Alterations of Specific Lymphocytic Subsets with Aging and Age-Related Metabolic and Cardiovascular Diseases
Abstract
:1. Introduction
2. Results
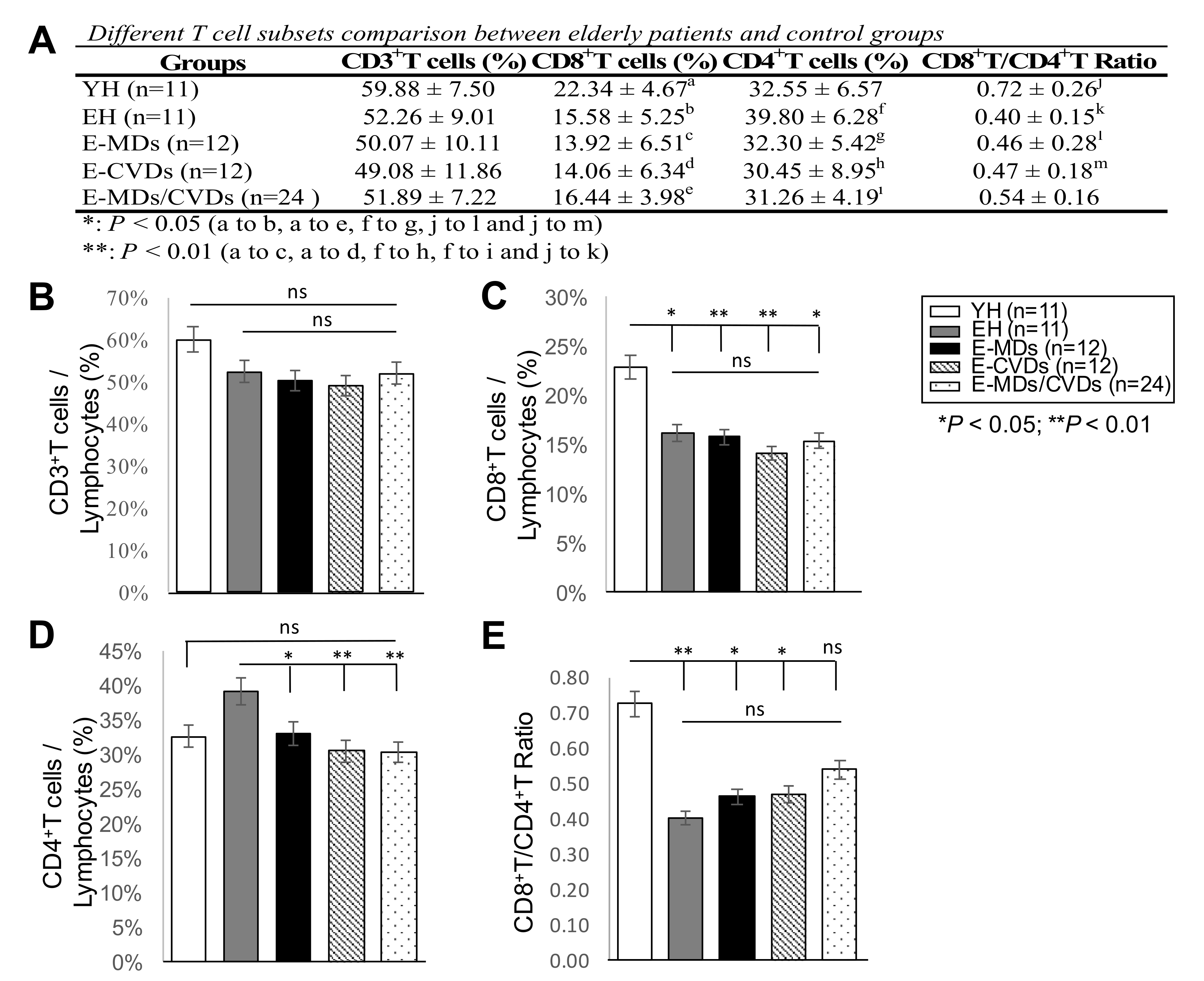
2.1. E-MD, E-CVD, and E-MD/CVD Groups Had Decreased CD4T Cell and Treg Cell Subsets Compared to EH
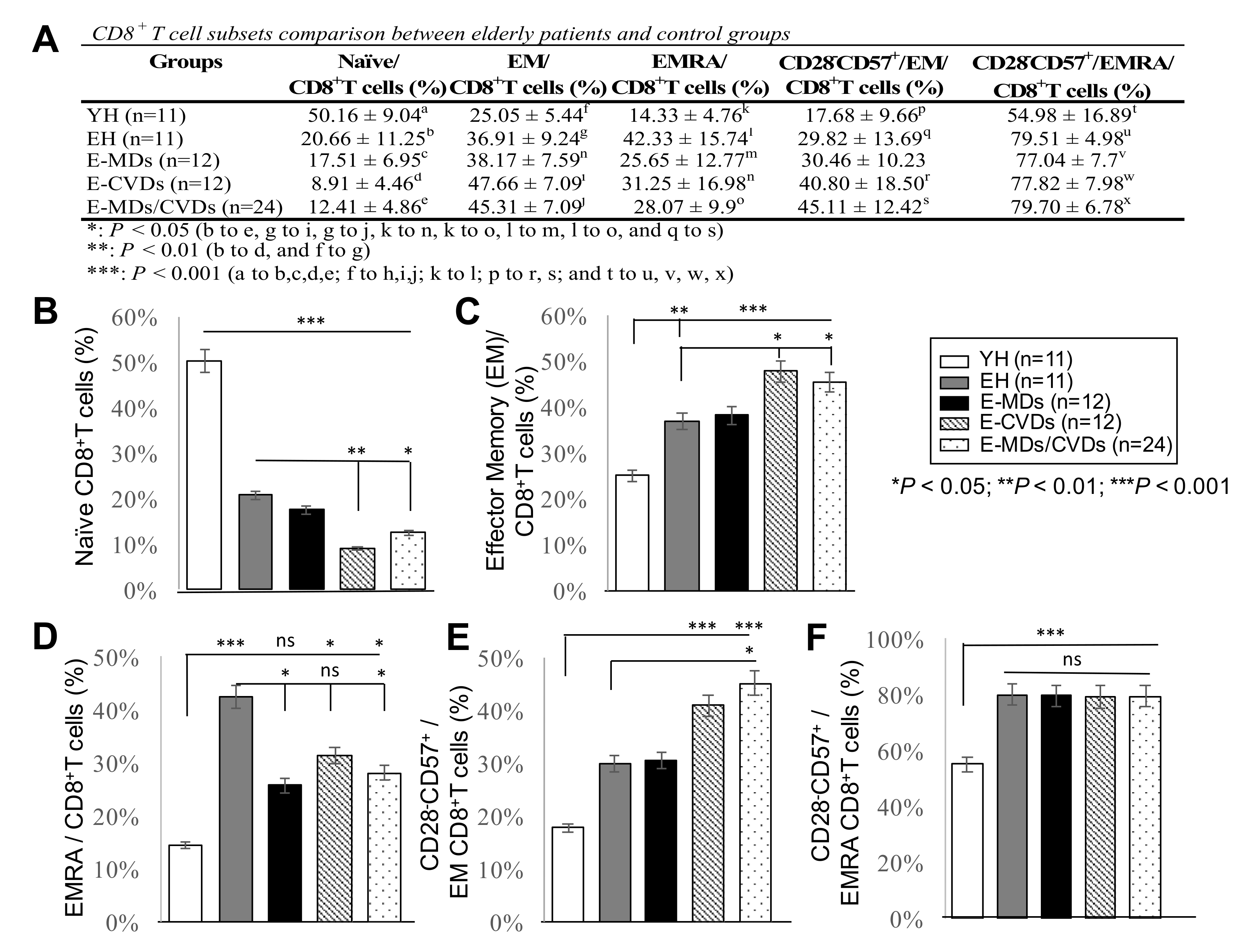
2.2. The Proportions of CD8 -TN Cells Were Reduced Whereas CD8-TEM Cells and CD8-TEMRA Were Increased in Most Elderly Groups Compared to YH, Together with Increased Frequencies of CD28-CD57+CD8+ Cells
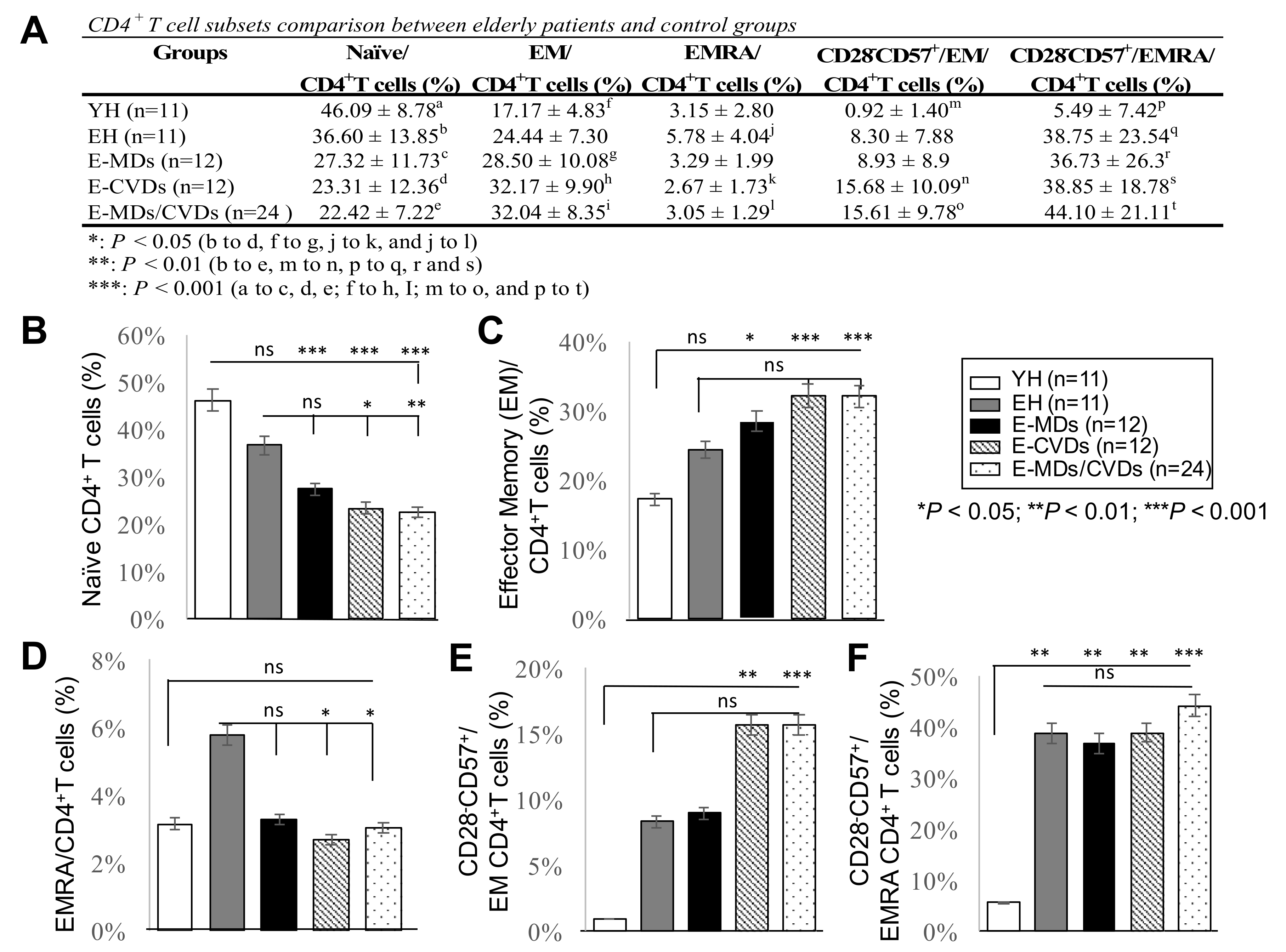
2.3. The Proportions of CD4-TN Cells Were Reduced While CD4-TEM Cells Were Increased in Most Elderly Groups, Together with Increased Frequencies of Cd28−Cd57+Cd8+ Cells, While Increased Cd4-Temra Cells Were Not Observed for Elderly Groups Compared to Yh
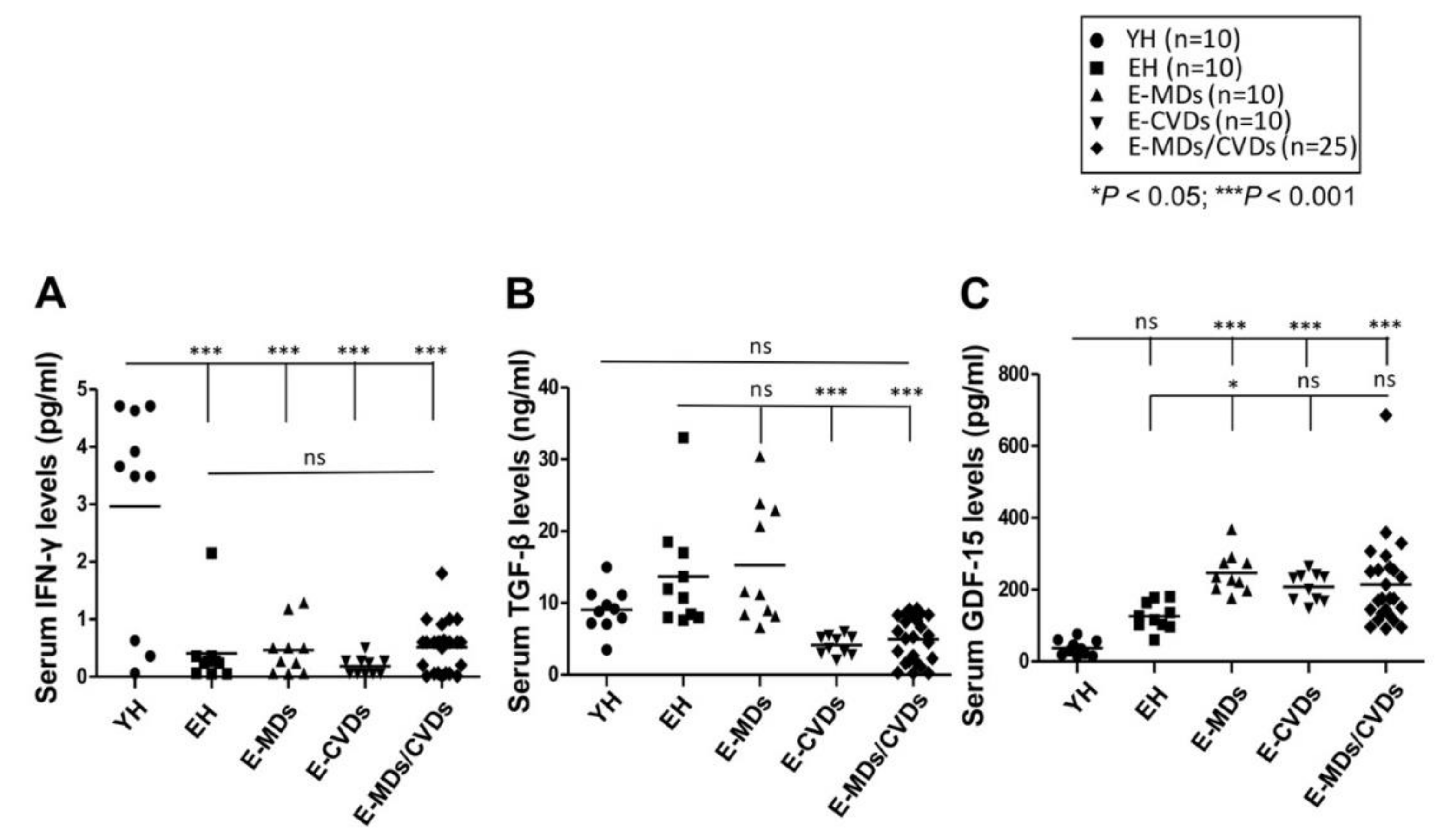
2.4. Serum Levels of Ifn-γ Were Reduced with Aging While Gdf-15 Levels Were Increased in Elderly Diseases Groups Compared to Yh Controls
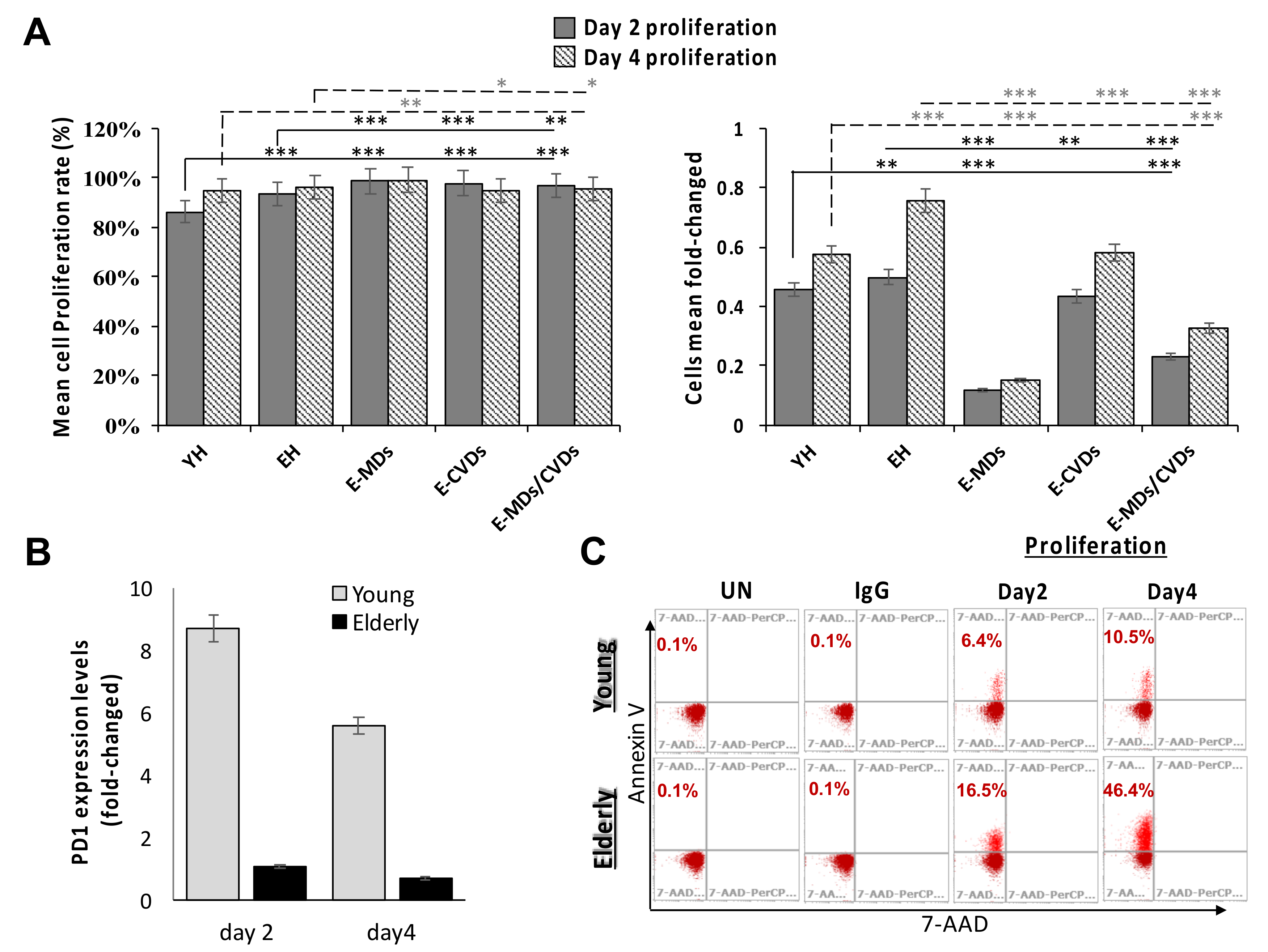
2.5. T-Cell Proliferation Activities Were Relatively High in Aged People and These Proliferated T-Cells Tended to Trigger Apoptosis Following Stimulation Due to a True Senescence Phenomenon
3. Discussion
4. Materials and Methods
4.1. Outpatients
4.2. Study Design
4.3. Methods
4.3.1. Blood Sample Collection
4.3.2. Flow Cytometry and Antibodies
4.3.3. Quantitation of Serum IFN-γ, TGF-β, and GDF-15 Levels
4.3.4. Proliferation Assay
4.3.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Accardi, G.; Caruso, C. Immune-inflammatory responses in the elderly: An update. Immun. Ageing 2018, 15, 1–4. [Google Scholar] [CrossRef]
- Thomas, R.; Wang, W.; Su, D.-M. Contributions of Age-Related Thymic Involution to Immunosenescence and Inflammaging. Immun. Ageing 2020, 17, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeske, S.S.; Schuler, P.J.; Doescher, J.; Theodoraki, M.N.; Laban, S.; Brunner, C.; Hoffmann, T.K.; Wigand, M.C. Age-related changes in T lymphocytes of patients with head and neck squamous cell carcinoma. Immun. Ageing 2020, 17, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, B.F.; Markert, M.L.; Sempowski, G.; Patel, D.D.; Hale, L.P. The Role of the Thymus in Immune Reconstitution in Aging, Bone Marrow Transplantation, and HIV-1 Infection. Annu. Rev. Immunol. 2000, 18, 529–560. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.T.; Memon, S.A.; Cepeda, R.; Jones, E.C.; Chow, C.K.; Kasten-Sportes, C.; Odom, J.; Vance, B.A.; Christensen, B.L.; Mackall, C.L.; et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J. Clin. Investig. 2005, 115, 930–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goronzy, J.J.; Lee, W.-W.; Weyand, C.M. Aging and T-cell diversity. Exp. Gerontol. 2007, 42, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumova, E.N.; Yassai, M.B.; Demos, W.; Reed, E.; Unruh, M.; Haribhai, D.; Williams, C.B.; Naumov, Y.N.; Gorski, J. Age-Based Dynamics of a Stable Circulating Cd8 T Cell Repertoire Component. Front. Immunol. 2019, 10, 1717. [Google Scholar] [CrossRef] [Green Version]
- Yanes, R.E.; Gustafson, C.E.; Weyand, C.M.; Goronzy, J.J. Lymphocyte generation and population homeostasis throughout life. Semin. Hematol. 2017, 54, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Jagger, A.T.; Shimojima, Y.; Goronzy, J.J.; Weyand, C.M. Regulatory T cells and the immune aging process: A mini-review. Gerontology 2013, 60, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Fessler, J.; Ficjan, A.; Duftner, C.; Dejaco, P.D.C. The Impact of Aging on Regulatory T-Cells. Front. Immunol. 2013, 4, 231. [Google Scholar] [CrossRef] [Green Version]
- Van Der Geest, K.S.M.; Abdulahad, W.H.; Tete, S.M.; Lorencetti, P.G.; Horst, G.; Bos, N.A.; Kroesen, B.-J.; Brouwer, E.; Boots, A.M.H. Aging disturbs the balance between effector and regulatory CD4+ T cells. Exp. Gerontol. 2014, 60, 190–196. [Google Scholar] [CrossRef]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Bolton, C.; Smith, P.A. The influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE). Ageing Res. Rev. 2018, 41, 64–81. [Google Scholar] [CrossRef]
- Costantini, E.; D’Angelo, C.; Reale, M. The Role of Immunosenescence in Neurodegenerative Diseases. Mediat. Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Wijeyekoon, R.S.; Scott, K.M.; Hayat, S.; Barker, R.A.; Jones, J.L. Abnormalities of age-related T cell senescence in Parkinson’s disease. J. Neuroinflammation 2018, 15, 166. [Google Scholar] [CrossRef]
- Seeger, T.; Haffez, F.; Fischer, A.; Koehl, U.; Leistner, D.M.; Seeger, F.H.; Boon, R.A.; Zeiher, A.M.; Dimmeler, S. Immunosenescence-associated microRNAs in age and heart failure. Eur. J. Hear. Fail. 2013, 15, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, G. Immunosenescence and cancer. Biogerontology 2017, 18, 717–721. [Google Scholar] [CrossRef]
- Trintinaglia, L.; Bandinelli, L.P.; Grassi-Oliveira, R.; Petersen, L.E.; Anzolin, M.; Correa, B.L.; Schuch, J.; Bauer, M.E. Features of Immunosenescence in Women Newly Diagnosed With Breast Cancer. Front. Immunol. 2018, 9, 1651. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.T.; Park, S.; Shin, E.-C.; Lee, W.-W. T cell senescence and cardiovascular diseases. Clin. Exp. Med. 2015, 16, 257–263. [Google Scholar] [CrossRef]
- Youn, J.-C.; Yu, H.T.; Lim, B.J.; Koh, M.J.; Lee, J.; Chang, D.-Y.; Choi, Y.-S.; Lee, S.-H.; Kang, S.-M.; Jang, Y.; et al. Immunosenescent CD8+ T Cells and C-X-C Chemokine Receptor Type 3 Chemokines Are Increased in Human Hypertension. Hypertension 2013, 62, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Chou, J.P.; Effros, R.B. T cell replicative senescence in human aging. Curr. Pharm. Des. 2013, 19, 1680–1698. [Google Scholar] [PubMed]
- Sheikh, V.; Zamani, A.; Mahabadi-Ashtiyani, E.; Tarokhian, H.; Borzouei, S.; Alahgholi-Hajibehzad, M. Decreased regulatory function of CD4+ CD25+ CD45RA+ T cells and impaired IL-2 signalling pathway in patients with type 2 diabetes mellitus. Scand. J. Immunol. 2018, 88, e12711. [Google Scholar] [CrossRef] [Green Version]
- Dock, J.N.; Effros, R.B. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging Dis. 2011, 2, 382–397. [Google Scholar] [PubMed]
- Callender, L.A.; Carroll, E.C.; Beal, R.W.J.; Chambers, E.S.; Nourshargh, S.; Akbar, A.N.; Henson, S.M. Human CD8+ EMRA T cells display a senescence-associated secretory phenotype regulated by p38 MAPK. Aging Cell 2017, 17, e12675. [Google Scholar] [CrossRef] [PubMed]
- Bandrés, E.; Merino, J.; Vazquez, B.; Inogés, S.; Moreno, C.; Subirá, M.; Sánchez-Ibarrola, A. The Increase of IFN-γ Production through Aging Correlates with the Expanded CD8+highCD28−CD57+ Subpopulation. Clin. Immunol. 2000, 96, 230–235. [Google Scholar] [CrossRef]
- Xu, W.; Larbi, A. Markers of T Cell Senescence in Humans. Int. J. Mol. Sci. 2017, 18, 1742. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, D.I.; Uldrich, A.P.; McCluskey, J.; Rossjohn, J.; Moody, D.B. The burgeoning family of unconventional T cells. Nat. Immunol. 2015, 16, 1114–1123. [Google Scholar] [CrossRef]
- Haynes, L.; Swain, S.L. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin. Immunol. 2012, 24, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Becklund, B.R.; Purton, J.F.; Ramsey, C.; Favre, S.; Vogt, T.K.; Martin, C.E.; Spasova, D.S.; Sarkisyan, G.; Leroy, E.; Tan, J.T.; et al. The aged lymphoid tissue environment fails to support naïve T cell homeostasis. Sci. Rep. 2016, 6, 30842. [Google Scholar] [CrossRef]
- Hale, J.S.; Boursalian, T.E.; Turk, G.L.; Fink, P.J. Thymic output in aged mice. Proc. Natl. Acad. Sci. USA 2006, 103, 8447–8452. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, H.; Clise-Dwyer, K.; Huston, G.E.; Duso, D.K.; Buck, A.L.; Johnson, L.L.; Haynes, L.; Swain, S.L. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc. Natl. Acad. Sci. USA 2009, 106, 18333–18338. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Myeloid-derived suppressor cells (MDSC): An important partner in cellular/tissue senescence. Biogerontology 2018, 19, 325–339. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Zhang, Q. Increased serum level of growth differentiation factor 15 (GDF-15) is associated with coronary artery disease. Cardiovasc. Ther. 2016, 34, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinti, M.; Appay, V.; Campisi, J.; Frasca, D.; Fülöp, T.; Sauce, D.; Larbi, A.; Weinberger, B.; Cossarizza, A. Aging of the immune system: Focus on inflammation and vaccination. Eur. J. Immunol. 2016, 46, 2286–2301. [Google Scholar] [CrossRef] [PubMed]
- Gruver, A.L.; Hudson, L.L.; Sempowski, G.D. Immunosenescence of ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef]
- Yan, J.; Greer, J.M.; Hull, R.; O’Sullivan, J.; Henderson, R.D.; Read, S.; McCombe, P.A. The effect of ageing on human lymphocyte subsets: Comparison of males and females. Immun. Ageing 2010, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elisia, I.; Lam, V.; Hofs, E.; Li, M.Y.; Hay, M.; Cho, B.; Brooks-Wilson, A.; Rosin, M.; Bu, L.; Jia, W.; et al. Effect of age on chronic inflammation and responsiveness to bacterial and viral challenges. PLoS ONE 2017, 12, e0188881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansoni, P.; Vescovini, R.; Fagnoni, F.; Biasini, C.; Zanni, F.; Zanlari, L.; Telera, A.; Lucchini, G.; Passeri, G.; Monti, D.; et al. The immune system in extreme longevity. Exp. Gerontol. 2008, 43, 61–65. [Google Scholar] [CrossRef]
- Hodes, R.J.; Hathcock, K.S.; Weng, N.-P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002, 2, 699–706. [Google Scholar] [CrossRef]
- Tario, J.D.; Humphrey, K.; Bantly, A.D.; Muirhead, K.A.; Moore, J.S.; Wallace, P.K. Optimized Staining and Proliferation Modeling Methods for Cell Division Monitoring using Cell Tracking Dyes. J. Vis. Exp. 2012, 2012, 4287. [Google Scholar] [CrossRef] [Green Version]

| Variables of Interest | YH (n = 19) | EH (n = 16) | E-MDs (n = 14) | E-CVDs (n = 14) | E-MDs/CVDs (n = 40) |
|---|---|---|---|---|---|
| Age (years), (mean ± SD) | 31.2 ± 5.8 | 67.1 ± 5.7 | 68.2 ± 8.3 | 77.6 ± 11.7 | 71.2 ± 9.9 |
| Sex (% female) | 52.6 | 44 | 64.3 | 42.9 | 52.5 |
| BMI (Male) | 21.38 ± 4.58 a | 23.93 ± 4.79 b | 25.32 ± 2.71 c | 25.89 ± 1.94 d | 26.79 ± 2.17 e |
| (Female) | 22.09 ± 2.52 f | 22.27 ± 0.5 g | 25.29 ± 2.20 h | 25.08 ± 4.01 i | 27.38 ± 4.94 j |
| WBC (10^9/L) | 6.4 ± 1.6 | 5.5 ± 1.3 k | 6.4 ± 1.5 l | 6.5 ± 1.5 m | 6.3 ± 1.4 n |
| Neutrophil (%) | 59.0 ± 8.6 | 60.6 ± 9.1 | 55.1 ± 6.9 | 59.1 ± 10.5 | 58.1 ± 8.1 |
| Monocyte (%) | 6.0 ± 1.8 | 6.4 ± 2.0 | 6.1 ± 3.5 | 5.7 ± 1.3 | 5.8 ± 1.5 |
| Lymphocyte (%) | 31.3 ± 7.7 | 30.8 ± 9.1 | 35.6 ± 7.1 | 31.8 ± 9.8 | 33.1 ± 8 |
| Diagnosis | no | no | Hyperlipidemia | COPD | Hypertension + hyperlipidemia |
| Hyperuricemia | Hypertension, | Hypertension + hyperuricemia | |||
| TypeII DM | Arrhythmia | TypeII DM + hypertension | |||
| TypeII DM + hypertension + hyperlipidemia | |||||
| Hypertension + hyperlipidemia + CAD + asthma | |||||
| TypeII DM + hypertension + hyperlipidemia + coronary syndrome + CAD | |||||
| TypeII DM + hypertension + hyperlipidemia + hyperuricemia | |||||
| Prescriptions | no | no | Statin | CCB | ARB + statin + Ezetimibe |
| Statin + BI + DDP4I | β-blocker | CCB + ARB + Diuretic + statin + BI + DDP4I | |||
| Ezetimibe + Fenofibrate | ARB | CCB + ARB + β-blocker + statin | |||
| Vytorin | Diuretic + ARB | ARB + SU + BI | |||
| Acarbose + BI + Pioglitazone | β-blocker + Diuretic + CCB + ARB | ARB + CCB + Diuretic | |||
| ARB + Diuretic + CCB + β-blocker + statin + Febuxostat | |||||
| ARB + Diuretic + SU + Fenofibrate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.J.; Liao, Y.J.; Tram, V.T.N.; Lin, C.H.; Liao, K.C.; Liu, C.L. Alterations of Specific Lymphocytic Subsets with Aging and Age-Related Metabolic and Cardiovascular Diseases. Life 2020, 10, 246. https://doi.org/10.3390/life10100246
Chen YJ, Liao YJ, Tram VTN, Lin CH, Liao KC, Liu CL. Alterations of Specific Lymphocytic Subsets with Aging and Age-Related Metabolic and Cardiovascular Diseases. Life. 2020; 10(10):246. https://doi.org/10.3390/life10100246
Chicago/Turabian StyleChen, Ying Jen, Yi Jen Liao, Van Thi Ngoc Tram, Chung Hao Lin, Kuo Chen Liao, and Chao Lien Liu. 2020. "Alterations of Specific Lymphocytic Subsets with Aging and Age-Related Metabolic and Cardiovascular Diseases" Life 10, no. 10: 246. https://doi.org/10.3390/life10100246






