Electropolishing Parametric Optimization of Surface Quality for the Fabrication of a Titanium Microchannel Using the Taguchi Method
Abstract
1. Introduction
2. Experimental Design
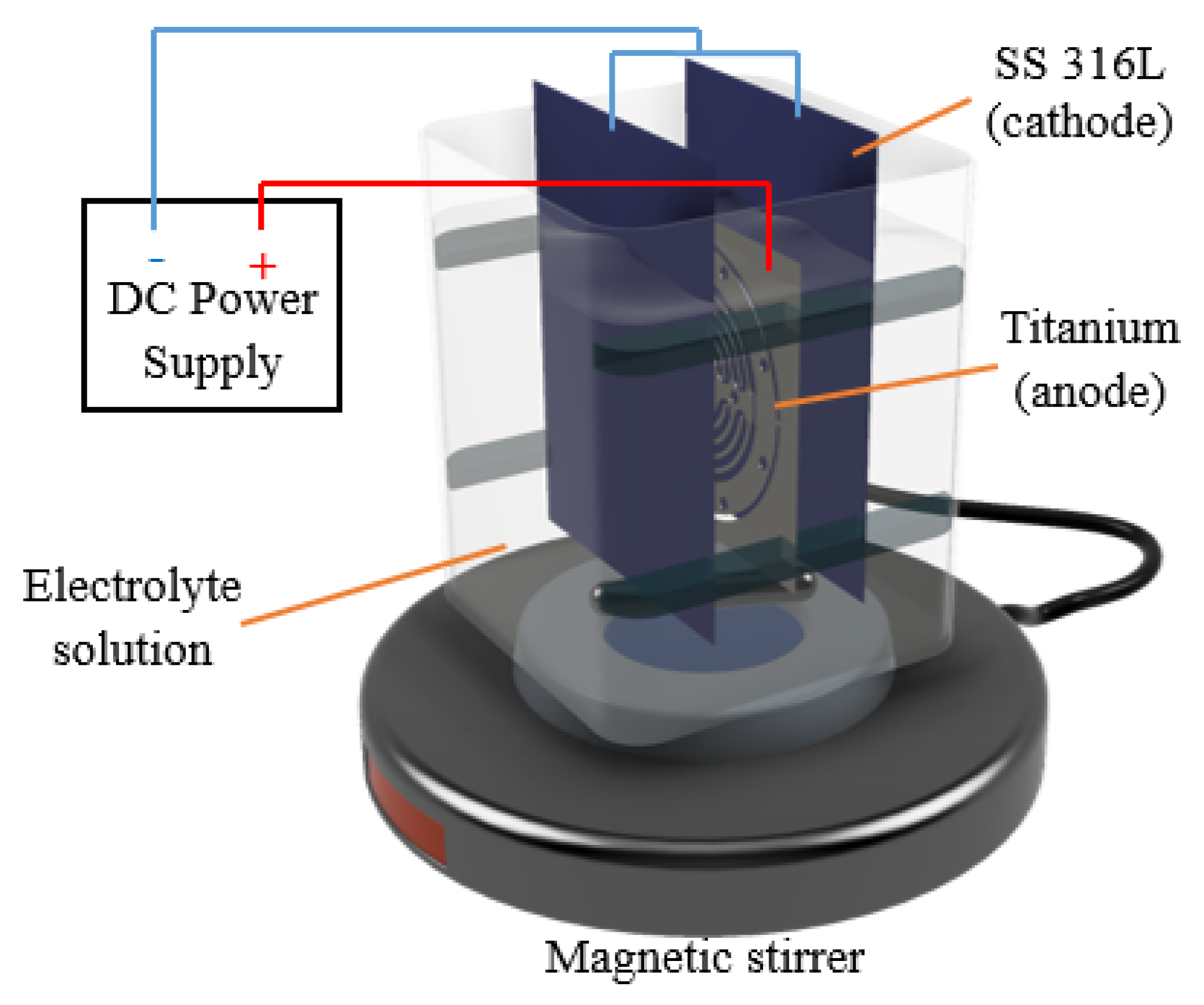
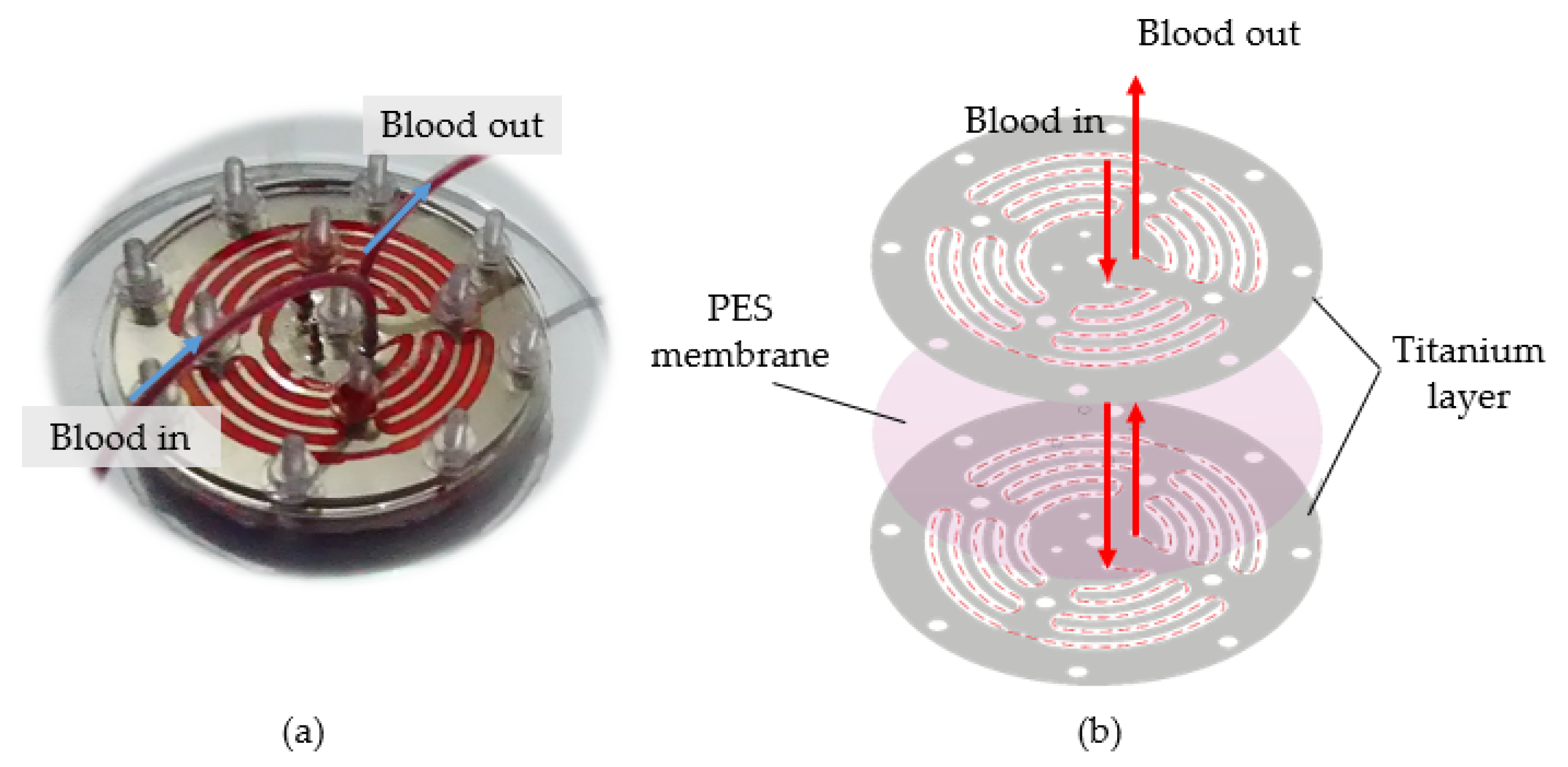
2.1. Material and Methods
2.2. Configuration of Experimental Factors and Their Levels
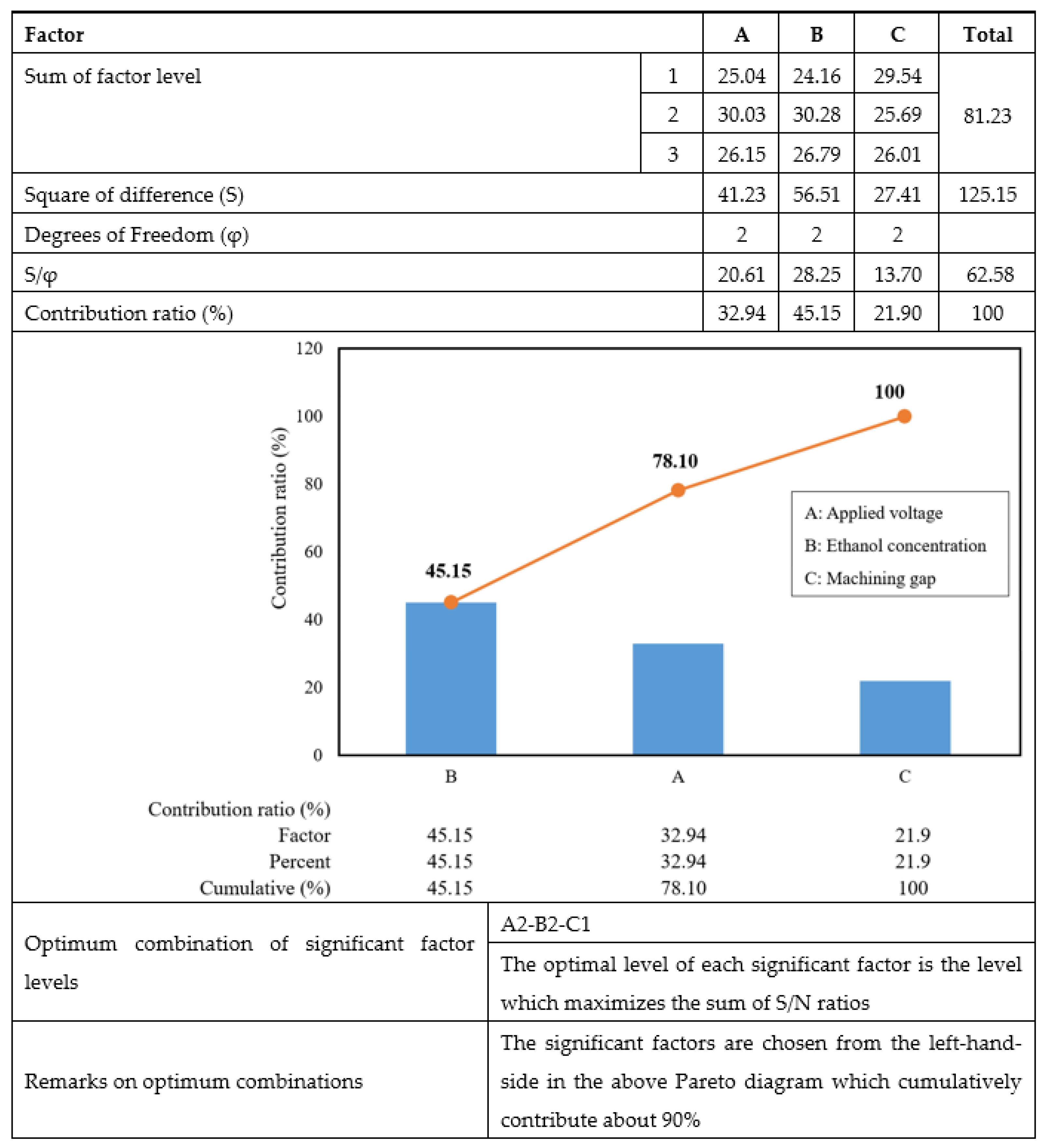
2.3. Pareto ANOVA
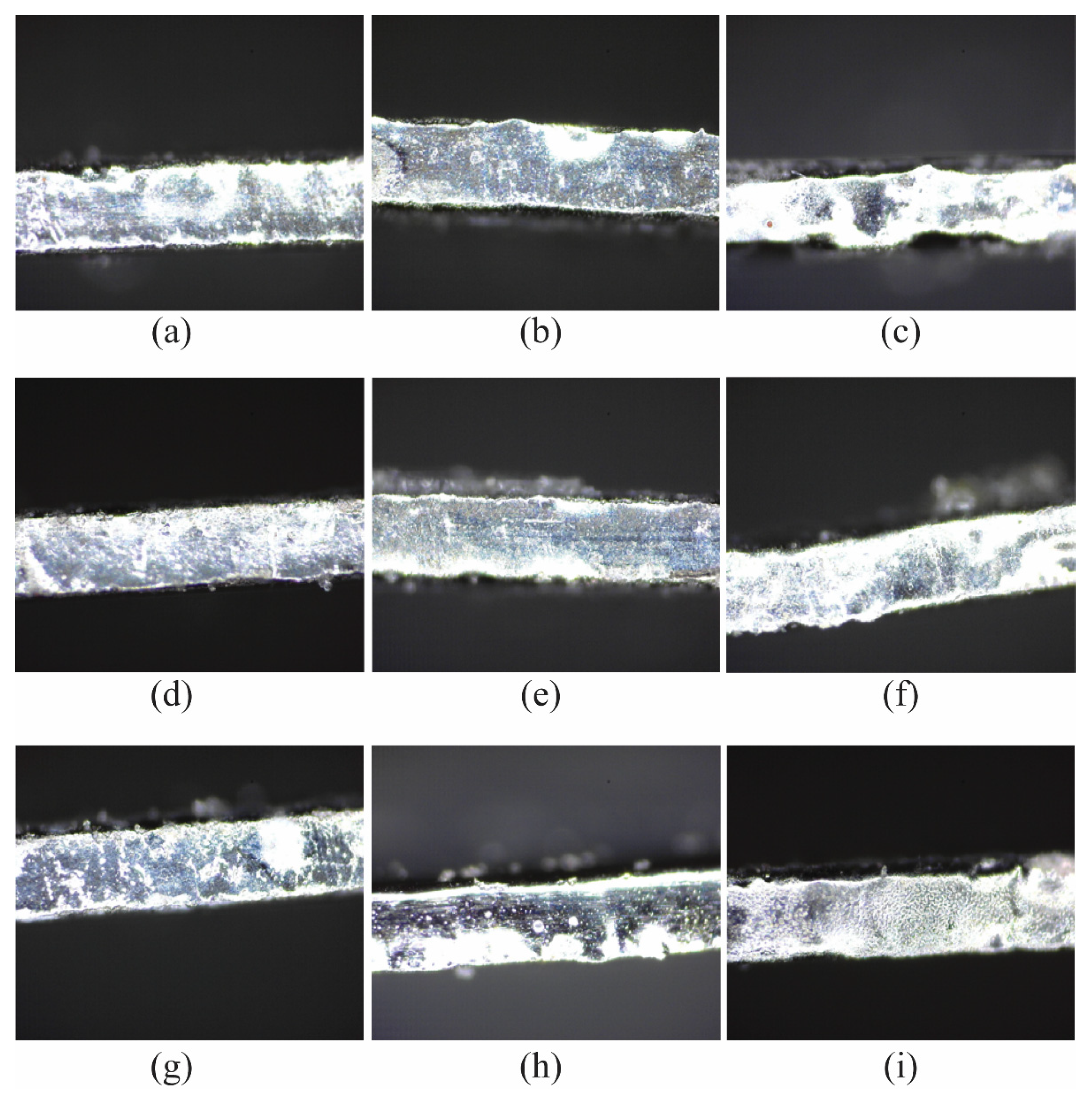
3. Results and Discussion
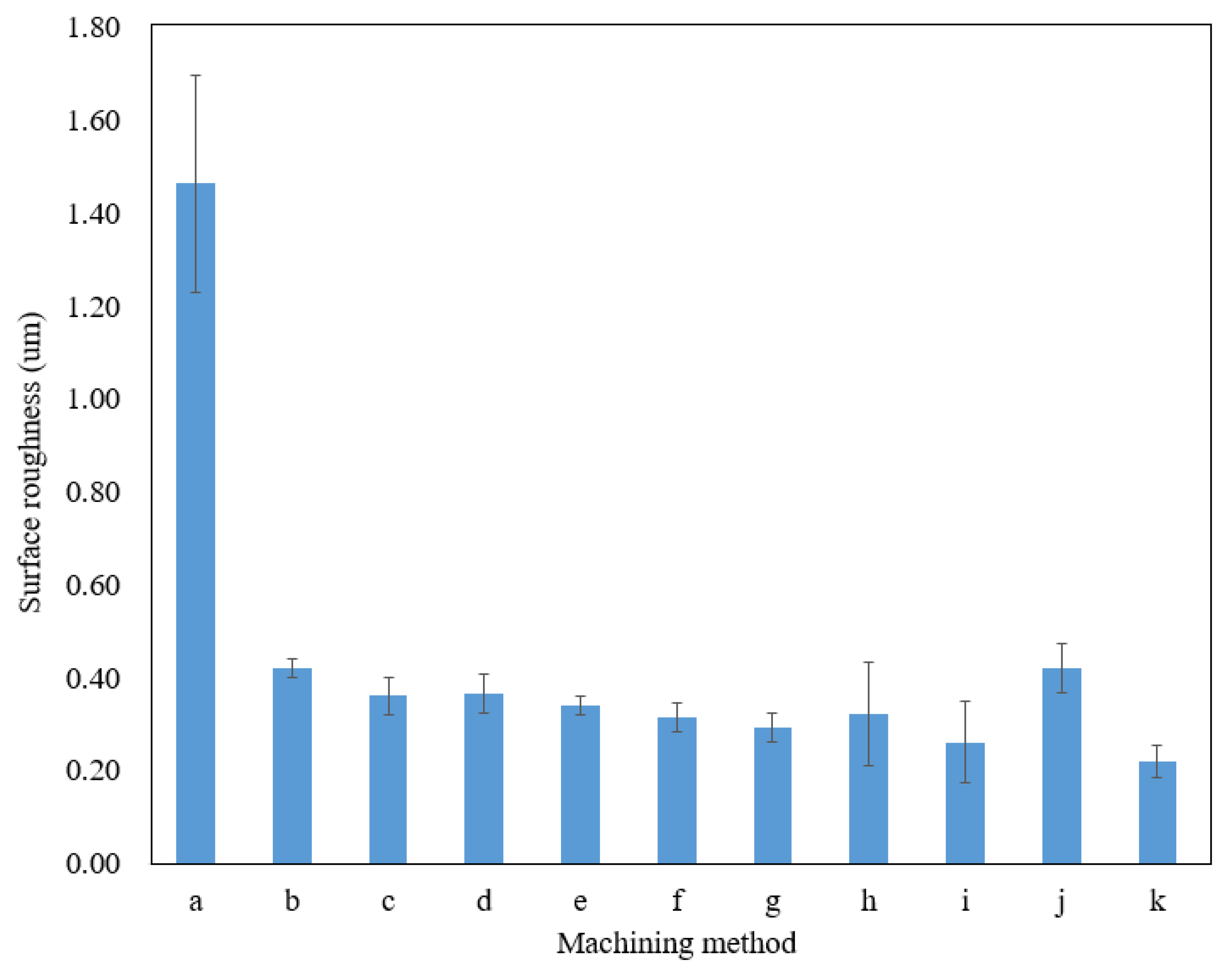
3.1. Combination of Optimal Levels for Each Factor and Verification Test
3.2. Effect of Ethanol Concentration on Surface Roughness
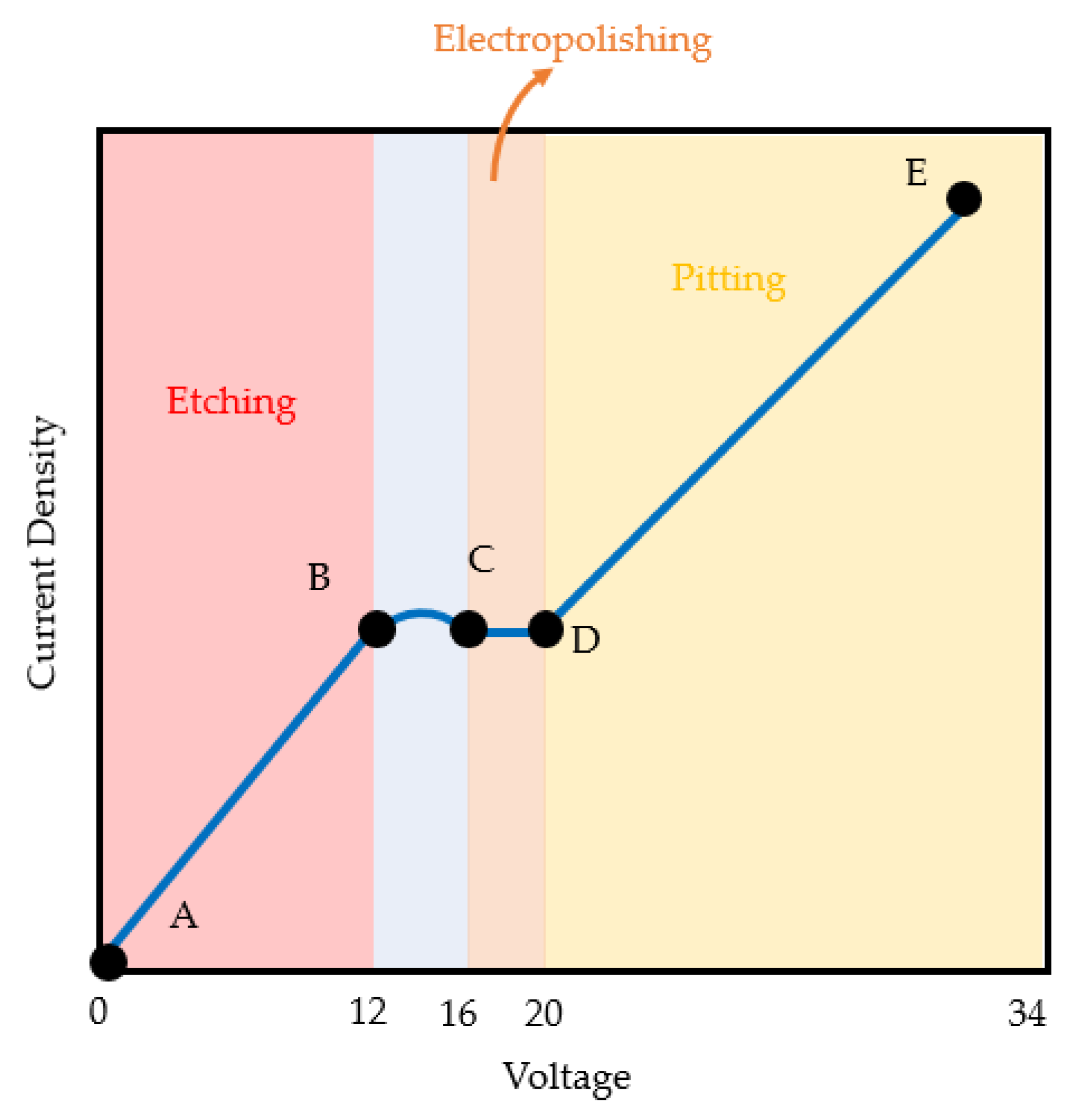
3.3. Effect of Applied Voltage on Surface Roughness
3.4. Effect of Machining Gap on Surface Roughness
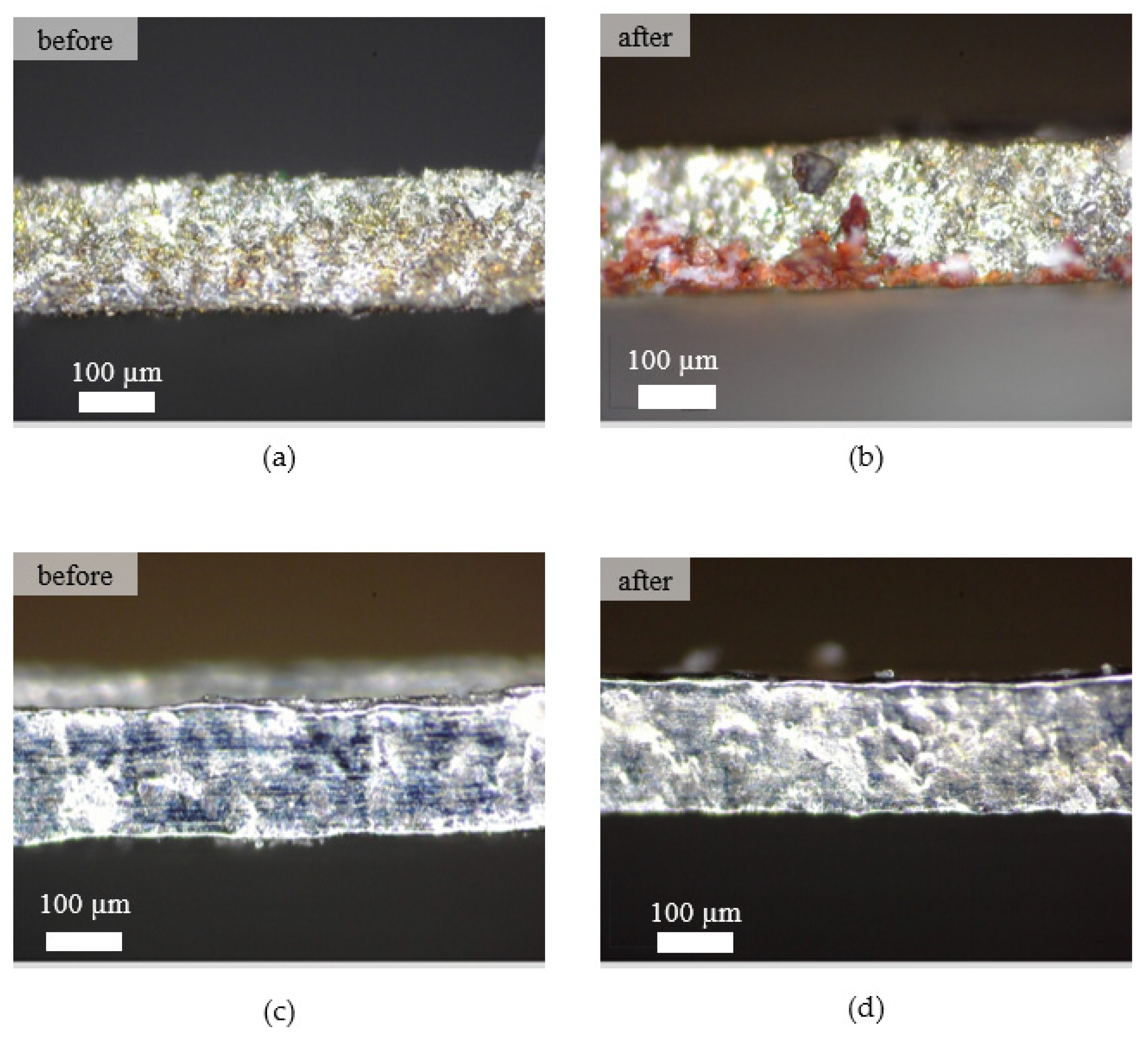
3.5. Microfluidic Fouling Experiments
4. Conclusions
- Through the Pareto ANOVA and from the percentages of the contribution of the microfluidic channel produced to surface roughness, the ethanol concentration in the electrolyte solution was proved to be the most significant EP process parameter, followed by applied voltage and machining gap.
- The optimum factor level combinations by which to achieve high surface quality of the microfluidic channel were an applied voltage of 20 V, the addition of ethanol at a concentration of 20 vol.%, and a machining gap of 10 mm.
- Utilizing the optimum factor level combinations for the verification test, the percentage improvement of surface roughness of the microfluidic channel was 85%.
- In vitro experiments confirmed that surface machined by the optimum machining parameters significantly lessened the biofouling on the sidewall of the microfluidic channel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGeough, J.A. Principles of Electrochemical Machining; CRC Press: Boca Raton, FL, USA, 1974. [Google Scholar]
- Jeykrishnan, J.; Ramnath, B.V.; Elanchezhian, C.; Akilesh, S. Parametric analysis on Electro-chemical machining of SKD-12 tool steel. Mater. Today Proc. 2017, 4, 3760–3766. [Google Scholar] [CrossRef]
- Prihandana, G.S.; Mahardika, M.; Nishinaka, Y.; Ito, H.; Kanno, Y.; Miki, N. Electropolishing of Microchannels and its Application to Dialysis System. Procedia CIRP 2013, 5, 164–168. [Google Scholar] [CrossRef][Green Version]
- Rajurkar, K.P.; Sundaram, M.M.; Malshe, A.P. Review of electrochemical and electrodischarge machining. Procedia CIRP 2013, 6, 13–26. [Google Scholar] [CrossRef]
- Prihandana, G.S.; Mahardika, M.; Sar, S.; Hamdi, M.; Wong, Y.S.; Mitsui, K. Workpiece vibration aided nano-graphite powder suspended dielectric fluid in micro-electrical discharge machining (μ-EDM) processes. In Proceedings of the 5th International Conference on Leading Edge Manufacturing in 21st Century, LEM 2009, Osaka, Japan, 2–4 December 2009. [Google Scholar]
- Sar, S.; Prihandana, G.S.; Mahardika, M.; Hamdi, M.; Mitsui, K. Simple model of micro-electrical discharge machining. In Proceedings of the 5th International Conference on Leading Edge Manufacturing in 21st Century, LEM 2009, Osaka, Japan, 2–4 December 2009. [Google Scholar]
- Joshi, S.S.; Marla, D. Electrochemical Micromachining; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Khundrakpam, N.; Brar, G.S.; Devi, M.B. Optimizing the process parameters of ECM using Taguchi method. Mater. Today Proc. 2020, 26, 1373–1379. [Google Scholar] [CrossRef]
- Setyawan, M.A.; Mahardika, M.; Sriani, T.; Prihandana, G.F.; Miki, N. Precision Electropolishing on Fabricating SS 316L Microchannel—A Taguchi Approach. Int. J. Eng. Appl. 2021, 9, 209–216. [Google Scholar] [CrossRef]
- Shimakura, M. Efficient polishing method of titanium. Soc. Titan. Alloy. Dent. 2007, 5, 32. [Google Scholar]
- Cai, C.; Zhao, X.; Yang, L.; Wang, R.; Qin, Q.; Chenc, D.; Zhang, E. A novel biomedical titanium alloy with high antibacterial property and low elastic modulus. J. Mater. Sci. Technol. 2021, 81, 13–25. [Google Scholar] [CrossRef]
- Klocke, F.; Zeis, M.; Klink, A.; Veselovac, D. Technological and economical comparison of roughing strategies via milling, sinking-EDM, wire-EDM and ECM for titanium- and nickel-based blisks. CIRP J. Manuf. Sci. Technol. 2013, 6, 198–203. [Google Scholar] [CrossRef]
- Zahiruddin, M.; Kunieda, M. Comparison of energy and removal efficiencies between micro and macro EDM. CIRP Ann. Manuf. Technol. 2012, 61, 187–190. [Google Scholar] [CrossRef]
- Khosrozadeh, B.; Shabgard, M. Effects of hybrid electrical discharge machining processes on surface integrity and residual stresses of Ti-6Al-4V titanium alloy. Int. J. Adv. Manuf. Technol. 2017, 93, 1999–2011. [Google Scholar] [CrossRef]
- Chalisgaonkar, R.; Kumar, J.; Pant, P. Prediction of machining characteristics of finish cut WEDM process for pure titanium using feed forward back propagation neural network. Mater. Today Proc. 2020, 25, 592–601. [Google Scholar] [CrossRef]
- Mitchell-Smith, J.; Speidel, A.; Clare, A.T. Advancing electrochemical jet methods through manipulation of the angle of address. J. Mater. Process. Technol. 2018, 255, 364–372. [Google Scholar] [CrossRef]
- Zou, H.; Yue, X.; Luo, H.; Liu, B.; Zhang, S. Electrochemical micromachining of micro hole using micro drill with non-conductive mask on the machined surface. J. Manuf. Process. 2020, 59, 366–377. [Google Scholar] [CrossRef]
- Zhan, S.; Zhao, Y. Intentionally-induced dynamic gas film enhances the precision of electrochemical micromachining. J. Mater. Process. Technol. 2021, 291, 117049. [Google Scholar] [CrossRef]
- Sharma, V.; Patel, D.S.; Agrawal, V.; Jain, V.K.; Ramkumar, J. Investigations into machining accuracy and quality in wire electrochemical micromachining under sinusoidal and triangular voltage pulse condition. J. Manuf. Process. 2021, 62, 348–367. [Google Scholar] [CrossRef]
- Bi, X.; Zeng, Y.; Qu, N. Wire electrochemical micromachining of high-quality pure-nickel microstructures focusing on different machining indicators. Precis. Eng. 2020, 61, 14–22. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, T.H. An evaluation of the machinability of nitinol shape memory alloy by electrochemical polishing. J. Mech. Sci. Technol. 2011, 25, 963–969. [Google Scholar] [CrossRef]
- Mwangi, J.M.; Nguyen, L.T.; Bui, V.D.; Berger, T.; Zeidler, H.; Schubert, A. Nitinol manufacturing and micromachining: A review of processes and their suitability in processing medical-grade nitinol. J. Manuf. Process. 2019, 38, 355–369. [Google Scholar] [CrossRef]
- Anasane, S.; Bhattacharyya, B. Experimental investigation on suitability of electrolytes for electrochemical micromachining of titanium. Int. J. Adv. Manuf. Technol. 2016, 86, 2147–2160. [Google Scholar] [CrossRef]
- Deng, T.; Zhu, Z.; Li, X.; Ma, T.; Wang, Q. Experimental study on electrochemical etching for titanium printed circuit heat exchanger channels. J. Mater. Process. Technol. 2020, 282, 116669. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.; Luo, Z.; Zhao, C.; Ao, S.; Gao, F.; Sun, Y. Electrochemical micromachining on titanium using the NaCl-containing ethylene glycol electrolyte. J. Mater. Process. Technol. 2018, 255, 784–794. [Google Scholar] [CrossRef]
- Ao, S.; Li, K.; Liu, W.; Qin, X.; Wang, T.; Dai, Y.; Luo, A. Electrochemical micromachining of NiTi shape memory alloy with ethylene glycol–NaCl electrolyte containing ethanol. J. Manuf. Process. 2020, 53, 223–228. [Google Scholar] [CrossRef]
- Wang, J.; Torres-Sanchez, C.; Borgman, J.M.; Zani, L.; Conway, P.P. Template-free, microscale dimple patterning of pure titanium surface through anodic dissolution using non-aqueous ethylene glycol-TiCl4 electrolytes. Surf. Coat. Technol. 2020, 404, 126555. [Google Scholar] [CrossRef]
- To, N.; Sanada, I.; Ito, H.; Prihandana, G.S.; Morita, S.; Kanno, Y.; Miki, N. Water-Permeable Dialysis Membranes for Multi-Layered Microdialysis System. Front. Bioeng. Biotechnol. 2015, 3, 70. [Google Scholar] [CrossRef]
- Sheshadri, R.; Nagaraj, M.; Lakshmikanthan, A.; Chandrashekarappa, M.P.G.; Pimenov, D.Y.; Giasin, K.; Prasad, R.V.S.; Wojciechowski, S. Experimental investigation of selective laser melting parameters for higher surface quality and microhardness properties: Taguchi and super ranking concept approaches. J. Mater. Res. Technol. 2021, 14, 2586–2600. [Google Scholar] [CrossRef]
- Kishore Kumar, M.S.; Gurudatt, B.; Reddappa, H.N.; Suresh, R. Parametric Optimization of Cutting Parameters for Micro-Machining of Titanium Grade-12 Alloy Using Statistical Techniques. Int. J. Lightweight Mater. Manuf. 2022, 5, 74–83. [Google Scholar]
- Verma, V.; Sahu, R. Process parameter optimization of die-sinking EDM on Titanium grade—V alloy (Ti6Al4V) using full factorial design approach. Mater. Today Proc. 2017, 4, 1893–1899. [Google Scholar] [CrossRef]
- Koyee, R.D.; Eisseler, R.; Schmauder, S. Application of Taguchi coupled Fuzzy Multi Attribute Decision Making (FMADM) for optimizing surface quality in turning austenitic and duplex stainless steels. Measurement 2014, 58, 375–386. [Google Scholar] [CrossRef]
- Selvaraj, D.P.; Chandramohan, P.; Mohanraj, M. Optimization of surface roughness, cutting force and tool wear of nitrogen alloyed duplex stainless steel in a dry turning process using Taguchi method. Measurement 2014, 49, 205–215. [Google Scholar] [CrossRef]
- Tajima, K.; Hironaka, M.; Chen, K.; Nagamatsu, Y.; Kakagawa, H.; Kozono, Y. Electropolishing of CP titanium and its alloys in an alcoholic solution-based electrolyte. Dent. Mater. J. 2008, 27, 258–265. [Google Scholar] [CrossRef]
- Said, M.; Eng, C.W.; Hixon, A.E.; Marks, N.E. Quantifying surface roughness on UO2 fuel pellets using optical techniques. Forensic Sci. Int. 2020, 316, 110470. [Google Scholar] [CrossRef]
- Kumar, S.P.L. Experimental investigations and empirical modeling for optimization of surface roughness and machining time parameters in micro end milling using Genetic Algorithm. Measurement 2018, 124, 386–394. [Google Scholar] [CrossRef]
- Godlewska, E.; Mitoraj, M.; Leszczynska, K. Hot corrosion of Ti-6Al-8Ta (at. %) intermetallic alloy. Corros. Sci. 2014, 78, 63–70. [Google Scholar] [CrossRef]
- Yuzhakov, V.V.; Chang, H.; Miller, A.E. Pattern formation during electropolishing. Phys. Rev. B 1997, 56, 12608–12624. [Google Scholar] [CrossRef]
- Kim, D.; Son, K.; Sung, D.; Kim, Y.; Chung, W. Effect of added ethanol in ethylene glycol-NaCl electrolyte on titanium Electropolishing. Corros. Sci. 2015, 98, 494–499. [Google Scholar] [CrossRef]
- Lee, E.-S. Machining Characteristics of the Electropolishing of Stainless Steel (STS316L). Int. J. Adv. Manuf. Technol. 2000, 16, 591–599. [Google Scholar] [CrossRef]
- Milner, K.R.; Snyder, A.J.; Siedlecki, C.A. Sub-micron texturing for reducing platelet adhesion to polyurethane biomaterials. J. Biomed. Mater. Res. A 2006, 76, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Bauer, J.W.; Siedlecki, C.A. Proteins, platelets, and blood coagulation at biomaterial interfaces. Colloids Surf B Biointerfaces 2014, 124, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Strnad, J.; Strnad, Z.; Šestak, J.; Urban, K.; Povýšil, C. Bio-activated titanium surface utilizable for mimetic bone implantation in dentistry—Part III: Surface characteristics and bone-implant contact formation. J. Phys. Chem. Solids 2007, 68, 841–845. [Google Scholar] [CrossRef]
- Eliasa, C.N.; Oshidab, Y.; Henrique, J.; Limad, C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242. [Google Scholar] [CrossRef] [PubMed]


| Mechanical Properties | Ti (99.5%) |
|---|---|
| Hardness, Brinell | 70 |
| Hardness, Vickers | 60 |
| Tensile Strength, Ultimate | 220 MPa |
| Tensile Strength, Yield | 140 MPa |
| Elongation at Break | 54% |
| Modulus of Elasticity | 116 GPa |
| Poisson’s Ratio | 0.34 |
| Shear Modulus | 43.0 GPa |
| Mechanical Properties | SS 316L |
|---|---|
| Hardness, Rockwell B | 79 |
| Tensile Strength, Ultimate | 560 MPa |
| Tensile Strength, Yield | 290 MPa |
| Elongation at Break | 50% |
| Tensile Modulus | 193 GPa |
| Component Elements | SS 316L |
|---|---|
| Carbon, C | ≤0.030% |
| Chromium, Cr | 16–18% |
| Iron, Fe | 61.9–72% |
| Manganese, Mn | ≤2.0% |
| Molybdenum, Mo | 2.0–3.0% |
| Nickel, Ni | 10–14% |
| Phosphorus, P | ≤0.045% |
| Silicon, Si | ≤1.0% |
| Sulfur, S | ≤0.030% |
| Carbon, C | ≤0.030% |
| Machining Parameters | |
|---|---|
| Pulse on-time (µs) | 4 |
| Pulse off-time (µs) | 4 |
| Voltage (V) | 50 |
| Flushing pressure (bar) | 8 |
| Control Factor | Levels | |||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| A | Applied voltage (V) | 15 | 20 | 25 |
| B | Ethanol concentration (vol.%) | 15 | 20 | 25 |
| C | Machining gap (mm) | 10 | 20 | 30 |
| Exp. No | Configuration of Machining Parameters | |||
|---|---|---|---|---|
| A | B | C | ||
| 1 | A1 | B1 | C1 | |
| 2 | A1 | B2 | C2 | |
| 3 | A1 | B3 | C3 | |
| 4 | A2 | B1 | C2 | |
| 5 | A2 | B2 | C3 | |
| 6 | A2 | B3 | C1 | |
| 7 | A3 | B1 | C3 | |
| 8 | A3 | B2 | C1 | |
| 9 | A3 | B3 | C2 | |
| Exp. No | Control Factor | Ra (µm) | Mean | |||||
|---|---|---|---|---|---|---|---|---|
| Noise Factor | ||||||||
| A | B | C | N0 | N1 | N2 | |||
| 1 | 15 | 15 | 10 | 0.44 | 0.42 | 0.40 | 0.42 | 7.53 |
| 2 | 15 | 20 | 20 | 0.32 | 0.36 | 0.40 | 0.36 | 8.84 |
| 3 | 15 | 25 | 30 | 0.40 | 0.32 | 0.38 | 0.37 | 8.68 |
| 4 | 20 | 15 | 20 | 0.32 | 0.34 | 0.36 | 0.34 | 9.36 |
| 5 | 20 | 20 | 30 | 0.28 | 0.34 | 0.32 | 0.31 | 10.05 |
| 6 | 20 | 25 | 10 | 0.30 | 0.32 | 0.26 | 0.29 | 10.62 |
| 7 | 25 | 15 | 30 | 0.34 | 0.42 | 0.52 | 0.43 | 7.27 |
| 8 | 25 | 20 | 10 | 0.36 | 0.22 | 0.20 | 0.26 | 11.39 |
| 9 | 25 | 25 | 20 | 0.44 | 0.36 | 0.46 | 0.42 | 7.49 |
| Factor | |||
|---|---|---|---|
| A | B | C | |
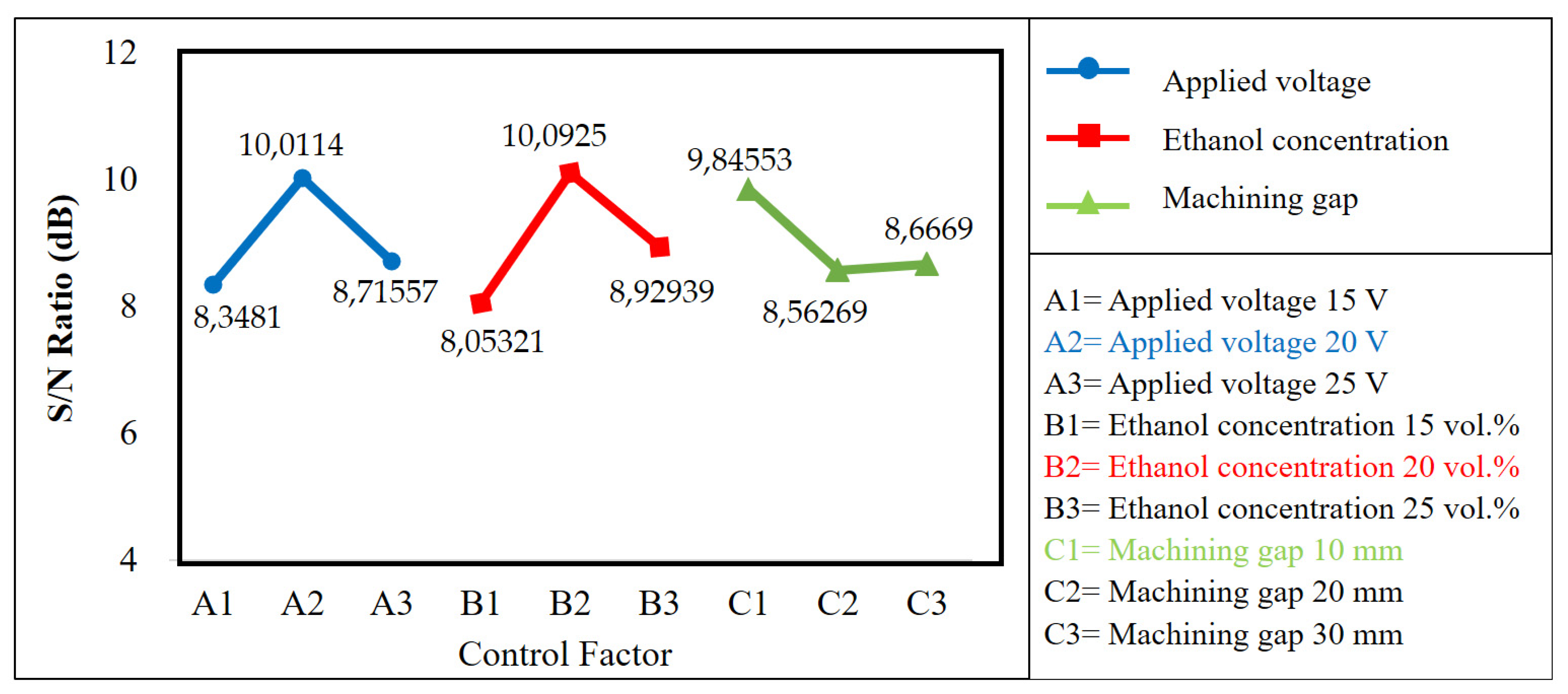
| Level 1 | 8.35 | 8.05 | 9.85 |
| Level 2 | 10.01 | 10.09 | 8.56 |
| Level 3 | 8.72 | 8.93 | 8.67 |
| Max–Min | 1.67 | 2.04 | 1.28 |
| Average | 9.02 | 9.03 | 9.03 |
| Factor | Level |
|---|---|
| A. Applied voltage | 20 Volt |
| B. Ethanol concentration | 20 vol.% |
| C. Machining gap | 10 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahardika, M.; Setyawan, M.A.; Sriani, T.; Miki, N.; Prihandana, G.S. Electropolishing Parametric Optimization of Surface Quality for the Fabrication of a Titanium Microchannel Using the Taguchi Method. Machines 2021, 9, 325. https://doi.org/10.3390/machines9120325
Mahardika M, Setyawan MA, Sriani T, Miki N, Prihandana GS. Electropolishing Parametric Optimization of Surface Quality for the Fabrication of a Titanium Microchannel Using the Taguchi Method. Machines. 2021; 9(12):325. https://doi.org/10.3390/machines9120325
Chicago/Turabian StyleMahardika, Muslim, Martin Andre Setyawan, Tutik Sriani, Norihisa Miki, and Gunawan Setia Prihandana. 2021. "Electropolishing Parametric Optimization of Surface Quality for the Fabrication of a Titanium Microchannel Using the Taguchi Method" Machines 9, no. 12: 325. https://doi.org/10.3390/machines9120325
APA StyleMahardika, M., Setyawan, M. A., Sriani, T., Miki, N., & Prihandana, G. S. (2021). Electropolishing Parametric Optimization of Surface Quality for the Fabrication of a Titanium Microchannel Using the Taguchi Method. Machines, 9(12), 325. https://doi.org/10.3390/machines9120325







