Design and Analysis of a Portable Robot for Venous Blood Sampling
Abstract
1. Introduction
2. Structure Design
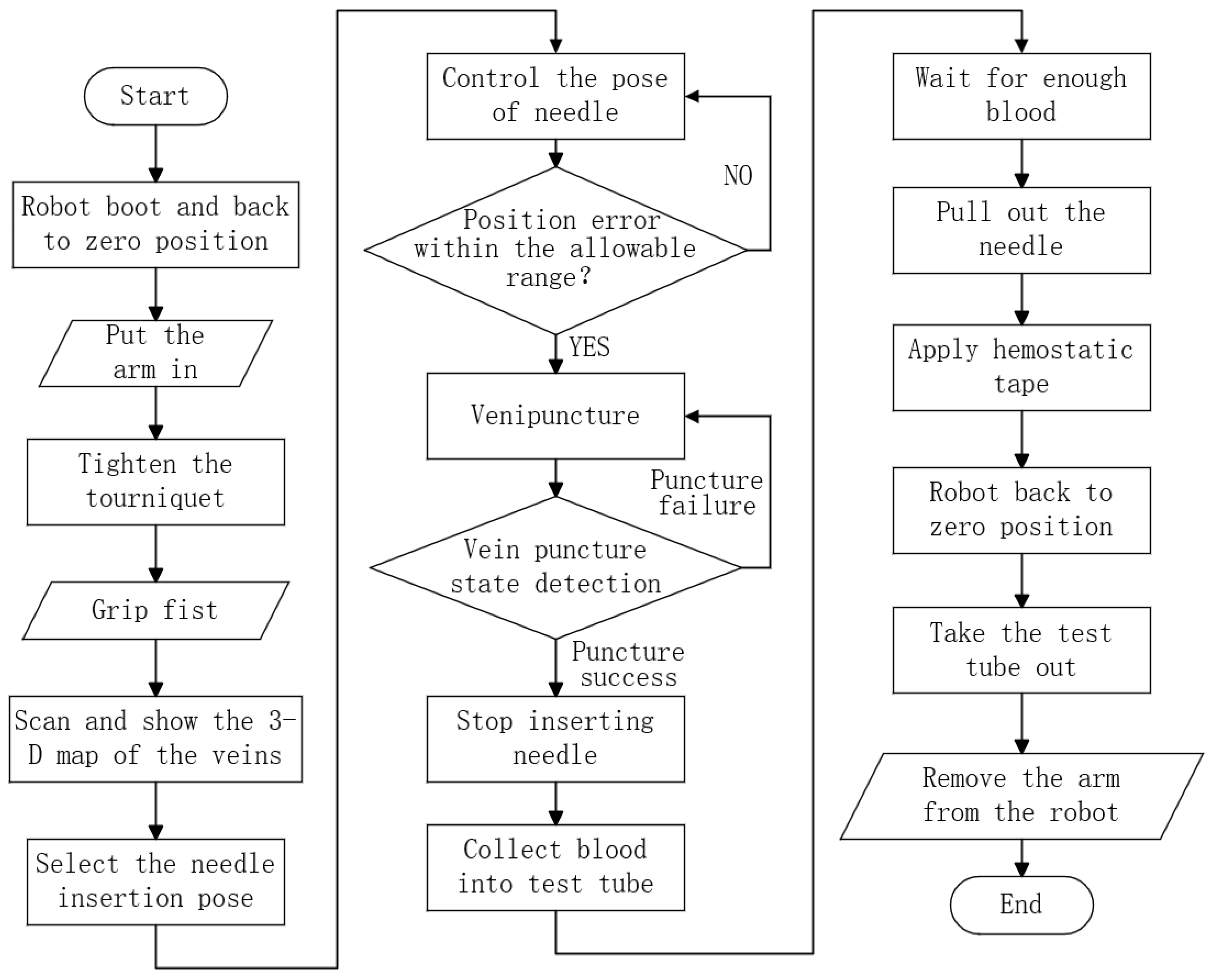
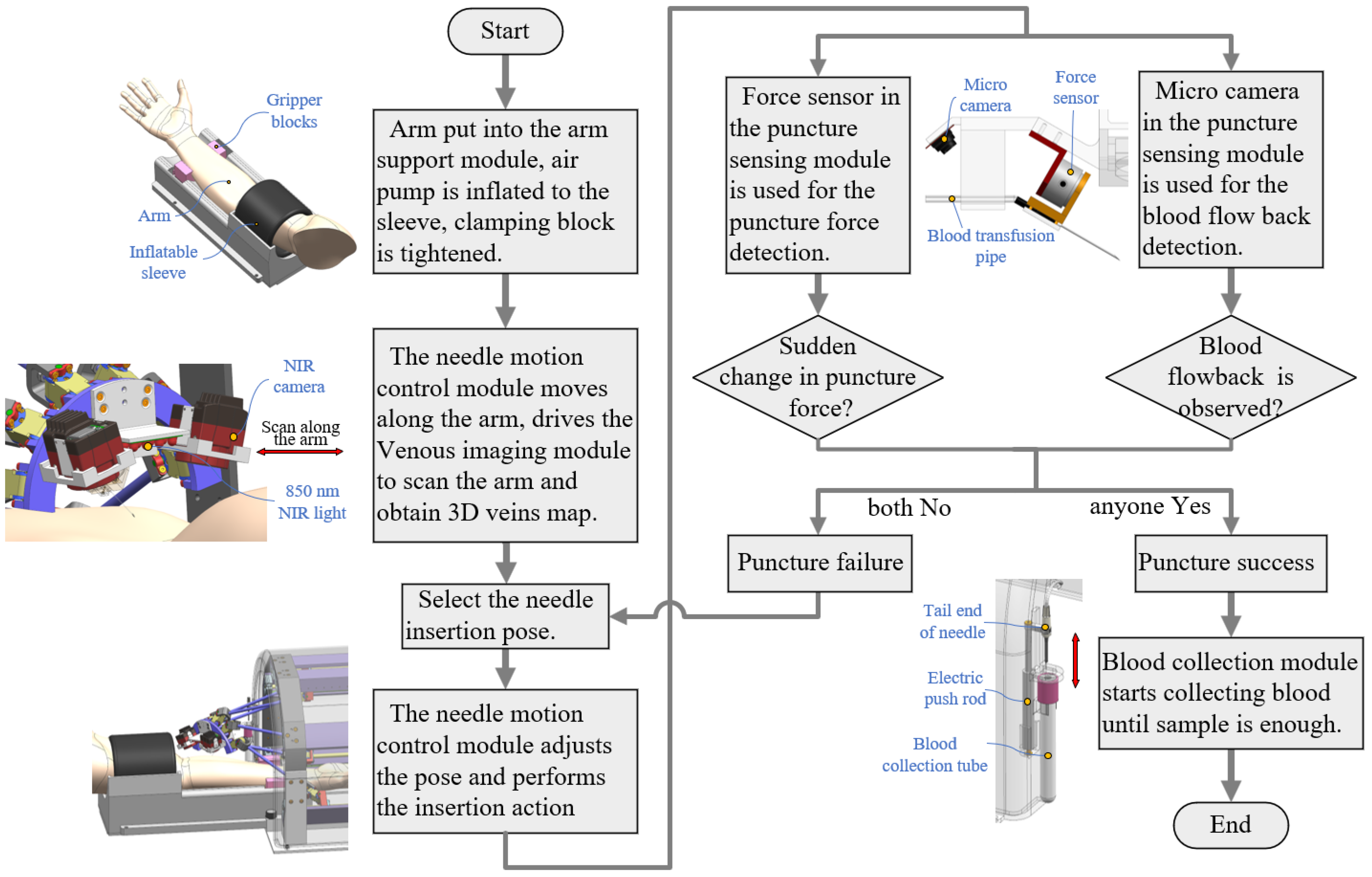
2.1. Robot Workflow Analysis and Module Division
- 1.
- Needle motion control module, which enables the blood sampling needle to perform venipuncture actions at the specified position and direction;
- 2.
- Arm support module, which provides pneumatic pressure for the vein and secures the arm to minimize movement, thereby increasing the venipuncture success rate;
- 3.
- Venous imaging module, which performs imaging of the elbow veins and uses the venous image information to guide the motion control module and position the blood collection needle accurately at the puncture site;
- 4.
- Puncture sensing module, which uses sensors to detect the penetration status of the vascular wall and determine whether the blood collection needle has successfully entered the venous vessel;
- 5.
- Blood collection module, which collects the patient’s blood into the blood collection tube.
2.2. Structural Design of Complete Machine and Modules
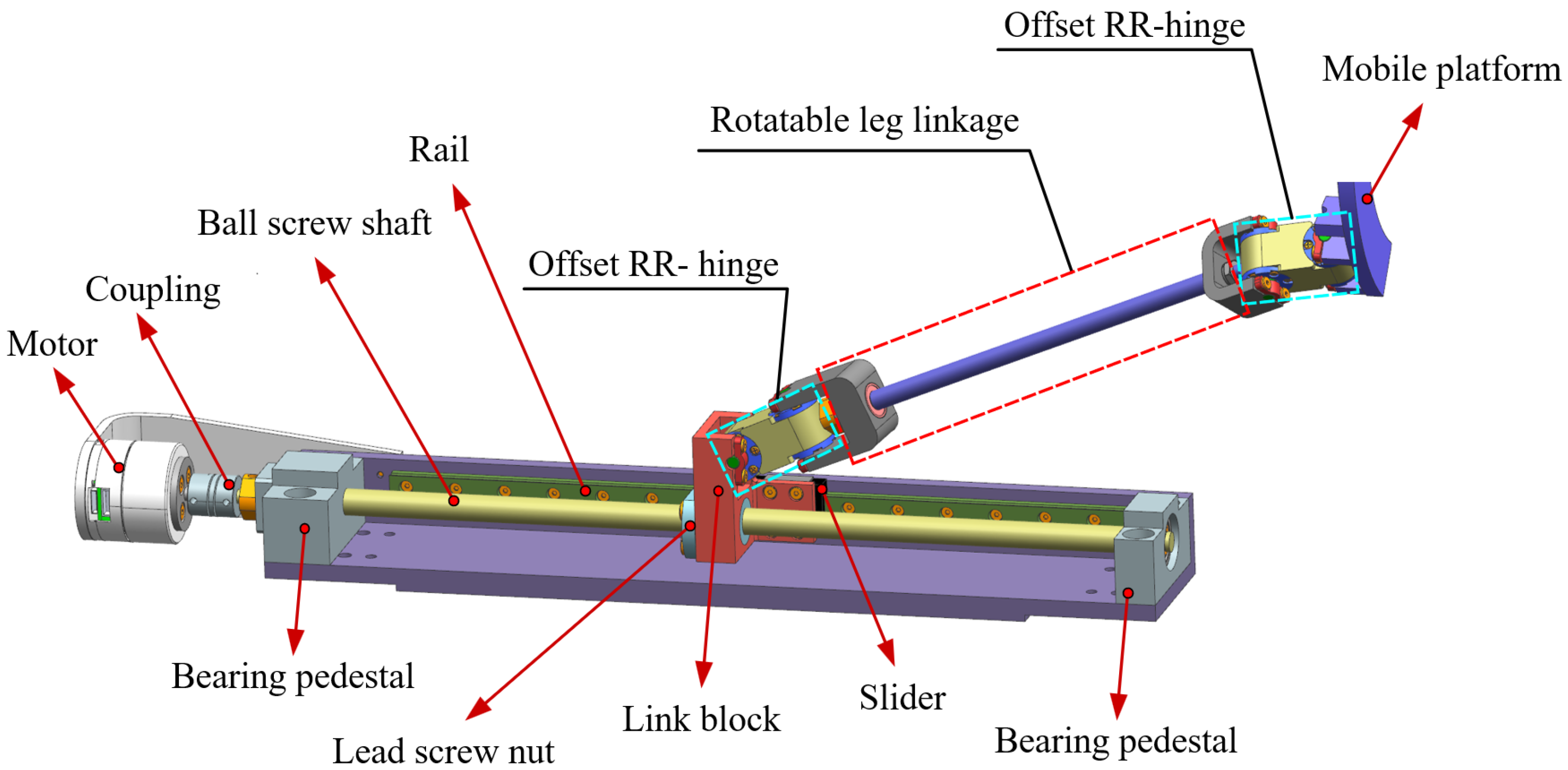
2.2.1. Needle Motion Control Module
2.2.2. Blood Collection Module
2.2.3. Arm Support Module
2.2.4. Venous Imaging Module
2.2.5. Puncture Sensing Module
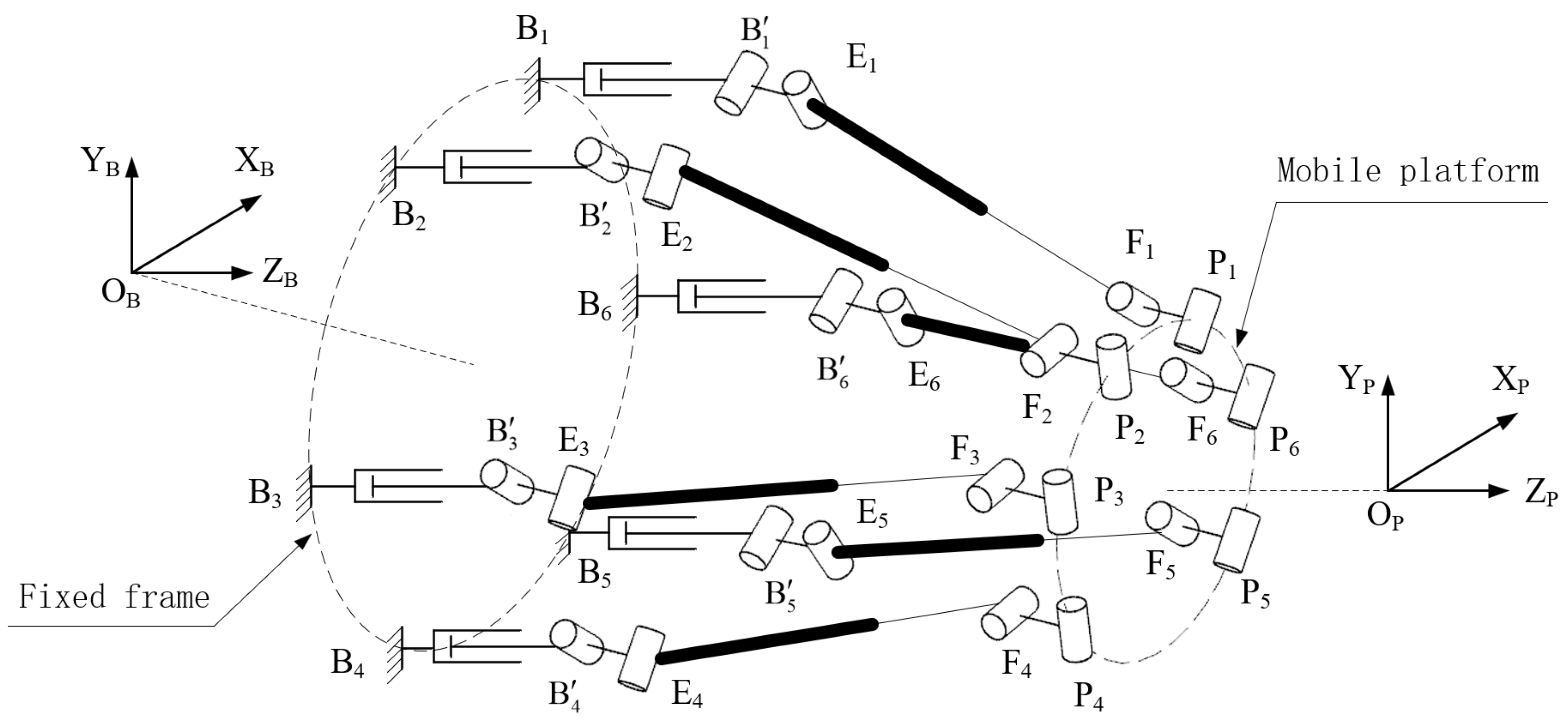
3. Kinematic Analysis of Needle Motion Control Module
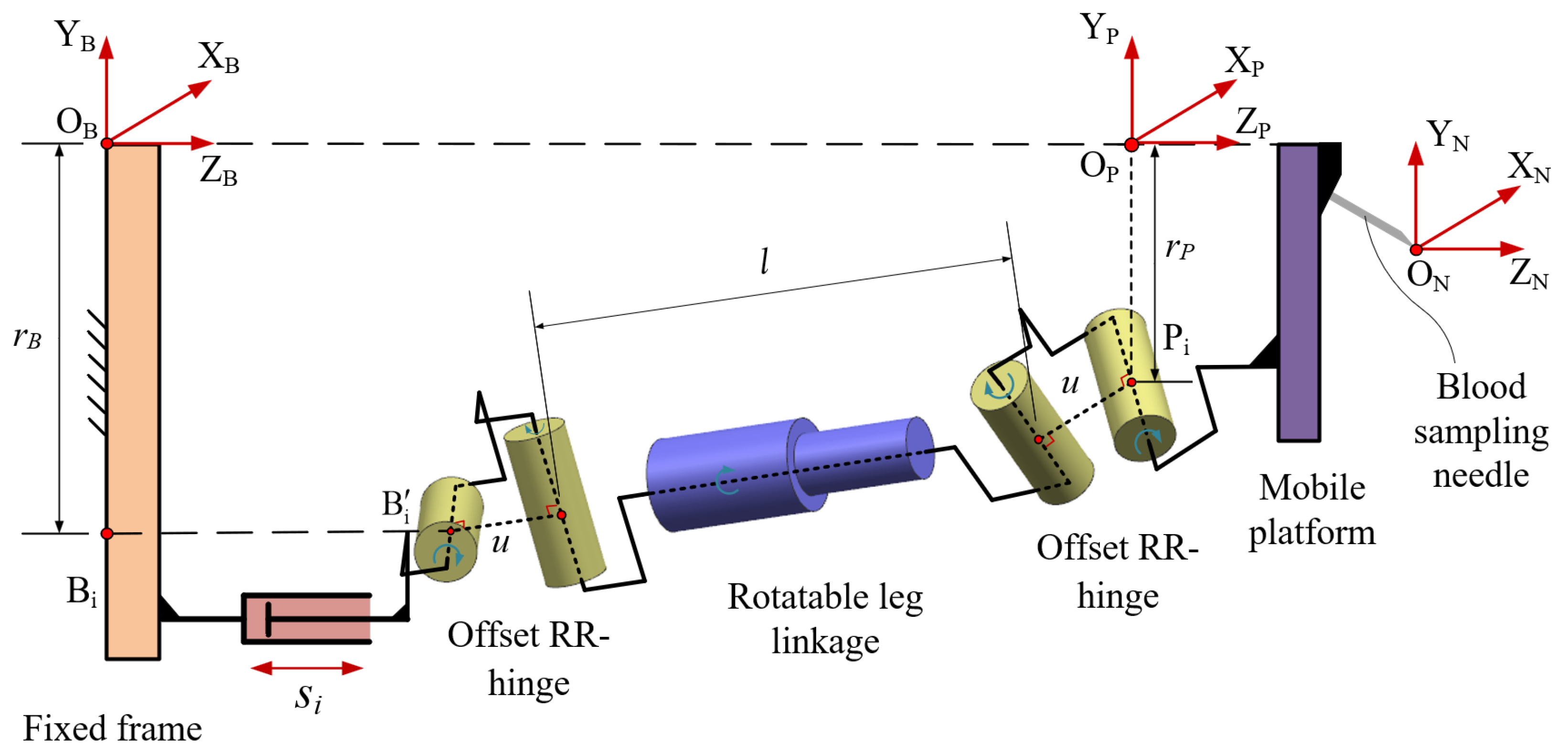
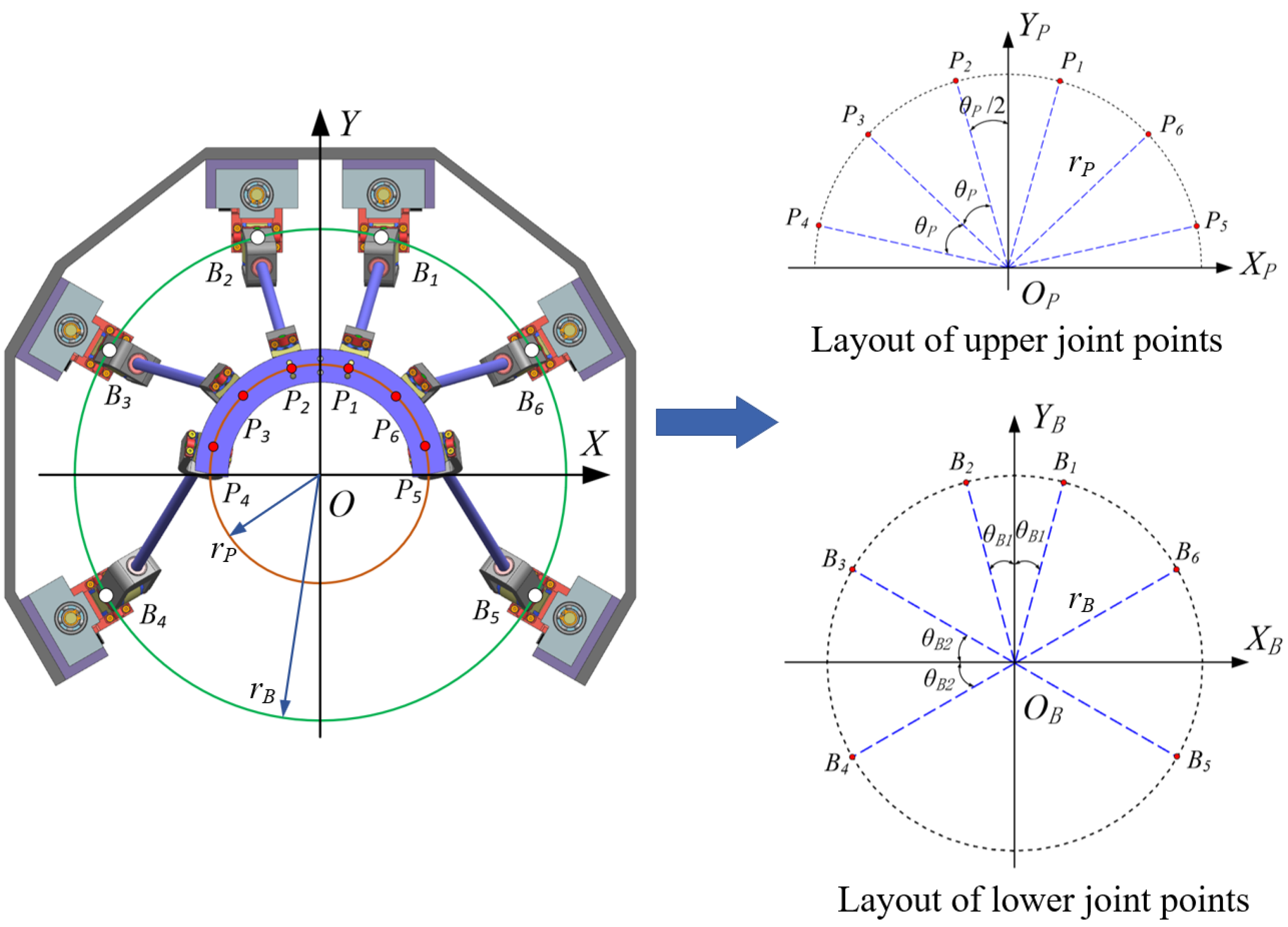
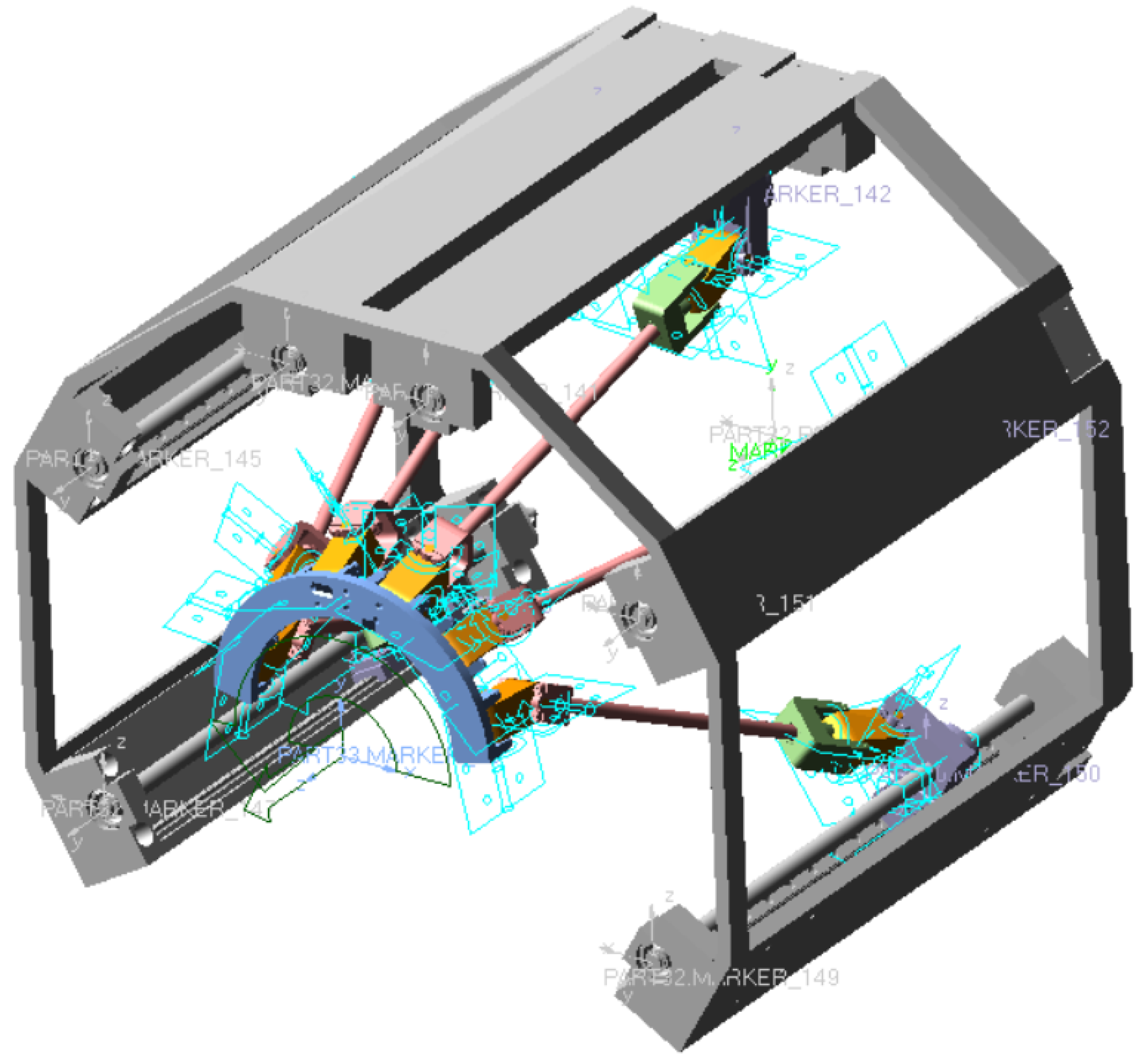
3.1. Parameters of Mechanism
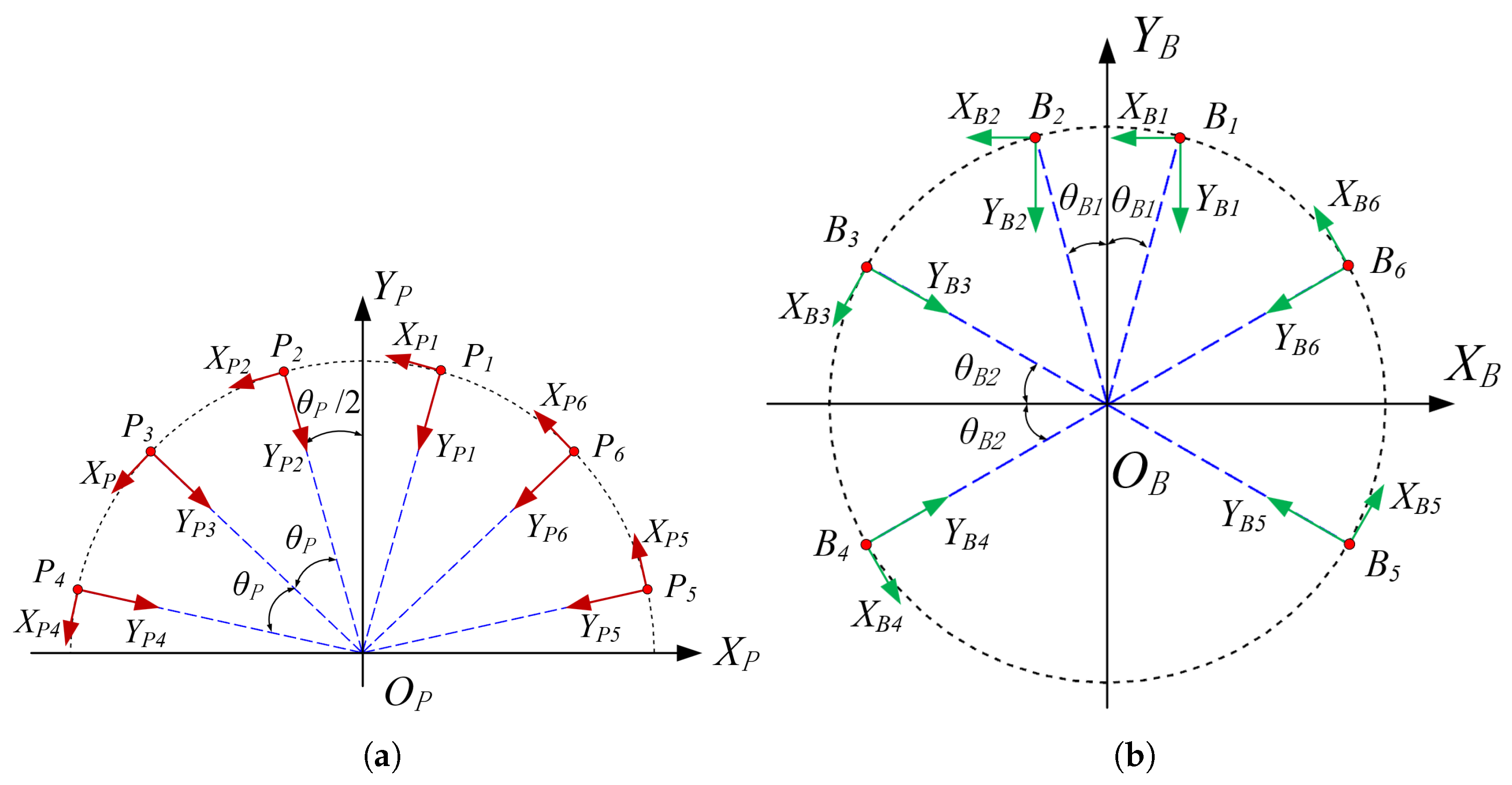
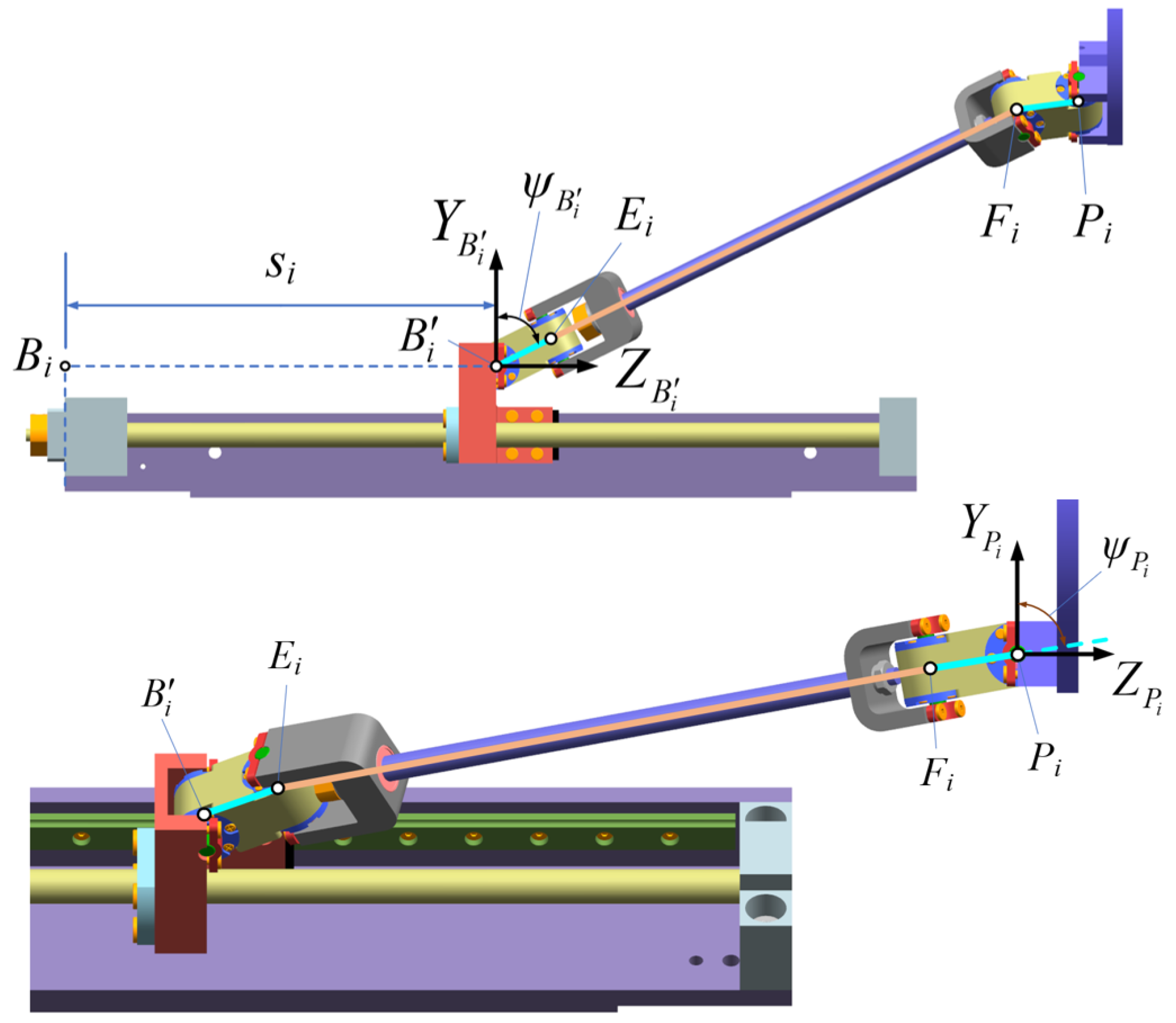
3.2. Definition of the Local Coordinate Systems for the Joint Points
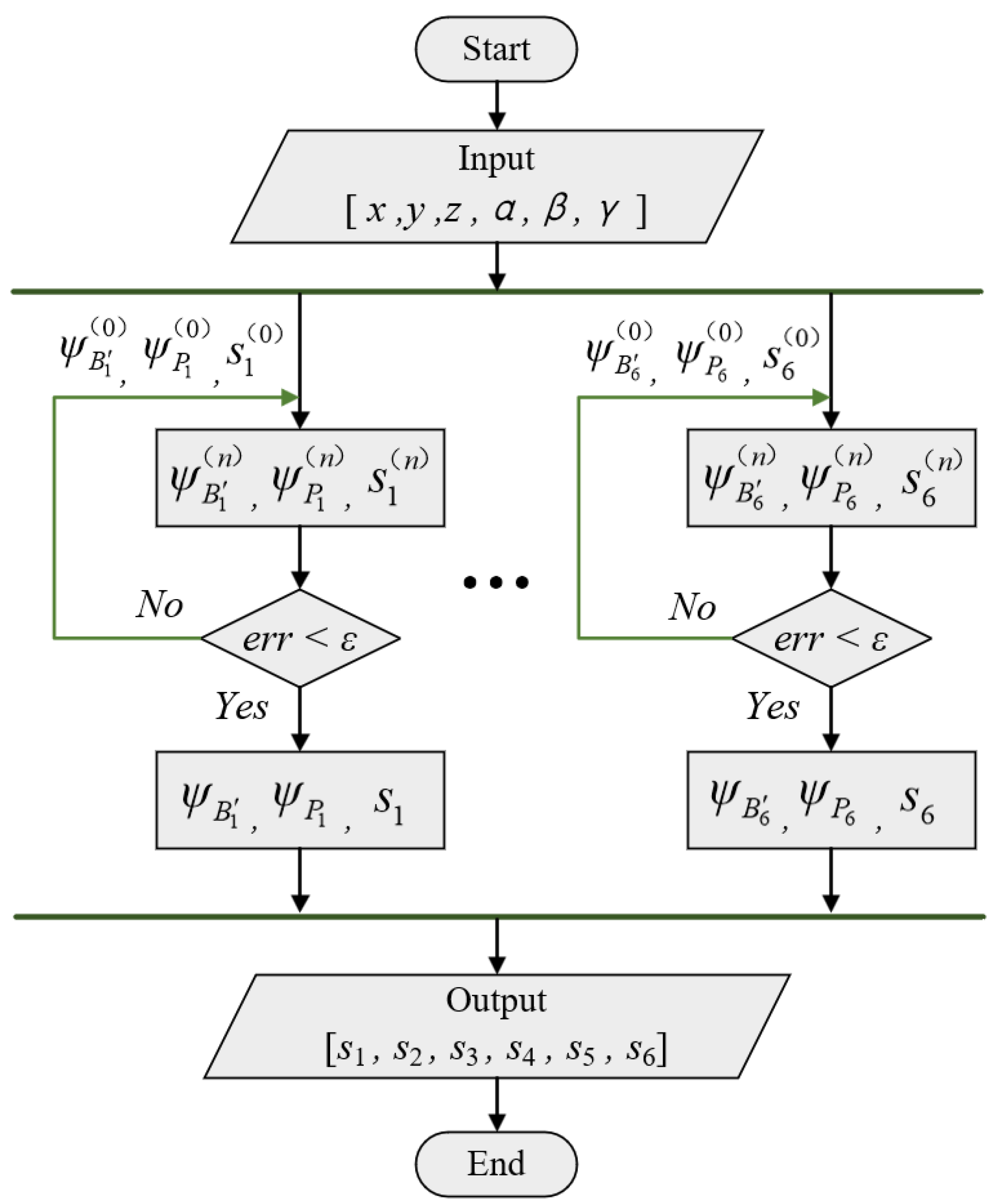
3.3. Analysis of Inverse Kinematics
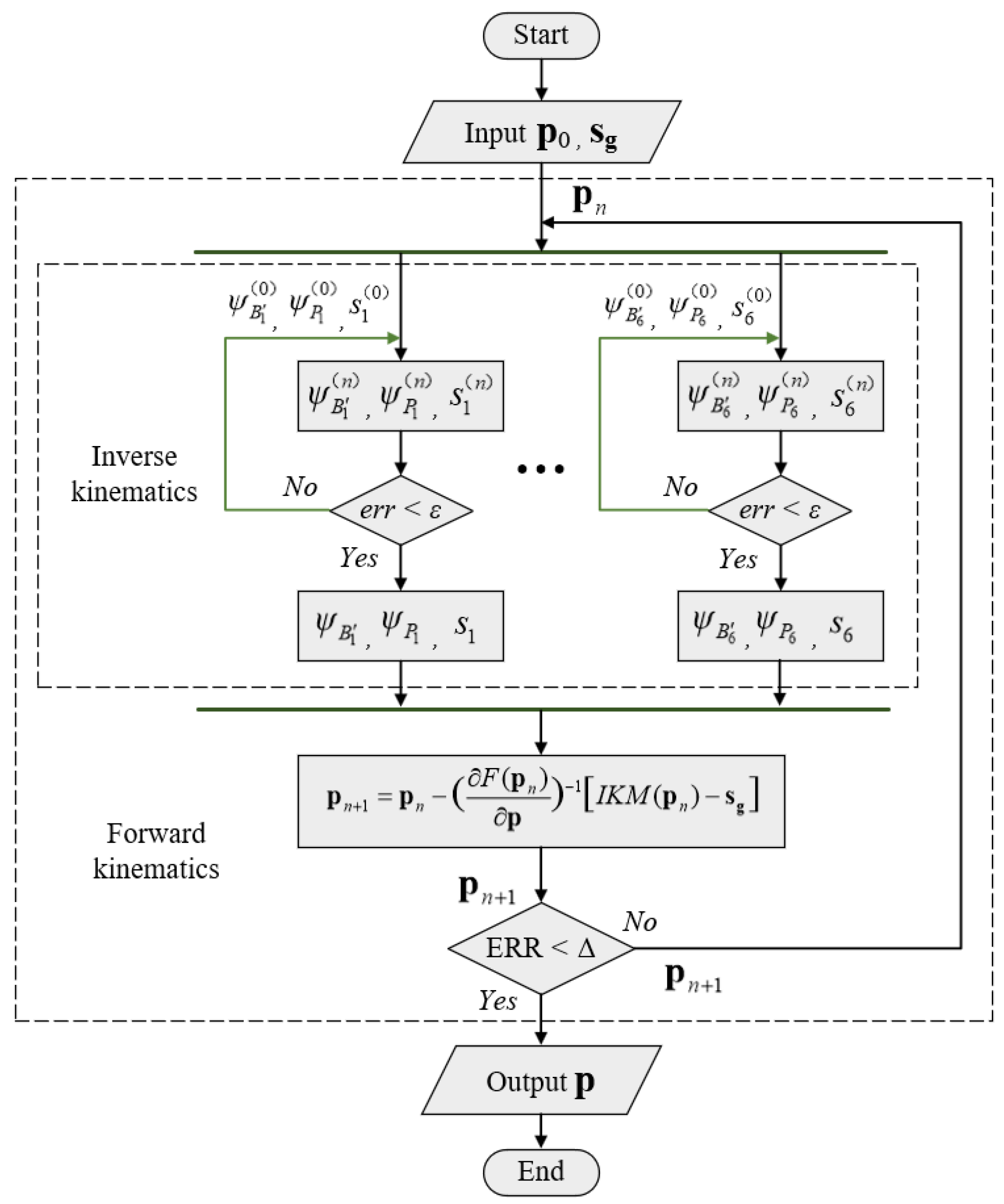
3.4. Analysis of Forward Kinematics
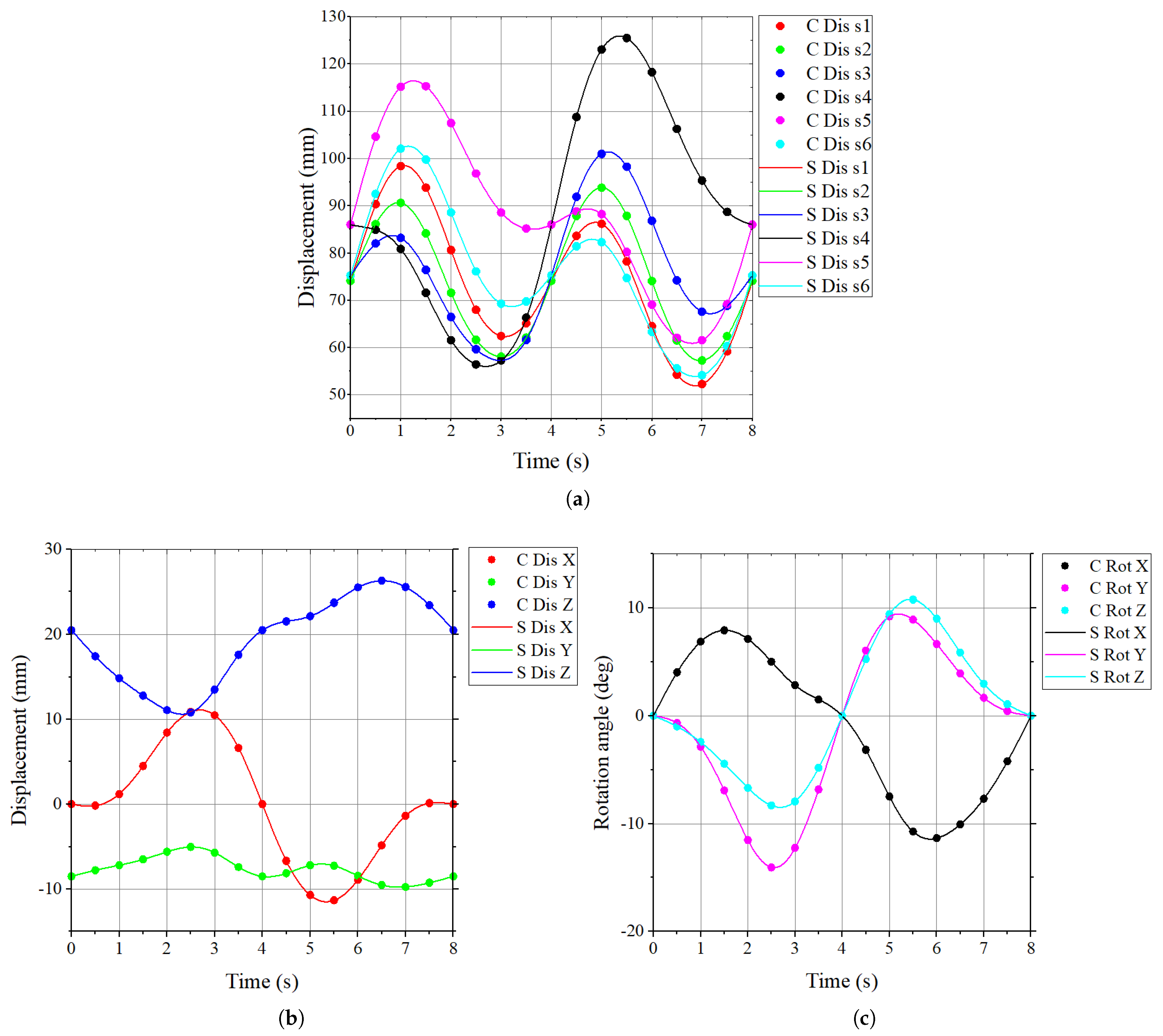
3.5. Kinematic Simulation Verification
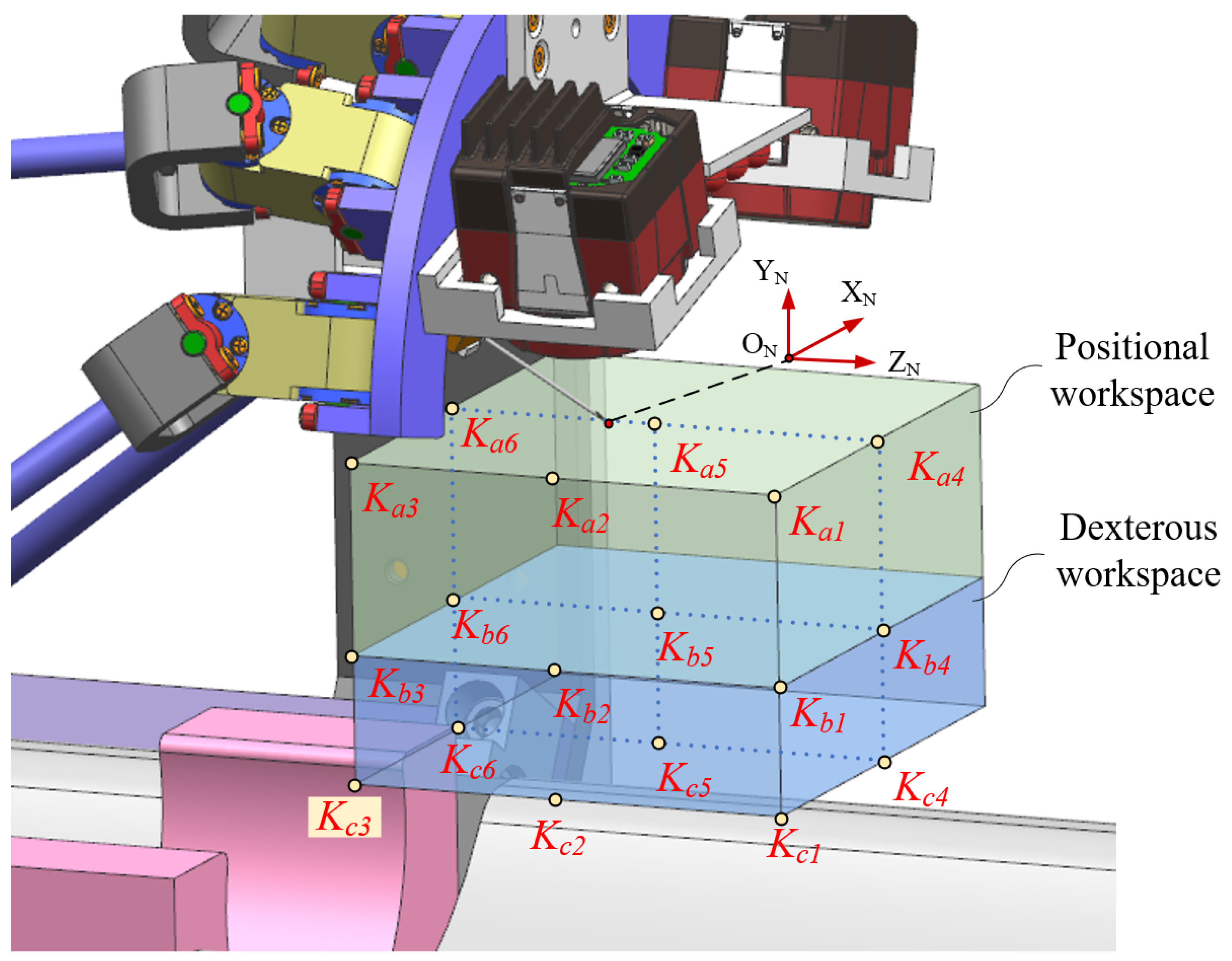
4. Workspace Validation
4.1. Factors Influencing the Workspace
- 1.
- Constraint on slider travel. Because of the length limitations of the lead screw and the guide rail, the travel of the slider is constrained. Specifically, the slider displacement must satisfy , where and are the minimum and maximum displacements of the i-th slider, respectively. For the blood sampling robot described in this paper, , .
- 2.
- Constraint on revolute pair angles. The rotation angle of the revolute pair is constrained by the structural limitations of the hinges, and there is a specific range within which the rotation angle can vary. If this range is exceeded, then motion interference may occur between the different components. In the case of the blood sampling robot described in this paper, the hinge points on the moving platform are more compact than those of conventional 6-DOF parallel robots. As a result, this motion interference may arise between adjacent offset hinges. It is difficult to use numerical methods to solve for the angular ranges of the hinges when such interference occurs. Therefore, a special pose simulation verification approach is used to ensure that the robot operates without interference within the required workspace.
- 3.
- Motion interference constraint between components. During the movement of the robot’s active parts (e.g., the moving platform, the linkage, the end effector), interference with other components (e.g., the frame, the arm brackets) may occur. This interference can affect the working space. Like the constraints on the rotational angles, this constraint can also be validated through a special pose simulation verification approach.
4.2. Special Pose Calculation and Simulation Verification
5. Accuracy Test
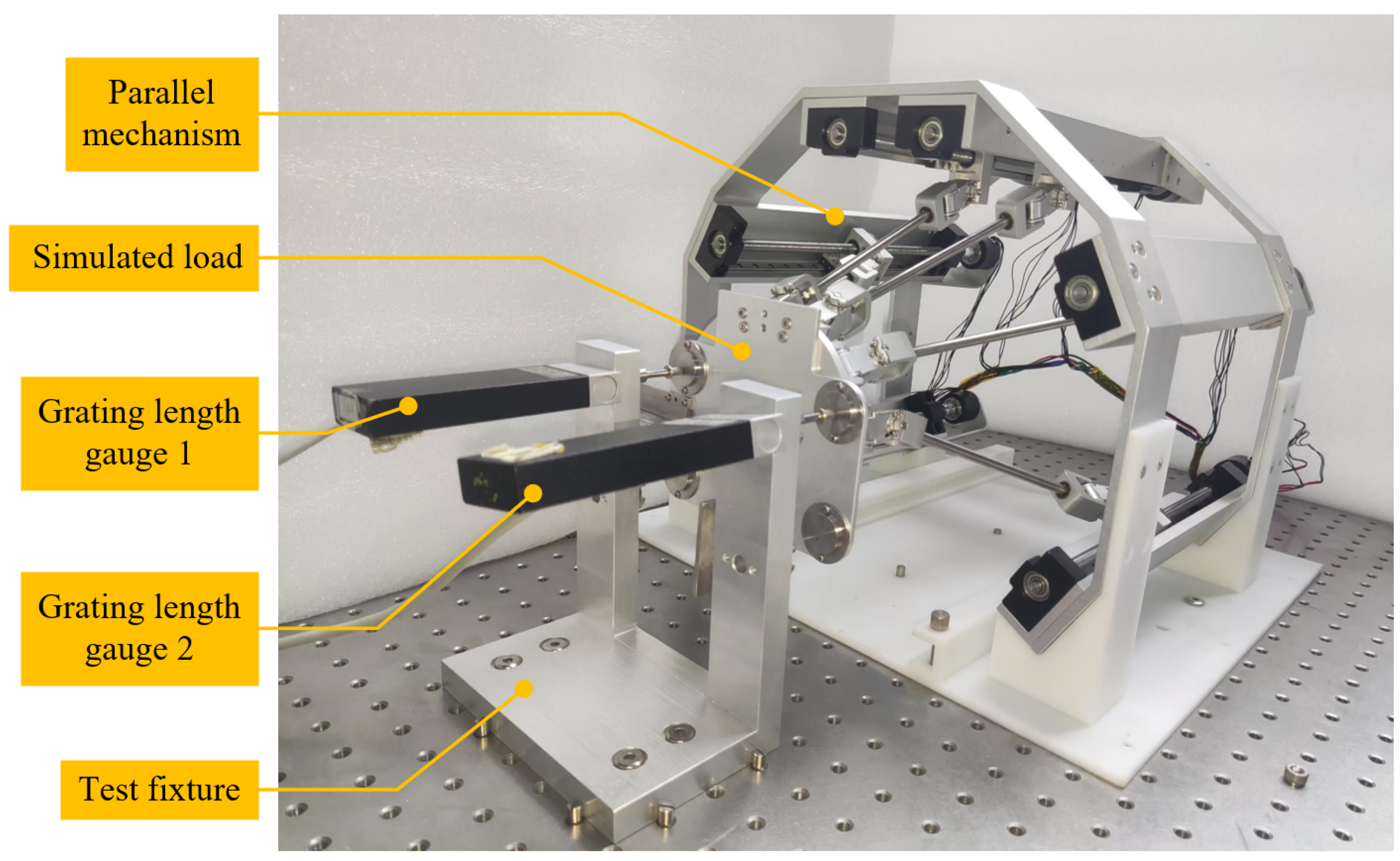
5.1. Test System Construction
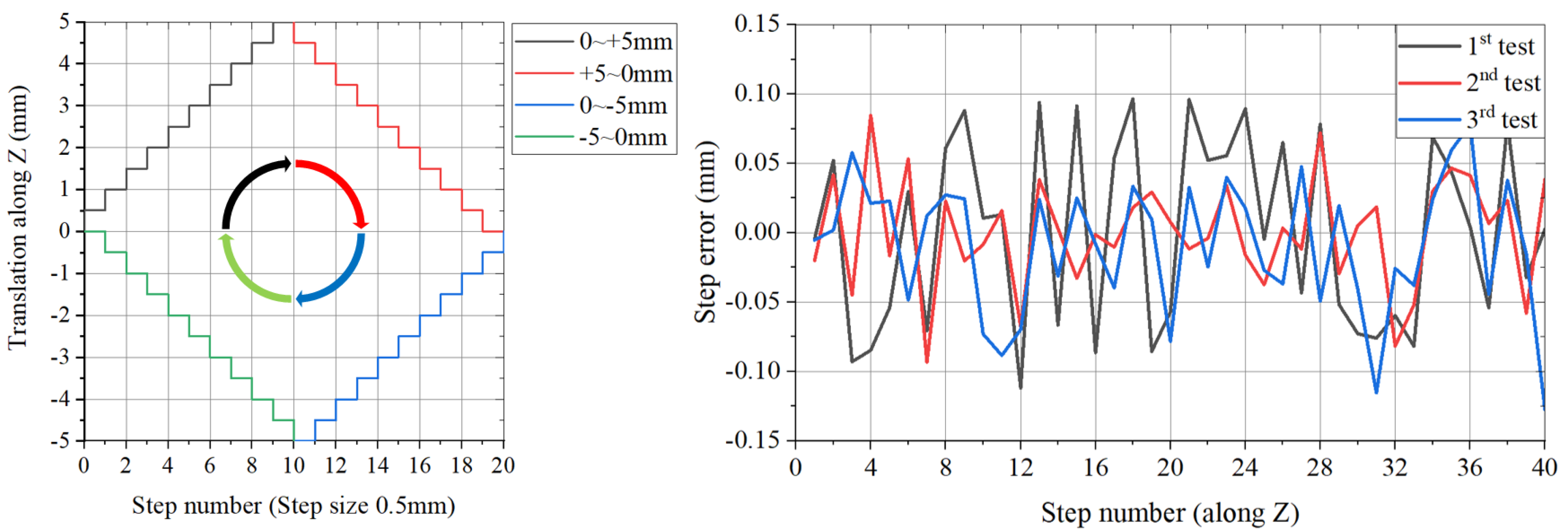
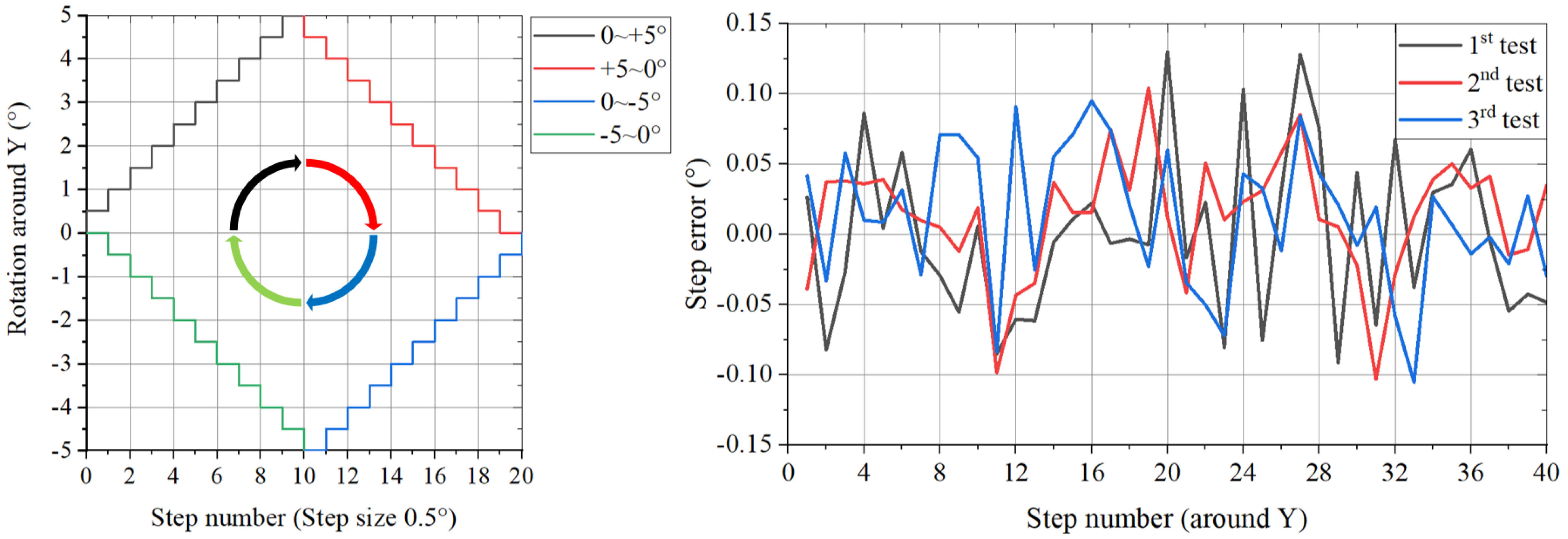
5.2. Motion Accuracy Testing
6. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kidd, B.A.; Hoffman, G.; Zimmerman, N.; Li, L.; Morgan, J.W.; Glowe, P.K.; Botwin, G.J.; Parekh, S.; Babic, N.; Doust, M.W.; et al. Evaluation of direct-to-consumer low-volume lab tests in healthy adults. J. Clin. Investig. 2016, 126, 2773. [Google Scholar] [CrossRef]
- Lillis, K.A.; Jaffe, D.M. Prehospital intravenous access in children. Ann. Emerg. Med. 1992, 21, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jiang, H. Current situation of medical resource allocation and service utilization in Chinese hospitals based on structural change analysis. Mod. Hosp. 2024, 24, 664–669+673. [Google Scholar]
- Katsogridakis, Y.L.; Seshadri, R.; Sullivan, C.; Waltzman, M.L. Veinlite transillumination in the pediatric emergency department—A therapeutic interventional trial. Pediatr. Emerg. Care 2008, 24, 83–88. [Google Scholar] [CrossRef]
- Xiong, X.; Xiong, Y.L.; Liu, G.F. Systematic review and meta-analysis: Safety of ultrasound-guided peripheral venipuncture and catheterization. Ann. Pallliat. Med. 2021, 10, 11721–11732. [Google Scholar] [CrossRef]
- Dreyer, J.; Huenges, K.; Cremer, J.; Haneya, A. A new device for vein localization and effect of application of disinfectant spray on its efficiency. Curr. Dir. Biomed. Eng. 2017, 3, 127–130. [Google Scholar] [CrossRef][Green Version]
- Zivanovic, A.; Davies, B.L. A robotic system for blood sampling. IEEE Trans. Inf. Technol. Biomed. 2000, 4, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kudashov, I.; Shchukin, S.; Al-harosh, M.; Shcherbachev, A. Smart Bio-Impedance-Based Sensor for Guiding Standard Needle Insertion. Sensors 2022, 22, 665. [Google Scholar] [CrossRef]
- Perry, T.S. Profile: Veebot drawing blood faster and more safely than a human can. IEEE Spectr. 2013, 50, 23. [Google Scholar] [CrossRef]
- Fang, X. MagicNurse: “AI+ Medical” to create a fully automatic intelligent blood collection Magic Nurse. Robot Ind. 2021, 2, 56–60. [Google Scholar]
- Kim, S.; Choi, S.; Yeom, K. Design and development of robotic system for precise vascular access and autonomous venipuncture. Electron. Lett. 2024, 60, e13121. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Yeom, K. An injection-point determination algorithm for intelligent injection robot systems. IEICE Electron. Express 2024, 21, 20230283. [Google Scholar] [CrossRef]
- Uchiyama, M. Structures and characteristics of parallel manipulators. Adv. Robot. 1994, 8, 545–557. [Google Scholar] [CrossRef]
- Shoham, M.; Burman, M.; Joskowicz, L.; Batkilin, E.; Kunicher, Y. Bone-mounted miniature robot for surgical procedures: Concept and clinical applications. IEEE Trans. Robot. Autom. 2013, 19, 893–901. [Google Scholar] [CrossRef]
- Nakano, T.; Sugita, N.; Ueta, T.; Tamaki, Y.; Mitsuishi, M. A parallel robot to assist vitreoretinal surgery. Int. J. Comput. Assist. Radiol. Surg. 2009, 4, 517–526. [Google Scholar] [CrossRef]
- Gough, V.E. Contribution to discussion to papers on research in automobile stability and control and in tire performance. Proc. Automot. Div. Inst. Mech. Eng. 1957, 180, 392–394. [Google Scholar]
- Stewart, D. A platform with six degrees of freedom. Proc. Inst. Mech. Eng. C. J. Mech. Eng. Sci. 1965, 180, 371–386. [Google Scholar] [CrossRef]
- Seide, K.; Wolter, D.; Kortmann, H.R. Fracture reduction and deformity correction with the hexapod Ilizarov fixator. Clin. Orthop. Relat. Res. 1999, 10, 186–195. [Google Scholar] [CrossRef]
- Takemura, H.; Onodera, T.; Ming, D.; Mizoguchi, H. Design and Control of a Wearable Stewart Platform-Type Ankle-Foot Assistive Device Regular Paper. Int. J. Adv. Robot. Syst. 2012, 9, 202–208. [Google Scholar] [CrossRef]
- Grossmann, K.; Kauschinger, B. Eccentric universal joints for parallel kinematic machine tools: Variants and kinematic transformations. Prod. Eng.-Res. Dev. 2012, 6, 521–529. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Y. Analyses of kinematics, statics, and workspace of a 3-RR(P)under-barRR parallel manipulator and its three isomeric mechanisms. Proc. Inst. Mech. Eng. Part C-J. Eng. Mech. Eng. Sci. 2008, 222, 1829–1837. [Google Scholar] [CrossRef]
- Li, P.Y.; Han, H.; Liu, C.L.; Ren, B.; Wu, Q.W.; Xu, Z.B. Workspace analysis of axial offset joint based on parameterization. Robotica 2023, 41, 2882–2906. [Google Scholar] [CrossRef]
- Ji, P.; Wu, H.T. Kinematics analysis of an offset 3-UPU translational parallel robotic manipulator. Robot. Auton. Syst. 2023, 42, 117–123. [Google Scholar] [CrossRef]
- Cai, Z.X.; Xie, B. Robotics, 3rd ed.; Tsinghua University Press: Beijing, China, 2015; pp. 45–46. [Google Scholar]
- Dalvand, M.M.; Shirinzadeh, B. Kinematics analysis of 6-DOF parallel micro-manipulators with offset U-joints: A case study. Int. J. Intell. Mechatron. Robot. 2012, 2, 28–40. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Han, H.; Xu, Z.B.; Han, C.Y.; Yu, Y.; Mao, A.L.; Wu, Q.W. Kinematics analysis and performance testing of 6-RR-RP-RR parallel platform with offset RR-hinges based on Denavit-Hartenberg parameter method. Proc. Inst. Mech. Eng. Part C-J. Eng. Mech. Eng. Sci. 2021, 235, 3519–3533. [Google Scholar] [CrossRef]
- Morell, A.; Tarokh, M.; Acosta, L. Solving the forward kinematics problem in parallel robots using Support Vector Regression. Eng. Appl. Artif. Intell. 2013, 26, 1698–1706. [Google Scholar] [CrossRef]
- Ogden-Grable, H.; Gill, G.W. Phlebotomy puncture juncture—Preventing phlebotomy errors—Potential for harming your patients. Lab. Med. 2005, 36, 430–433. [Google Scholar] [CrossRef]
- China NHCotPsRo. Guidelines of venous blood specimen collection. Chin. Pract. J. Rural. Dr. 2020, 27, 7–11. [Google Scholar]
- GB/T12642; Industrial Robots—Performance Criteria and Related Test Methods. AQSIQ, SAC: Beijing, China, 2013.



| mm | mm | mm | mm | mm | |||
|---|---|---|---|---|---|---|---|
| 55 | 125 | 31 | 15 | 30 | 20 | 170 | (0, , 20.5) |
| Prescribed Motion of the Needle (for Inverse Kinematics) | Prescribed Motion of the Sliders (for Forward Kinematics) |
|---|---|
| Robot | Veebot | MagicNurse | VeniBot | This Paper |
|---|---|---|---|---|
| Structure type | Series | Series | Series | Parallel |
| Monocular NIR imaging | Monocular NIR imaging | Binocular NIR imaging | ||
| Sense scheme | – | +US imaging | +US imaging | +Puncture force detection |
| +Blood flow back detection | ||||
| Size (mm) | About | About | About | 390 × 378 × 325 |
| 1500 × 1000 × 1000 | 1500 × 1500 × 1500 | 600 × 600 × 600 | ||
| Portability | × | × | ✓ | ✓ |
| Accuracy | High | High | Low | Medium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Han, H.; Man, G.; Feng, Z.; Yu, P.; Xu, Z. Design and Analysis of a Portable Robot for Venous Blood Sampling. Machines 2025, 13, 203. https://doi.org/10.3390/machines13030203
Xue H, Han H, Man G, Feng Z, Yu P, Xu Z. Design and Analysis of a Portable Robot for Venous Blood Sampling. Machines. 2025; 13(3):203. https://doi.org/10.3390/machines13030203
Chicago/Turabian StyleXue, Haoran, Hasiaoqier Han, Gang Man, Zeran Feng, Peng Yu, and Zhenbang Xu. 2025. "Design and Analysis of a Portable Robot for Venous Blood Sampling" Machines 13, no. 3: 203. https://doi.org/10.3390/machines13030203
APA StyleXue, H., Han, H., Man, G., Feng, Z., Yu, P., & Xu, Z. (2025). Design and Analysis of a Portable Robot for Venous Blood Sampling. Machines, 13(3), 203. https://doi.org/10.3390/machines13030203





