FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Testicular Tissues for Immunohistochemistry (IHC) Studies
2.3. Immunohistochemistry in Human Normal and Neoplastic Testicular Tissues
2.4. Scoring System
2.5. Protein Extraction from Testicular Tissues
2.6. Semen Samples and Spermatozoa Preparations
2.7. Processing of Ejaculated Sperm
2.8. Western Blot Analysis of Sperm Proteins
2.9. Immunofluorescence Assay
2.10. Imaging
2.11. Evaluation of Sperm Motility and Viability
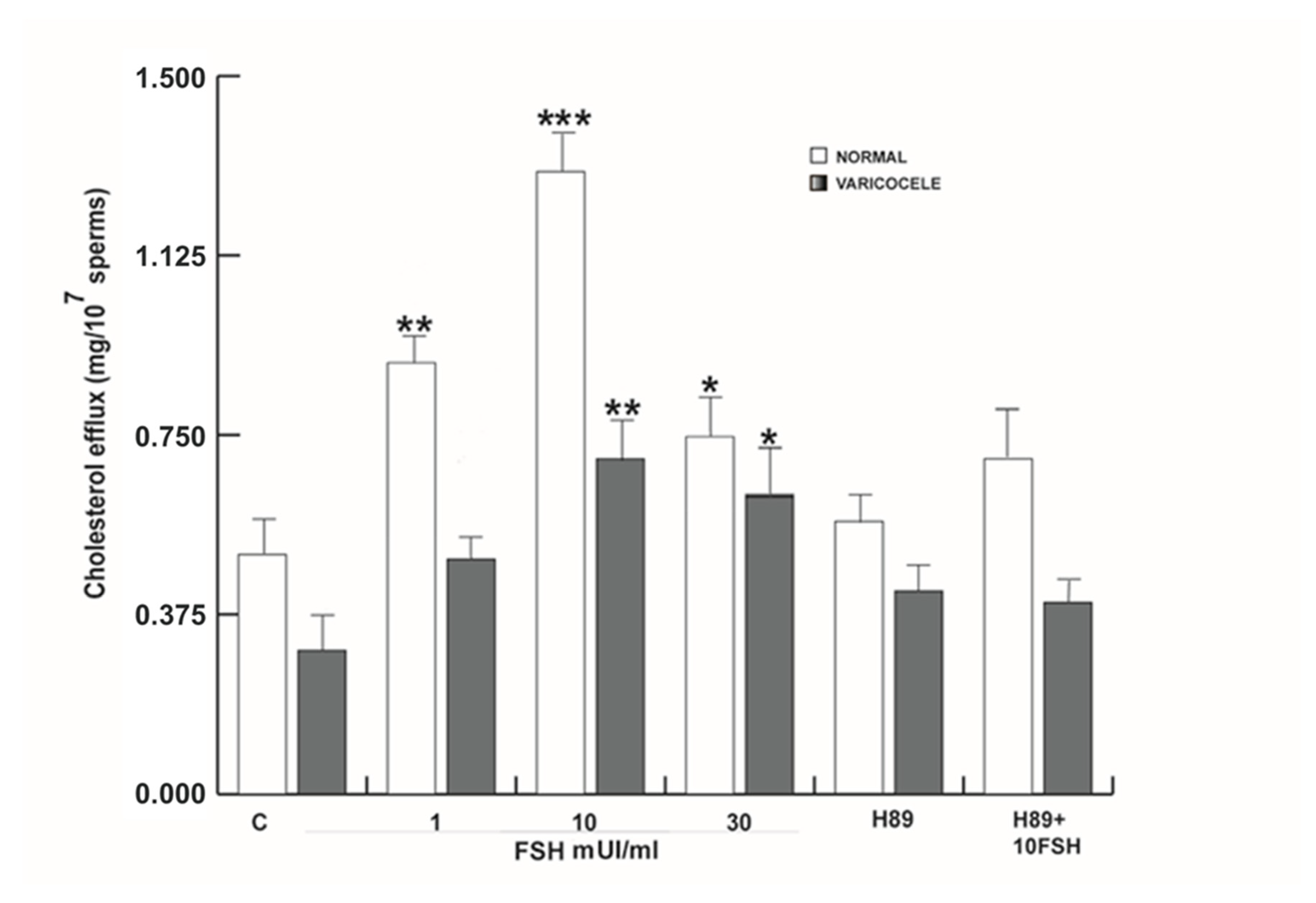
2.12. Measurement of Cholesterol in the Sperm Culture Medium
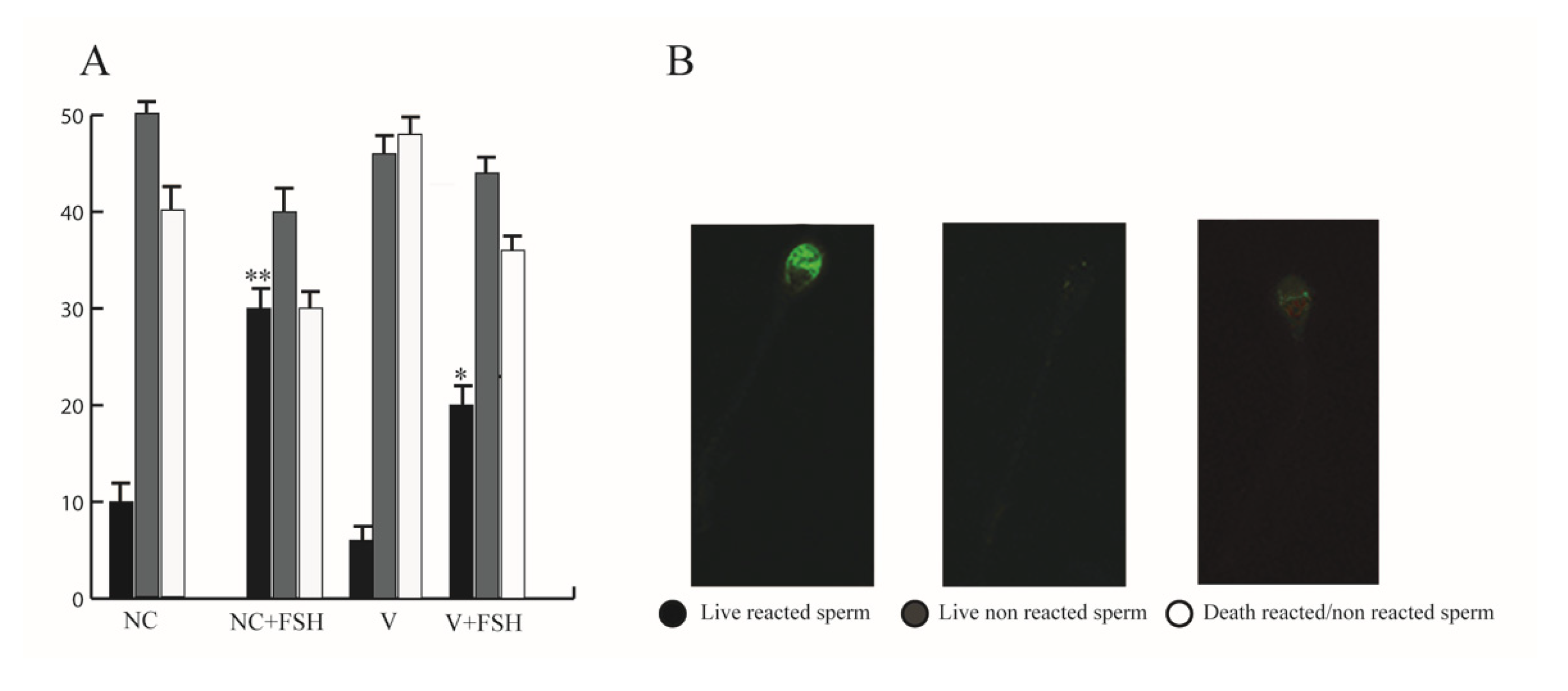
2.13. Acrosome Reaction
2.14. Statistical Analysis
3. Results
3.1. Morphological Analysis of Normal Human Tissues
3.2. FSH-R Immunolocalization in Normal Human Tissues
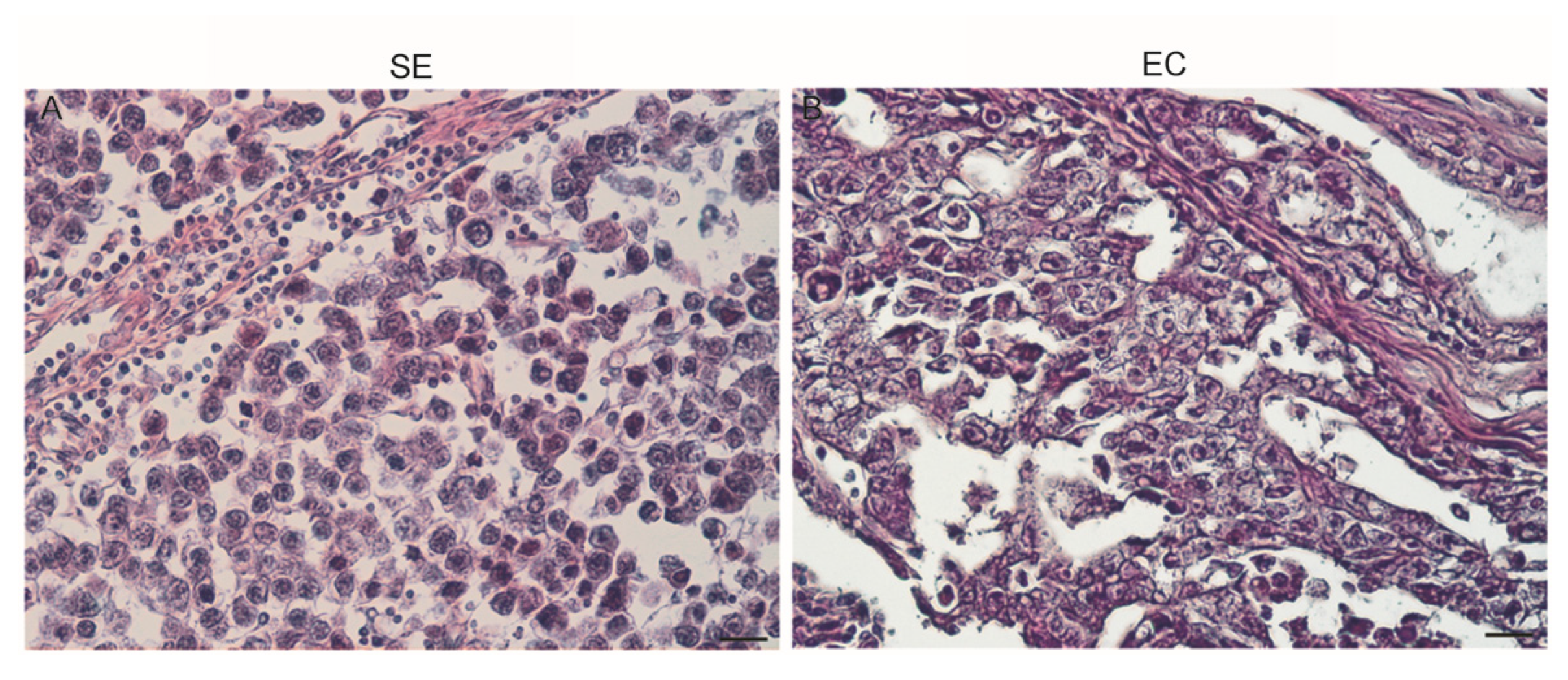
3.3. Morphology of Testicular Germ Cell Tumors
3.4. IHC for FSH-R Localization in Human Neoplastic Testicular Tissues
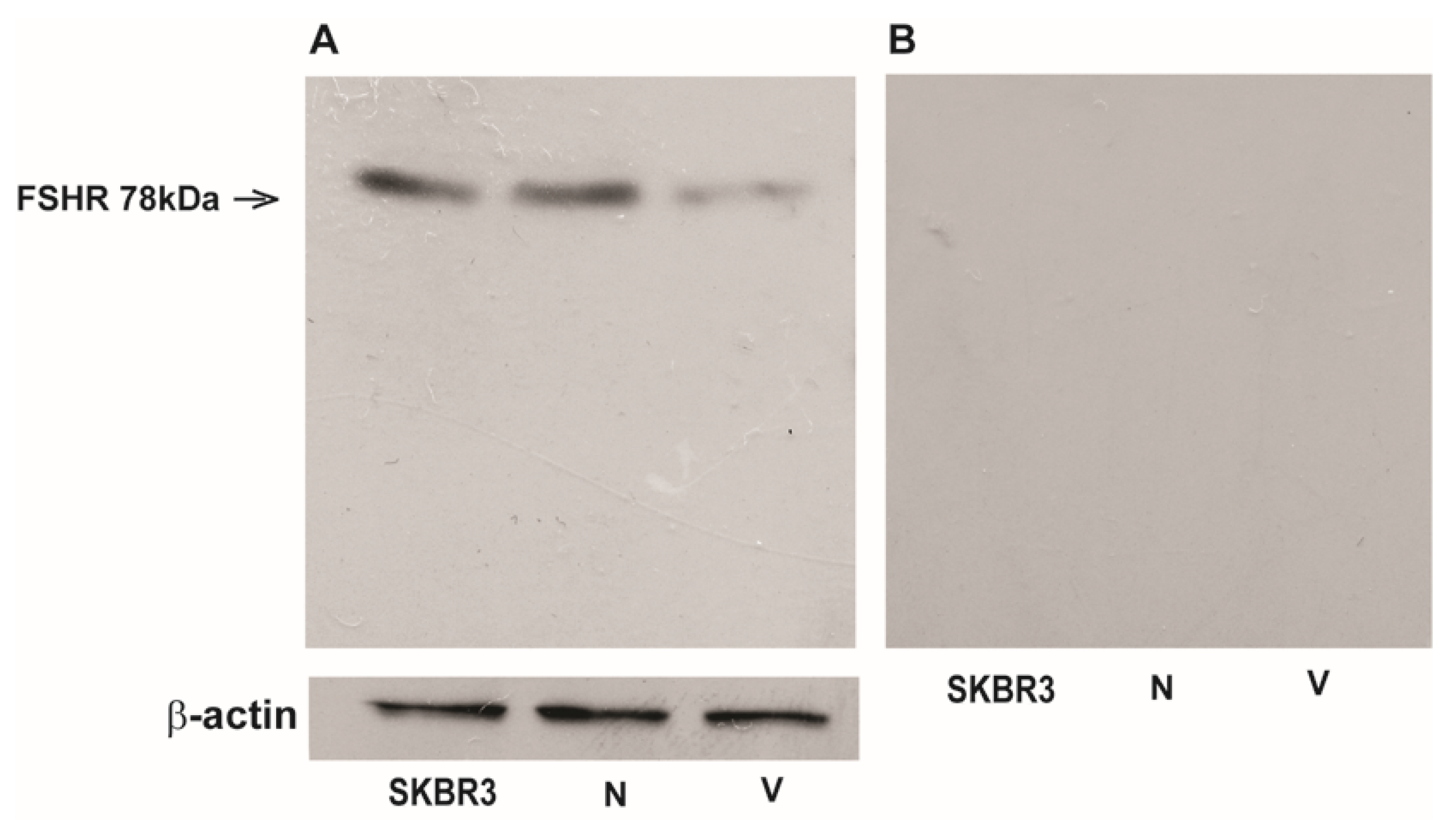
3.5. FSH-R Protein Expression in Human Normal Testicular Tissue, SE, and EC by Western Blot Analysis
3.6. FSH-R is Present in Human Ejaculated Spermatozoa
3.7. Immunofluorescence Assay
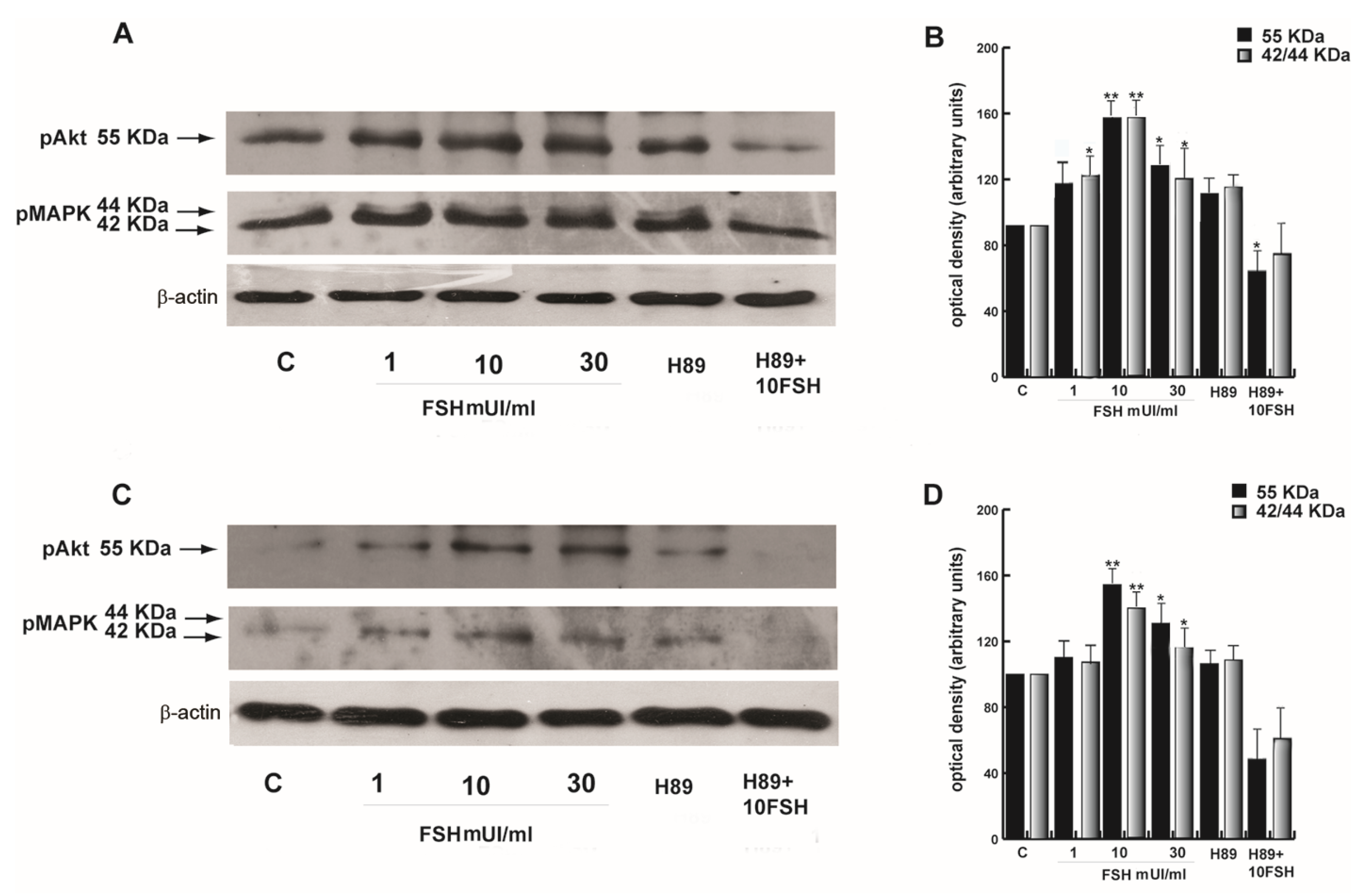
3.8. FSH-Induced Activation of Akt is Mediated by PKA in Human Sperm
3.9. FSH-R Inhibition Abrogates the Stimulatory Effect of FSH on Human Sperm Motility and Survival
3.10. FSH Induced Capacitation in Human Sperm
3.11. FSH Increased Acrosome Reaction in Human Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chappel, S.C.; Howles, C. Reevaluation of the roles of luteinizing hormone and follicle-stimulating hormone in the ovulatory process. Hum. Reprod. 1991, 6, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH directly regulates bone mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Z.; Zhu, L.L.; Peng, Y.; Liu, X.; Li, J.; Agrawal, M.; Robinson, L.J.; Iqbal, J.; Blair, H.C.; et al. Further evidence for direct pro-resorptive actions of FSH. Biochem. Biophys. Res. Commun. 2010, 394, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17. [Google Scholar] [CrossRef]
- Cannon, J.G.; Kraj, B.; Sloan, G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine 2011, 53, 141–144. [Google Scholar] [CrossRef]
- Stilley, J.A.; Christensen, D.E.; Dahlem, K.B.; Guan, R.; Santillan, D.A.; England, S.K.; Al-Hendy, A.; Kirby, P.A.; Segaloff, D.L. FSH receptor (FSHR) expression in human extragonadal reproductive tissues and the developing placenta; and the impact of its deletion on pregnancy in mice. Biol. Reprod. 2014, 91, 74. [Google Scholar] [CrossRef]
- Stilley, J.A.; Guan, R.; Duffy, D.M.; Segaloff, D.L. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J. Clin. Endocrinol. Metab. 2014, 99, E813–E820. [Google Scholar] [CrossRef]
- Song, Y.; Wang, E.S.; Xing, L.L.; Shi, S.; Qu, F.; Zhang, D.; Li, J.Y.; Shu, J.; Meng, Y.; Sheng, J.Z.; et al. Follicle-Stimulating Hormone Induces Postmenopausal Dyslipidemia Through Inhibiting Hepatic Cholesterol Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 254–263. [Google Scholar] [CrossRef]
- Casarini, L.; Moriondo, V.; Marino, M.; Adversi, F.; Capodanno, F.; Grisolia, C.; La Marca, A.; La Sala, G.B.; Simoni, M. FSHR polymorphism p.N680S mediates different responses to FSH in vitro. Mol. Cell. Endocrinol. 2014, 393, 83–91. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Marino, M. Impact of gene polymorphisms of gonadotropins and their receptors on human reproductive success. Reproduction 2015, 150, R175–R184. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.R.; Wang, Y.; Lu, N.; Matzuk, M.M. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat. Genet. 1997, 15, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Foresta, C. New genetic markers for male infertility. Curr. Opin. Obstet. Gynecol. 2014, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G.; Hai, M.T.; Ghinea, N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.; Desestret, V.; Antoine, M.; Fromont, G.; Huerre, M.; Sanson, M.; Camparo, P.; Pichon, C.; Planeix, F.; Gonin, J.; et al. Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer 2013, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Planeix, F.; Siraj, M.A.; Bidard, F.C.; Robin, B.; Pichon, C.; Sastre-Garau, X.; Antoine, M.; Ghinea, N. Endothelial follicle-stimulating hormone receptor expression in invasive breast cancer and vascular remodeling at tumor periphery. J. Exp. Clin. Cancer Res. 2015, 34, 12. [Google Scholar] [CrossRef]
- Rago, V.; Romeo, F.; Giordano, F.; Ferraro, A.; Andò, S.; Carpino, A. Identification of ERbeta1 and ERbeta2 in human seminoma; in embryonal carcinoma and in their adjacent intratubular germ cell neoplasia. Reprod. Biol. Endocrinol. 2009, 7, 56. [Google Scholar] [CrossRef]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The follicle stimulating hormone receptor: Biochemistry; molecular biology; physiology; and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [CrossRef]
- Gloaguen, P.; Crépieux, P.; Heitzler, D.; Poupon, A.; Reiter, E. Mapping the follicle-stimulating hormone-induced signaling networks. Front. Endocrinol. 2011, 2, 45. [Google Scholar] [CrossRef]
- Seger, R.; Hanoch, T.; Rosenberg, R.; Dantes, A.; Merz, W.E.; Strauss, J.F., III; Amsterdam, A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001, 276, 13957–13964. [Google Scholar] [CrossRef]
- Amsterdam, A.; Hanoch, T.; Dantes, A.; Tajima, K.; Strauss, J.F.; Seger, R. Mechanisms of gonadotropin desensitization. Mol. Cell. Endocrinol. 2002, 187, 69–74. [Google Scholar] [CrossRef]
- Conti, M. Specificity of the cyclic adenosine 3′,5′monophosphate signal in granulosa cell function. Biol. Reprod. 2002, 67, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell. Signal. 2006, 18, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.A.; Bonnet, B.; Weaver, B.A.; Watts, J.; Kluetzman, K.; Thomas, R.M.; Poli, S.; Mutel, V.; Campo, B. A negative allosteric modulator demonstrates biased antagonism of the follicle stimulating hormone receptor. Mol. Cell. Endocrinol. 2011, 333, 143–150. [Google Scholar] [CrossRef][Green Version]
- Guido, C.; Perrotta, I.; Panza, S.; Middea, E.; Avena, P.; Santoro, M.; Marsico, S.; Imbrogno, P.; Andò, S.; Aquila, S. Human sperm physiology: Estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J. Cell. Physiol. 2011, 226, 3403–3412. [Google Scholar] [CrossRef]
- Baccetti, B.; Collodel, G.; Costantino-Ceccarini, E.; Eshkol, A.; Gambera, L.; Moretti, E.; Strazza, M.; Piomboni, P. Localization of human follicle-stimulating hormone in the testis. FASEB J. 1998, 12, 1045–1054. [Google Scholar] [CrossRef]
- Heckert, L.L.; Griswold, M.D. The Expression of the Follicle-stimulating Hormone Receptor in Spermatogenesis. Recent Prog. Horm. Res. 2002, 57, 129–148. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Kawashima, Y.; Kodera, Y.; Singh, A.; Matsumoto, M.; Matsumoto, H. Efficient extraction of proteins from formalin-fixed paraffin-embedded tissues requires higher concentration of tris(hydroxymethyl)aminomethane. Clin. Proteom. 2014, 11, 4. [Google Scholar] [CrossRef]
- Rago, V.; Romeo, F.; Giordano, F.; Ferraro, A.; Carpino, A. Identification of G protein-coupled estrogen receptor (GPER) in human prostate: Expression site of the estrogen receptor in the benign and neoplastic gland. Andrology 2016, 4, 121–127. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Aquila, S.; Middea, E.; Catalano, S.; Marsico, S.; Lanzino, M.; Casaburi, I.; Barone, I.; Bruno, R.; Zupo, S.; Andò, S. Human sperm express a functional androgen receptor: Effects on PI3K/AKT pathway. Hum. Reprod. 2007, 22, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Rago, V.; Giordano, F.; Brunelli, E.; Zito, D.; Aquila, S.; Carpino, A. Identification of G protein-coupled estrogen receptor in human and pig spermatozoa. J. Anat. 2014, 224, 732–736. [Google Scholar] [CrossRef]
- Aquila, S.; Guido, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Baffa, M.F.; Andò, S.; Bifulco, M. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. Br. J. Pharmacol. 2010, 159, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Cappello, A.R.; Guido, C.; Santoro, A.; Santoro, M.; Capobianco, L.; Montanaro, D.; Madeo, M.; Andò, S.; Dolce, V.; Aquila, S. The mitochondrial citrate carrier (CIC) is present and regulates insulin secretion by human male gamete. Endocrinology 2012, 153, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H. Induction of capacitation and acrosome reaction of boar spermatozoa by L-arginine and nitric oxide synthesis associated with the anion transport system. Reproduction 2002, 124, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Giordano, F.; Guido, C.; Rago, V.; Carpino, A. Nitric oxide involvement in the acrosome reaction triggered by leptin in pig sperm. Reprod. Biol. Endocrinol. 2011, 9, 133. [Google Scholar] [CrossRef]
- Guido, C.; Panza, S.; Santoro, M.; Avena, P.; Panno, M.L.; Perrotta, I.; Giordano, F.; Casaburi, I.; Catalano, S.; De Amicis, F.; et al. Estrogen receptor beta (ERβ) produces autophagy and necroptosis in human seminoma cell line through the binding of the Sp1 on the phosphatase and tensin homolog deleted from chromosome 10 (PTEN) promoter gene. Cell Cycle 2012, 11, 2911–2921. [Google Scholar] [CrossRef]
- Castagliola, S.; Panneels, V.; Bonomi, M.; Koch, J.; Many, M.C.; Smits, G.; Vassart, G. Tyrosine sulfation is required for agonist recognition by glycoprotein hormone receptors. EMBO J. 2002, 21, 504–513. [Google Scholar] [CrossRef]
- Uchida, S.; Uchida, H.; Maruyama, T.; Kajitani, T.; Oda, H.; Miyazaki, K.; Kagami, M.; Yoshimura, Y. Molecular analysis of a mutated FSH receptor detected in a patient with spontaneous ovarian hyperstimulation syndrome. PLoS ONE 2013, 8, e75478. [Google Scholar] [CrossRef]
- Travis, A.J.; Kopf, G.S. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J. Clin. Invest. 2002, 110, 731–736. [Google Scholar] [CrossRef]
- Heindel, J.J.; Rothenberg, R.; Robison, G.A.; Steinberger, A. LH and FSH stimulation of cyclic AMP in specific cell types isolated from the testes. J. Cyclic Nucleotide Res. 1975, 1, 69. [Google Scholar]
- Meachem, S.J.; Ruwanpura, S.M.; Ziolkowski, J.; Ague, J.M.; Skinner, M.K.; Loveland, K.L. Developmentally distinct in vivo effects of FSH on proliferation and apoptosis during testis maturation. J. Endocrinol. 2005, 186, 429–446. [Google Scholar] [CrossRef]
- Mariani, S.; Salvatori, L.; Basciani, S.; Arizzi, M.; Franco, G.; Petrangeli, E.; Spera, G.; Gnessi, L. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate; benign prostatic hyperplasia and prostate cancer. J. Urol. 2006, 175, 2072–2077. [Google Scholar] [CrossRef]
- Grazul-Bilska, A.T.; Reyaz, A.; Valkov, V.; Dorsam, S.T.; Redmer, D.A. Follicle stimulating hormone receptor protein is expressed in ovine uterus during the estrous cycle and utero-placenta during early pregnancy: An immunohistochemical study. Acta Histochem. 2018, 120, 420–428. [Google Scholar] [CrossRef]
- Chung, H.H.; Lee, J.C.; Minn, I. Follicle-stimulating hormone receptor in gynecological cancers. Mol. Cell. Toxicol. 2018, 14, 1–7. [Google Scholar] [CrossRef]
- Hon, C.A.; Gardner, P.J.; Leuschen, P. Immunocytochemical staining of isolated rat Sertoli cells for anti FSH β. Am. J. Anat. 1983, 167, 441–450. [Google Scholar] [CrossRef]
- Kliesch, S.; Pennttila, T.L.; Gromoll, J.; Saunders, P.T.K.; Nieschlag, E.; Parvinen, M. FSH receptor mRNA is expressed stage-dependently during rat spermatogenesis. Mol. Cell. Endocr. 1992, 84, R45–R49. [Google Scholar] [CrossRef]
- Bockers, T.M.; Nieschlag, E.; Kreutz, M.R.; Bergmann, M. Localization of follicle-stimulating hormone (FSH) immunoreactivity and hormone receptor mRNA in testicular tissue of fertile men. Cell Tissue Res. 1994, 278, 595–600. [Google Scholar] [CrossRef]
- Shimizu, A.; Tsutsui, K.; Kawashima, S. Autoradiographic study of binding and internalization of follicle-stimulating hormone in the mouse testis minces in vitro. Endocrinol. Jpn. 1987, 34, 431–442. [Google Scholar] [CrossRef]
- Shimizu, A.; Kawashima, S. Kinetic study of internalization and degradation of 131I-labeled follicle-stimulating hormone in mouse Sertoli cells and its relevance to other systems. J. Biol. Chem. 1989, 264, 13632–13638. [Google Scholar]
- The Human Protein Atlas. FSHR. Available online: https://www.proteinatlas.org/ENSG00000170820-FSHR (accessed on 12 October 2020).
- Hess, R.A. Estrogen in the adult male reproductive tract: A review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef]
- Cornwall, G.A.; von Horsten, H.H.; Swartz, D.; Johnson, S.; Chau, K.; Whelly, S. Extracellular quality control in the epididymis. Asian J. Androl. 2007, 9, 500–507. [Google Scholar] [CrossRef]
- Swider-Al-Amawi, M.; Kolasa, A.; Sikorski, A.; Marchlewicz, M.; Baranowska-Bosiacka, I.; Wiszniewska, B. The immunoexpression of FSH-R in the ductuli efferentes and the epididymis of men and rat: Effect of FSH on the morphology and steroidogenic activity of rat epididymal epithelial cells in vitro. J. Biomed. Biotechnol. 2010, 2010, 506762. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Danilovich, N.; Morales, C.R.; Sairam, M.R. Qualitative and quantitative decline in spermatogenesis of the follicle-stimulating hormone receptor knockout (FORKO) mouse. Biol. Reprod. 2000, 62, 1146–1159. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Babu, P.S.; Morales, C.R.; Sairam, R.M. Delay in sexual maturity of the follicle-stimulating hormone receptor knockout male mouse. Biol. Reprod. 2001, 65, 522–531. [Google Scholar] [CrossRef]
- Grover, A.; Smith, C.E.; Gregory, M.; Cyr, D.G.; Sairam, M.R.; Hermo, L. Effects of FSH receptor deletion on epididymal tubules and sperm morphology; numbers; and motility. Mol. Reprod. Dev. 2005, 72, 135–144. [Google Scholar] [CrossRef]
- Pawlikowski Łódź, M. Expression of follicle stimulating hormone receptors in intra-tumoral vasculature and in tumoral cells—The involvement in tumour progression and the perspectives of application in cancer diagnosis and therapy. Endokrynol. Pol. 2018, 69, 192–198. [Google Scholar]
- Chrusciel, M.; Ponikwicka-Tyszko, D.; Wolczynski, S.; Huhtaniemi, I.; Rahman, N.A. Extragonadal FSHR Expression and Function-Is It Real? Front. Endocrinol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Reiter, E.; Crepieux, P. FSH receptor signaling: Complexity of interactions and signal diversity. Endocrinology 2018, 159, 3020–3035. [Google Scholar] [CrossRef]
- Gonzalez-Robayna, I.J.; Falender, A.E.; Ochsner, S.; Firestone, G.L.; Richards, J.S. Follicle-stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): Evidence for A kinase-independent signaling by FSH in granulosa cells. Mol. Endocrinol. 2000, 14, 1283–1300. [Google Scholar] [CrossRef]

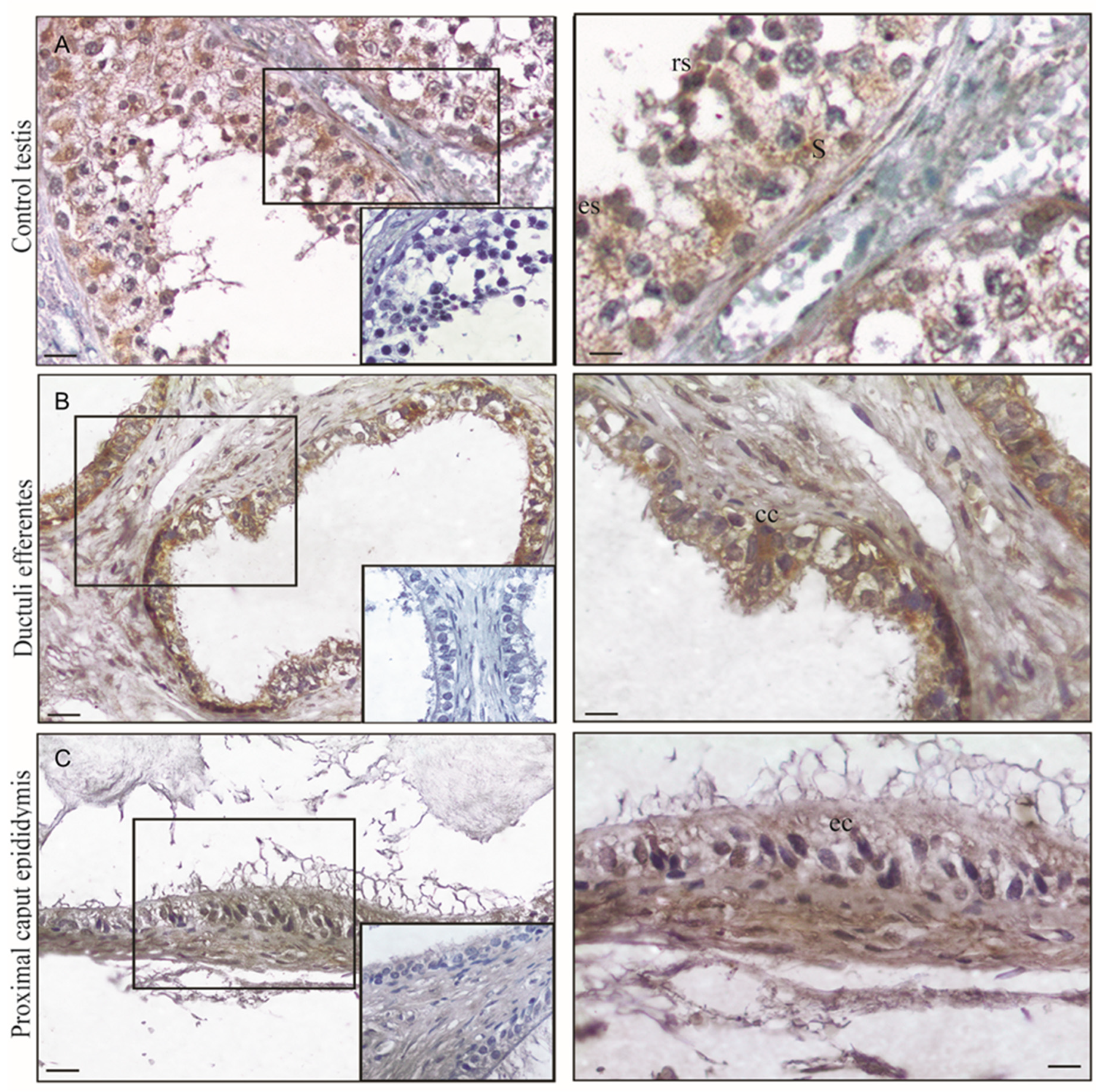




| Normal Testes | Ductuli Efferentes | Proximal Epididymus |
|---|---|---|
| Germ cells + | Epithelial cells +++ | Epithelial cells ++ |
| Sertoli cells +++ | Muscle cells + | Muscle cells + |
| Leydig cells − | Stromal cells − | Stromal cells − |
| FSH-R | ERb | |
|---|---|---|
| Seminoma | 1 | 8 |
| Embryonal carcinoma | 6 ** | 4 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panza, S.; Giordano, F.; De Rose, D.; Panno, M.L.; De Amicis, F.; Santoro, M.; Malivindi, R.; Rago, V.; Aquila, S. FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders. Life 2020, 10, 336. https://doi.org/10.3390/life10120336
Panza S, Giordano F, De Rose D, Panno ML, De Amicis F, Santoro M, Malivindi R, Rago V, Aquila S. FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders. Life. 2020; 10(12):336. https://doi.org/10.3390/life10120336
Chicago/Turabian StylePanza, Salvatore, Francesca Giordano, Daniela De Rose, Maria Luisa Panno, Francesca De Amicis, Marta Santoro, Rocco Malivindi, Vittoria Rago, and Saveria Aquila. 2020. "FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders" Life 10, no. 12: 336. https://doi.org/10.3390/life10120336
APA StylePanza, S., Giordano, F., De Rose, D., Panno, M. L., De Amicis, F., Santoro, M., Malivindi, R., Rago, V., & Aquila, S. (2020). FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders. Life, 10(12), 336. https://doi.org/10.3390/life10120336







