Non-Criteria Antiphospholipid Antibodies: Risk Factors for Endothelial Dysfunction in Women with Pre-Eclampsia
Abstract
:1. Introduction
2. Results
2.1. Anthropometric Factors, Personal, Family and Obstetric History. Pregnancy Data
2.2. Association between Antiphospholipid Antibodies and Pre-Eclampsia
2.3. Main Parameters of Cardiovascular Involvement (PWV and ABI)
2.4. Relationship between aPLs and Cardiovascular Parameters
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. ELISA Serum Antiphospholipid Antibodies
4.3. Lupus Anticoagulant Test
4.4. PWV and ABI Determination
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| aβ2GPI | Antiβ2glycoprotein-I antibodies |
| ABI | Ankle-brachial index |
| aCL | Anticardiolipin antibodies |
| aPL | Antiphospholipid antibodies |
| APS | Antiphospholipid syndrome |
| aPS/PT | Antiphosphatidylserine-prothrombin complex antibodies |
| aPT | Antiprothrombin antibodies |
| APTT | Activated Partial Thromboplastin Time |
| BMI | Body mass index |
| DBP | Diastolic blood pressure |
| LA | Lupus anticoagulant |
| NS-PE | Non-severe pre-eclampsia |
| OAPS | Obstetric antiphospholipid syndrome |
| PE | Pre-eclampsia |
| PWV | Pulse wave velocity |
| RVVT | Russell Viper Venom Time |
| SBP | Systolic blood pressure |
| S-PE | Severe pre-eclampsia |
References
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Homer, C.S.E.; Brown, M.A.; Mangos, G.; Davis, G.K. Non-proteinuric pre-eclampsia: A novel risk indicator in women with gestational hypertension. J. Hypertens. 2008, 26, 295–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hnat, M.D.; Sibai, B.M.; Caritis, S.; Hauth, J.; Lindheimer, M.D.; MacPherson, C.; Van Dorsten, J.P.; Landon, M.; Miodovnik, M.; Paul, R.; et al. Perinatal outcome in women with recurrent preeclampsia compared with women who develop preeclampsia as nulliparas. Am. J. Obstet. Gynecol. 2002, 186, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Sarno, L.; Maruotti, G.M.; Saccone, G.; Sirico, A.; Mazzarelli, L.L.; Martinelli, P. Pregnancy outcome in proteinuria-onset and hypertension-onset preeclampsia. Hypertens. Pregnancy 2015, 34, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Chesley, L.C.; Cooper, D.W. Genetics of hypertension in pregnancy: Possible single gene control of pre-eclampsia and eclampsia in the descendants of eclamptic women. BJOG Int. J. Obstet. Gynaecol. 1986, 93, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P.; George, E.M.; Palei, A.C.; Spradley, F.T.; Granger, J.P. Recent advances in the understanding of the pathophysiology of preeclampsia. Hypertension 2013, 62, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J. Endothelial Dysfunction in Preeclampsia. Semin. Reprod. Med. 1998, 16, 5–15. [Google Scholar] [CrossRef]
- Taylor, R.N.; De Groot, C.J.M.; Cho, Y.K.; Lim, K.H. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin. Reprod. Endocrinol. 1998, 16, 17–31. [Google Scholar] [CrossRef]
- Wadhwani, P.; Saha, P.K.; Kalra, J.K.; Gainder, S.; Sundaram, V. A study to compare maternal and perinatal outcome in early vs. late onset preeclampsia. Obstet. Gynecol. Sci. 2020, 63, 270–277. [Google Scholar] [CrossRef]
- De Jesus, G.R.; Agmon-Levin, N.; Andrade, C.A.; Andreoli, L.; Chighizola, C.B.; Porter, T.F.; Salmon, J.; Silver, R.M.; Tincani, A.; Branch, D.W. 14th International Congress on Antiphospholipid Antibodies Task Force Report on Obstetric Antiphospholipid Syndrome. Autoimmun. Rev. 2014, 13, 795–813. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derkesen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Jara, L.J.; Medina, G.; Vera-Lastra, O.; Amigo, M.C. Accelerated atherosclerosis, immune response and autoimmune rheumatic diseases. Autoimmun. Rev. 2006, 5, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Pahor, A.; Hojs, R.; Holc, I.; Ambrožič, A.; Čučnik, S.; Kveder, T.; Rozman, B. Antiphospholipid antibodies as a possible risk factor for atherosclerosis in patients with rheumatoid arthritis. Immunobiology 2006, 211, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Vlachoyiannopoulos, P.G.; Samarkos, M. Peripheral vascular disease in antiphospholipid syndrome. Thromb. Res. 2004, 114, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, M.L.; Amengual, O.; Atsumi, T.; Binder, W.L.; De Laat, B.; Forastiero, R.; Kutteh, W.H.; Lambert, M.; Matsubayashi, H.; Murthy, V.; et al. “Non-criteria” aPL tests: Report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus 2011, 20, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Nayfe, R.; Uthman, I.; Aoun, J.; Aldin, E.S.; Merashli, M.; Khamashta, M.A. Seronegative antiphospholipid syndrome. Rheumatology 2013, 52, 1358–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zohoury, N.; Bertolaccini, M.L.; Rodriguez-Garcia, J.L.; Shums, Z.; Ateka-Barrutia, O.; Sorice, M.; Norman, G.L.; Khamashta, M. Closing the serological gap in the antiphospholipid syndrome: The value of “non-criteria” antiphospholipid antibodies. J. Rheumatol. 2017, 44, 1597–1602. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Zheng, H.; Yin, Y.F.; Hu, Q.Y.; Teng, J.L.; Sun, Y.; Liu, H.L.; Cheng, X.B.; Ye, J.N.; Su, Y.T.; et al. Antiphosphatidylserine/prothrombin antibodies (aPS/PT) as potential diagnostic markers and risk predictors of venous thrombosis and obstetric complications in antiphospholipid syndrome. Clin. Chem. Lab. Med. 2018, 56, 614–624. [Google Scholar] [CrossRef] [Green Version]
- Sciascia, S.; Amigo, M.C.; Roccatello, D.; Khamashta, M. Diagnosing antiphospholipid syndrome: “extra-criteria” manifestations and technical advances. Nat. Rev. Rheumatol. 2017, 13, 548–560. [Google Scholar] [CrossRef]
- Marozio, L.; Curti, A.; Botta, G.; Canuto, E.M.; Salton, L.; Tavella, A.M.; Benedetto, C. Anti-prothrombin antibodies are associated with adverse pregnancy outcome. Am. J. Reprod. Immunol. 2011, 66, 404–409. [Google Scholar] [CrossRef]
- Von Landenberg, P.; Matthias, T.; Zaech, J.; Schultz, M.; Lorber, M.; Blank, M.; Shoenfeld, Y. Antiprothrombin antibodies are associated with pregnancy loss in patients with the antiphospholipid syndrome. Am. J. Reprod. Immunol. 2003, 49, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.; Torricelli, M.; Scaccia, V.; Fineschi, D.; Pescaglini, M.; Gasparri, L.; Florio, P.; Petraglia, F. Increased plasma concentrations of antiprothrombin antibodies in women with recurrent spontaneous abortions. Clin. Chem. 2007, 53, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Žigon, P.; Čučnik, S.; Ambrožič, A.; Kveder, T.; Šemrl, S.S.; Rozman, B.; Božič, B. Detection of antiphosphatidylserine/prothrombin antibodies and their potential diagnostic value. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura-Ogasawara, M.; Atsumi, T.; Ozaki, Y.; Koike, T.; Suzumori, K. Phosphatidylserine-dependent antiprothrombin antibodies are not useful markers for high-risk women with recurrent miscarriages. Fertil. Steril. 2004, 82, 1440–1442. [Google Scholar] [CrossRef]
- Tsutsumi, A.; Atsumi, T.; Yamada, H.; Kato, E.H.; Ichikawa, K.; Fujimoto, S.; Koike, T. Anti-phosphatidylserine/prothrombin antibodies are not frequently found in patients with unexplained recurrent miscarriages. Am. J. Reprod. Immunol. 2001, 46, 242–244. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Liu, N.; Yang, X.; Jia, R.L.; Mu, R.; Su, Y.; Li, Z.G. Diagnostic value of antibodies to phosphatidylserine/prothrombin complex for antiphospholipid syndrome in Chinese patients. Clin. Rheumatol. 2017, 36, 401–406. [Google Scholar] [CrossRef]
- Morales, J.M.; Serrano, M.; Martínez-Flores, J.A.; Pérez, D.; Castro, M.J.; Sánchez, E.; García, F.; Rodríguez-Antolín, A.; Alonso, M.; Gutierrez, E.; et al. The presence of pretransplant antiphospholipid antibodies iga anti-b-2-glycoprotein i as a predictor of graft thrombosis after renal transplantation. Transplantation 2017, 101, 597–607. [Google Scholar] [CrossRef]
- Arrebola-Moreno, A.L.; Laclaustra, M.; Kaski, J.C. Noninvasive assessment of endothelial function in clinical practice. Rev. Esp. Cardiol. 2012, 65, 80–90. [Google Scholar] [CrossRef]
- Naka, K.K.; Tweddel, A.C.; Doshi, S.N.; Goodfellow, J.; Henderson, A.H. Flow-mediated changes in pulse wave velocity: A new clinical measure of endothelial function. Eur. Heart J. 2006, 27, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Torrado, J.; Bia, D.; Zócalo, Y.; Valls, G.; Lluberas, S.; Craiem, D.; Armentano, R.L. Reactive hyperemia-related changes in carotid-radial pulse wave velocity as a potential tool to characterize the endothelial dynamics. In Proceedings of the 31st Annual International Conference of the IEEE Engineering in Medicine and Biology Society: Engineering the Future of Biomedicine, IEEE Computer Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 1800–1803. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J. Hypertens. 2018, 36, 1956–2041. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Cockcroft, J.; Van Bortel, L.; Boutouyrie, P.; Giannattasio, C.; Hayoz, D.; Pannier, B.; Vlachopoulos, C.; Wilkinson, I.; Struijker-Boudier, H. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur. Heart J. 2006, 27, 2588–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, T.W.; Staessen, J.A.; Torp-Pedersen, C.; Rasmussen, S.; Thijs, L.; Ibsen, H.; Jeppesen, J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 2006, 113, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Oyama-Kato, M.; Ohmichi, M.; Takahashi, K.; Suzuki, S.; Henmi, N.; Yokoyama, Y.; Kurachi, H. Change in pulse wave velocity throughout normal pregnancy and its value in predicting pregnancy-induced hypertension: A longitudinal study. Am. J. Obstet. Gynecol. 2006, 195, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.B.; Burgmann, M.; Neubauer, A.; Zeisler, H.; Sanani, R.; Gottsauner-Wolf, M.; Schiessl, B.; Andreas, M. Augmentation index and pulse wave velocity in normotensive and pre-eclamptic pregnancies. Acta Obstet. Gynecol. Scand. 2013, 92, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, C.M.; Lekakis, J.P.; Stamatelopoulos, K.S.; Papaioannou, T.G.; Alevizaki, M.K.; Cimponeriu, A.T.; Kanakakis, J.E.; Papapanagiotou, A.; Kalofoutis, A.T.; Stamatelopoulos, S.F. Ankle-brachial index as a predictor of the extent of coronary atherosclerosis and cardiovascular events in patients with coronary artery disease. Am. J. Cardiol. 2000, 86, 615–618. [Google Scholar] [CrossRef]
- Sikkink, C.J.J.M.; Van Asten, W.N.J.C.; Van Hof, M.A.; Van Langen, H.; Van Der Vliet, J.A. Decreased ankle/brachial indices in relation to morbidity and mortality in patients with peripheral arterial disease. Vasc. Med. 1997, 2, 169–173. [Google Scholar] [CrossRef] [Green Version]
- McKenna, M.; Wolfson, S.; Kuller, L. The ratio of ankle and arm arterial pressure as an independent predictor of mortality. Atherosclerosis 1991, 87, 119–128. [Google Scholar] [CrossRef]
- Yasuda, M.; Takakuwa, K.; Tokunaga, A.; Tanaka, K. Prospective Studies of the Association Between Anticardiolipin Antibody and Outcome of Pregnancy. Obstet. Gynecol. 1995, 86, 555–559. [Google Scholar] [CrossRef]
- Lockwood, C.J.; Romero, R.; Feinberg, R.F.; Clyne, L.P.; Coster, B.; Hobbins, J.C. The prevalence and biologic significance of lupus anticoagulant and anticardiolipin antibodies in a general obstetric population. Am. J. Obstet. Gynecol. 1989, 161, 369–373. [Google Scholar] [CrossRef]
- Tsapanos, V.; Kanellopoulos, N.; Cardamakis, E.; Fotopoulos, A.; Schinas, V.; Kondakis, X.; Tzingounis, V. Anticardiolipin antibodies levels in healthy pregnant and non-pregnant woman. Arch. Gynecol. Obstet. 2000, 263, 111–115. [Google Scholar] [CrossRef]
- Allen, J.Y.; Tapia-Santiago, C.; Kutteh, W.H. Antiphospholipid antibodies in patients with preeclampsia. Am. J. Reprod. Immunol. 1996, 36, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yoshimura, S.; Geshi, Y.; Sasamori, Y.; Okinaga, S.; Kobayashi, T.; Mori, H. Measurement of antiphospholipid antibody by ELISA using purified beta 2-glycoprotein I in preeclampsia. Clin. Exp. Immunol. 1993, 94, 196–200. [Google Scholar] [CrossRef]
- Taylor, P.V.; Skerrow, S.M.; Redman, C.W.G. Pre-eclampsia and anti-phospholipid antibody. BJOG Int. J. Obstet. Gynaecol. 1991, 98, 604–606. [Google Scholar] [CrossRef] [PubMed]
- Dekker, G.A.; de Vries, J.I.P.; Doelitzsch, P.M.; Huijgens, P.C.; von Blomberg, B.M.E.; Jakobs, C.; van Geijn, H.P. Underlying disorders associated with severe early-onset preeclampsia. Am. J. Obstet. Gynecol. 1995, 173, 1042–1048. [Google Scholar] [CrossRef]
- Kurki, T.; Ailus, K.; Palosuo, T.; Ylikorkala, O. Antibodies to oxidized low-density lipoprotein, cardiolipin, and phosphatidyl serine fail to predict the risk of preeclampsia. Hypertens. Pregnancy 1996, 15, 251–256. [Google Scholar] [CrossRef]
- Martínez-Abundis, E.; González-Ortiz, M.; Cortés-Llamas, V.; Salazar-Páramo, M. Anticardiolipin antibodies and the severity of preeclampsia-eclampsia. Gynecol. Obstet. Investig. 1999, 48, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Von Tempelhoff, G.F.; Heilmann, L.; Spanuth, E.; Kunzmann, E.; Hommel, G. Incidence of the Factor V Leiden-mutation, coagulation inhibitor deficiency, and elevated antiphospholipid-antibodies in patients with preeclampsia or HELLP-Syndrome. Thromb. Res. 2000, 100, 363–365. [Google Scholar] [CrossRef]
- Heilmann, L.; Schneider, D.M.; Tempelhoff, C.F.; Kuse, S. Antiphospholipid-antibodies and other thrombophilic defects in patients with a history of early onset severe preeclampsia or HELLP-syndrome. Geburtshilfe Frauenheilkd. 2000, 60, 95–100. [Google Scholar] [CrossRef]
- Branch, D.W.; Porter, T.F.; Rittenhouse, L.; Caritis, S.; Sibai, B.; Hogg, B.; Lindheimer, M.D.; Klebanoff, M.; MacPherson, C.; VanDorsten, J.P.; et al. Antiphospholipid antibodies in women at risk for preeclampsia. Am. J. Obstet. Gynecol. 2001, 184, 825–834. [Google Scholar] [CrossRef]
- Lee, R.M.; Brown, M.A.; Branch, D.W.; Ward, K.; Silver, R.M. Anticardiolipin and anti-β2-glycoprotein-I antibodies in preeclampsia. Obstet. Gynecol. 2003, 102, 294–300. [Google Scholar]
- Out, H.J.; Bruinse, H.W.; Christiaens, G.C.M.L.; van Vliet, M.; de Groot, P.G.; Nieuwenhuis, H.K.; Derksen, R.H.W.M. A prospective, controlled multicenter study on the obstetric risks of pregnant women with antiphospholipid antibodies. Am. J. Obstet. Gynecol. 1992, 167, 26–32. [Google Scholar] [CrossRef]
- Lynch, A.; Marlar, R.; Murphy, J.; Davila, G.; Santos, M.; Rutledge, J.; Emlen, W. Antiphospholipid antibodies in predicting adverse pregnancy outcome: A prospective study. Ann. Intern. Med. 1994, 120, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, M.; Hedelin, G.; Kutnahorsky, R.; Lehmann, M.; Viville, B.; Langer, B.; Fleury, A.; M’Barek, M.; Treisser, A.; Wiesel, M.L.; et al. Antiphospholipid antibodies and preeclampsia: A case-control study. Obstet. Gynecol. 2001, 97, 29–34. [Google Scholar] [CrossRef]
- Fernández-Fresnedo, G.; López-Hoyos, M.; San Segundo, D.; Crespo, J.; Ruiz, J.C.; De Francisco, A.L.M.; Arias, M. Clinical significance of antiphospholipid antibodies on allograft and patient outcome after kidney transplantation. Transplant. Proc. 2005, 37, 3710–3711. [Google Scholar] [CrossRef] [PubMed]
- López-Escribano, H.; Miñambres, E.; Labrador, M.; Bartolomé, M.J.; López-Hoyos, M. Induction of cell death by sera from patients with acute brain injury as a mechanism of production of autoantibodies. Arthritis Rheum. 2002, 46, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Tsutsumi, A.; Ichikawa, K.; Kato, E.; Koike, T.; Fujimoto, S. IgA-class anti-beta2-glycoprotein I in women with unexplained recurrent spontaneous abortion. Arthritis Rheum. 1999, 42, 2727–2728. [Google Scholar] [CrossRef]
- Lee, R.M.; Branch, D.W.; Silver, R.M. Immunoglobulin A anti-β2-glycoprotein antibodies in women who experience unexplained recurrent spontaneous abortion and unexplained fetal death. Am. J. Obstet. Gynecol. 2001, 185, 748–753. [Google Scholar] [CrossRef]
- Sciascia, S.; Sanna, G.; Murru, V.; Roccatello, D.; Khamashta, M.A.; Bertolaccini, M.L. Anti-prothrombin (aPT) and anti-phosphatidylserine/prothrombin (aPS/PT) antibodies and the risk of thrombosis in the antiphospholipid syndrome a systematic review. Thromb. Haemost. 2013, 111, 354–364. [Google Scholar] [CrossRef]
- Pengo, V.; Del Ross, T.; Ruffatti, A.; Bison, E.; Cattini, M.G.; Pontara, E.; Testa, S.; Legnani, C.; Pozzi, N.; Peterle, D.; et al. Lupus anticoagulant identifies two distinct groups of patients with different antibody patterns. Thromb. Res. 2018, 172, 172–178. [Google Scholar] [CrossRef]
- Atsumi, T.; Amengual, O.; Yasuda, S.; Koike, T. Antiprothrombin antibodies—Are they worth assaying? Thromb. Res. 2004, 114, 533–538. [Google Scholar] [CrossRef]
- Ghirardello, A.; Bizzaro, N.; Zampieri, S.; Iaccarino, L.; Bassi, N.; Tozzoli, R.; Ruffatti, A.; Villalta, D.; Tonutti, E.; Doria, A. Biological and clinical relevance of anti-prothrombin antibodies. Ann. N. Y. Acad. Sci. 2007, 1109, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Radin, M.; Foddai, S.G.; Cecchi, I.; Rubini, E.; Schreiber, K.; Roccatello, D.; Bertolaccini, M.L.; Sciascia, S. Antiphosphatidylserine/Prothrombin Antibodies: An Update on Their Association with Clinical Manifestations of Antiphospholipid Syndrome. Thromb. Haemost. 2020, 120, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, A.; Ruffatti, A.; Mattia, E.; Meneghel, L.; Tonello, M.; Salvan, E.; Pengo, V.; Punzi, L. Relationship between antiphosphatidylserine/prothrombin and conventional antiphospholipid antibodies in primary antiphospholipid syndrome. Clin. Chem. Lab. Med. 2015, 53, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Abou-Nassar, K.; Carrier, M.; Ramsay, T.; Rodger, M.A. The association between antiphospholipid antibodies and placenta mediated complications: A systematic review and meta-analysis. Thromb. Res. 2011, 128, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Do Prado, A.D.; Piovesan, D.M.; Staub, H.L.; Horta, B.L. Association of anticardiolipin antibodies with preeclampsia: A systematic review and meta-analysis. Obstet. Gynecol. 2010, 116, 1433–1443. [Google Scholar] [CrossRef]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and future cardiovascular health. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. Br. Med. J. 2007, 335, 974–977. [Google Scholar] [CrossRef] [Green Version]
- Lampinen, K.H.; Rönnback, M.; Kaaja, R.J.; Groop, P.H. Impaired vascular dilatation in women with a history of pre-eclampsia. J. Hypertens. 2006, 24, 751–756. [Google Scholar] [CrossRef]
- Asmar, R.; Benetos, A.; Topouchian, J.; Laurent, P.; Pannier, B.; Brisac, A.M.; Target, R.; Levy, B.I. Assessment of arterial distensibility by automatic pulse wave velocity measurement: Validation and clinical application studies. Hypertension 1995, 26, 485–490. [Google Scholar] [CrossRef]
- Belch, J.J.F.; Topol, E.J.; Agnelli, G.; Bertrand, M.; Califf, R.M.; Clement, D.L.; Creager, M.A.; Easton, J.D.; Gavin, J.R.; Greenland, P.; et al. Critical issues in peripheral arterial disease detection and management: A call to action. Arch. Intern. Med. 2003, 163, 884–892. [Google Scholar] [CrossRef]
- Khalil, A.; Jauniaux, E.; Cooper, D.; Harrington, K. Pulse wave analysis in normal pregnancy: A prospective longitudinal study. PLoS ONE 2009, 4, e6134. [Google Scholar] [CrossRef] [PubMed]
- Hausvater, A.; Giannone, T.; Sandoval, Y.H.G.; Doonan, R.J.; Antonopoulos, C.N.; Matsoukis, I.L.; Petridou, E.T.; Daskalopoulou, S.S. The association between preeclampsia and arterial stiffness. J. Hypertens. 2012, 30, 17–33. [Google Scholar] [CrossRef]
- Avni, B.; Frenkel, G.; Shahar, L.; Golik, A.; Sherman, D.; Dishy, V. Aortic stiffness in normal and hypertensive pregnancy. Blood Press. 2010, 19, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Chowienczyk, P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: A systematic review. Hypertension 2009, 54, 1328–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowkes, G.; Fowkes, F.G.R.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; et al. Ankle brachial index combined with Framingham risk score to predict cardiovascular events and mortality: A meta-analysis. J. Am. Med. Assoc. 2008, 300, 197–208. [Google Scholar]
- Eskenazi, B. A Multivariate Analysis of Risk Factors for Preeclampsia. J. Am. Med. Assoc. 1991, 266, 237. [Google Scholar] [CrossRef]
- Lehmann, K. Eklampsien i Danmark i Aarene; Busck: Copenhagen, Denmark, 1933; pp. 1918–1927. [Google Scholar]
- Thomopoulos, C.; Tsioufis, C.; Michalopoulou, H.; Makris, T.; Papademetriou, V.; Stefanadis, C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J. Hum. Hypertens. 2013, 27, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Sibai, B.M.; Mercer, B.; Sarinoglu, C. Severe preeclampsia in the second trimester: Recurrence risk and long-term prognosis. Am. J. Obstet. Gynecol. 1991, 165, 1408–1412. [Google Scholar] [CrossRef]
- Schieve, L.A.; Handler, A.; Hershow, R.; Persky, V.; Davis, F. Urinary tract infection during pregnancy: Its association with maternal morbidity and perinatal outcome. Am. J. Public Health 1994, 84, 405–410. [Google Scholar] [CrossRef] [Green Version]
- England, L.; Zhang, J. Smoking and risk of preeclampsia: A systematic review. Front. Biosci. 2007, 12, 2471–2483. [Google Scholar] [CrossRef] [Green Version]
- Mattace-Raso, F.U.S.; Hofman, A.; Verwoert, G.C.; Wittemana, J.C.M.; Wilkinson, I.; Cockcroft, J.; McEniery, C.; Yasmina; Laurent, S.; Boutouyrie, P.; et al. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘Establishing normal and reference values’. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar]
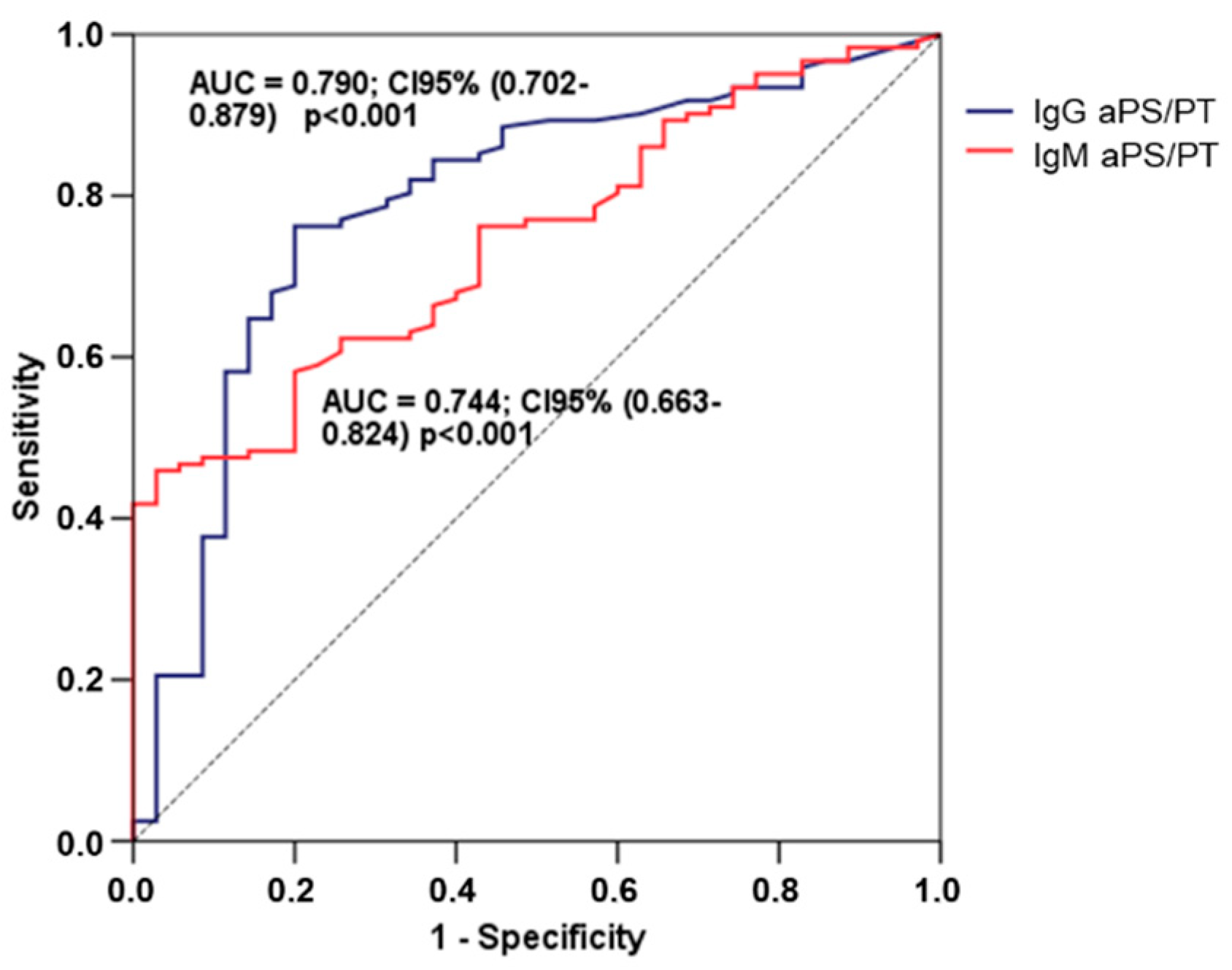
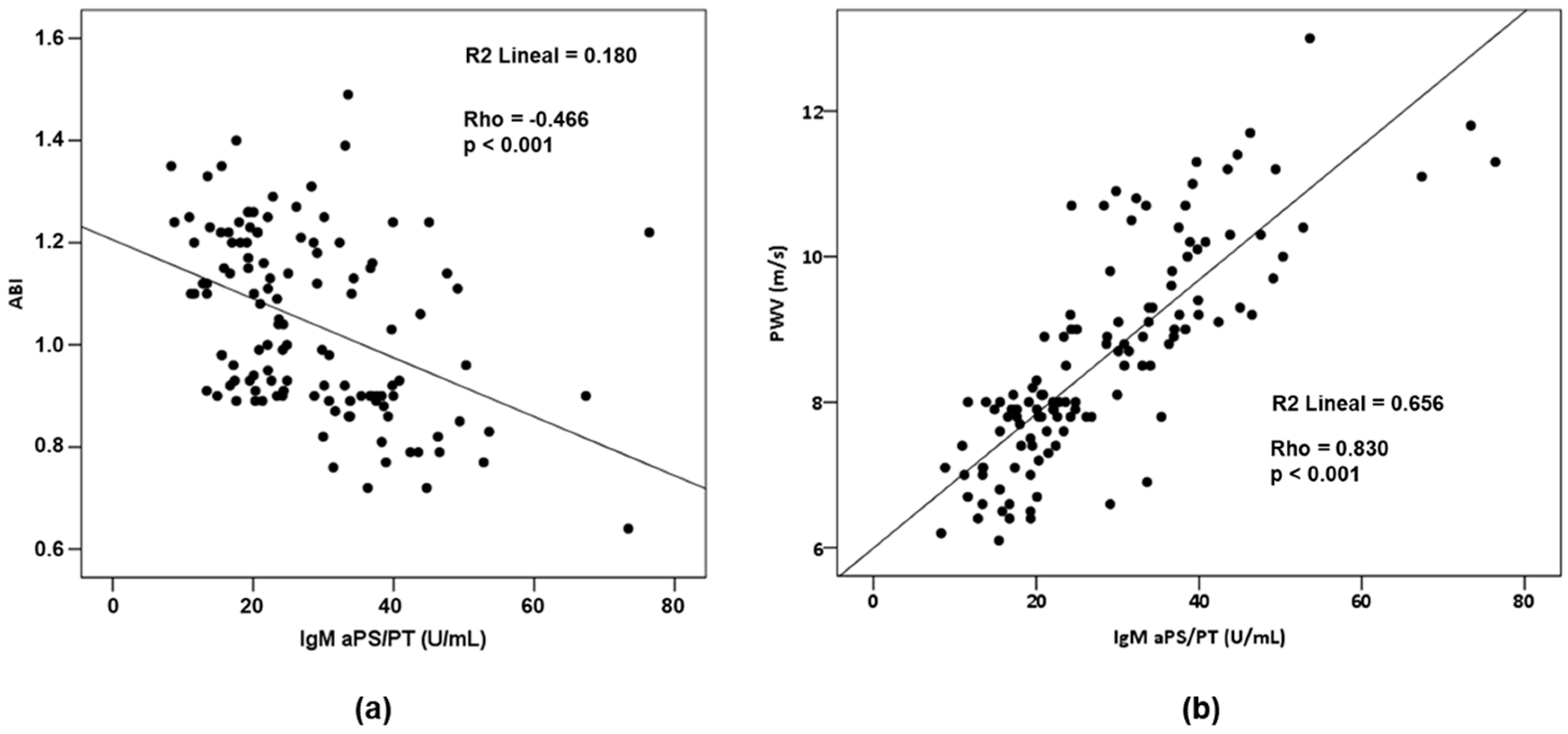
| Analysis Group | Severity of PE | ||||||
|---|---|---|---|---|---|---|---|
| Total n = 157 | Control n = 35 | PE n = 122 | p | NS-PE n = 56 | S-PE n = 66 | p | |
| Classical aPLs | 31 (19.7%) | 8 (22.9%) | 23 (18.9%) | 0.600 | 8 (14.3%) | 15 (22.7%) | 0.235 |
| LA | 5 (3.2%) | 2 (5.7%) | 3 (2.5%) | 0.309 | 2 (3.6%) | 1 (1.5%) | 0.593 |
| IgG aCL | 9 (5.7%) | 3 (8.6%) | 6 (4.9%) | 0.418 | 2 (3.6%) | 4 (6.1%) | 0.686 |
| IgM aCL | 6 (3.8%) | 1 (2.9%) | 5 (4.1%) | 0.736 | − | 5 (7.6%) | − |
| IgG aβ2GPI | 19 (12.1%) | 2 (5.7%) | 17 (13.9%) | 0.248 | 6 (10.7%) | 11 (16.7%) | 0.344 |
| IgM aβ2GPI | 14 (8.9%) | 3 (8.6%) | 11 (9.0%) | 0.935 | 5 (8.9%) | 6 (9.1%) | 0.975 |
| Unconventional aPLs | 75 (47.8%) | 8 (22.9%) | 67 (54.9%) | 0.001 | 21 (37.5%) | 46 (69.7%) | <0.001 |
| IgA aCL | 4 (2.5%) | − | 4 (3.3%) | − | 1 (1.8%) | 3 (4.5%) | 0.624 |
| IgA aβ2GPI | 10 (6.4%) | 1 (2.9%) | 9 (7.4%) | 0.460 | 2 (3.6%) | 7 (10.6%) | 0.177 |
| IgG aPS/PT | 11 (7.0%) | 1 (2.9%) | 10 (8.2%) | 0.458 | 4 (7.1%) | 6 (9.1%) | 0.752 |
| IgM aPS/PT | 63 (40.1%) | 5 (14.3%) | 58 (47.5%) | <0.001 | 16 (28.6%) | 42 (63.6) | <0.001 |
| PE OR (CI 95%) | p | S-PE OR (CI 95%) | p | |
|---|---|---|---|---|
| Classical aPLs | 0.8 (0.3–1.9) | 0.600 | 1.8 (0.7–4.5) | 0.238 |
| LA | 0.4 (0.1–2.6) | 0.348 | 0.4 (0.0–4.7) | 0.478 |
| IgG aCL | 0.6 (0.1–2.3) | 0.418 | 1.7 (0.3–9.9) | 0.531 |
| IgM aCL | 1.5 (0.2–12.9) | 0.737 | − | − |
| IgG aβ2GPI | 2.7 (0.6–12.2) | 0.204 | 1.7 (0.6–4.8) | 0.348 |
| IgM aβ2GPI | 1.1 (0.3–4.0) | 0.935 | 1.0 (0.3–3.5) | 0.975 |
| Unconventional aPLs | 4.1 (1.7–9.8) | 0.001 | 3.8 (1.8–8.1) | <0.001 |
| IgA aCL | − | − | 2.6 (0.3–25.9) | 0.410 |
| IgA aβ2GPI | 2.7 (0.3–22.1) | 0.353 | 3.2 (0.6–16.1) | 0.157 |
| IgG aPS/PT | 3.0 (0.4–24.6) | 0.298 | 1.3 (0.3–4.9) | 0.697 |
| IgM aPS/PT | 5.4 (2.0–14.9) | 0.001 | 4.4 (2.0–9.4) | <0.001 |
| Analysis Group | Severity of PE | |||||
|---|---|---|---|---|---|---|
| Control n = 35 | PE n = 122 | p | NS-PE n = 56 | S-PE n = 66 | p | |
| BMI (Kg/m2) | 23.1 (20.0–27.8) | 27.0 (24.2–30.0) | 0.001 | 27.9 (24.2–30.9) | 26.8 (24.1–29.5) | 0.342 |
| SBP (mm Hg) | 122.0 (112.0–129.0) | 120.0 (111.0–126.0) | 0.166 | 121.0 (111.0–128.0) | 119.0 (110.8–124.0) | 0.256 |
| DBP (mm Hg) | 72.0 (71.0–80.0) | 76.0 (71.0–84.0) | 0.132 | 76.0 (70.3–82.8) | 77.0 (71.8–85.0) | 0.666 |
| PWV (m/s) | 7.7 (7.2–8.0) | 8.2 (7.6–9.5) | <0.001 | 8.0 (7.6–8.9) | 9.0 (7.8–10.2) | 0.015 |
| ABI | 1.16 (1.00–1.30) | 1.04 (0.90–1.26) | <0.001 | 1.11 (0.97–1.36) | 0.94 (0.89–1.24) | <0.001 |
| Altered PWV | Altered ABI | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||
| OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | OR (CI 95%) | p | |
| Cardiovascular risk factors | ||||||||
| Age | 0.82 (0.66–1.02) | 0.073 | 0.75 (0.54–1.05) | 0.098 | 1.00 (0.93–1.10) | 0.841 | 1.04 (0.95–1.12) | 0.412 |
| BMI | 1.03 (0.89–1.18) | 0.720 | 1.05 (0.87–1.28) | 0.608 | 1.01 (0.93–1.10) | 0.762 | 1.01 (0.94–1.10) | 0.723 |
| Diabetes | 0.14 (0.01–1.77) | 0.130 | 0.21 (0.03–1.81) | 0.158 | 1.25 (0.11–14.24) | 0.857 | 1.18 (0.09–12.51) | 0.726 |
| Smoking | 0.86 (0.10–7.56) | 0.894 | 0.82 (0.24–5.43) | 0.921 | 3.21 (0.93–11.17) | 0.066 | 0.98 (0.24–3.93) | 0.976 |
| Unconventional aPLs | ||||||||
| IgA aCL | 1.04 (0.80–1.34) | 0.782 | 0.92 (0.69–1.23) | 0.560 | 1.00 (0.88–1.14) | 0.990 | 0.97 (0.85–1.10) | 0.623 |
| IgA aβ2GPI | 1.04 (0.89–1.21) | 0.654 | 0.93 (0.78–1.13) | 0.474 | 1.08 (1.01–1.15) | 0.023 | 1.10 (1.02–1.18) | 0.012 |
| IgG aPS/PT | 1.08 (0.92–1.27) | 0.360 | 1.16 (0.88–1.53) | 0.281 | 1.06 (1.00–1.14) | 0.071 | 1.08 (1.00–1.16) | 0.051 |
| IgM aPS/PT | 1.33 (1.10–1.59) | 0.002 | 1.38 (1.10–1.73) | 0.006 | 1.08 (1.04–1.13) | <0.001 | 1.09 (1.04–1.14) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belmar Vega, L.; Fernández Fresnedo, G.; Irure Ventura, J.; Orallo Toural, V.; Heras Vicario, M.; Ruiz San Millán, J.C.; Rodrigo, E.; López Hoyos, M. Non-Criteria Antiphospholipid Antibodies: Risk Factors for Endothelial Dysfunction in Women with Pre-Eclampsia. Life 2020, 10, 241. https://doi.org/10.3390/life10100241
Belmar Vega L, Fernández Fresnedo G, Irure Ventura J, Orallo Toural V, Heras Vicario M, Ruiz San Millán JC, Rodrigo E, López Hoyos M. Non-Criteria Antiphospholipid Antibodies: Risk Factors for Endothelial Dysfunction in Women with Pre-Eclampsia. Life. 2020; 10(10):241. https://doi.org/10.3390/life10100241
Chicago/Turabian StyleBelmar Vega, Lara, Gema Fernández Fresnedo, Juan Irure Ventura, Victoria Orallo Toural, Milagros Heras Vicario, Juan Carlos Ruiz San Millán, Emilio Rodrigo, and Marcos López Hoyos. 2020. "Non-Criteria Antiphospholipid Antibodies: Risk Factors for Endothelial Dysfunction in Women with Pre-Eclampsia" Life 10, no. 10: 241. https://doi.org/10.3390/life10100241
APA StyleBelmar Vega, L., Fernández Fresnedo, G., Irure Ventura, J., Orallo Toural, V., Heras Vicario, M., Ruiz San Millán, J. C., Rodrigo, E., & López Hoyos, M. (2020). Non-Criteria Antiphospholipid Antibodies: Risk Factors for Endothelial Dysfunction in Women with Pre-Eclampsia. Life, 10(10), 241. https://doi.org/10.3390/life10100241






