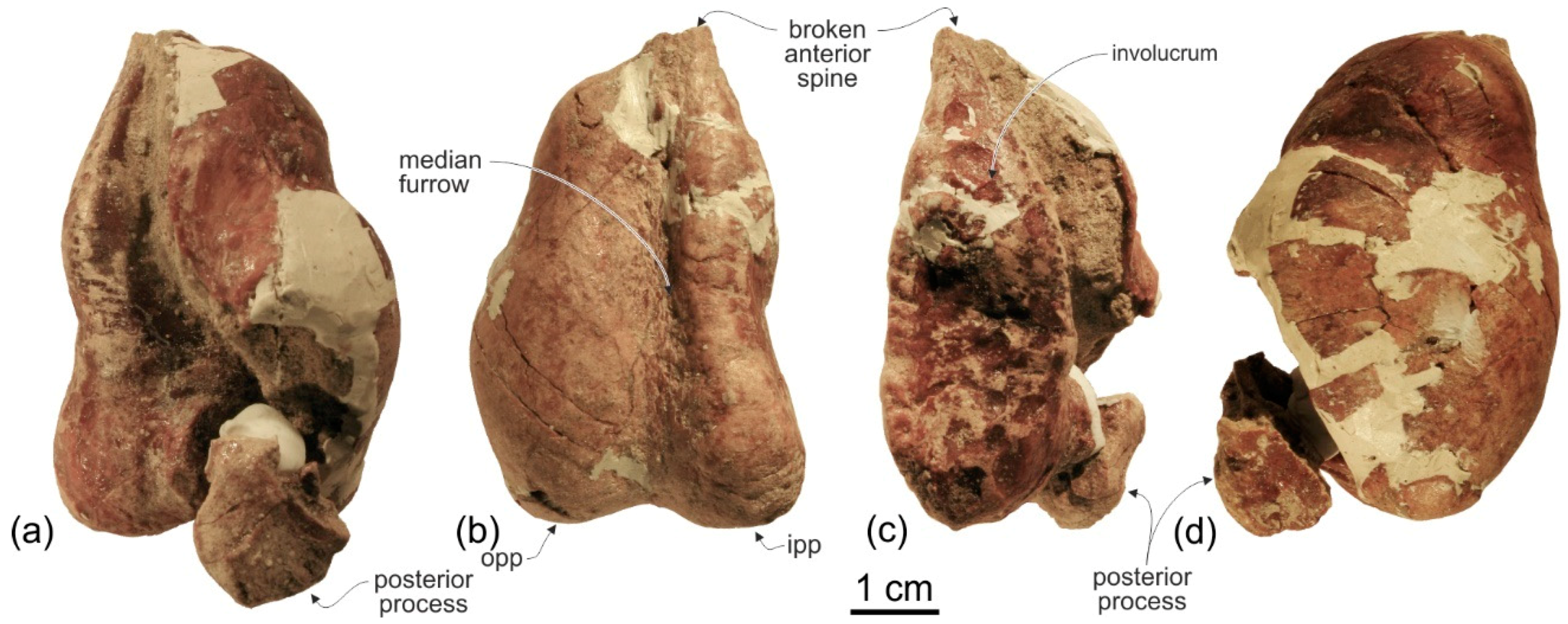
Tympanic bulla. Both tympanic bullae are preserved in situ, well exposed on the ventral surface of the skull (
Figure 3,
Figure 7a,b). They are also visible, partially covered by the mandibles, with the skull in lateral view (
Figure 4,
Figure 5,
Figure 7c,d). As in the platanistids
Platanista,
Pomatodelphis, and
Zarhachis, the median furrow is partially filled with spongy bone, and, unlike the squalodelphinids for which the tympanic bulla is preserved (
Notocetus,
Phocageneus, and
Squalodelphis), it does not extend anteriorly on the anterior spine (
Figure 7a,b). The outer posterior prominence is slightly shorter than the inner posterior prominence, a condition shared with the allodelphinids
Allodelphis, Ninjadelphis, and
Zarhinocetus, whereas in the squalodelphinids
Notocetus,
Phocageneus, and
Squalodelphis the outer and inner posterior prominences have approximately the same posterior extent, and in the platanistids
Platanista,
Pomatodelphis, and
Zarhachis the outer posterior prominence extends farther posteriorly than the inner prominence.
The anterior spine is thin and very long. A more or less elongated anterior spine is present in all platanistoids whose tympanic bulla is preserved, but we observed an extreme elongation as in
Ensidelphis (anterior spine ca 27% of the total length of the bulla) only in the tympanic bulla associated with the skull MUSM 603 (Figure 7 in [
24]). Interestingly enough, the tympanic bulla MUSM 603 shares other features with
Ensidelphis (e.g., spongy bones in the median furrow, outer posterior prominence posteriorly shorter than the inner posterior prominence), supporting a congeneric referral of the two specimens (see below). The partly exposed lateral surface of the tympanic bulla of
Ensidelphis (
Figure 4,
Figure 5,
Figure 7c,d) reveals a high and inflated outer lip, as in other platanistoids (e.g., [
92]). The conical process is moderately developed and almost in contact posteriorly with the elongated sigmoid process, the latter having its distal portion posterodorsally bent. The lateral furrow is clearly visible. A small portion of the posterior process of the tympanic bulla, articulated with the squamosal and the exoccipital, is also visible.
Mandible
The mandibles are tightly articulated with the cranium, the right and the left mandibular condyles being inside their respective mandibular fossae on the ventral surface of the zygomatic processes of the squamosals (
Figure 3). The two mandibles are fused together as in all other Platanistoidea having mandibles preserved and in the Eurhinodelphinoidea (Eurhinodelphinidae + Eoplatanistidae), but not in the longirostral homodont odontocete
Chilcacetus [
57]. In particular, along the 150 mm-long anterior portion of the symphysis the mandibles are ankylosed, with the medial suture being invisible in ventral view. The long mandibular symphysis represents 61% of the total length of the mandibles. Posterior to the symphysis the two mandibular rami draw an angle of 25° in ventral view. The same proportions of the symphysis and a similar angle between the rami are observed in all allodelphinids, whereas the platanistids
Araeodelphis,
Platanista, Pomatodelphis, and
Zarhachis have a more elongated symphysis (>65%) and a consequently larger angle between the mandibular rami (roughly 60°). Among other platanistoids the mandible is only well known in the squalodelphinids
Notocetus and
Squalodelphis, both having a shorter symphysis (40% and 43%, respectively) and an angle between the mandibular rami equal to 38°. These values are consistent with a rostrum significantly shorter than in
Ensidelphis.
The symphysis of
Ensidelphis is dorsoventrally flattened and it is longitudinally furrowed by two deep lateral grooves, one for each mandible, running from 65 mm from the anterior end of the mandible to the posterior end of the symphysis (
Figure 4a,b,
Figure 5a,b). Such grooves are present in all platanistoids whose mandible is preserved, with the exception of the squalodelphinids
Huaridelphis,
Notocetus, and
Squalodelphis.
In lateral view the height of the postsymphyseal portion of the mandible increases gradually posteriorly, with both the dorsal and ventral margins forming a low angle with the horizontal axis of the mandible. Among other platanistoids with a mandible preserved, a similar shape of the mandibular rami in lateral view is observed in the allodelphinid Goedertius and the squalodelphinid Notocetus, whereas a more abrupt posterior elevation of the dorsal margin is present in Zarhinocetus and, to an even greater extent, in Platanista and Zarhachis. The mandible of Squalodelphis is too damaged to assess this feature.
Dentition
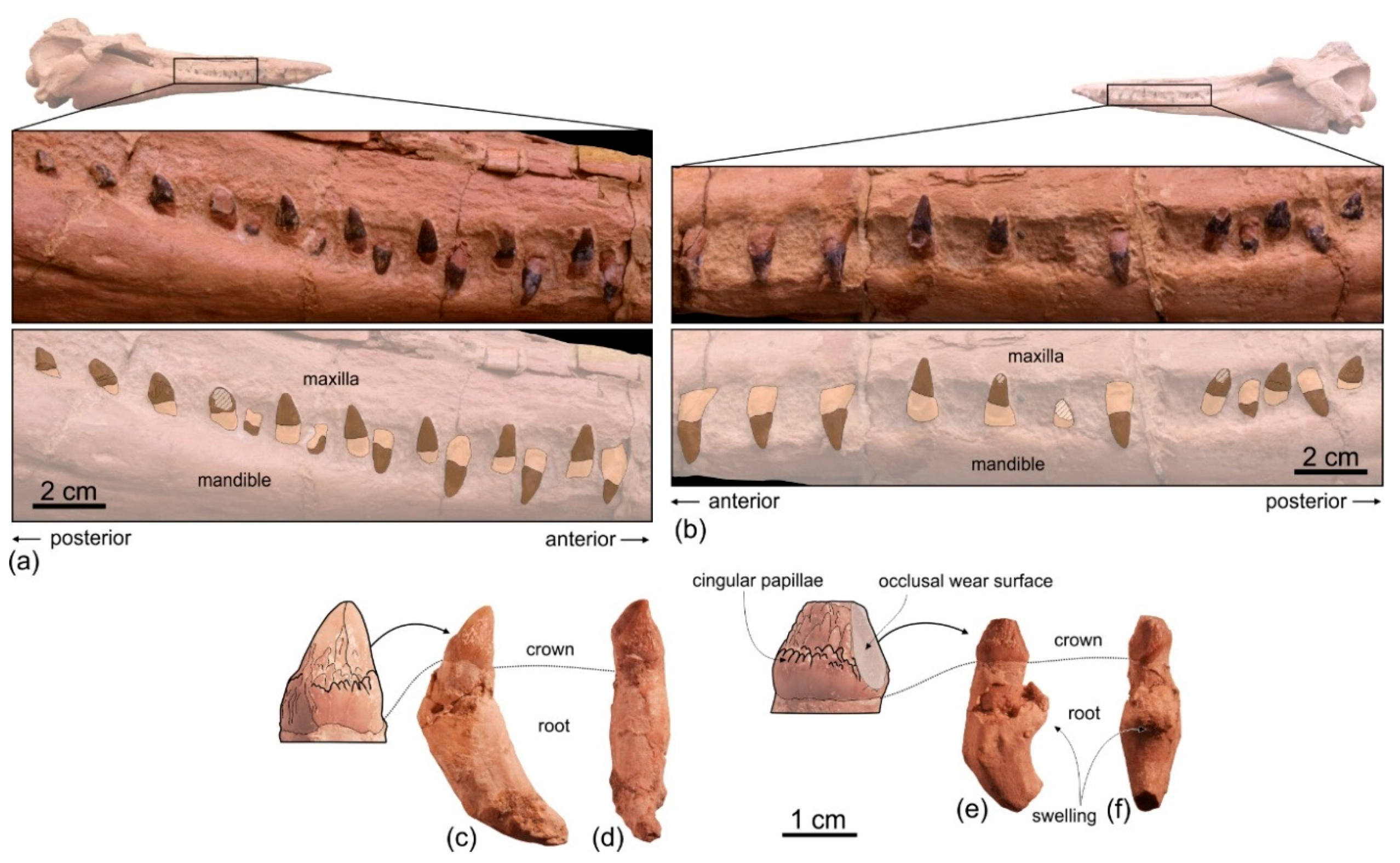
In ventral view on the right side of the rostrum, 54 alveoli are visible, the six anteriormost being in the premaxilla (
Figure 5a,b). However, this value does not represent the total tooth count of the upper right quadrant, considering that the posterior portion of the upper right alveolar row is covered by the mandible. The complete alveolar row is instead exposed on the left mandible (
Figure 4a,b); here 62 alveoli are counted and two additional alveoli are estimated to have been originally present in a 28 mm-long reconstructed mid-length portion of the rostrum, resulting in a total tooth count for each mandible of about 64. Since the exposed alveoli on the rostrum have roughly the same longitudinal length and the same spacing as on the mandible, approximately 64 teeth could also be inferred for each upper quadrant. Therefore, the total tooth count of
Ensidelphis is estimated at 256. A tooth count > 200 characterizes all hyper-longirostrine platanistoids, with a remarkable count of about 315 teeth in
Zarhachis [
111].
The alveoli are small and roughly circular, only slightly longer than transversely wide. Their transverse width varies between 2.4 and 4.4 mm, with an average value of 3.5 mm. The ratio between the transverse width for alveoli at mid rostrum length and the BZW is 0.018. Similarly, low values (<0.02) are observed in all other platanistoids, except in squalodelphinids (ratio > 0.03). The interalveolar septa range between 2.3 and 8.0 mm in length, with an average value of 5.5 mm. On each tooth row, the 6–8 anteriormost alveoli (for premaxillary teeth) are slightly smaller and less spaced than more posterior alveoli. However, with the exception of a few cases, interalveolar septa are longer than the adjacent alveoli, suggesting that when the mouth was closed interlocking teeth of the upper and lower tooth rows did not systematically contact each other. It is significant to underline that for example in the extant longirostrine dolphin Pontoporia blainvillei interalveolar spaces increase with the age of the animal (C.M. personal observation); therefore, in Ensidelphis riveroi this character could also be subject to ontogenetic variation.
Six teeth are preserved in their alveoli on the right mandible (
Figure 5a,b,
Figure 8): two in the anterior part of the symphyseal portion (sixth and seventh teeth from the apex) and four in the postsymphyseal portion. All preserved teeth are single rooted, with a simple conical crown lacking accessory denticles or cingula. However, it cannot be excluded that accessory denticles and/or cingula were present in the lost posteriormost teeth, as in some other platanistoids (e.g.,
Notocetus and
Phocageneus). The crowns of the two preserved anterior teeth are broken and only their basal portion is preserved; their diameter is 4.3 mm. The best-preserved posterior tooth is slender, having a diameter at the base of the crown of 2.8 mm; the height of its almost complete crown is 4.6 mm.
Cervical Vertebrae
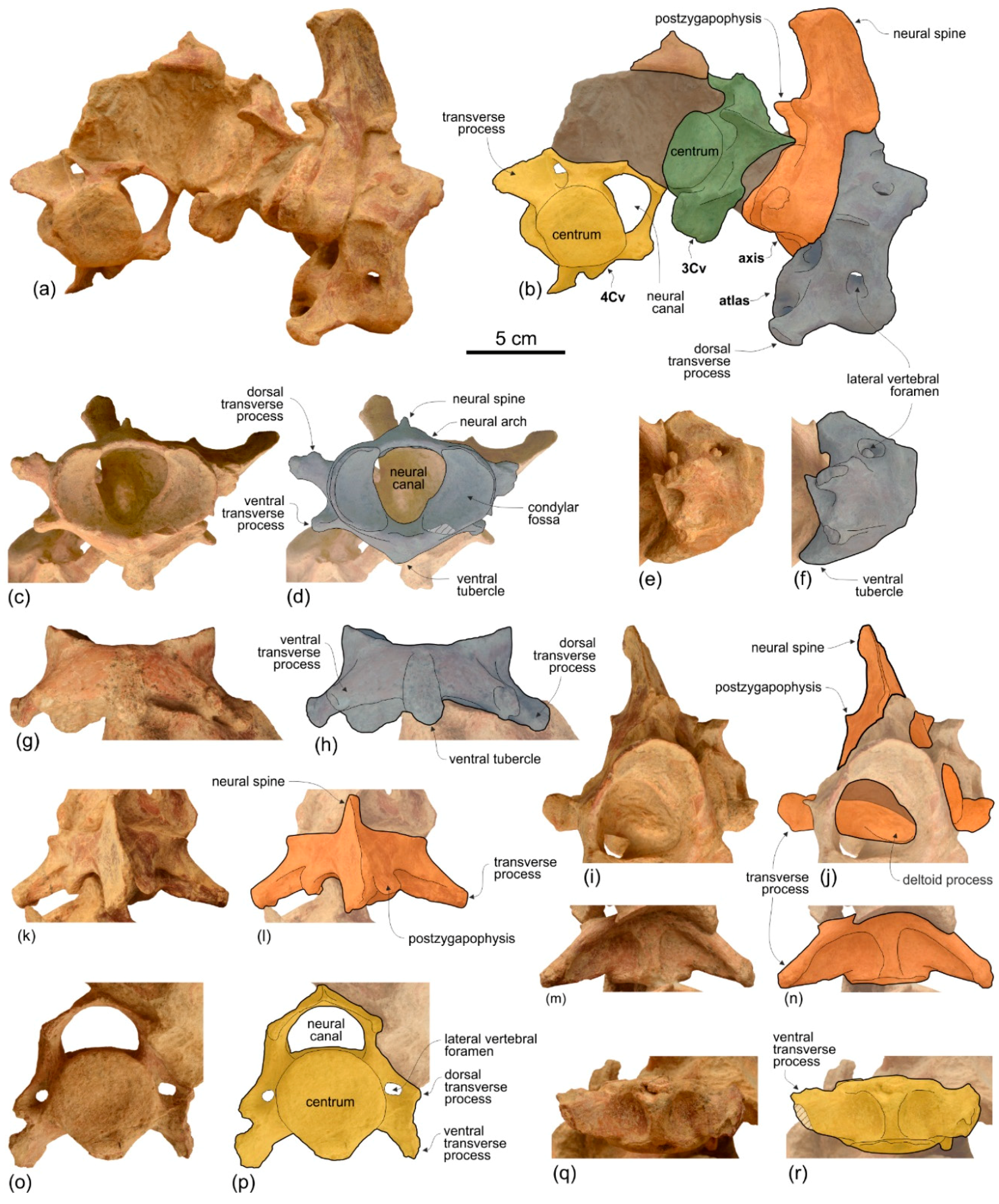
None of the atlas, axis, and two other cervicals were fused (
Figure 9). They were kept attached by sediment in their position as found in the field, which is not in anatomical connection but strictly associated. We think it is plausible that the original anatomical sequence was maintained and that, therefore, the two vertebrae posterior to the axis represent the third and fourth cervicals.
Atlas. The dorsal transverse processes of the atlas are slightly more elongated and robust than the ventral transverse processes (
Figure 9c–h). As far as the degree of reduction of the ventral transverse processes is concerned,
Ensidelphis is intermediate between squalodelphinids (
Huaridelphis,
Macrosqualodelphis, Notocetus, and
Phocageneus), all having an extreme reduction of this process, and the other platanistoids, all having similarly elongated dorsal and ventral transverse processes. As in
Macrosqualodelphis and
Notocetus the dorsal transverse processes are widely visible in anterior view, unlike in other platanistoids whose atlas is preserved. This is due to the less posteriorly projected condition of these processes. The neural canal is transversely compressed (ratio between width and height = 0.81), as in
Macrosqualodelphis (ratio 0.76), whereas it is circular or slightly dorsoventrally compressed in other platanistoids with the atlas preserved. The neural arch is low, pierced by large lateral vertebral foramina for the vertebrarterial canal, and with a transversally thin and short neural spine. The ventral tubercle is robust with a posteroventrally directed pointed tip. Measurements of the cervical vertebrae of MUSM 3898 are provided in
Table 2.
Axis. Among Platanidelphidi the axis is only known in
Araeodelphis (USNM 16569),
Huaridelphis (MUSM 1403, incomplete),
Platanista, and USNM 206006, an undescribed platanistid from the Calvert Formation (U.S.A.) showing affinities with
Pomatodelphis and
Zarhachis. The transverse processes of
Ensidelphis are similar to those of
Huaridelphis, being short and robust, triangular in dorsal and ventral view, and posteriorly projected (
Figure 9i–n). The transverse processes of
Araeodelphis,
Platanista, and USNM 206006 are slender, longer, and posteroventrally projected. The neural arch and the neural spine of
Ensidelphis are massive and anteroposteriorly thick in lateral view, whereas they are thin in
Araeodelphis and USNM 206006, and dorsoventrally short and overall triangular in
Platanista.
Third and fourth cervicals. Among Platanidelphidi, the cervical vertebrae posterior to the axis are only known in
Araeodelphis (USNM 16569),
Huaridelphis (MUSM 1403, incomplete),
Phocageneus (USNM 21036, only the third and fifth)
Platanista, and
Notocetus (only one in MUSM 1395). Better preserved than the third, the fourth cervical of
Ensidelphis shows the closest similarities with the third cervical of
Phocageneus, both having an almost circular centrum in anterior view, a wider than high neural canal, and a low and transversely wide medial keel (
Figure 9o–r). However, the ventral transverse processes in C4 of
Ensidelphis are more ventrally projected and the transverse foramina for the vertebrarterial canal are smaller than in the C3 of
Phocageneus. Cervicals of the allodelphinids
Allodelphis,
Goedertius, and
Ninjadelphis differ from the cervicals of
Ensidelphis and of the other Platanidelphidi by having significantly more anteroposteriorly elongated centra [
121].
Platanidelphidi indet.
Referred specimen, locality, and age. MUSM 3899 is an incomplete cranium with several missing parts including the anterior portion of the rostrum and the right orbital region. Moreover, several areas are damaged, such as the premaxillae on the rostrum, the vertex, and the supraoccipital, and the posterior half of the neurocranium is shifted to the left in respect to the sagittal plane, as clearly seen in ventral view. On the palatal surface of the rostrum 21 and 24 small alveoli for single-rooted teeth are counted on the right and left side, respectively. Earbones and teeth are not preserved. Zamaca locality, Western Ica Valley, Ica Region, Peru (
Figure 1a,b). Geographic coordinates: 14°37'28.77" S, 75°38'22.92" W; 345 m above sea level. This specimen was reported in the Zamaca fossil map of Di Celma et al. [
22] with the field number ZM 128 and provisionally referred to “Platanistoidea indet.” From the Chilcatay Fm, 38.1 m above the contact with the underlying Otuma Formation, in the Ct1a facies association of the Ct1 allomember [
21,
22] (
Figure 1d). The age of this portion of the Ct1a facies association can be constricted between 19.00 ± 0.25 Ma and 18.08 ± 0.07 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
Brief Description and Comparison
The long hamular fossa of the pterygoid sinus extending anteriorly on the palatal surface of the rostrum, the cranium distinctly shorter than wide, and the anterior portion of the zygomatic process of the squamosal tightly appressed to the postorbital process of the frontal are all derived characters allowing us to refer MUSM 3899 to the Platanistoidea superfamily (
Figure 10). In particular, MUSM 3899 belongs to the Platanidelphidi clade in having: (1) Asymmetry of the premaxillae on the rostrum at some distance anterior to the premaxillary foramina, with the right premaxilla being distinctly narrower than the left in dorsal view; (2) posterior dorsal infraorbital foramen along the vertex more medial than the lateralmost margin of the premaxilla in the cranium; (3) deep fossa in the frontal on the orbit roof, at the level of the frontal groove, presumably for an orbital lobe of the pterygoid sinus; (4) vertex distinctly shifted to the left compared to the sagittal plane of the skull; (5) palatine not exposed anterior to the pterygoid; (6) dorsoventrally thick zygomatic process of the squamosal; and (7) straight ventral edge of the zygomatic process in lateral view.
Within the Platanidelphidi MUSM 3899 shares affinities with Ensidelphis in its small size (BZW equals 183 cm in MUSM 3899 and 196 cm in the holotype of Ensidelphis riveroi; bicondylar width equals 79 cm in MUSM 3899 and 80 cm in the holotype of E. riveroi), the narrow and possibly elongated rostrum, the shape of the zygomatic process of the squamosal (with half-circle shaped dorsal margin and straight anteroventral margin), the moderately transversely wide dorsal opening of the mesorostral groove in the rostrum base area, the transversely wide and anteriorly located premaxillary foramen, the limited posterior extension of the ascending processes of the premaxillae, and the small maxillary alveoli (the average of the transverse diameter for the 21 posteriormost alveoli is 3.8 m in both MUSM 3899 and Ensidelphis). Moreover MUSM 3899 shares with Ensidelphis the absence of any of the derived characters distinguishing both the squalodelphinids and the platanistids within the Platanidelphidi. Nevertheless, MUSM 3899 differs from Ensidelphis in the dorsoventrally thinner supraorbital process of the frontal, the transversely narrower vertex (20% and 25% of the BZW in MUSM 3899 and Ensidelphis, respectively), the right and left posterior dorsal infraorbital foramina being more laterally located, and the apparent absence of the peculiar temporal swelling on the medial wall of the temporal fossa (but this area is badly preserved on both sides of MUSM 3899's cranium). Moreover, MUSM 3899 differs from Ensidelphis in the narrower space between the alveoli (the average length of interalveolar septa between the 21 posteriormost alveoli is 3.0 m in MUSM 3899 and 4.6 in the holotype of E. riveroi), although this character, as outlined above, could be subject to intraspecific ontogenetic variation. Based on these observations and considering the fragmentary state of the specimen, we assign MUSM 3899 to an indeterminate basal Platanidelphidi, pending the discovery of more complete specimens.
Squalodelphinidae Dal Piaz, 1917
Emended diagnosis. The Squalodelphinidae have the following synapomorphies, absent in other members of the Platanidelphidi clade: (1) Deep, V-shaped, left antorbital notch, related to an anteriorly pointed left antorbital process; (2) left-side torsion of the rostrum with the longitudinal axis of the neurocranium forming an angle of about 5° with the main axis of the rostrum in dorsal view, generating asymmetry of the posterior portion of the rostrum; (3) pars cochlearis of the periotic square-shaped in ventral view; (4) large and thin-edged aperture of the cochlear aqueduct of the periotic; (5) median furrow of the tympanic affecting the whole length of the bone, including the anterior spine; (6) apical extension of the manubrium of the malleus; (7) strong development of the dorsal transverse process of the atlas and extreme reduction of its ventral process.
Type genus. Squalodelphis Dal Piaz, 1917
Other genera included.Furcacetus gen. nov., Huaridelphis, Macrosqualodelphis, Medocinia, Notocetus, Phocageneus.
Furcacetus, gen. nov.
LSID: zoobank.org:act: 7B69F853-98CE-4824-88AC-3294D1B0580D
Type and only known species. Furcacetus flexirostrum, sp. nov.
Diagnosis. As for the type and only referred species.
Etymology. From ‘furca’, fork in Latin, and ‘cetus’, whale in Latin. For the procumbent anterior upper teeth, which, together with the rostrum, look like a fork. Gender masculine.
Furcacetus flexirostrum, sp. nov.
LSID: zoobank.org:act: B290D90E-D943-4768-956F-28DD1DF75988
Holotype and only referred specimen. MUSM 487 consists of a cranium damaged in some parts; in particular the left lateral and the posteroventral portions of the neurocranium are missing. Eight broken teeth are inside their alveoli on the rostrum (five on the maxilla and three on the premaxilla). The incomplete right periotic is still articulated to the cranium.
Type locality. MUSM 487 was collected several years ago from layers of the Chilcatay Fm in the Zamaca-Ullujaya area, western Ica Valley, Ica Region, southern Peru (
Figure 1a,b). Approximate geographic coordinates: 14°36’ S, 75°38’ W.
Type horizon. The exact horizon of the Chilcatay Fm where the holotype of
Furcacetus flexirostrum, MUSM 487, was discovered is unknown. Nevertheless, the entire stratigraphical sequence of the Chilcatay Fm exposed at Zamaca and Ullujaya localities has been roughly constricted through radiometric dating of ash layers to an interval between 19.25 ± 0.05 and 18.02 ± 0.07 Ma (early Burdigalian) [
89].
Diagnosis. Furcacetus is a small odontocete having an asymmetrical cranium with a narrow and moderately elongated rostrum (about 68% of the CBL), bearing about 25 single-rooted teeth in each upper quadrant. Its rostrum differs from that of all other Platanistoidea s.s., as defined above, in having a sigmoid shape in lateral view and bearing procumbent anterior teeth. Furcacetus shares with the other platanistoids the elongated hamular fossa of the pterygoid sinus extending anteriorly on the palatal surface of the rostrum and the neurocranium being shorter than wide (ratio < 0.90). Furcacetus belongs to the Platanidelphidi clade in having: asymmetry of the premaxillae on the rostrum at some distance anterior to the premaxillary foramina (ca 18 cm in this case), with the right premaxilla distinctly narrower than the left in dorsal view; posterior dorsal infraorbital foramen along the vertex more medial than the lateralmost margin of the premaxilla on the cranium; deep fossa in the frontal on orbit roof, at the level of the frontal groove; vertex distinctly shifted to the left compared to the sagittal plane of the skull; and thick zygomatic process of the squamosal (ratio between the maximum distance from the anteroventral margin of the zygomatic process to the posterodorsal margin, in lateral view, and the vertical distance from the lower margin of the occipital condyles to the vertex of the skull > 0.35). Furcacetus is referred to the Squalodelphinidae in having: pars cochlearis of the periotic square-shaped in ventral view; and longitudinal axis of the neurocranium forming an angle of about 5° with the main axis of the rostrum in dorsal view, generating asymmetry in the posterior portion of the rostrum.
It differs from all other squalodelphinids in having a greater asymmetry of the ascending processes of the premaxillae, the left process being significantly wider and more posteriorly extended than the right; and in having about 25 teeth for upper quadrant, a tooth count that is greater than in Notocetus (22–23) and Squalodelphis (15), and lower than in Dilophodelphis (ca 35) and Huaridelphis (28–30) (exact tooth count unknown in Macrosqualodelphis, Medocinia, and Phocageneus). It shares with Macrosqualodelphis and Notocetus the anteroposteriorly elongated temporal fossa (ratio between horizontal width and vertical height = 1.42) and the correspondingly elongated zygomatic process of the squamosal. It shares with Dilophodelphis and Huaridelphis a deep, V-shaped right antorbital notch drawing an angle of about 60°. It shares with Notocetus the transverse widening of the premaxillae in the anterior portion of the rostrum. It shares with Dilophodelphis and Medocinia a marked dorsoventral thickening of the preorbital process of the frontal. It differs from Medocinia, and Squalodelphis in the narrower transverse opening of the mesorostral groove near the rostrum base and in the lesser transverse widening of the premaxilla at rostrum base.
Etymology. From ‘flexus’, bent in Latin, and ‘rostrum’. For the sinusoidal shape of the rostrum in lateral view of the cranium.
Description and Comparison
Ontogeny. The closed sutures of the cranial bones, the medial fusion of the frontals on the vertex, and the well-individualized alveoli on the rostrum suggest that the holotype and only referred specimen of Furcacetus flexirostrum MUSM 487 was an adult animal.
Total body length estimate. Estimating the BZW of the holotype MUSM 487 (left zygomatic process of the squamosal lost) at 240 mm, we used the equation proposed by Pyenson and Sponberg [
110] for stem Platanistoidea to obtain an approximate TBL of 234 cm for
Furcacetus flexirostrum, a value slightly lower than the TBL of
Notocetus vanbenedeni (237–255 cm based on the BZW of MUSM 3896, and MUSM 3897).
Cranium
General morphology. By adding about 20 mm to the length for the missing part of the occipital condyles, the cranium of
Furcacetus could have reached a CBL of roughly 585 mm, 67% of which being occupied by the rostrum (
Table 3). These values are in the range of
Notocetus (CBL = 580–634 mm; rostrum = 62%–68% of CBL). CBL of other squalodelphinids is either greater (
Macrosqualodelphis: > 770 mm;
Squalodelphis: 640 mm) or lesser (
Huaridelphis: 494 mm;
Dilophodelphis: 440) than in
Furcacetus, but in all of them the rostrum is moderately elongated (63–70% of CBL), as in
Furcacetus.
The rostrum of
Furcacetus has a peculiar sinusoidal shape in lateral view (
Figure 13a–d): from its base, it curves upward until about 50 mm from the apex, where it curves downward until its anterior end. The 50 mm downward-bent anterior portion of the rostrum is formed by the premaxillae only and hosted procumbent incisors (
Figure 13e,f). Among other squalodelphinids, we also observed a curved (but not sinusoidal) rostrum in the cranium MUSM 1403 of
Huaridelphis raimondii (but not in the holotype) and in the crania MUSM 1395 and MUSM 3897 of
Notocetus vanbenedeni (but not in the holotype and the other referred specimens). Among extant odontocetes, although not associated with procumbent incisors a sinusoidal shape of the rostrum was observed in a few crania of
Pontoporia blainvillei [
114], whereas a rostrum raising anterodorsally, but lacking the anterior downward counter-curvature and, again, the procumbent incisors, was noted in one cranium of the brevirostrine pontoporiid
Brachydelphis mazeasi [
122] and in some crania of
Platanista gangetica [
113,
123] and of few delphinid species (e.g.,
Delphinus delphis MZUF 12484, G.B. personal observation;
Delphinus capensis, Figure 2 in [
124] ).
The rostrum of
Furcacetus is dorsoventrally compressed in its anterior and posterior portions, whereas it becomes more laterally compressed towards mid-length. A deep transverse concavity, involving both the maxillae and the premaxillae, occurs on the dorsal surface of the cranium around the base of the rostrum (
Figure 11,
Figure 13c). This concavity is laterally overhung by the elevated antorbital regions, which, as in all Platanidelphidi, are distinctly higher than the dorsal margin of the rostrum base in lateral view.
The right antorbital notch is deep and V-shaped, drawing an angle of about 60°, as in Dilophodelphis and Huaridelphis, whereas other squalodelphinids have a more open right antorbital notch. The left antorbital notch is not preserved and, consequently, it is not possible to check if the right and left antorbital notches were asymmetrical, as in most other platanistoids.
As mentioned above, the original BZW of Furcacetus is estimated at 240 mm, suggesting that the neurocranium was proportionally short (79% of BZW), a condition observed in all Platanistoidea.
The asymmetry of the cranium of Furcacetus is remarkable, as in most other Platanidelphidi. This asymmetry concerns: (1) The vertex and the bony nares, being shifted to the left side; (2) the right bony naris being transversely broader and more posteriorly located than the left; (3) the left premaxilla being markedly more posteriorly extended than the left; (4) the left frontal at the vertex being anteroposteriorly shorter than the right; and (5) the missing left nasal being probably originally smaller than the right. Moreover, as in all other squalodelphinids the asymmetry of the cranium of Furcacetus involves also the posterior portion of the rostrum due to the left lateral shift of the rostrum (main axis of the rostrum in dorsal view forming an angle of about 5° with the longitudinal axis of the neurocranium).
The temporal fossa is significantly elevated (ratio between the vertical height of the fossa, in lateral view, and the vertical distance from the lower margin of the occipital condyles to the vertex of the skull = 0.70), as in
Ensidelphis,
Macrosqualodelphis,
Notocetus, and
Platanista. The temporal fossa of
Furcacetus is also anteroposteriorly elongated (ratio between the horizontal length and vertical height = 1.42), similarly to
Macrosqualodelphis and
Notocetus [
26].
Premaxilla. The anterior portion of the rostrum is formed by the premaxilla alone for 50 mm (
Figure 11), a condition also observed in all squalodelphinids having the apex of the rostrum preserved (
Dilophodelphis,
Huaridelphis,
Notocetus, and
Squalodelphis), and in
Ensidelphis. Partially related to this feature, in dorsal view the lateral premaxilla-maxilla suture is laterally bent and the premaxilla widens transversely towards the apex of the rostrum. A similar widening is observed in
Notocetus, as well as in squalodontids; in the latter it is similarly associated with procumbent premaxillary teeth (e.g., [
98]).
As outlined above, this anterior premaxillary portion of the rostrum is curved downward, dorsoventrally compressed, and bears three alveoli on each side (
Figure 13).
The dorsal surface of the anterior portion of the rostrum is poorly preserved, but a foramen that pierces the left premaxilla is clearly visible 55 mm posteriorly to the apex. Foramina piercing the premaxillae on the anterior portion of the rostrum are also observed in Araeodelphis, Dilophodelphis, and Notocetus.
The dorsal opening of the mesorostral groove is very narrow (maximum transverse width = 2 mm) on the 160 mm-long anterior portion of the rostrum; the mesorostral groove is fully closed dorsally, in the middle portion of the rostrum, for a tract 75 mm long, then the premaxillae gradually diverge towards the base of the rostrum, although the opening remains narrow for the whole posterior tract of the mesorostral groove, reaching a maximum transverse width of 8 mm. Among other squalodelphinids, a similarly narrow opening of the mesorostral groove near the rostrum base is present in Huaridelphis, Macrosqualodelphis, and Notocetus, whereas Medocinia and Squalodelphis display a wider opening.
The premaxilla–maxilla suture is distinct along the whole rostral length, but it is not located in a deep lateral groove as in the platanistids Platanista, Pomatodelphis, and Zarhachis.
The right and the left premaxillae on the rostrum maintain almost the same transverse width for their whole anteroposterior extension, with the right premaxilla only slightly narrower than the left near rostrum mid-length. The asymmetry of the premaxillae, a peculiar feature of the Platanidelphidi, is more pronounced in other squalodelphinids, as for example Notocetus.
At the base of the rostrum the premaxilla is transversely wider than the maxilla as in most other squalodelphinids, but not as much as in Medocinia and Squalodelphis. At this level each premaxilla exhibits a marked medial slope, bounding a deep medial depression that extends posteriorly on the neurocranium, with the premaxillary sac fossae also ventromedially sloping.
The premaxillary foramina (one on each premaxilla) are located roughly at the level of the right antorbital notch, a condition observed, among other platanistoids, in Dilophodelphis, Macrosqualodelphis, Platanista, and the Notocetus skulls from the Chilcatay Fm.
The weakly excavated premaxillary sac fossa is laterally delimited by a wide and deep posterolateral sulcus that ends posteriorly where the premaxilla reaches it maximum transverse width. The anteromedial sulcus is shallower than the posterolateral sulcus and, as in Huaridelphis, Macrosqualodelphis, and Notocetus, is significantly elongated, extending about 120 mm anterior to the premaxillary foramen. The posteromedial sulcus is not clearly discernible.
The anterior limit of the bony nares is defined by an angle of the medial margin of each premaxilla, the angle of the right premaxilla being 20 mm posterior to the angle of the left, generating the marked asymmetry of the bony nares mentioned above.
The right and left ascending processes of the premaxilla are strongly asymmetrical. The right process ends with a posteromedial point that contacts the anterolateral angle of the right nasal. The left process extends significantly more posteriorly, far beyond the nares; it was probably medially in contact with the lost left nasal, and reaches posteriorly the frontal. Among other squalodelphinids, an asymmetry of the ascending processes of the premaxillae is also observed in Dilophodelphis, Huaridelphis, and Notocetus, but less marked than in Furcacetus, being limited to a more pointed end of the right ascending process (left process not significantly longer than the right). Unlike in other squalodelphinids and some platanistids (e.g., Zarhachis), there is no trace of a longitudinal groove on the posterior portion of the ascending processes of the premaxillae of Furcacetus MUSM 487.
In ventral view (
Figure 12), on the rostrum the premaxilla–maxilla suture runs obliquely from the posterior margin of the third incisor to a medial point located 138 mm anterior to the right antorbital notch. Consequently, the premaxillae display a narrow and elongated ventral exposure between the palatal processes of the maxillae.
Maxilla. The rostral portions of the maxillae are not well preserved, with some missing parts, especially for the left maxilla (
Figure 11). However, it is clearly discernible that the transverse width of the dorsal exposure of the maxilla remains narrow for the entire length of the rostrum. Moreover, for most of its anteroposterior extension on the rostrum, the maxilla appears to slope laterally. Approaching the antorbital notch, the maxilla first becomes flat and horizontal, then slopes medially, forming, with the premaxilla, the deep dorsomedian depression at the base of the rostrum.
The only dorsal infraorbital foramen discernible in MUSM 487 is a small posterior foramen piercing the ascending process of the right maxilla very close to the posterior end of the right premaxilla and more medial than the lateralmost margin of the premaxilla. A similar position of the posterior dorsal infraorbital foramen is observed in all the known skulls of Platanidelphidi for which this area is well preserved.
Apparently, the maxilla displays a limited anterolateral extension above the preorbital process of the frontal, although the maxilla–frontal suture it is not clearly discernible on the right side of the cranium and the left side is incompletely preserved. Above the orbit, the right maxilla exhibits a longitudinal bulge posterolateral to the antorbital notch (
Figure 13c). A similar bulge is present in other squalodelphinids, but less marked, except in
Dilophodelphis (thickening significantly greater than in
Furcacetus) and
Squalodelphis (thickening similar to
Furcacetus). It is important to note that the comparison was made using the right side of the neurocranium and that in other squalodelphinids the right preorbital + orbital region is less elevated than the left. It is therefore probable that the thickening on the partly preserved left side of MUSM 487 was even greater than that of the right side. Be that as it may, the thickening observed in
Furcacetus does not produce an individualized crest forming an acute angle in cross section as observed in the platanistids
Platanista,
Pomatodelphis, and
Zarhachis. As in other squalodelphinids, a marked asymmetry characterizes the posteromedial portion of the ascending processes of the maxillae lateral to the vertex, the right maxilla being significantly anteroposteriorly longer than the left maxilla. Both posteromedial portions of the maxillae slope steeply laterally from the vertex, with the right maxilla being almost vertical.
In ventral view (
Figure 12), the palatal surface of the rostrum is mainly formed by the maxillae. It is flat along its anterior two thirds, becoming weakly transversely convex towards the base of the rostrum. Badly preserved and partly covered by sediment, the lateral portions of the palatal surface of the maxillae display relatively large alveoli, some of them being filled by broken teeth, as described in detail below.
Presphenoid and cribriform plate. The nasal septum separating the asymmetrical nares is well ossified and elevated (
Figure 11). Its posterodorsal margin (medial portion of the cribriform plate) is in contact with the right nasal.
Nasal. Only the right nasal is preserved. In dorsal view (
Figure 11), it is weakly inflated, rectangular, with a main axis that is obliquely oriented in respect to the frontal plane of the skull. Similar features are observed in
Huaridelphis,
Macrosqualodelphis, and
Notocetus. In lateral view (
Figure 13a,b), the nasal of
Furcacetus appears to slope anteriorly (at least in its anterior portion) as in
Huaridelphis, but not in
Macrosqualodelphis and
Notocetus, having the dorsal surface of the nasal roughly horizontal. The lost left nasal was probably smaller than the right, judging by the size and the shape of the depressed area between the right nasal and the medial margin of the posterior portion of the left premaxilla. If this interpretation is correct, the asymmetry of the nasals in
Furcacetus is opposite to the asymmetry observed in the other squalodelphinids, all of them having the left nasal slightly larger than the right. The nasal–frontal suture is sinusoidal, with a weak anteromedial convexity, resembling the holotype of
Huaridelphis raimondii MUSM 1396 and differing from
Macrosqualodelphis (suture roughly straight),
Notocetus (suture distinctly anteromedially pointed), and
Medocinia (suture posteromedially pointed).
Frontal. At the vertex, the lateral margin of the right frontal is longitudinally more elongated than the left (
Figure 11), a condition shared with
Dilophodelphis,
Huaridelphis,
Notocetus, and
Squalodelphis. The medial suture between the frontals at the vertex is not discernible. In lateral view (
Figure 13a,b), the dorsal surface of the frontals at the vertex appears horizontal, as in
Notocetus, but not in
Huaridelphis and
Macrosqualodelphis, both having frontals anteroventrally sloping.
On the anterolateral portion of the neurocranium, the frontal is widely exposed dorsally, since the maxilla only partially covers the supraorbital and preorbital processes, a condition also observed in
Dilophodelphis, among other squalodelphinids. In lateral view both these processes appear dorsoventrally thickened. The thickening of the preorbital process is greater than in all other squalodelphinids except
Dilophodelphis, Medocinia, and the possibly related skull USNM 475596 [
24,
126]. A more developed preorbital process is also seen in the platanistids
Pomatodelphis and
Zarhachis. The postorbital process of the frontal of
Furcacetus is robust and trapezoidal in lateral view, similar to that of
Notocetus. The medial portion of the ventral surface of the frontal of
Furcacetus is excavated in the orbit region by a deep and obliquely oriented fossa (
Figure 12). A similar fossa, probably corresponding to an extension of the pterygoid sinus in the orbit region, has been observed in all other Platanidelphidi.
Supraoccipital. The supraoccipital is poorly preserved. The eroded nuchal crest is roughly straight in dorsal view (
Figure 11). The supraoccipital slopes posteriorly from the vertex with its posterodorsal surface drawing, in lateral view, an angle of ca 50° with the horizontal plane (
Figure 13a,b). A similar inclination of the supraoccipital is observed in
Notocetus, whereas
Huaridelphis and
Macrosqualodelphis display a lower inclination and
Squalodelphis an almost vertical supraoccipital.
Palatine. The palatines are not discernible on the ventral surface of the skull (
Figure 12). Their anterior portions are probably fully covered by the pterygoids, as generally observed in other Platanidelphidi. Lateral to the pterygoids, the ventral surface of the skull is damaged and partially covered by sediment; it does not show any trace of the lateral exposure of the palatines.
Pterygoid. On the posterior palatal surface of the rostrum (
Figure 12), the right and left pterygoids are medially sutured and each is excavated by a pterygoid sinus fossa extending about 30 mm anterior to the right antorbital notch. An elongated hamular fossa of the pterygoid sinus, extending anterior to the rostrum base, is a derived feature shared by all platanistoids. The lateral lamina of the pterygoid runs posterolaterally, reaching the anterior margin of the falciform process of the squamosal.
Jugal–Lacrimal. There is no trace of the jugals and lacrimals. Nevertheless, the ventral surface of the well-preserved preorbital process of the right frontal is marked by a deep oblique groove that represents the suture for the missing lacrimal (
Figure 12). This suture indicates that the lacrimal was anteroposteriorly narrow along the lateral wall of the antorbital notch, as in all other platanistoids.
Squamosal. In lateral view (
Figure 13a,b), the zygomatic process of the squamosal is robust and displays a convex dorsal margin, two features shared with all Platanidelphidi. More specifically, the dorsal margin of the zygomatic process of
Furcacetus is more similar to that of
Medocinia, contrasting with those, more regularly arched, of other squalodelphinids. The anterodorsal surface of the zygomatic process of
Furcacetus tightly contacts the postorbital process of the frontal, a feature related to the anterodorsal extension of the zygomatic process and shared with all platanistoids and eurhinodelphinids. The anteroventral margin of the zygomatic process it not preserved in the holotype of
Furcacetus flexirostrum. The anteroposterior elongation of the process is significant, similar to that observed in
Macrosqualodelphis and
Notocetus, but not in
Dilophodelphis,
Huaridelphis and
Ensidelphis, all having a shorter zygomatic process. In ventral view, posteromedial to the mandibular fossa a longitudinally elongated tympanosquamosal recess is visible. The falciform process projects anteromedially and articulates with the lateral lamina of the pterygoid.
Parietal. In lateral view (
Figure 13a,b), the parietal is widely exposed in the temporal fossa. There is no trace of the peculiar temporal swelling observed in
Ensidelphis.
Exoccipital. The whole left exoccipital is lost and the right paroccipital process is badly preserved (
Figure 13c). A shallow dorsal condyloid fossa is visible dorsolateral to the broken right occipital condyle.
Basioccipital. The basioccipital is damaged, but the right basioccipital crest is partially preserved (
Figure 12). The angle between right and left basioccipital crests in ventral view is estimated to about 40°.
Vomer. The vomer is exposed in ventral view (
Figure 12), posterior and medial to each choana, and on the palatal surface of the rostrum, between the maxillae, for a tract extending from roughly 100 to 150 mm anterior to the right antorbital notch.
Periotic. The incomplete right periotic is preserved in articulation with the corresponding squamosal (
Figure 12). The posterior process is lost and the pars cochlearis is damaged. As in all other squalodelphinids, the anterior process is elongate and not transversely thickened; it does not display the peculiar marked anteromedial bending that characterizes platanistids, and its ventral surface is excavated by an elongate and deep anterior bullar facet. The posterior portion of the pars cochlearis is lost, but its well-preserved anterior wall is rectilinear, suggesting that originally this part of the periotic was square-shaped in dorsal and ventral view, as in all other squalodelphinids.
Dentition
The estimated tooth count for each upper quadrant is 25 (
Figure 12). Excluding the longirostrine platanistoids, all having smaller and more numerous teeth, the tooth count of
Furcacetus is slightly higher than in
Notocetus (18−23), significantly higher than in
Squalodelphis (15), and lower than in
Araeodelphis (ca 50),
Dilophodelphis (ca 35), and
Huaridelphis (28−30).
The transverse diameter of the preserved alveoli ranges from 4.5 to 7.2 mm and the spacing between the alveoli increases posteriorly; the premaxillary alveoli are almost in contact and the alveoli at rostrum mid-length are about 15 mm from each other. The posterior alveoli are either not preserved or covered by hard sediment. The ratio between the maximum transverse width for alveoli at rostrum mid-length and the estimated BZW is 0.025, a value similar to
Huaridelphis (0.026) and smaller than in
Macrosqualodelphis (0.042) and
Notocetus (0.040). Judging by the marked oblique orientation of the axis of the three roots embedded in the premaxillae, the incisors (premaxillary teeth) were anterolaterally procumbent (
Figure 13e,f), a condition absent in all other Platanistoidea s.s. and more similar to
Waipatia and squalodontids. The maximum diameter of the root of these incisors is 7 mm. The posteriormost preserved tooth, located about 100 mm anterior to the right antorbital notch, displays a small proximal portion of crown (
Figure 13g,h). In particular, only the lateral surface of the crown is well preserved, showing cusp-like cingular nodules and enamel ornamented with longitudinal striations. The maximum diameter of the root and crown of this tooth is 6.0 and 6.5 mm, respectively. About 70 mm anterior to this tooth, along the upper alveolar groove, another tooth has a root with a diameter of 6.0 mm and a crown without accessory denticles (
Figure 13a,b).
Notocetus Moreno, 1892
Type and only included species. Notocetus vanbenedeni Moreno, 1892.
Notocetus vanbenedeniMoreno, 1892
Holotype. MLP 5-5, skull including the mandible but without ear bones; early Miocene of Chubut Province, Argentina [
127,
128,
129].
Previously referred specimens. AMNH 9485, skull including the right tympanic bulla, mandibles and some vertebrae and ribs; lower Miocene, Santa Cruz Province, Argentina [
129]; AMNH 29026, fragmentary skull including the squamosal, part of the exoccipital, the right periotic, tympanic bulla and malleus, several teeth, a scapula and fragments of vertebrae and ribs; lower Miocene, Chubut Province, Argentina [
92]. MUSM 1395, incomplete cranium with associated periotics and without teeth, and one cervical vertebra of the same animal [
25]; Ullujaya, Western Ica Valley, Ica Region, Peru, from an unknow horizon of the Chilcatay Fm. The age of the Chilcatay Fm exposed at Ullujaya can be constricted between 19.00 ± 0.25 Ma and 18.02 ± 0.07 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
New referred specimens, localities, and ages. MUSM 3896 (
Figure 14a–e,
Figure 15a,b), an almost complete skull including the cranium (only the jugals are missing, the dorsal surface of the vertex is covered by a concretion, and the dorsal surface of the rostrum at mid-length is damaged), fused mandibles in articulation with the cranium, articulated ear bones, and presumably all upper and lower teeth in their respective alveoli. Zamaca locality, western Ica Valley, Ica Region, Peru. Geographic coordinates: 14°37'28.77" S, 75°38'22.92" W; 340 m above sea level. This specimen was reported in the Zamaca fossil map of Di Celma et al. [
22] with the field number ZM 47 and provisionally referred to “
Notocetus sp.”. From the Chilcatay Fm, 7 m above the contact with the underlying Otuma Formation, in the Ct1c facies association of the Ct1 allomember [
21,
22]. The age of the Ct1c facies association is constricted between 19.25 ± 0.08 Ma and 19.00 ± 0.28 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89]. More details for this age are reported above in the horizon and age description of the holotype of
Ensidelphis riveroi found ca 3 m above MUSM 3896 in the same locality.
MUSM 3897 (
Figure 14f–k,
Figure 15c–f), a cranium with eroded vertex, lacking jugals and ear bones, and without teeth in situ; incomplete mandibles fused, but not articulated to the cranium and with two teeth inside alveoli; and 4 detached teeth, all belonging to the same animal. Zamaca locality, Western Ica Valley, Ica Region, Peru. Geographic coordinates: 14°37'18.05" S, 75°38'34.55" W; 340 m above sea level. This specimen was recently discovered and consequently it was not reported in the previously published Zamaca fossil map [
22]. From the Chilcatay Fm, 35 m above the contact with the underlying Otuma Formation, in the upper portion of the Ct1a facies association of the Ct1 allomember [
21,
22]. The age of this portion of the Ct1a facies association can be constricted between 19.00 ± 0.25 Ma and 18.08 ± 0.07 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
MUSM 1484 (
Figure 16 and
Figure 17) consists of the left tympanic bulla, the incomplete fused mandibles, one tooth, the atlas, the right and left humeri, the left radius and the left ulna of a single individual. Other bones of the same animal, including some vertebrae and ribs are still in the field (Figure 8h–i in [
20]); the cranium is not preserved. Ullujaya locality, western Ica Valley, Ica Region, Peru. Geographic coordinates: 14°34'50.8" S, 75°38'44.9" W; 325 m above sea level. This specimen was reported in the Ullujaya fossil map [
21] with the field number O4 and provisionally referred to “Squalodelphinidae indet.” From the Chilcatay Fm, 27.9 m above the base of the exposed section at Ullujaya, in the Ct1a facies association of the Ct1 allomember [
21,
22]. The age of the Chilcatay Fm exposed at Ullujaya can be constricted between 19.00 ± 0.25 Ma and 18.02 ± 0.07 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
Brief Description and Comparison of the Newly Referred Specimens
MUSM 3896 and MUSM 3897 fall in the size, shape, and measurements ranges of variation of the skulls previously referred to
Notocetus vanbenedeni (
Table 3). In fact, both skulls show the diagnostic characters of
N. vanbenedeni as redefined by Bianucci et al. [
25] for the description of MUSM 1395. The only significant difference is observed in the tooth count for the upper quadrant of the skull MUSM 3897, being smaller (18) and out of the range of variation (21–23) of the two Argentinian specimens (tooth count unknown in MUSM 1395, 1484, and 3896). Associated with a larger size of the alveoli, this difference could be due to intraspecific variation as observed in the extant
Platanista gangetica, for which the tooth count of the upper quadrant varies from 26 to 39 [
23].
This new Zamaca material confirms that
Notocetus was a squalodelphinid with a powerful feeding apparatus characterized by robust mandibles, large, interlocking, and moderately heterodont teeth, and anteroposteriorly elongated temporal fossa and zygomatic process of the squamosal. These characters are particularly evident in MUSM 3896 (
Figure 14a–e), the only skull of the species that preserves the complete mandibles firmly articulated to the cranium (and probably all the teeth and ear bones in place). For these features
Notocetus is found to be intermediate between the slightly smaller
Furcacetus and the significantly larger
Macrosqualodelphis.
On the ventral surface of the tympanic bulla of MUSM 3896, the wide and deep median furrow extends clearly on the anterior spine (
Figure 14e). Such an anterior extension of the median furrow is present in
N. vanbenedeni AMNH 29026 from Argentina and is considered a synapomorphy of the family Squalodelphinidae. By contrast, the median furrow of the well-preserved tympanic bulla of MUSM 1484 apparently does not extend anteriorly along the anterior spine (
Figure 16a–d). However, some longitudinal striations on the ventral surface of the anterior spine could represent spongy bone filling the anterior portion of the median furrow, suggesting that this character could be subject to intraspecific variation, possibly due to ontogenesis. Both tympanic bullae of MUSM 1484 and MUSM 3896 display an elongated anterior spine (although incomplete in MUSM 1484) associated with a marked anterolateral convexity, a derived character observed in all platanistoids. Moreover, the inner and outer posterior prominences of both tympanic bullae have roughly the same posterior extent, a condition shared with the squalodelphinids
Notocetus,
Phocageneus, and
Squalodelphis. In lateral view the tympanic bulla of MUSM 1484 is almost identical to that of
Notocetus vanbenedeni AMNH 29026, figured by Muizon ([
92], Figures. 4a,7a).
The preservation of a complete set of teeth implanted in their alveoli in MUSM 3896 (
Figure 15a,b), together with the four detached teeth of MUSM 3897 (
Figure 15c–f;
Table 4), is relevant since, to date, teeth of
Notocetus were only known in the fragmentary specimen AMNH 29026 [
92]. The Peruvian teeth confirm the observations made by Muizon [
92] on AMNH 29026: the two posterior teeth embedded in the left mandible of MUSM 3896 have a low triangular crown with two-three small posterior accessory denticles and cusp-like cingular nodules on the lateral surface; moving to the anterior portion of the mandibles and rostrum of MUSM 3896, tooth crowns become gradually higher, forming a conical point, slightly recurved posteriorly, and lacking the posterior denticles; their enamel is ornamented only by thin longitudinal striations. The four detached teeth of MUSM 3897 have a fusiform root, proportionally large compared to the crown. The two best-preserved teeth have a low triangular crown with weak anterior and posterior carinae and several cusp-like cingular nodules and papillae forming a cingulum near the base of the crown. One of these two teeth displays a wide wear surface on the posterolingual surface of the crown, probably due to the contact with the opposite tooth (attritional wear facet). The same tooth has a peculiar swelling on the posterior surface of the root. The only preserved tooth of MUSM 1484 (
Figure 16h) fully overlaps in size and shape with the described anterior teeth of
N. vanbendeni [
92]. This tooth is fusiform, weakly curved, and its crown is conical, weakly mesiodistally compressed, and bearing a posterior carina. The enamel is ornamented by thin anastomosed longitudinal grooves and ridges, more marked on the labial surface of the crown, where an ectocingulum is also visible.
As in the mandibles of the Argentinian specimens, in MUSM 3896 (
Figure 14a,c,d), MUSM 3897 (
Figure 14g,i,k), and MUSM 1484 (
Figure 16e–g) the mandibular symphysis is fused, the symphyseal portion is markedly dorsoventrally flattened, and, as in
Huaridelphis and
Squalodelphis, lacking the pair of lateral grooves observed in members of the families Allodelphinidae and Platanistidae, and in the basal Platanidelphidi
Ensidelphis. The symphyseal portion of the complete mandibles of MUSM 3896 represents 40% of the total mandibular length, measured parallel to the sagittal plane, a value close to
Squalodelphis (42%), the only other squalodelphinid for which complete mandibles are known. The angle between the two mandibles equals 38° in both MUSM 3896 and MUSM 3897, similar to
Squalodelphis but significantly smaller than in all platanistids (> 50°). Embrasure pits are observed between and lateral to the alveoli on the mandibles of MUSM 3897 (
Figure 14g) and MUSM 1484 (
Figure 16f–g). In lateral view, posterior to the alveolar row, the robust ramus (preserved in MUSM 3896 and MUSM 3897) raises posterodorsally and the coronoid process is significantly elevated. The angular process is prominent, separated by a wide notch from the mandibular condyle.
The almost complete atlas of MUSM 1484 (
Figure 16i,j) is close in shape to the atlas of the specimen of
N. vanbenedeni AMNH 9485, described by True [
125], in the extreme reduction of the ventral transverse processes, the neural arch being proportionally lower, and the roughly circular neural canal.
Description of the Forelimb Bones of MUSM 1484
The humerus, radius, and ulna, preserved in MUSM 1484 (
Figure 17;
Table 5), are here described in detail because up to now these bones were unknown in
Notocetus vanbenedeni. Among other squalodelphinids the forelimb was described only in
Macrosqualodelphis ukupachai, whereas among other platanistoids these bones are known in the extant
Platanista gangetica and in the allodelphinids
Allodelphis pratti, A.
woodburnei,
Goedertius oregonensis, and
Zarhinocetus errabundus ([
121], Figure 39). Comparisons (
Figure 18;
Table 6) were made also with the few other archaic odontocetes having some of these bones preserved (
Awamokoa tokarahi,
Kelloggia barbara,
Otekaikea huata,
Schizodelphis sp.,
Squalodon bellunensis,
S. calvertensis, and with derived archaeocetes (e.g.,
Cynthiacetus peruvianus).
Humerus. The humerus of MUSM 1484 (
Figure 17) is similar in shape but smaller than in
Macrosqualodelphis ukupachai; among platanistoids it shares only with
M. ukupachai the large, hemispherical and posterolaterally protruding head, the lesser tubercle being higher than the head, the salient and distally elongated deltopectoral crest, and the large and deep fossa for insertion of M. infraspinatus. In particular, the large, hemispherical and posterolaterally protruding head may be a diagnostic character of squalodelphinids since it was not observed in the humerus of any other cetacean, whereas the lesser tubercle being higher than the head is also observed in
Kelloggia barbara [
130]. Moreover, the humeri of MUSM 1484 and
M. ukupachai are clearly anteroposteriorly wider than allodelphinid humeri. In fact, the ratio between the anteroposterior width at mid-length and the proximodistal length (B/A in
Table 6) is 0.41 and 0.46 in MUSM 1848 and
M. ukupachai, respectively, whereas in allodelphinids it ranges from 0.26 in
Zarhinocetus errabundus to 0.31 in
Allodelphis woodburnei. A humerus as robust as in MUSM 1848 and
M. ukupachai is instead observed in
Platanista gangetica (B/A = 0.36–0.41), although the humerus of the only extant platanistid differs from all other platanistoids in lacking the delctopectoral crest and in its pronounced distal anteroposterior widening.
Radius. The radius of MUSM 1484 (
Figure 17c) is a mediolaterally flat bone that distally widens anteroposteriorly, more so than in
M. ukupachai but to a lesser degree than in
P. gangetica. The proximodistal length of the radius of MUSM 1484 is significantly smaller than the proximodistal length of the humerus, with a ratio (C/A in
Table 6) = 0.69, lower than in
M. ukupachai (0.77), but higher than in
P. gangetica (0.50–0.56), whereas in the allodelphinid
Ninjadelphis ujiharai the two bones almost have the same length (C/A = 0.96).The ratio between the anteroposterior width at mid-length and the proximodistal length (D/C) is relatively high (0.51), if compared to
N. ujiharai (0.24) and some archaic odontocetes, whereas it is similar to
M. ukupachai (0.49) and lower than in
P. gangetica (0.68–0.74).
The radius of MUSM 1484 is articulated with the ulna only for a small proximal portion of its posterior margin; distal to this articulation, the interosseous space between the radius and ulna is very broad, a condition similarly observed in M. ukupachai and P. gangetica. The articular surface for the first carpals is distally convex and arched in lateral view, as in P. gangetica.
Ulna. The ulna of MUSM 1484 (
Figure 17c), is proximodistally shorter than the radius and, as in
M. ukupachai and
P. gangetica, at its mid-length it is anteroposteriorly narrower than the radius. Like the radius, it is mediolaterally flattened and the ratio between its anteroposterior width at mid-length and its proximodistal length (F/E in
Table 6) has a value (0.51) higher than in allodelphinids and other archaic odontocetes, but lower than in
P. gangetica (0.72–0.87) (ratio not computable for the ulna of
M. ukupachai due to its incompleteness). The olecranon of the ulna of MUSM 1484 is even smaller than in
M. ukupachai, clearly less developed anteroposteriorly and proximally than in allodelphinids (plesiomorphic condition), whereas the olecranon is completely lacking in
Platanista.
cf.Notocetussp.
Referred specimen, locality, and age. MUSM 1485 consists of a right isolated tympanic bulla. Ullujaya locality, Western Ica Valley, Ica Region, Peru. Exact locality and stratigraphical horizon unknown. Approximate geographic coordinates: 14°35’ S, 75°38’ W. The age of the Chilcatay Fm exposed at Ullujaya can be constricted between 19.00 ± 0.25 Ma and 18.02 ± 0.07 Ma (early Burdigalian) on the basis of two volcanic ash layer samples dated by
40Ar/
39Ar [
89].
Brief Description and Comparison
Like MUSM 1484 described above this tympanic bulla is similar in size and shape to those referred to
Notocetus vanbenedeni. The median furrow (
Figure 19b) is only moderately deep, but it extends anteriorly on the preserved portion of the broken anterior spine, a feature that, as outlined above, has been observed in all squalodelphinid tympanic bullae (
Phocageneus,
Notocetus, and
Squalodelphis). Here also, as in the other squalodelphinids, the inner and outer posterior prominences have approximately the same posterior extent. Considering the incompleteness of this specimen, we cautiously refer it to cf.
Notocetus sp.
Platanistidae Gray, 1846
Emended diagnosis. The Platanistidae are characterized by the following synapomorphies, absent in the other members of the Platanidelphidi clade: (1) extremely elongated mandibular symphysis (>65% of the total length of the mandible) and wide angle (>50°) between the mandibular rami; (2) distinct dorsal crest in the antorbital-supraorbital region (character absent in Araeodelphis); (3) hook-like articular process on the lateral surface of the periotic (periotic unknown in Araeodelphis); (4) outer posterior prominence of the tympanic bulla posteriorly longer than the inner posterior prominence (tympanic bulla unknown in Araeodelphis).
Platanistidae indet. aff.Araeodelphis
Referred specimen. MUSM 631, fragmentary skull consisting of rostrum and fused symphyseal portion of mandibles.
Locality and horizon. Western Ica Valley, Ica Region, Zamaca, Peru, Chilcatay Fm. Exact locality and stratigraphical horizon unknown. Approximate geographic coordinates: 14°37’ S, 75°38’ W. The entire stratigraphical sequence of the Chilcatay Fm exposed at Zamaca can be roughly constricted between 19 and 18 Ma (early Burdigalian), considering that a volcanic ash layer located 4 m above the contact between the Chilcatay Fm and the underlying Otuma Formation in the Zamaca area gave an Ar/Ar age of 19.25 ± 0.05 Ma, and a volcanic ash layer from the nearby locality of Ullujaya, located below the contact between the Chilcatay and Pisco formations, gave an age of 18.02 ± 0.07 Ma [
89].
Brief Description and Comparison
MUSM 631 shows marked affinities with the rostrum and associated mandibles of the early diverging platanistid
Araeodelphis natator (holotype USNM 10478 and referred cranium USNM 526604 [
108,
132]), from the late early Miocene (Burdigalian) of Maryland (USA). Shared characters between MUSM 631 and
A. natator are the following:
- (1)
Rostrum and mandibles elongated and narrow, with lateral margins parallel in dorsal view (
Figure 20a,b), and strongly dorsoventrally compressed in lateral view (
Figure 20e,f), especially in their anterior half;
- (2)
premaxilla fused to the maxilla in the anteriormost portion of the rostrum;
- (3)
premaxilla-maxilla suture along the rostrum outlined by a distinct sulcus (more excavated in the anterior half of the rostrum), but without the deep lateral groove featuring more derived platanistids;
- (4)
mesorostral canal dorsally closed or very narrow for the whole rostrum length;
- (5)
oblique sulci on the dorsal surface of the premaxillae; similar sulci are also present in the longirostral odontocete Chilcacetus, but not in other platanistoids;
- (6)
firmly ankylosed mandibular symphysis being extremely elongated (
Figure 20g–j), a distinctive character of the platanistids within the platanistoids; indeed, even if it is not possible to calculate the ratio between the length of the symphysis and the total length of the mandible, the extreme elongation of MUSM 631's symphysis is evidenced by the fact that the posterior end of the symphysis almost reaches the pterygoid-maxilla suture;
- (7)
ventral surface of the symphyseal portion of each mandible marked by a deep longitudinal groove; this character is also present in all other platanistoids with the exception of squalodelphinids;
- (8)
symphyseal portion of the mandible bearing a similar number of teeth: 39–40 for MUSM 631 and 38 for USNM 10478;
- (9)
similar tooth count for each upper quadrant (
Figure 20g,h); in fact Godfrey et al. [
108] estimated approximately 50 teeth for each quadrant on the rostrum of USNM 526604, a number close to that counted (55) for the almost complete rostrum of MUSM 631; USNM 10478 has 47 alveoli for each upper quadrant, but the rostrum is not as complete as in MUSM 631;
- (10)
presence of a medial trough between the maxillae on the palatal surface of the rostrum.
Significant differences between MUSM 631 and Araeodelphis natator are:
- (1)
the size, MUSM 631 being larger than USNM 10478 (the length of the mandibular symphyseal portion is 415 mm in MUSM 631 contra 291 mm in USNM 10478; the transverse width of the fused mandibles at the posterior end of the symphysis is 57 mm in MUSM 631 contra 50 mm in USNM 10478) (
Table 7);
A. natator USNM 526604 is even smaller than the holotype (approximately 10%–15% smaller, according to Godfrey et al. [
108]);
- (2)
the mandibular alveoli being proportionally larger and not as many, compared to the upper alveoli in MUSM 631, contra the same size and number of alveoli in lower and upper quadrant in A. natator USNM 10478; in fact the transverse diameters of alveoli in MUSM 631 range between 4 and 7 mm in the mandible and 2 and 5.5 mm in the rostrum and there are 40 alveoli in the symphyseal portion of the mandible, for about 53 in the corresponding rostral portion;
- (3)
the angle formed by the mandibular rami is apparently more acute in MUSM 631 than in A. natator USNM 10478; the wide angle (>50°) between the mandibles is an important feature related to the extreme elongation of the symphysis shared by all platanistoids, including Araeodelphis; however, the two mandibular rami of MUSM 631 are fragmentary and show some post-mortem fractures that could have changed their original orientation; it is therefore probable that the two mandibles originally formed a wide angle, as expected considering the very long symphysis; and
- (4)
on the dorsal surface of the rostrum of A. natator USNM 526604 the lateral margin of the premaxilla is laterally convex near the antorbital notch, a feature not observed in MUSM 631; however this convexity of the premaxilla could have been originally present in the missing posterior portion of the rostrum of MUSM 631 (the same could be true for A. natator USNM 10478, apparently lacking a premaxillary convexity, but with a rostrum incomplete at its base).
Considering the affinities and differences above mentioned, it is possible that MUSM 631 either belongs to: (1) A large specimen of A. natator, considering that the size of the mandible and of the alveoli are subject to ontogenetic variation; (2) an undescribed, larger species of Araeodelphis; or (3) an unknow genus and species of basal platanistid. Pending the discovery of a more complete specimen, MUSM 631 is here assigned to Platanistidae indet. aff. Araeodelphis.