Is Malignant Potential of Barrett’s Esophagus Predictable by Endoscopy Findings?
Abstract
1. Introduction
2. Possibility of WLE Findings for Predicting Neoplastic Progression
2.1. Length of BE
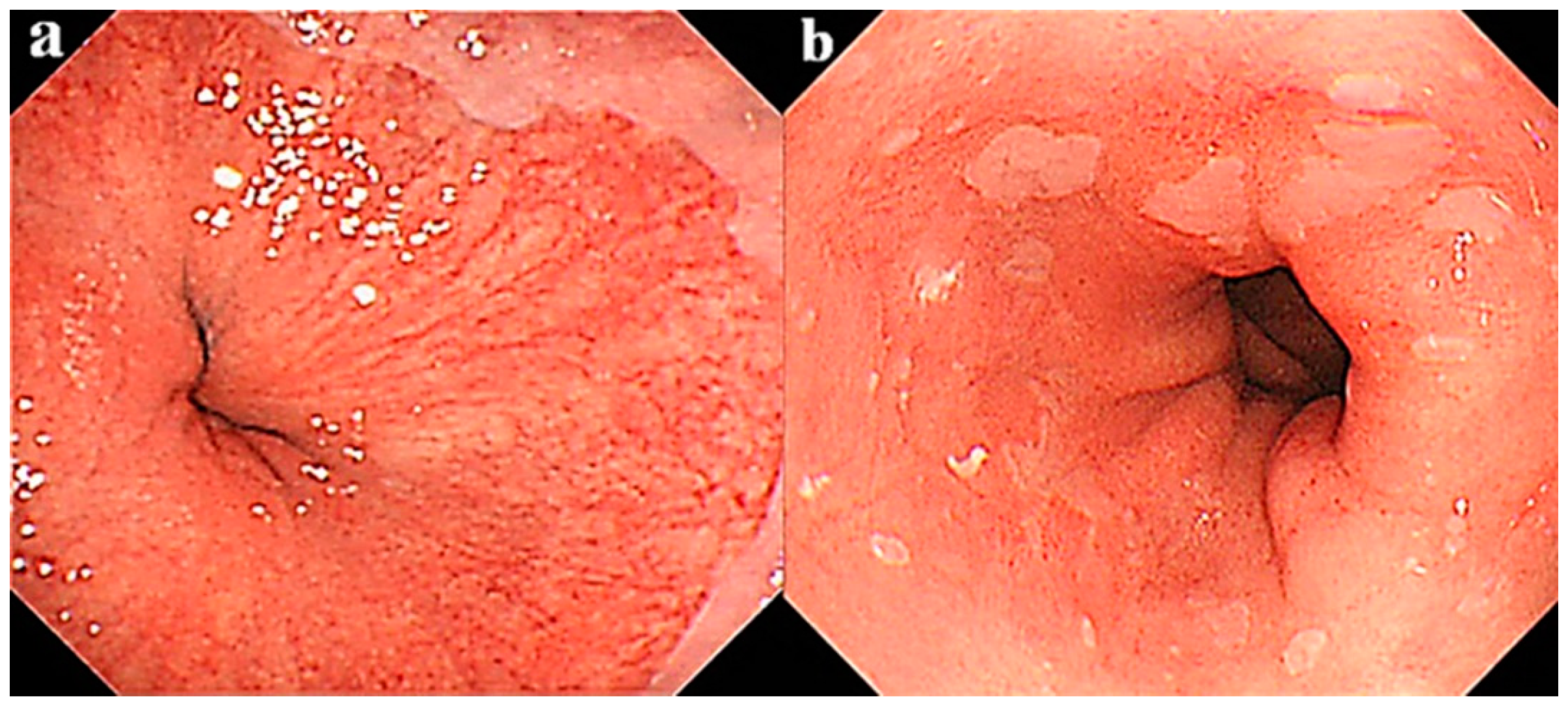
2.2. Other Endoscopic Findings
3. Endoscopic Detection of Intestinal Metaplasia
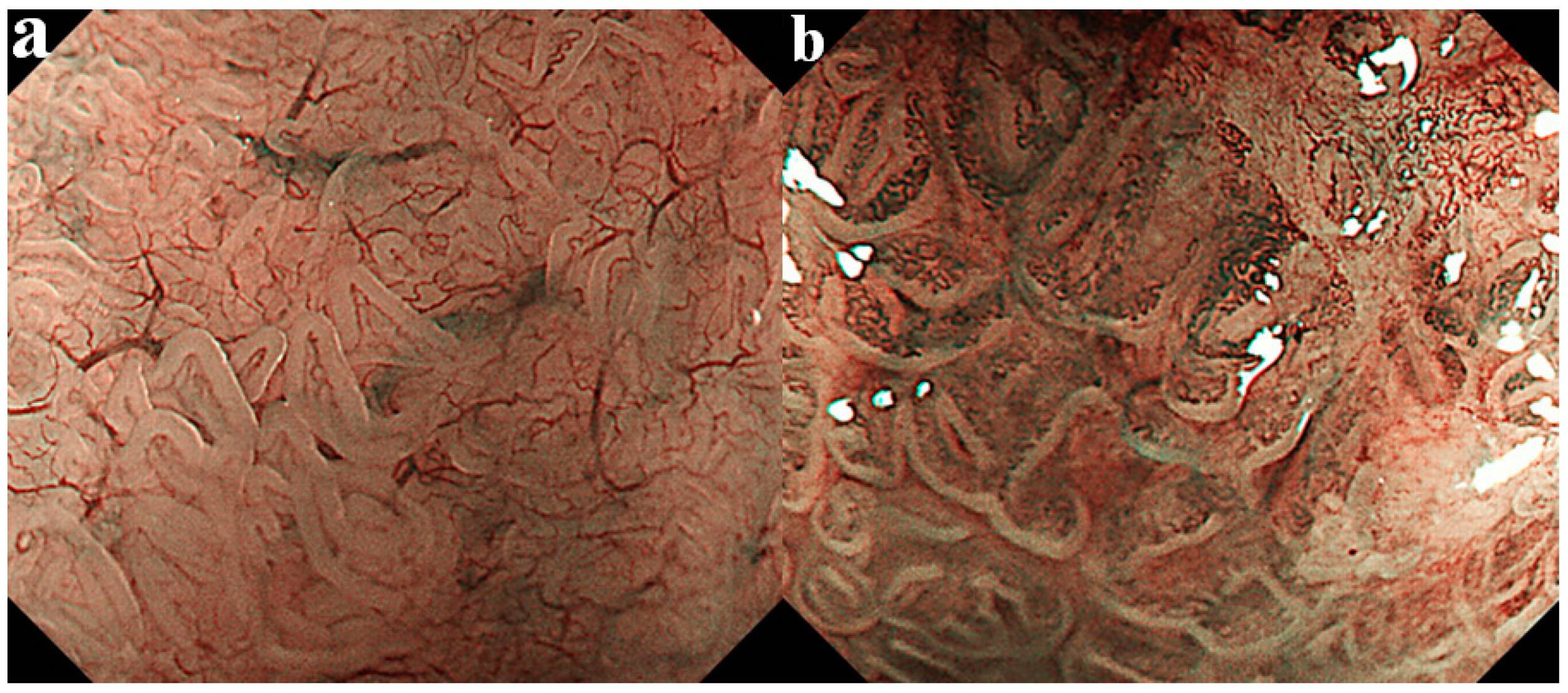
3.1. Detection by IEE
3.2. Detection by NBI Endoscopy
4. Endoscopic Findings Related to COX-2 Protein Expression and Cellular Proliferation
4.1. Assessment by Esophageal Palisade Vessels
4.2. Assessment by Mucosal Pattern
4.3. Assessment of Micro-Vascular Pattern
5. Endoscopic Findings Showing Possible Predictive Biomarker Information Regarding Initiation and Progression of EAC
5.1. Molecular Biomarker Associated with Pathogenesis of EAC Progression
5.1.1. Genetic Instability
5.1.2. Growth Factors
5.1.3. Other Markers
5.2. Possible Endoscopic Identification of Molecular Biomarker in Association with EAC Progression
6. What Is an Efficient Method for Surveillance of BE?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Caygill, C.P.; Royston, C.; Charlett, A.; Wall, C.M.; Gatenby, P.A.; Ramus, J.R.; Watson, A.; Winslet, M.; Bardhan, K.D. Mortality in Barrett’s esophagus: Three decades of experience at a single center. Endoscopy 2012, 44, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef]
- Verbeek, R.E.; Leenders, M.; Ten Kate, F.J.; van Hillegersberg, R.; Vleggaar, F.P.; van Baal, J.W.; Van Oijen, M.G.H.; Siersema, P.D. Surveillance of Barrett’s esophagus and mortality from esophageal adenocarcinoma: A population-based cohort study. Am. J. Gastroenterol. 2014, 109, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Pech, O.; Behrens, A.; May, A.; Nachbar, L.; Gossner, L.; Rabenstein, T.; Manner, H.; Guenter, E.; Huijsmans, J.; Vieth, M.; et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut 2008, 57, 1200–1206. [Google Scholar] [CrossRef]
- Desai, T.K.; Krishnan, K.; Samala, N.; Singh, J.; Cluley, J.; Perla, S.; Howden, C.W. The incidence of oesophageal adenocarcinoma in non-dysplastic Barrett’s oesophagus: A meta-analysis. Gut 2012, 61, 970–976. [Google Scholar] [CrossRef]
- Kroep, S.; Lansdorp-Vogelaar, I.; Rubenstein, J.H.; de Koning, H.J.; Meester, R.; Inadomi, J.M.; Van Ballegooijen, M. An accurate cancer incidence in Barrett’s esophagus: A best estimate using published data and modeling. Gastroenterology 2015, 149, 577–585. [Google Scholar] [CrossRef]
- Desai, M.; Lieberman, D.A.; Kennedy, K.F.; Hamade, N.; Thota, P.; Parasa, S.; Gorrepati, V.S.; Bansal, A.; Gupta, N.; Gaddam, S.; et al. Increasing prevalence of high-grade dysplasia and adenocarcinoma on index endoscopy in Barrett’s esophagus over the past 2 decades: Data from a multicenter U.S. consortium. Gastrointest. Endosc. 2019, 89, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.C.; di Pietro, M.; Ragunath, K.; Ang, Y.; Kang, J.Y.; Watson, P.; Trudgill, N.; Patel, P.; Kaye, P.; Sanders, S.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut 2014, 63, 7–42. [Google Scholar] [CrossRef]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B. American College of Gastroenterology; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar] [CrossRef]
- Qumseya, B.; Sultan, S.; Bain, P.; Jamil, L.; Jacobson, B.; Anandasabapathy, S.; Agrawal, D.; Buxbaum, J.L.; Fishman, D.S.; Gurudu, S.R.; et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest. Endosc. 2019, 90, 335–359. [Google Scholar] [CrossRef]
- Cooper, S.; Menon, S.; Nightingale, P.; Trudgill, N.J. Risk factors for the development of oesophageal adenocarcinoma in Barrett’s oesophagus: A UK primary care retrospective nested case-control study. United Eur. Gastroenterol. J. 2014, 2, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Borah, B.; Heien, H.; Das, A.; Chak, A.; Iyer, P.G. Rates and predictors of progression to esophageal carcinoma in a large population-based Barrett’s esophagus cohort. Gastrointest. Endosc. 2016, 84, 40–46. [Google Scholar] [CrossRef]
- Petrick, J.L.; Falk, R.T.; Hyland, P.L.; Caron, P.; Pfeiffer, R.M.; Wood, S.N.; Dawsey, S.M.; Abnet, C.C.; Taylor, P.R.; Guillemette, C.; et al. Association between circulating levels of sex steroid hormones and esophageal adenocarcinoma in the FINBAR Study. PLoS ONE 2018, 13, e0190325. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, M.G.; Cantwell, M.M.; Murray, L.J.; Anderson, L.A.; Abnet, C.C.; FINBAR Study Group. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. Int. J. Cancer 2011, 129, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.H.; Morgenstern, H.; Chey, W.D.; Murray, J.; Scheiman, J.M.; Schoenfeld, P. Protective role of gluteofemoral obesity in erosive oesophagitis and Barrett’s oesophagus. Gut 2014, 63, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Visceral adipose tissue attacks beyond the liver: Esophagogastric junction as a new target. Gastroenterology 2010, 139, 1823–1826. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hashmi, A.; Garcia, J.; Richardson, P.; Alsarraj, A.; Fitzgerald, S.; Vela, M.; Shaib, Y.; Abraham, N.S.; Velez, M.; et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barrett’s oesophagus: A case-control study. Gut 2014, 63, 220–229. [Google Scholar] [CrossRef]
- Amano, Y.; Nakahara, R.; Yuki, T.; Murakami, D.; Ujihara, T.; Tomoyuki, I.; Sagami, R.; Suehiro, S.; Katsuyama, Y.; Hayasaka, K.; et al. Relationship between Barrett’s esophagus and colonic diseases: A role for colonoscopy in Barrett’s surveillance. J. Gastroenterol. 2019, 54, 984–993. [Google Scholar] [CrossRef]
- Lagergren, J.; Bergström, R.; Lindgren, A.; Nyrén, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999, 340, 825–831. [Google Scholar] [CrossRef]
- Sikkema, M.; Looman, C.W.; Steyerberg, E.W.; Kerkhof, M.; Kastelein, F.; van Dekken, H.; Van Vuuren, A.J.; Bode, W.A.; Van Der Valk, H.; Ouwendijk, R.J.T.; et al. Predictors for neoplastic progression in patients with Barrett’s Esophagus: A prospective cohort study. Am. J. Gastroenterol. 2011, 106, 1231–1238. [Google Scholar] [CrossRef]
- Kastelein, F.; Spaander, M.C.; Steyerberg, E.W.; Biermann, K.; Valkhoff, V.E.; Kuipers, E.J.; Bruno, M. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin. Gastroenterol. Hepatol. 2013, 11, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.A.Z.; de Caestecker, J.; Love, S.B.; Reilly, G.; Watson, P.; Sanders, S.; Ang, Y.; Morris, D.; Bhandari, P.; Brooks, C.; et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): A randomised factorial trial. Lancet 2018, 392, 400–408. [Google Scholar] [CrossRef]
- Altaf, K.; Xiong, J.J.; La Iglesia, D.; Hickey, L.; Kaul, A. Meta-analysis of biomarkers predicting risk of malignant progression in Barrett’s oesophagus. Br. J. Surg. 2017, 104, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, V.T.; van Olphen, S.H.; Biermann, K.E.; Looijenga, L.H.J.; Bruno, M.B.; Spaander, M.C.W. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186305. [Google Scholar] [CrossRef]
- Stachler, M.D.; Camarda, N.D.; Deitrick, C.; Kim, A.; Agoston, A.T.; Odze, R.D.; Hornick, J.L.; Nag, A.; Thorner, A.R.; Ducar, M.; et al. Detection of mutations in Barrett’s esophagus before progression to high-grade dysplasia or adenocarcinoma. Gastroenterology 2018, 155, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Majka, J.; Rembiasz, K.; Migaczewski, M.; Budzynski, A.; Ptak-Belowska, A.; Pabianczyk, R.; Urbanczyk, K.; Urbanczyk, K.; Matlok, M.; Brzozowski, T. Cyclooxygenase-2 (COX-2) is the key event in pathophysiology of Barrett’s esophagus. Lesson from experimental animal model and human subjects. J. Physiol. Pharmacol. 2010, 61, 409–418. [Google Scholar]
- Sharma, P.; Dent, J.; Armstrong, D.; Bergman, J.J.; Gossner, L.; Hoshihara, Y.; Jankowski, J.A.; Junghard, O.; Lundell, L.; Tytgat, G.N.J.; et al. The development and validation of an endoscopic grading system for Barrett’s esophagus: The Prague C & M criteria. Gastroenterology 2006, 131, 1392–1399. [Google Scholar]
- Rudolph, R.E.; Vaughan, T.L.; Storer, B.E.; Haggitt, R.C.; Rabinovitch, P.S.; Levine, D.S.; Reid, B.J. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann. Intern. Med. 2000, 132, 612–620. [Google Scholar] [CrossRef]
- Cameron, A.J.; Souto, E.O.; Smyrk, T.C. Small adenocarcinomas of the esophagogastric junction: Association with intestinal metaplasia and dysplasia. Am. J. Gastroenterol. 2002, 97, 1375–1380. [Google Scholar] [CrossRef]
- Weston, A.P.; Sharma, P.; Mathur, S.; Banerjee, S.; Jafri, A.K.; Cherian, R.; McGregor, D.; Hassanein, R.S.; Hall, M. Risk stratification of Barrett’s esophagus: Updated prospective multivariate analysis. Am. J. Gastroenterol. 2004, 99, 1657–1666. [Google Scholar] [CrossRef]
- Rugge, M.; Zaninotto, G.; Parente, P.; Zanatta, L.; Cavallin, F.; Germanà, B.; Macrì, E.; Galliani, E.; Iuzzolino, P.; Iuzzolino, P.; et al. Barrett’s esophagus and adenocarcinoma risk: The experience of the North-Eastern Italian Registry (EBRA). Ann. Surg. 2012, 256, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Pohl, H.; Wrobel, K.; Bojarski, C.; Voderholzer, W.; Sonnenberg, A.; Rösch, T.; Baumgart, D.C. Risk factors in the development of esophageal adenocarcinoma. Am. J. Gastroenterol. 2013, 108, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Anaparthy, R.; Gaddam, S.; Kanakadandi, V.; Alsop, B.R.; Gupta, N.; Higbee, A.D.; Wani, S.B.; Singh, M.; Rastogi, A.; Bansal, A.; et al. Association between length of Barrett’s esophagus and risk of high-grade dysplasia or adenocarcinoma in patients without dysplasia. Clin. Gastroenterol. Hepatol. 2013, 11, 1430–1436. [Google Scholar] [CrossRef]
- Solanky, D.; Krishnamoorthi, R.; Crews, N.; Johnson, M.; Wang, K.; Wolfsen, H.; Fleischer, D.; Ramirez, F.C.; Katzka, D.; Buttar, N.; et al. Barrett esophagus length, nodularity, and low-grade dysplasia are predictive of progression to esophageal adenocarcinoma. J. Clin. Gastroenterol. 2019, 53, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, D.; Ness-Jensen, E.; Mattsson, F.; Lagergren, J. Clinical prediction model for tumor progression in Barrett’s esophagus. Surg. Endosc. 2019, 33, 2901–2908. [Google Scholar] [CrossRef]
- Coleman, H.G.; Bhat, S.K.; Murray, L.J.; McManus, D.T.; O’Neill, O.M.; Gavin, A.T.; Johnston, B.T. Symptoms and endoscopic features at barrett’s esophagus diagnosis: Implications for neoplastic progression risk. Am. J. Gastroenterol. 2014, 109, 527–534. [Google Scholar] [CrossRef]
- Hamade, N.; Vennelaganti, S.; Parasa, S.; Vennalaganti, P.; Gaddam, S.; Spaander, M.C.W.; Van Olphen, S.H.; Thota, P.N.; Kennedy, K.F.; Bruno, M.J.; et al. Lower Annual Rate of Progression of Short-Segment vs Long-Segment Barrett’s Esophagus to Esophageal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 864–868. [Google Scholar] [CrossRef]
- Bhat, S.; Coleman, H.G.; Yousef, F.; Johnston, B.T.; McManus, D.T.; Gavin, A.T.; Murray, L.J. Risk of malignant progression in Barrett’s esophagus patients: Results form a large population-based study. J. Natl. Cancer Inst. 2011, 103, 1049–1057. [Google Scholar] [CrossRef]
- Buttar, N.S.; Wang, K.K.; Sebo, T.J.; Riehle, D.M.; Krishnadath, K.K.; Lutzke, L.S.; Bs, M.A.A.; Petterson, T.M.; Burgart, L.J. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology 2001, 120, 1630–1639. [Google Scholar] [CrossRef]
- Prasad, G.A.; Bansal, A.; Sharma, P.; Wang, K.K. Predictors of progression in Barrett’s esophagus: Current knowledge and future directions. Am. J. Gastroenterol. 2010, 105, 1490–1502. [Google Scholar] [CrossRef]
- Hillman, L.C.; Chiragakis, L.; Clarke, A.C.; Kaushik, S.P.; Kaye, G.L. Barrett’s esophagus: Macroscopic markers and the prediction of dysplasia and adenocarcinoma. J. Gastroenterol. Hepatol. 2003, 18, 526–533. [Google Scholar] [CrossRef]
- Avidan, B.; Sonnenberg, A.; Schnell, T.G.; Chejfec, G.; Metz, A.; Sontag, S.J. Hiatal hernia size, Barrett’s length, and severity of acid reflux are all risk factors for esophageal adenocarcinoma. Am. J. Gastroenterol. 2002, 97, 1930–1936. [Google Scholar] [CrossRef]
- Spechler, S.J.; Sharma, P.; Souza, R.F.; Inadomi, J.M.; Shaheen, N.J. American Gastroenterological Association medical position statement on the management of Barrett’s esophagus. Gastroenterology 2011, 140, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J. New British Society of Gastroenterology (BSG) guidelines for the diagnosis and management of Barrett’s oesophagus. Gut 2006, 55, 442. [Google Scholar] [CrossRef] [PubMed]
- Canto, M.I.; Setrakian, S.; Willis, J.; Chak, A.; Petras, R.; Powe, N.R.; Sivak, M.V., Jr. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest. Endosc. 2000, 51, 560–568. [Google Scholar] [CrossRef]
- Endo, T.; Awakawa, T.; Takahashi, H.; Arimura, Y.; Itoh, F.; Yamashita, K.; Sasaki, S.; Yamamoto, H.; Tang, X.; Imai, K. Classification of Barrett’s epithelium by magnifying endoscopy. Gastrointest. Endosc. 2002, 55, 641–647. [Google Scholar] [CrossRef]
- Horwhat, J.D.; Maydonovitch, C.L.; Ramos, F.; Colina, R.; Gaertner, E.; Lee, H.; Wong, R.K. A randomized comparison of methylene blue-directed biopsy versus conventional four-quadrant biopsy for the detection of intestinal metaplasia and dysplasia in patients with long-segment Barrett’s esophagus. Am. J. Gastroenterol. 2008, 103, 546–554. [Google Scholar] [CrossRef]
- Ngamruengphong, S.; Sharma, V.K.; Das, A. Diagnostic yield of methylene blue chromoendoscopy for detecting specialized intestinal metaplasia and dysplasia in Barrett’s esophagus: A meta-analysis. Gastrointest. Endosc. 2009, 69, 1021–1028. [Google Scholar] [CrossRef]
- Guelrud, M.; Herrera, I.; Essenfeld, H.; Castro, J. Enhanced magnification endoscopy: A new technique to identify specialized intestinal metaplasia in Barrett’s esophagus. Gastrointest. Endosc. 2001, 53, 559–565. [Google Scholar] [CrossRef]
- Hamamoto, Y.; Endo, T.; Nosho, K.; Arimura, Y.; Sato, M.; Imai, K. Usefulness of narrow-band imaging endoscopy for diagnosis of Barrett’s esophagus. J. Gastroenterol. 2004, 39, 14–20. [Google Scholar] [CrossRef]
- Goda, K.; Tajiri, H.; Ikegami, M.; Urashima, M.; Nakayoshi, T.; Kaise, M. Usefulness of magnifying endoscopy with narrow band imaging for the detection of specialized intestinal metaplasia in columnar-lined esophagus and Barrett’s adenocarcinoma. Gastrointest. Endosc. 2007, 65, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bansal, A.; Mathur, S.; Wani, S.; Cherian, R.; McGregor, D.; Higbee, A.; Hall, S.; Weston, A. The utility of a novel narrow band imaging endoscopy system in patients with Barrett’s esophagus. Gastrointest. Endosc. 2006, 64, 167–175. [Google Scholar] [CrossRef]
- Singh, R.; Anagnostopoulos, G.K.; Yao, K.; Karageorgiou, H.; Fortun, P.J.; Shonde, A.; Garsed, K.; Kaye, P.V.; Hawkey, C.J.; Ragunath, K. Narrow-band imaging with magnification in Barrett’s esophagus: Validation of a simplified grading system of mucosal morphology patterns against histology. Endoscopy 2008, 40, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bergman, J.J.; Goda, K.; Kato, M.; Messmann, H.; Alsop, B.R.; Gupta, N.; Vennalaganti, P.; Hall, M.; Konda, V.; et al. Development and validation of a classification system to identify high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus using narrow-band imaging. Gastroenterology 2016, 150, 591–598. [Google Scholar] [CrossRef]
- Furuhashi, H.; Goda, K.; Shimizu, Y.; Kato, M.; Takahashi, M.; Dobashi, A.; Ikegami, M.; Shimoda, T.; Kato, M.; Sharma, P. Image assessment of Barrett’s esophagus using the simplified narrow band imaging classification. J. Gastroenterol. 2019, 54, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.B.; Dinis-Ribeiro, M.; Vieth, M.; Rabenstein, T.; Goda, K.; Kiesslich, R.; Haringsma, J.; Edebo, A.; Toth, E.; Soares, J.; et al. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow-band imaging: Accuracy and interobserver agreement of different classification systems (with videos). Gastrointest. Endosc. 2011, 73, 7–14. [Google Scholar] [CrossRef]
- Nunobe, S.; Nakanishi, Y.; Taniguchi, H.; Sasako, M.; Sano, T.; Kato, H.; Yamagishi, H.; Sekine, S.; Shimoda, T. Two distinct pathways of tumorigenesis of adenocarcinomas of the esophagogastric junction, related or unrelated to intestinal metaplasia. Pathol. Int. 2007, 57, 315–321. [Google Scholar] [CrossRef]
- Takubo, K.; Aida, J.; Naomoto, Y.; Sawabe, M.; Arai, T.; Shiraishi, H.; Matsuura, M.; Ell, C.; May, A.; Pech, O.; et al. Cardiac rather than intestinal-type background in endoscopic resection specimens of minute Barrett adenocarcinoma. Hum. Pathol. 2009, 40, 65–74. [Google Scholar] [CrossRef]
- Schnell, T.G.; Sontag, S.J.; Chejfec, G. Adenocarcinomas arising in tongues or short segments of Barrett’s esophagus. Dig. Dis. Sci. 1992, 37, 137–143. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Gossner, L.; Pech, O.; Muller, H.; Vieth, M.; Stolte, M.; Ell, C. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus (SSBE): Curative treatment using local endoscopic treatment techniques. Endoscopy 2002, 34, 604–610. [Google Scholar] [CrossRef]
- Aida, J.; Vieth, M.; Ell, C.; May, A.; Pech, O.; Hoshihara, Y.; Kumagai, Y.; Kawada, K.; Hishima, T.; Tateishi, Y.; et al. Palisade vessels as a new histologic marker of esophageal origin in ER specimens from columnar-lined esophagus. Am. J. Surg. Pathol. 2011, 35, 1140–1145. [Google Scholar] [CrossRef]
- Morris, C.D.; Armstrong, G.R.; Bigley, G.; Green, H.; Attwood, S.E. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am. J. Gastroenterol. 2001, 96, 990–996. [Google Scholar] [CrossRef]
- Kaur, B.S.; Triadafilopoulos, G. Acid-and bile-induced PGE2 release and hyperproliferation in Barrett’s esophagus are COX-2 and PKC-εdependent. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G327–G334. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Ishihara, S.; Kushiyama, Y.; Yuki, T.; Takahashi, Y.; Chinuki, D.; Miyake, T.; Miyaoka, Y.; Rumi, M.A.K.; Ishimura, N.; et al. Barrett’s oesophagus with predominant intestinal metaplasia correlates with superficial cyclo-oxygenase-2 expression, increased proliferation and reduced apoptosis: Changes that are partially reversed by non-steroidal anti-inflammatory drugs usage. Aliment. Pharmacol. Ther. 2004, 20, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Falk, G.W.; Buttar, N.S.; Foster, N.R.; Ziegler, K.L.A.; Demars, C.J.; Romero, Y.; Marcon, N.E.; Schnell, T.; Corley, D.A.; Sharma, P.; et al. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett’s esophagus. Gastroenterology 2012, 143, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, F.; Spaander, M.C.; Biermann, K.; Steyerberg, E.W.; Kuipers, E.J.; Bruno, M.J.; Probar-study Group. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett’s esophagus. Gastroenterology 2011, 141, 2000–2008. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.-Q.; Ding, X.-W.; Yang, R.-K.; Huang, S.-L.; Kastelein, F.; Bruno, M.; Yu, X.-J.; Zhou, D.; Zou, X.-P. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett’s esophagus: A meta-analysis. Br. J. Cancer 2014, 110, 2378–2388. [Google Scholar] [CrossRef]
- Ishimura, N.; Amano, Y.; Kinoshita, Y. Endoscopic definition of esophagogastric junction for diagnosis of Barrett’s esophagus: Importance of systematic education and training. Dig. Endosc. 2009, 21, 213–218. [Google Scholar] [CrossRef]
- Amano, Y.; Azumi, T.; Tsuboi, M.; Motoori, S. Reflux esophagitis and esophageal adenocarcinoma. Clin. Gastroenterol. 2015, 30, 1371–1380, (In Japanese with English Abstract). [Google Scholar]
- Amano, Y. Relationship between Barrett’s carcinogenesis and inflammation. BIO Clin. 2017, 6, 24–29, (In Japanese with English Abstract). [Google Scholar]
- Amano, Y.; Ishimura, N.; Furuta, K.; Takahashi, Y.; Chinuki, D.; Mishima, Y.; Moriyama, I.; Fukuhara, H.; Ishihara, S.; Adachi, K.; et al. Which landmark results in a more consistent diagnosis of Barrett’s esophagus, the gastric folds or the palisade vessels? Gastrointest. Endosc. 2006, 64, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Amano, Y.; Komazawa, Y.; Ishimura, N.; Ohara, S.; Aimi, M.; Fujishiro, H.; Ishihara, S.; Adachi, K.; Kinoshita, Y. Two cases of superficial cancer in Barrett’s esophagus detected with chromoendoscopy with crystal violet. Gastrointest. Endosc. 2004, 59, 143–146. [Google Scholar] [CrossRef]
- Amano, Y.; Kushiyama, Y.; Ishihara, S.; Yuki, T.; Miyaoka, Y.; Yoshino, N.; Ishimura, N.; Fujishiro, H.; Adachi, K.; Maruyama, R.; et al. Crystal violet chromoendoscopy with mucosal pit pattern diagnosis is useful for surveillance of short-segment Barrett’s esophagus. Am. J. Gastroenterol. 2005, 100, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Yuki, T.; Amano, Y.; Kushiyama, Y.; Takahashi, Y.; Ose, T.; Moriyama, I.; Fukuhara, H.; Ishimura, N.; Koshino, K.; Furuta, K.; et al. Evaluation of modified crystal violet chromoendoscopy procedure using new mucosal pit pattern classification for detection of Barrett’s dysplastic lesions. Dig. Liv. Dis. 2006, 38, 296–300. [Google Scholar] [CrossRef]
- Amano, Y.; Azumi, T.; Tsuboi, M.; Motoori, S.; Ishimura, N. Epidemiology of Barrett’s cancer in Japan: Presents and perspectives. Nihon Shokakibyo Gakkai Zasshi 2015, 112, 219–231, (In Japanese with English Abstract). [Google Scholar]
- Goda, K.; Fujisaki, J.; Ishihara, R.; Takeuchi, M.; Takahashi, A.; Takaki, Y.; Hirasawa, D.; Momma, K.; Amano, Y.; Yagi, K.; et al. Newly developed magnifying endoscopic classification of the Japan Esophageal Society to identify superficial Barrett’s esophagus-related neoplasms. Esophagus 2018, 15, 153–159. [Google Scholar] [CrossRef]
- Hermann, B.; Li, Y.; Ray, M.B.; Wo, J.M.; Martin, R.C., 2nd. Association of manganese superoxide dismutase expression with progression of carcinogenesis in Barrett esophagus. Arch. Surg. 2005, 140, 1204–1209. [Google Scholar] [CrossRef][Green Version]
- Von Rahden, B.H.; Stein, H.J.; Pühringer, F.; Koch, I.; Langer, R.; Piontek, G.; Siewert, J.R.; Höfler, H.; Sarbia, M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005, 65, 5038–5044. [Google Scholar] [CrossRef]
- Moriyama, N.; Amano, Y.; Mishima, Y.; Okita, K.; Takahashi, Y.; Yuki, T.; Ishimura, N.; Ishihara, S.; Kinoshita, Y. What is the clinical significance of stromal angiogenesis in Barrett’s esophagus? J. Gastroenterol. Hepatol. 2008, 23 (Suppl. S2), S210–S215. [Google Scholar] [CrossRef]
- Uno, G.; Ishimura, N.; Tada, Y.; Tamagawa, Y.; Yuki, T.; Matsushita, T.; Ishihara, S.; Amano, Y.; Maruyama, R.; Kinoshita, Y. Simplified classification of capillary pattern in Barrett esophagus using magnifying endoscopy with narrow band imaging: Implications for malignant potential and interobserver agreement. Medicine (Baltimore) 2015, 94, e405. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.B.; Wang, T.D. Emerging optical methods for surveillance of Barrett’s oesophagus. Gut 2015, 64, 1816–1823. [Google Scholar] [CrossRef]
- Lin, X.; Finkelstein, S.D.; Zhu, B.; Ujevich, B.J.; Silverman, J.F. Loss of heterozygosities in Barrett esophagus, dysplasia, and adenocarcinoma detected by esophageal brushing cytology and gastroesophageal biopsy. Cancer Cytopathol. 2009, 117, 57–66. [Google Scholar] [CrossRef]
- Fléjou, J.F. Barrett’s oesophagus: From metaplasia to dysplasia and cancer. Gut 2005, 54 (Suppl. 1), i6–i12. [Google Scholar]
- Fels Elliott, D.R.; Fitzgerald, R.C. Molecular markers for Barrett’s esophagus and its progression to cancer. Curr. Opin. Gastroenterol. 2013, 29, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.M.; Middleton, M.R.; Tomlinson, I. Genetic biomarkers of Barrett’s esophagus susceptibility and progression to dysplasia and cancer: A systematic review and meta-analysis. Dig. Dis. Sci. 2016, 61, 25–38. [Google Scholar] [CrossRef]
- Ross-Innes, C.S.; Chettouh, H.; Achilleos, A.; Galeano-Dalmau, N.; Debiram-Beecham, I.; Macrae, S.; Fessas, P.; Walker, E.; Varghese, S.; Evan, T.; et al. Risk stratification of Barrett’s oesophagus using a non-endoscopic sampling method coupled with a biomarker panel: A cohort study. Lancet Gastroenterol. Hepatol. 2017, 2, 23–31. [Google Scholar] [CrossRef]
- Tokuyama, M.; Geisler, D.; Deitrick, C.; Fasanella, K.E.; Chennat, J.S.; McGrath, K.M.; Pai, R.K.; Davison, J.M. Use of p53 immunohistochemistry in conjunction with routine histology improves risk stratification of patients with Barrett’s oesophagus during routine clinical care. Histopathology 2020. online ahead of print. [Google Scholar] [CrossRef]
- Sikkema, M.; Kerkhof, M.; Steyerberg, E.W.; Kusters, J.G.; Van Strien, P.M.H.; Looman, C.W.N.; Van Dekken, H.; Siersema, P.D.; Kuipers, E.J. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: A case-control study. Am. J. Gastroenterol. 2009, 104, 2673–2680. [Google Scholar] [CrossRef]
- Moinova, H.R.; LaFramboise, T.; Lutterbaugh, J.D.; Chandar, A.K.; Dumot, J.; Faulx, A.; Brock, W.; Cabrera, O.D.L.C.; Guda, K.; Sloan, A.E.; et al. Identifying DNA methylation biomarkers for non-endoscopic detection of Barrett’s esophagus. Sci. Transl. Med. 2018, 10, eaao5848. [Google Scholar] [CrossRef]
- Jin, Z.; Cheng, Y.; Gu, J.; Zheng, Y.; Mori, Y.; Olaru, A.; Paun, B.C.; Kan, T.; Hamilton, J.P.; Selaru, F.M.; et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 2009, 69, 4112–4115. [Google Scholar] [CrossRef]
- Duits, L.C.; Lao-Sirieix, P.; Wolf, W.A.; O’Donovan, M.; Galeano-Dalmau, N.; Meijer, S.L.; Offerhaus, G.J.A.; Redman, J.; Crawte, J.; Zeki, S.; et al. A biomarker panel predicts progression of Barrett’s esophagus to esophageal adenocarcinoma. Dis. Esophagus 2018, 32, 1–9. [Google Scholar] [CrossRef]
- Boerwinkel, D.F.; Di Pietro, M.; Liu, X.; Shariff, M.K.; Lao-Sirieix, P.; Walker, C.E.; Visser, M.; Donovan, M.O.; Kaye, P.; Bergman, J.J.G.H.M.; et al. Endoscopic trimodal imaging and biomarkers for neoplasia conjoined: A feasibility study in Barrett’s esophagus. Dis. Esophagus 2014, 27, 435–443. [Google Scholar] [CrossRef]
- Bird–Lieberman, E.L.; Dunn, J.M.; Coleman, H.G.; Lao–Sirieix, P.; Oukrif, D.; Moore, C.E.; Varghese, S.; Johnston, B.T.; Arthur, K.; McManus, D.T.; et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology 2012, 143, 927–935. [Google Scholar]
- Hadjinicolaou, A.V.; van Munster, S.N.; Achilleos, A.; Garcia, J.S.; Killcoyne, S.; Ragunath, K.; Bergman, J.J.; Fitzgerald, R.C.; Di Pietro, M. Aneuploidy in targeted endoscopic biopsies outperforms other tissue biomarkers in the prediction of histologic progression of Barrett’s oesophagus: A multi-centre prospective cohort study. EBioMedicine 2020, 56, 102765. [Google Scholar] [CrossRef]
- Timmer, M.R.; Martinez, P.; Lau, C.T.; Westra, W.M.; Calpe, S.; Rygiel, A.M.; Rosmolen, W.D.; Meijer, S.L.; Kate, F.J.W.T.; Dijkgraaf, M.G.W.; et al. Derivation of genetic biomarkers for cancer risk stratification in Barrett’s oesophagus: A prospective cohort study. Gut 2016, 65, 1602–1610. [Google Scholar] [CrossRef]
- Van Olphen, S.H.; Kate, F.J.C.T.; Doukas, M.; Kastelein, F.; Steyerberg, E.W.; Stoop, H.A.; Spaander, M.C.; Looijenga, L.H.J.; Bruno, M.J.; Biermann, K. Value of cyclin A immunohistochemistry for cancer risk stratification in Barrett esophagus surveillance: A multicenter case-control study. Medicine (Baltimore) 2016, 95, e5402. [Google Scholar] [CrossRef]
- Auvinen, M.I.; Sihvo, E.I.; Ruohtula, T.; Salminen, J.T.; Koivistoinen, A.; Siivola, P.; Rönnholm, R.; Rämö, J.O.; Bergman, M.; Salo, J.A. Incipient angiogenesis in Barrett’s epithelium and lymphangiogenesis in Barrett’s adenocarcinoma. J. Clin. Oncol. 2002, 20, 2971–2979. [Google Scholar] [CrossRef]
- Möbius, C.; Stein, H.; Becker, I.; Feith, M.; Theißen, J.; Gais, P.; Jütting, U.; Siewert, J. The ‘angiogenic switch’ in the progression from Barrett’s metaplasia to esophageal adenocarcinoma. Eur. J. Surg. Oncol. 2003, 29, 890–894. [Google Scholar] [CrossRef]
- Griffiths, E.A.; Pritchard, S.A.; McGrath, S.M.; Valentine, H.R.; Price, P.M.; Welch, I.M.; West, C.M. Increasing expression of hypoxiainducible proteins in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Br. J. Cancer 2007, 96, 1377–1383. [Google Scholar] [CrossRef]
- Huo, X.; Juergens, S.; Zhang, X.; Rezaei, D.; Yu, C.; Strauch, E.D.; Wang, J.-Y.; Cheng, E.; Meyer, F.; Wang, D.H.; et al. Deoxycholic acid causes DNA damage while inducing apoptotic resistance through NF-κB activation in benign Barrett’s epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G278–G286. [Google Scholar] [CrossRef]
- Buas, M.F.; He, Q.; Johnson, L.G.; Onstad, L.; Levine, D.M.; Thrift, A.P.; Gharahkhani, P.; Palles, C.; Lagergren, J.; Fitzgerald, R.C.; et al. Germline variation in inflammation-related pathways and risk of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut 2017, 66, 1739–1747. [Google Scholar] [CrossRef]
- Dhar, D.K.; Udagawa, J.; Ishihara, S.; Otani, H.; Kinoshita, Y.; Takasawa, S.; Okamoto, H.; Kubota, H.; Fujii, T.; Tachibana, M.; et al. Expression of regenerating gene I in gastric adenocarcinomas: Correlation with tumor differentiation status and patient survival. Cancer 2004, 100, 1130–1136. [Google Scholar] [CrossRef]
- Sekikawa, A.; Fukui, H.; Fujii, S.; Takeda, J.; Nanakin, A.; Hisatsune, H.; Seno, H.; Takasawa, S.; Okamoto, H.; Fujimori, T.; et al. REG Iα protein may function as a trophic and/or anti-apoptotic factor in the development of gastric cancer. Gastroenterology 2005, 128, 642–653. [Google Scholar] [CrossRef]
- Chinuki, D.; Amano, Y.; Ishihara, S.; Moriyama, N.; Ishimura, N.; Kazumori, H.; Kadowaki, Y.; Takasawa, S.; Okamoto, H.; Kinoshita, Y. REG I-alpha protein expression in Barrett’s esophagus. J. Gastroenterol. Hepatol. 2008, 23, 296–302. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Fitzgerald, R.C.; Vaughan, T.L.; Palles, C.; Gockel, I.; Tomlinson, I.; Buas, M.F.; May, A.; Gerges, C.; Anders, M.; et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: A large-scale meta-analysis. Lancet Oncol. 2016, 17, 1363–1373. [Google Scholar] [CrossRef]
- Murao, T.; Shiotani, A.; Yamanaka, Y.; Kimura, Y.; Tsutsui, H.; Matsumoto, H.; Kamada, T.; Manabe, N.; Hata, J.; Haruma, K. Usefulness of endoscopic brushing and magnified endoscopy with narrow band imaging (ME-NBI) to detect intestinal phenotype in columnar-lined esophagus. J. Gastroenterol. 2012, 47, 1108–1114. [Google Scholar] [CrossRef]
- Di Pietro, M.; Boerwinkel, D.F.; Shariff, M.K.; Liu, X.; Telakis, E.; Lao-Sirieix, P.; Walker, E.; Couch, G.; Mills, L.; Nuckcheddy-Grant, T.; et al. The combination of autofluorescence endoscopy and molecular biomarkers is a novel diagnostic tool for dysplasia in Barrett’s oesophagus. Gut 2015, 64, 49–56. [Google Scholar] [CrossRef]
- Thekkek, N.; Anandasabapathy, S.; Richards-Kortum, R. Optical molecular imaging for detection of Barrett’s-associated neoplasia. World. J. Gastroenterol 2011, 17, 53–62. [Google Scholar] [CrossRef]
- Sturm, M.B.; Joshi, B.P.; Lu, S.; Piraka, C.; Khondee, S.; Elmunzer, B.J.; Kwon, R.S.; Beer, D.G.; Appelman, H.D.; Turgeon, D.K.; et al. Targeted imaging of esophageal neoplasia with a fluorescently labeled peptide: First in-human results. Sci. Transl. Med. 2013, 8, 184. [Google Scholar] [CrossRef]
- Bird-Lieberman, E.L.; Neves, A.A.; Lao-Sirieix, P.; O’Donovan, M.; Novelli, M.; Lovat, L.B.; Eng, W.S.; Mahal, L.K.; Brindle, K.M.; Fitzgerald, R.C. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Med. 2012, 18, 315–321. [Google Scholar] [CrossRef]
- Joshi, B.P.; Duan, X.; Kwon, R.S.; Piraka, C.; Elmunzer, B.J.; Lu, S.; Rabinsky, E.F.; Beer, D.G.; Appelman, H.D.; Owens, S.R.; et al. Multimodal endoscope can quantify wide-field fluorescence detection of Barrett’s neoplasia. Endoscopy 2016, 48, A1–A13. [Google Scholar] [CrossRef]
- Karstensen, J.G.; Klausen, P.H.; Saftoiu, A.; Vilmann, P. Molecular confocal laser endomicroscopy: A novel technique for in vivo cellular characterization of gastrointestinal lesions. World, J. Gastroenterol. 2014, 20, 7794–7800. [Google Scholar] [CrossRef]
- Ghatwary, N.; Ahmed, A.; Grisan, E.; Jalab, H.; Bidaut, L.; Ye, X. In-vivo Barrett’s esophagus digital pathology stage classification through feature enhancement of confocal laser endomicroscopy. J. Med. Imaging (Bellingham) 2019, 6, 014502. [Google Scholar] [CrossRef]
- Di Pietro, M.; Bird–Lieberman, E.L.; Liu, X.; Nuckcheddy-Grant, T.; Bertani, H.; O’Donovan, M.; Fitzgerald, R.C. Autofluorescence-directed confocal endomicroscopy in combination with a three-biomarker panel can inform management decisions in Barrett’s esophagus. Am. J. Gastroenterol. 2015, 110, 1549–1558. [Google Scholar] [CrossRef]
- Neves, A.A.; Di Pietro, M.; O’Donovan, M.; Waterhouse, D.J.; Bohndiek, S.E.; Brindle, K.M.; Fitzgerald, R.C. Detection of early neoplasia in Barrett’s esophagus using lectin-based near-infrared imaging: An ex vivo study on human tissue. Endoscopy 2018, 50, 618–625. [Google Scholar] [CrossRef]
- Nagengast, W.B.; Hartmans, E.; Garcia-Allende, P.B.; Peters, F.T.M.; Linssen, M.D.; Koch, M.; Koller, M.; Tjalma, J.J.J.; Karrenbeld, A.; Jorritsma-Smit, A.; et al. Near-infrared fluorescence molecular endoscopy detects dysplastic oesophageal lesions using topical and systemic tracer of vascular endothelial growth factor A. Gut 2019, 68, 7–10. [Google Scholar] [CrossRef]
- Shiota, S.; Singh, S.; Anshasi, A.; El-Serag, H.B. Prevalence of Barrett’s Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 1907–1918. [Google Scholar] [CrossRef]
- Okita, K.; Amano, Y.; Takahashi, Y.; Mishima, Y.; Moriyama, N.; Ishimura, N.; Ishihara, S.; Kinoshita, Y. Barrett’s esophagus in Japanese patients: Its prevalence, form, and elongation. J. Gastroenterol. 2008, 43, 928–934. [Google Scholar] [CrossRef]
- Soh, Y.S.A.; Lee, Y.Y.; Gotoda, T.; Sharma, P.; Ho, K.Y.; Asian Barrett’s Consortium. Challenges to diagnostic standardization of Barrett’s esophagus in Asia. Dig. Endosc. 2019, 31, 609–618. [Google Scholar] [CrossRef]
- Masuda, M.; Okada, M.; Tangoku, A.; Doki, Y.; Endo, S.; Fukuda, H.; Hirata, Y.; Iwata, H.; Kobayashi, J.; Kumamaru, H.; et al. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 414–449. [Google Scholar]
- Matsuhashi, N.; Sakai, E.; Ohata, K.; Ishimura, N.; Fujisaki, J.; Shimizu, T.; Iijima, K.; Koike, T.; Endo, T.; Kikuchi, T.; et al. Surveillance of patients with long-segment Barrett’s esophagus: A multicenter prospective cohort study in Japan. J. Gastroenterol. Hepatol. 2017, 32, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Oyama, T.; Abe, S.; Takahashi, H.; Ono, H.; Fujisaki, J.; Kaise, M.; Goda, K.; Kawada, K.; Koike, T.; et al. Risk of metastasis in adenocarcinoma of the esophagus: A multicenter retrospective study in a Japanese population. J. Gastroenterol. 2017, 52, 800–808. [Google Scholar] [CrossRef]
- Sharma, P.; Hawes, R.H.; Bansal, A.; Gupta, N.; Curvers, W.; Rastogi, A.; Singh, M.; Hall, M.; Mathur, S.C.; Wani, S.; et al. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: A prospective, international, randomised controlled trial. Gut 2013, 62, 15–21. [Google Scholar] [CrossRef]
- Parasa, S.; Vennalaganti, S.; Gaddam, S.; Vennalaganti, P.; Young, P.; Gupta, N.; Thota, P.; Cash, B.; Mathur, S.; Sampliner, R.; et al. Development and Validation of a Model to Determine Risk of Progression of Barrett’s Esophagus to Neoplasia. Gastroenterology 2018, 154, 1282–1289. [Google Scholar] [CrossRef]

| WLE Findings | Risk Ratio (95% CI) | Study | |
|---|---|---|---|
| BE length | per every 1 cm | OR 1.39 (1.34–1.74) | Weston (2004) [30] |
| RR 1.11 (1.01–1.20) | Sikkema (2011) [20] | ||
| RR 1.16 (1.03–1.30) | Rugge (2012) [31] | ||
| OR 1.19 (1.09–1.30) | Pohl (2013) [32] | ||
| OR 1.21 (1.12–1.30) | Anaparthy (2013) [33] | ||
| HR 1.16 (1.04–1.30) | Solanky (2019) [34] | ||
| 3–8 cm vs. ≥8 cm | OR 2.3 (1.4–3.9) vs. 4.3 (2.5–7.2) | Holmberg (2019) [35] | |
| LSBE vs. SSBE | OR 2.69 (1.48–4.88) | Pohl (2013) [32] | |
| HR 7.1 (1.72–29.04) | Coleman (2014) [36] | ||
| No evidence | Bhat (2011) [38] | ||
| SSBE vs. LSBE | HR 0.32 (0.18–0.57) | Hamade (2019) [37] | |
| Barrett’s ulcer | RR 7.60 (2.63–21.9) | Rugge (2012) [31] | |
| HR 1.72 (1.08–2.76) | Coleman (2014) [36] | ||
| Esophagitis | RR 3.5 (1.3–9.5) | Sikkema (2011) [20] | |
| No evidence | Coleman (2014) [36] | ||
| Nodularity | HR 4.98 (1.80–11.7) | Solanky (2019) [34] | |
| Stricture | No evidence | Coleman (2014) [36] | |
| Hiatal hernia | ≥6 cm vs. none | OR 17.30 (2.58–115.93) | Weston (2004) [30] |
| ≥6 cm vs. ≤2 cm | OR 8.55 (1.18–61.56) | ||
| ≥6 cm vs. 3–5 cm | No evidence | ||
| presence vs. absence | OR 1.2 (1.04–1.39) | Avidan (2002) [42] | |
| No evidence | Sikkema (2011) [20] | ||
| No evidence | Pohl (2013) [32] | ||
| No evidence | Coleman (2014) [36] | ||
| Esophagitis, Ulcer, Nodularity, or Stricture | one marker | HR 6.7 (1.3–35) | Hillman (2003) [41] |
| two or more markers | HR 14.1 (2.02–102) | ||
| Esophagitis + ulcer | HR 8.9 (1.1–75) | ||
| Nodularity + stricture | HR 17.1 (1.8–162) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amano, Y.; Ishimura, N.; Ishihara, S. Is Malignant Potential of Barrett’s Esophagus Predictable by Endoscopy Findings? Life 2020, 10, 244. https://doi.org/10.3390/life10100244
Amano Y, Ishimura N, Ishihara S. Is Malignant Potential of Barrett’s Esophagus Predictable by Endoscopy Findings? Life. 2020; 10(10):244. https://doi.org/10.3390/life10100244
Chicago/Turabian StyleAmano, Yuji, Norihisa Ishimura, and Shunji Ishihara. 2020. "Is Malignant Potential of Barrett’s Esophagus Predictable by Endoscopy Findings?" Life 10, no. 10: 244. https://doi.org/10.3390/life10100244
APA StyleAmano, Y., Ishimura, N., & Ishihara, S. (2020). Is Malignant Potential of Barrett’s Esophagus Predictable by Endoscopy Findings? Life, 10(10), 244. https://doi.org/10.3390/life10100244





