Effect of Hyperbaric Oxygen Therapy on Acute Liver Injury and Survival in a Rat Cecal Slurry Peritonitis Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Polymicrobial Sepsis Model
2.3. Hyperbaric Oxygen Therapy (HBOT)
2.4. Experimental Protocols
2.5. Laboratory Analyses
2.6. Cytokine Analyses
2.7. Quantitative Analyses of Intracellular ROS Generation and Maganase Superoxide
2.8. Western Blot Anlaysis
2.9. Histological Hepatic Injury Severity Score
2.10. Statistical Analysis
3. Results
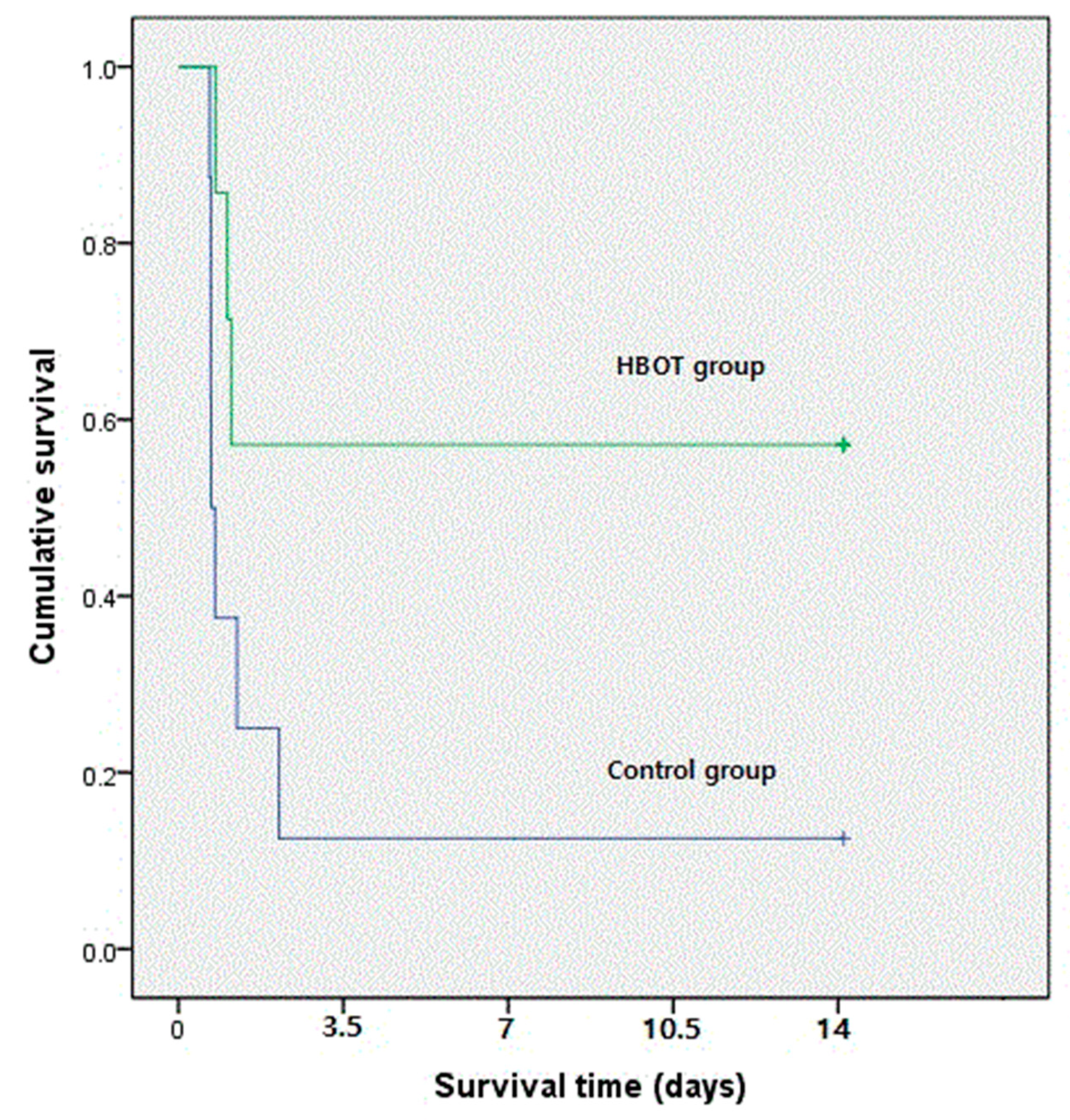
3.1. Survival Rate
3.2. Laboratory Analyses
3.3. Sepsis-Related Cytokines
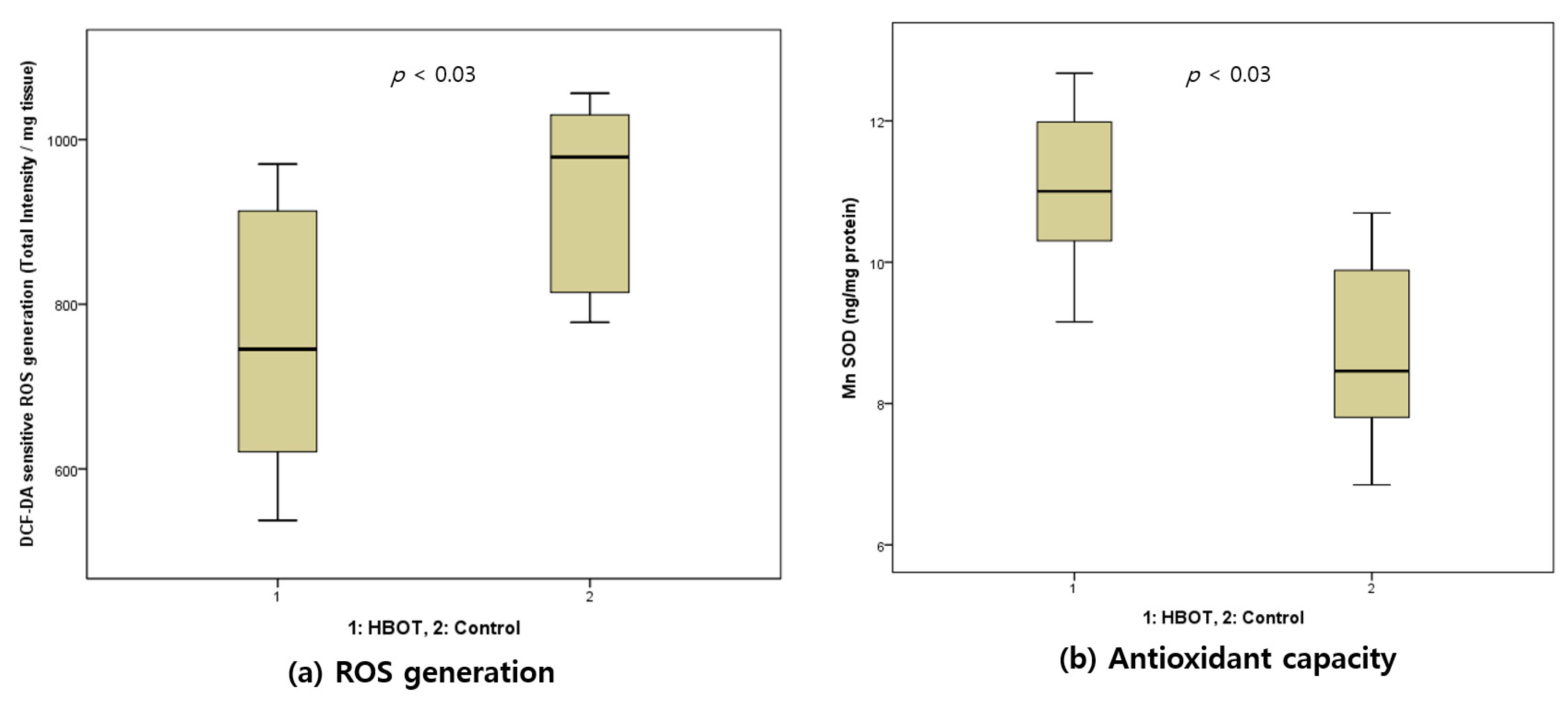
3.4. Intracellular ROS Generation and Manganese SOD
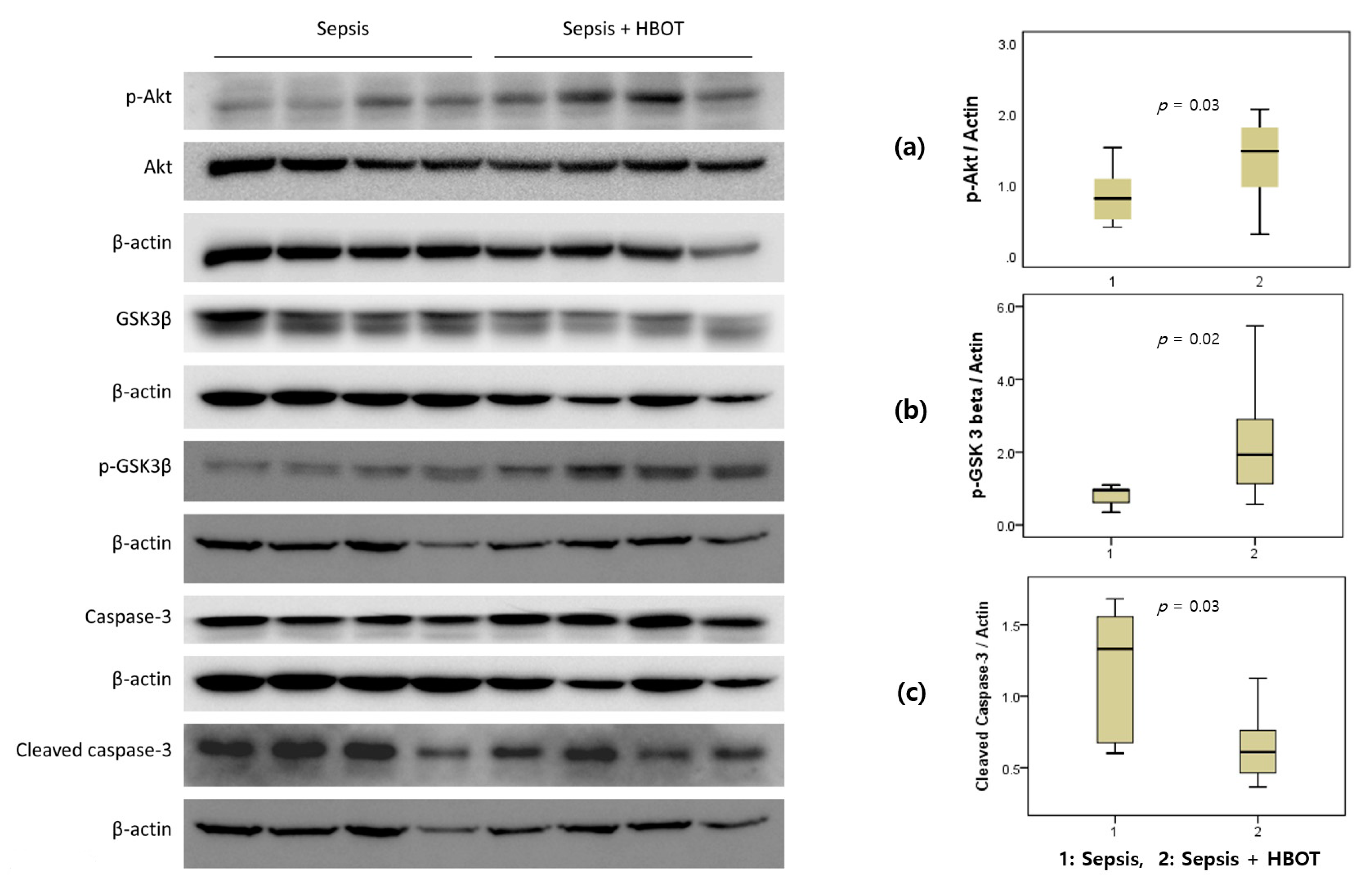
3.5. Western Blot Analysis
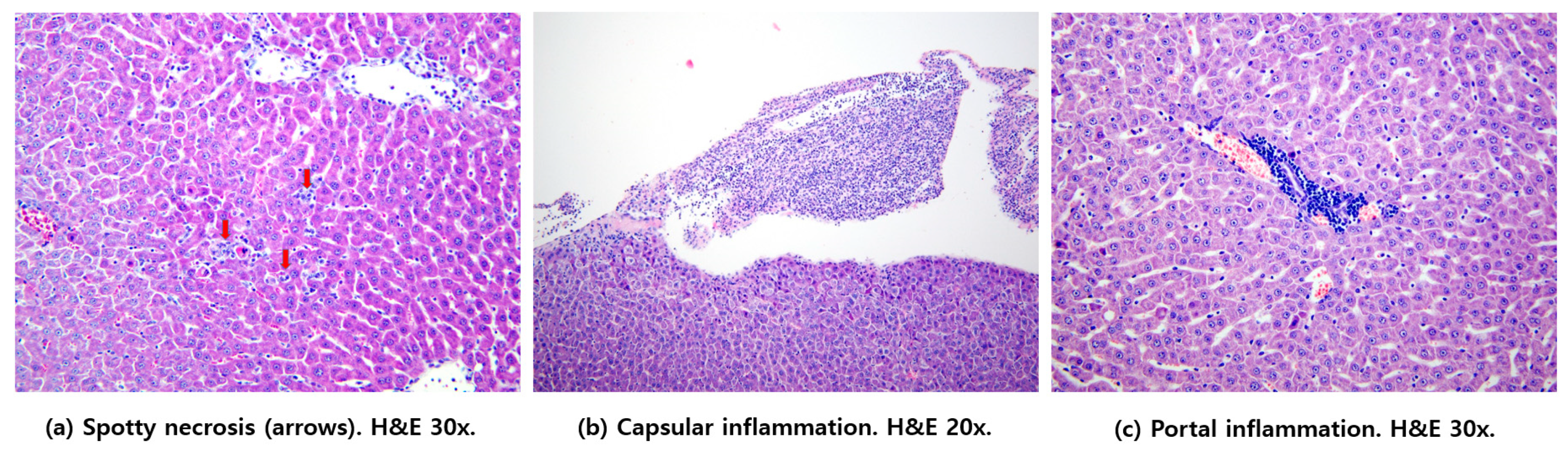
3.6. Histological Hepatic Injury Scores
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.M.; Annane, D.; Bauer, M.M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.J.; Coopersmith, C.C.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Lee, H.; Ahn, S. Epidemiology of sepsis in Korea: A population-based study of incidence, mortality, cost and risk factors for death in sepsis. Clin. Exp. Emerg. Med. 2019, 6, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.M.; Evans, L.E.; Rhodes, A. The surviving sepsis campaign bundle: 2018 update. Intensiv. Care Med. 2018, 44, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Pravda, J. Metabolic theory of septic shock. World J. Crit. Care Med. 2014, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Lundy, D.J.; Trzeciak, S. Microcirculatory dysfunction in sepsis. Crit. Care Nurs. Clin. N. Am. 2011, 23, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Thom, S.R.; Lauermann, M.W.; Hart, G.B. Intermittent hyperbaric oxygen therapy for reduction of mortality in experimental polymicrobial sepsis. J. Infect. Dis. 1986, 154, 504–510. [Google Scholar] [CrossRef]
- Oter, S.; Edremitlioğlu, M.; Korkmaz, A.; Coskun, O.; Kiliç, D.; Kısa, Ü.; Yaren, H.; Bilgic, H.; Kisa, U. Effects of hyperbaric oxygen treatment on liver functions, oxidative status and histology in septic rats. Intensiv. Care Med. 2005, 31, 1262–1268. [Google Scholar] [CrossRef]
- Buras, J.A.; Holt, D.; Orlow, D.; Belikoff, B.; Pavlides, S.; Reenstra, W.R. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit. Care Med. 2006, 34, 2624–2629. [Google Scholar] [CrossRef]
- Weber, S.U.; Koch, A.; Kankeleit, J.; Schewe, J.-C.; Siekmann, U.; Stüber, F.; Hoeft, A.; Schröder, S. Hyperbaric oxygen induces apoptosis via a mitochondrial mechanism. Apoptosis 2009, 14, 97–107. [Google Scholar] [CrossRef]
- Muth, C.M.; Radermacher, P.; Cuzzocrea, S. Hyperbaric oxygen and sepsis: Time to recognize. Intensiv. Care Med. 2005, 31, 1150–1152. [Google Scholar] [CrossRef]
- Frey, G.; Lampl, L.; Radermacher, P.; Bock, K.H. Hyperbaric oxygenation. An area for the anesthetist? Anaesthesist 1998, 47, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Granowitz, E.V.; Skulsky, E.J.; Benson, R.M.; Wright, J.; Garb, J.L.; Cohen, E.R.; Smithline, E.C.; Brown, R.B. Exposure to increased pressure or hyperbaric oxygen suppresses interferon-gamma secretion in whole blood cultures of healthy humans. Undersea Hyperb. Med. 2002, 29, 216–225. [Google Scholar] [PubMed]
- Poff, A.M.; Kernagis, D.; D’Agostino, D.P. Hyperbaric environment: Oxygen and cellular damage versus protection. Compr. Physiol. 2016, 7, 213–234. [Google Scholar] [PubMed]
- Almzaiel, A.J.; Billington, R.; Smerdon, G.; Moody, A.J. Effects of hyperbaric oxygen treatment on antimicrobial function and apoptosis of differentiated hl-60 (neutrophil-like) cells. Life Sci. 2013, 93, 125–131. [Google Scholar] [CrossRef]
- Godman, C.A.; Joshi, R.; Giardina, C.; Perdrizet, G.; Hightower, L.E. Hyperbaric oxygen treatment induces antioxidant gene expression. Ann. N. Y. Acad. Sci. 2010, 1197, 178–183. [Google Scholar] [CrossRef]
- Bhutani, S.; Vishwanath, G. Hyperbaric oxygen and wound healing. Indian J. Plast. Surg. 2012, 45, 316–324. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, K.; Jo, Y.H.; Lee, J.H.; Hwang, J.E. Dose-dependent mortality and organ injury in a cecal slurry peritonitis model. J. Surg. Res. 2016, 206, 427–434. [Google Scholar] [CrossRef]
- Regel, G.; Grotz, M.; Weltner, T.; Sturm, J.A.; Tscherne, H. Pattern of organ failure following severe trauma. World J. Surg. 1996, 20, 422–429. [Google Scholar] [CrossRef]
- Thom, S.R. Oxidative stress is fundamental to hyperbaric oxygen therapy. J. Appl. Physiol. 2009, 106, 988–995. [Google Scholar] [CrossRef]
- Mizrahi, S.; Dolberg, L.; Jacobsohn, W.Z. Alteration in aminotransferase levels in rats after acute hepatic injury. Isr. J. Med. Sci. 1987, 23, 188–192. [Google Scholar]
- Woźnica, E.A.; Inglot, M.; Woźnica, R.K.; Łysenko, L. Liver dysfunction in sepsis. Adv. Clin. Exp. Med. 2018, 27, 547–551. [Google Scholar] [CrossRef] [PubMed]
- De Brito, R.C.; Lucena-Silva, N.; Torres, L.C.; Luna, C.F.; Correia, J.B.; da Silva, G.A. The balance between the serum levels of IL-6 and IL-10 cytokines discriminates mild and severe acute pneumonia. BMC Pulm. Med. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Kong, L.; Fink, M.P.; Weissfeld, L.A.; Yealy, D.M.; Pinsky, M.R. Understanding the inflammatory cytokine response in pneumonia and sepsis: Results of the genetic and inflammatory markers of sepsis (genims) study. Arch. Intern. Med. 2007, 167, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.H.; Smith, M.D.; Weverling, G.J.; Suputtamongkol, Y.; Angus, B.; Chaowagul, W.; White, N.J.; Van Deventer, S.J.H.; Prins, J.M. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J. Infect. Dis. 2000, 181, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase b. Nature 1995, 378, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-S.; Ueng, S.W.N.; Niu, C.-C.; Yuan, L.-J.; Yang, C.-Y.; Chen, W.-J.; Lee, M.S.; Chen, J.-K. Hyperbaric oxygen promotes osteogenic differentiation of bone marrow stromal cells by regulating wnt3a/beta-catenin signaling--an in vitro and in vivo study. Stem Cell Res. 2014, 12, 260–274. [Google Scholar] [CrossRef]
- Maurer, U.; Preiss, F.; Brauns-Schubert, P.; Schlicher, L.; Charvet, C. GSK-3—At the crossroads of cell death and survival. J. Cell Sci. 2014, 127, 1369–1378. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Chang, K.C.; Cobb, J.P.; Buchman, T.G.; Korsmeyer, S.J.; Karl, I.E. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 14541–14546. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Chang, K.C.; Swanson, P.E.; Tinsley, K.W.; Hui, J.J.; Klender, P.; Xanthoudakis, S.; Roy, S.; Black, C.; Grimm, E.; et al. Caspase inhibitors improve survival in sepsis: A critical role of the lymphocyte. Nat. Immunol. 2000, 1, 496–501. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.G.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef]
- Weislow, O.S.; Pakman, L.M. Inhibition of pseudomonas aeruginosa by hyperbaric oxygen: Interaction with mouse peritoneal exudate cells. Infect. Immun. 1974, 10, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Halbach, J.L.; Prieto, J.M.; Wang, A.W.; Hawisher, D.; Cauvi, D.M.; Reyes, T.; Okerblom, J.; Ramirez-Sanchez, I.; Villarreal, F.; Patel, H.H.; et al. Early hyperbaric oxygen therapy improves survival in a model of severe sepsis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R160–R168. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
| Parameter | HBOT Group | Control Group | p-Value |
|---|---|---|---|
| pH | 7.4 (7.31–7.42) | 7.3 (7.2–7.4) | 0.25 |
| PCO2 (mmHg) | 35.2 (25.0–37.6) | 26.9 (19.4–45.8) | 0.74 |
| PO2 (mmHg) | 103.3 (92.2–114.63) | 94.3 (58.8–112.3) | 0.44 |
| HCO3 (mmol/L) | 18.2 (15.6–22.0) | 15.7 (11.4–18.0) | 0.10 |
| Lactate (mmol/L) | 2.8 (2.3–3.9) | 5.9 (3.5–6.6) | 0.02 |
| Base excess (mmol/L) | −6.3 (−9.5 to −3.2) | −10.6 (−14.7 to −7.6) | 0.03 |
| Albumin (g/dL) | 3.8 (3.6–4.0) | 3.8 (3.6–3.9) | 0.91 |
| ALT (IU/L) | 34.0 (31.0–38.0) | 46.5 (37.3–163.8) | 0.02 |
| Amylase (IU/L) | 730.0 (634.0–1194.0) | 976.0 (742.5–1137.0) | 0.27 |
| Glucose (mg/dL) | 121.0 (115.0–137.0) | 101.5 (65.3–111.8) | 0.04 |
| Variable | HBOT Group | Control Group | p-Value |
|---|---|---|---|
| IL-6 (pg/mL) | 4.5 × 103 (3.7 × 103–8.2 × 103) | 23 × 103 (88 × 103–298 × 103) | 0.01 |
| IL-10 (pg/mL) | 3.3 × 103 (3.0 × 103–4.2 × 103) | 4.3 × 103 (3.6 × 103–6.6 × 103) | 0.04 |
| IL-6/IL-10 ratio | 1.5 (1.1–1.9) | 3.9 (1.8–5.6) | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.W.; Choi, S.; Song, H.; Lee, M.J.; Kwon, J.E.; Lee, H.A.R.; Kim, K. Effect of Hyperbaric Oxygen Therapy on Acute Liver Injury and Survival in a Rat Cecal Slurry Peritonitis Model. Life 2020, 10, 283. https://doi.org/10.3390/life10110283
Yang HW, Choi S, Song H, Lee MJ, Kwon JE, Lee HAR, Kim K. Effect of Hyperbaric Oxygen Therapy on Acute Liver Injury and Survival in a Rat Cecal Slurry Peritonitis Model. Life. 2020; 10(11):283. https://doi.org/10.3390/life10110283
Chicago/Turabian StyleYang, Hee Won, Sangchun Choi, Hakyoon Song, Min Ji Lee, Ji Eun Kwon, Han A. Reum Lee, and Kyuseok Kim. 2020. "Effect of Hyperbaric Oxygen Therapy on Acute Liver Injury and Survival in a Rat Cecal Slurry Peritonitis Model" Life 10, no. 11: 283. https://doi.org/10.3390/life10110283
APA StyleYang, H. W., Choi, S., Song, H., Lee, M. J., Kwon, J. E., Lee, H. A. R., & Kim, K. (2020). Effect of Hyperbaric Oxygen Therapy on Acute Liver Injury and Survival in a Rat Cecal Slurry Peritonitis Model. Life, 10(11), 283. https://doi.org/10.3390/life10110283






