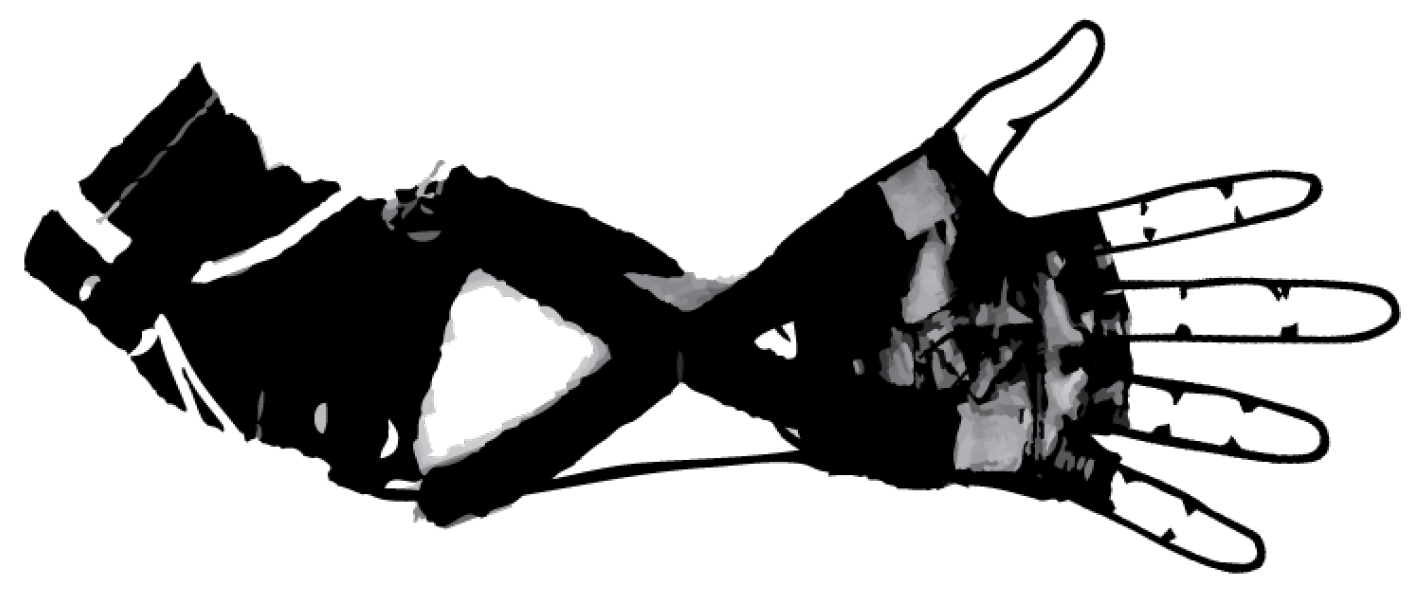
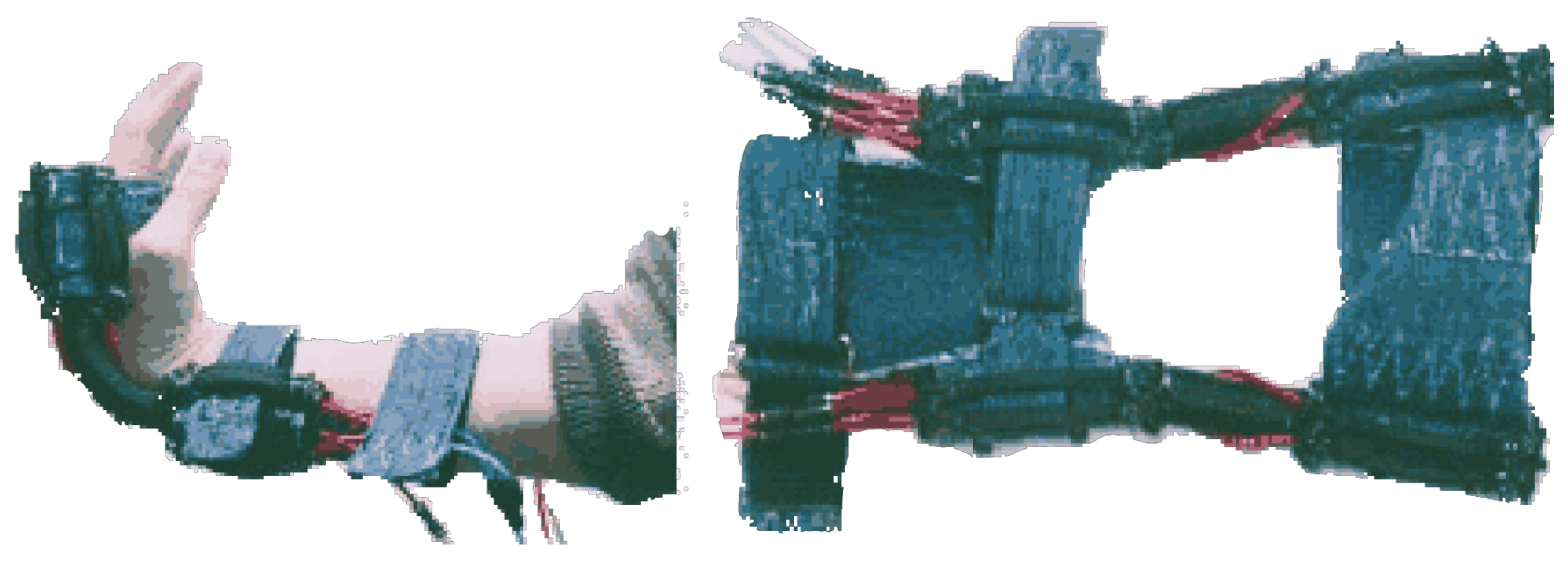
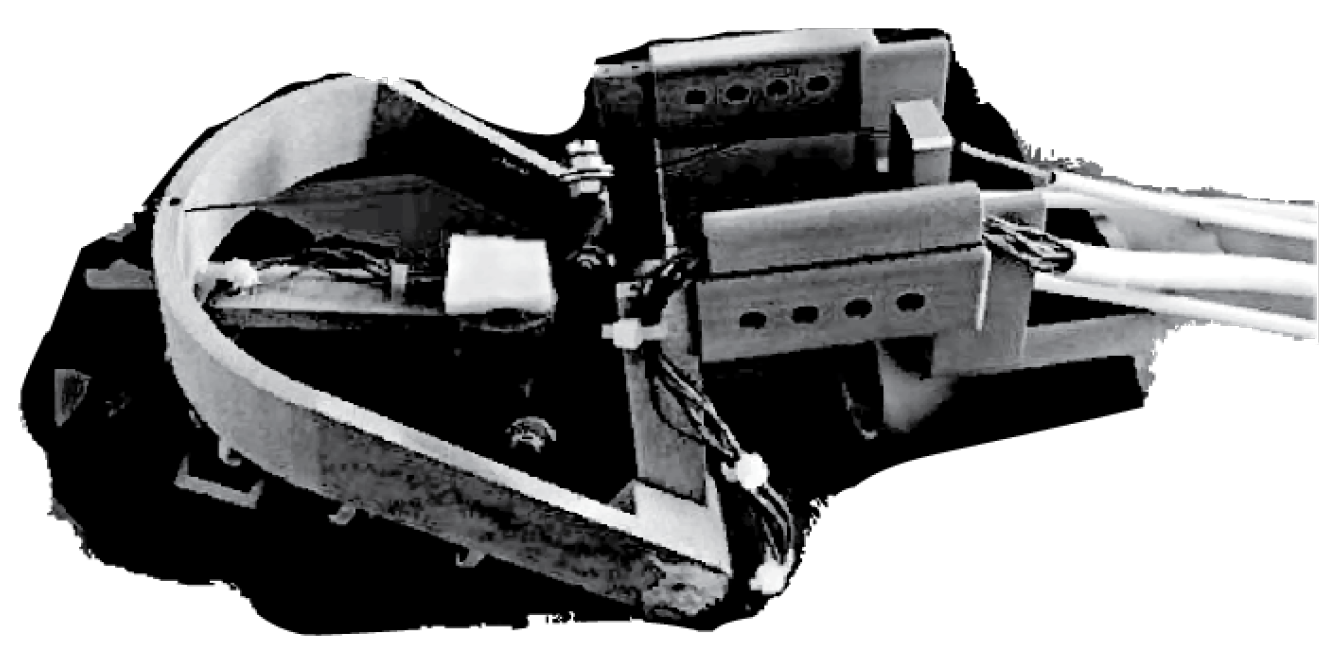
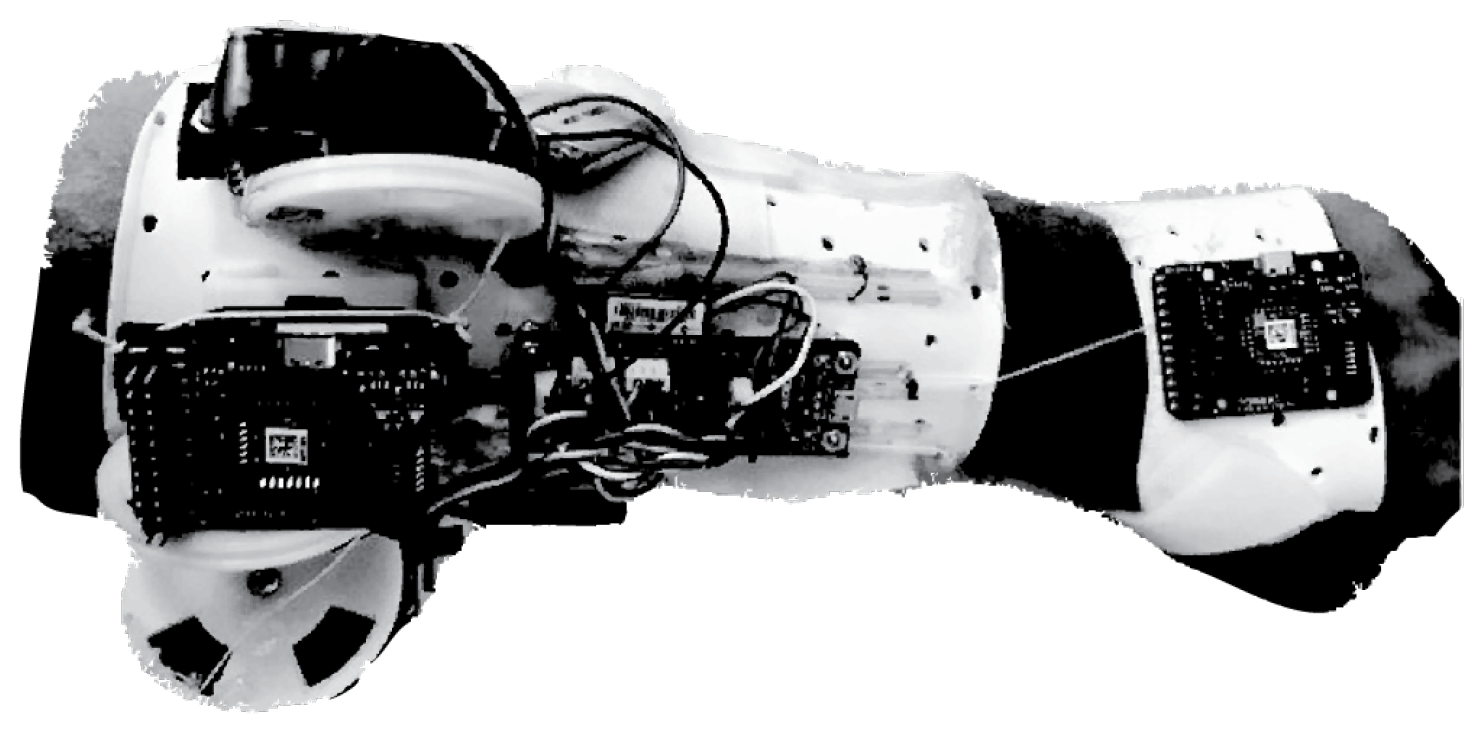
State of the Art in Wearable Wrist Exoskeletons Part II: A Review of Commercial and Research Devices
Abstract
1. Introduction
2. Materials and Methods
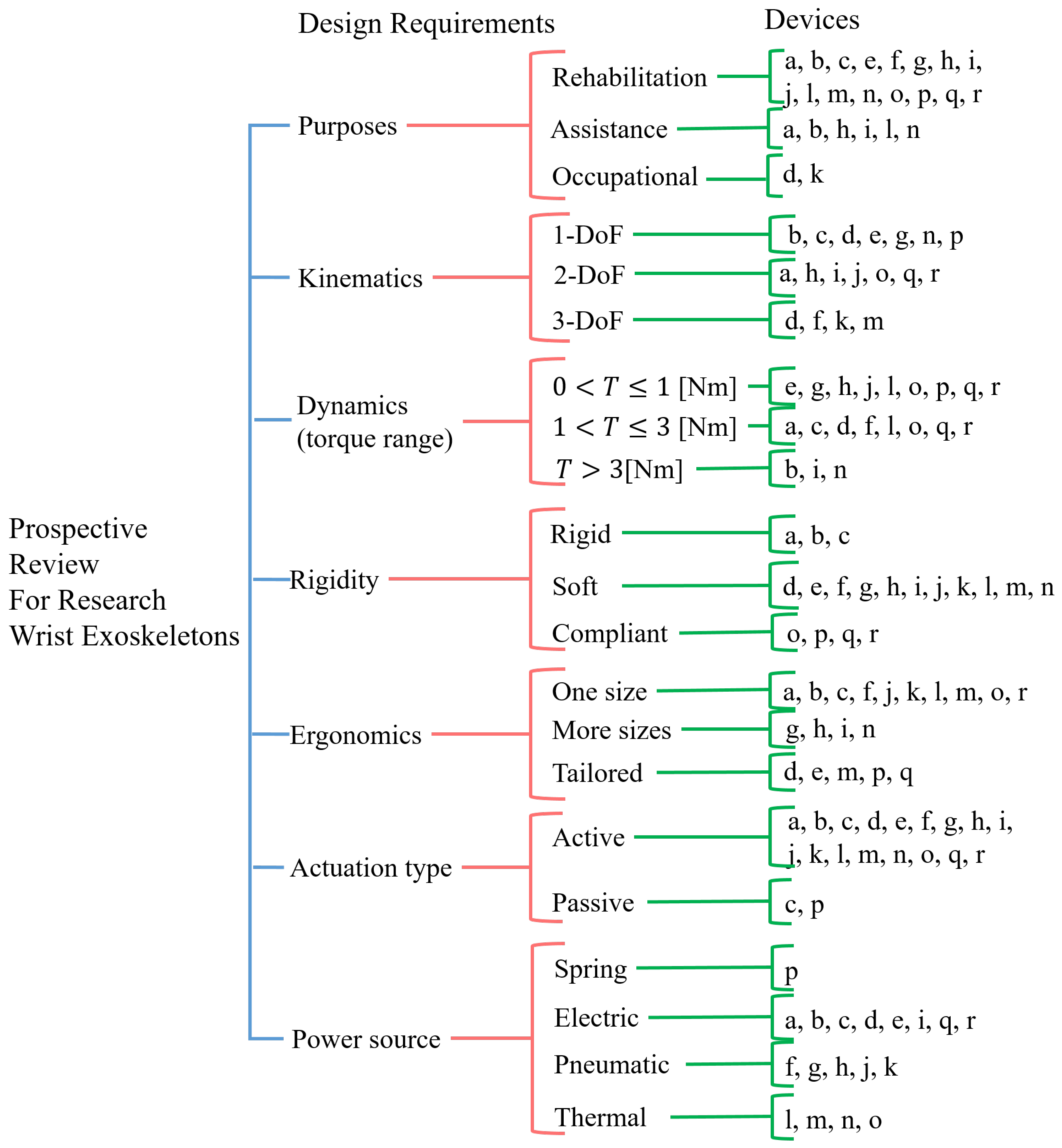
- Upper limb exoskeletons, which include the wrist in their designs;
- Devices able to relieve pain or mitigate fatigue by supporting at least one wrist movement;
- Devices intended for rehabilitation, assistance, and occupational purposes;
- Portable devices;
- All studies must be accessible by the authors in English.
- Prosthesis or exoskeletons that do not allow free wrist movements;
- Military devices;
- Fixed/grounded devices;
- Studies in other languages or with insufficient information, which made the analysis unclear.
3. Research and Pre-Commercial Devices
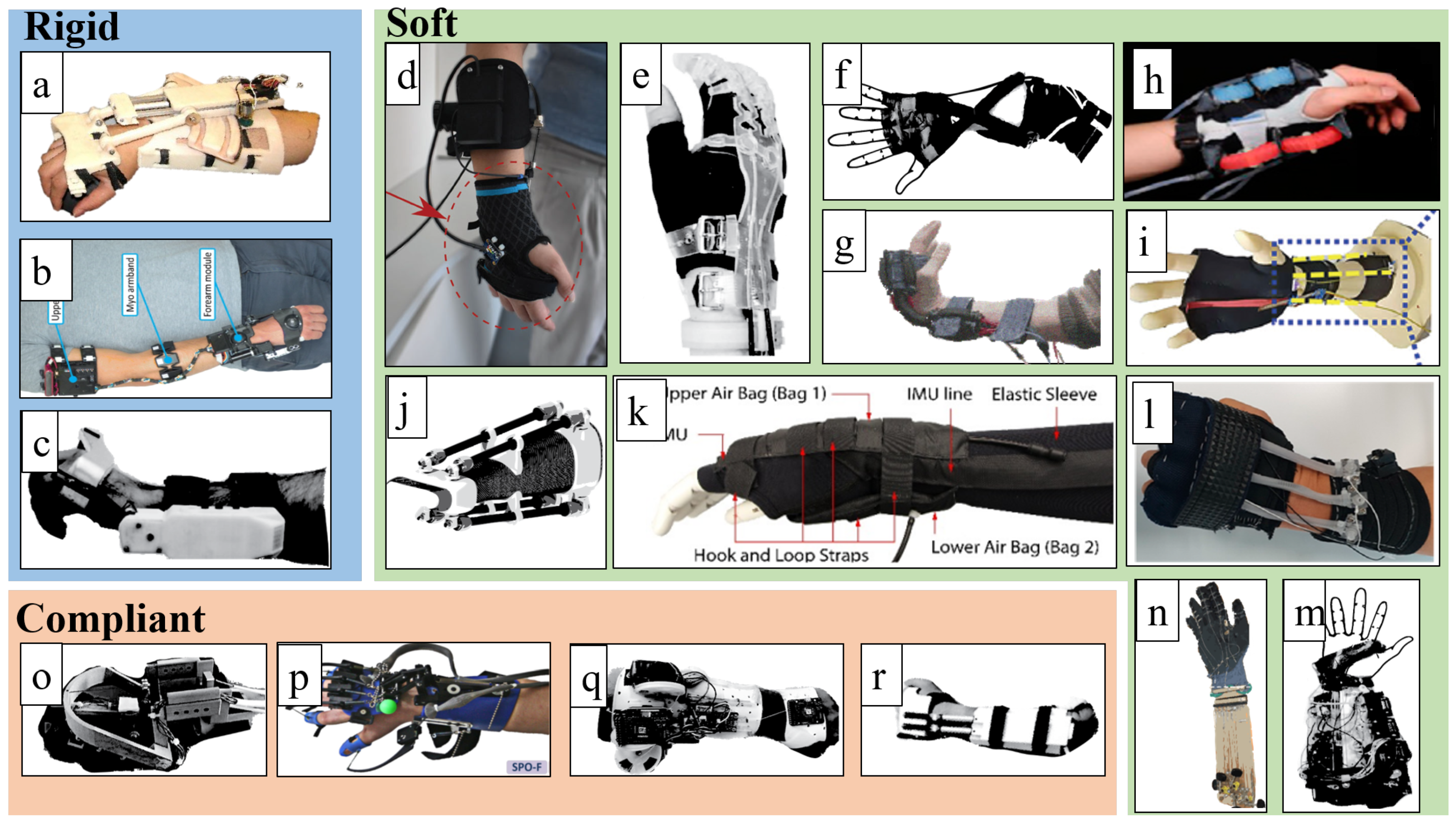
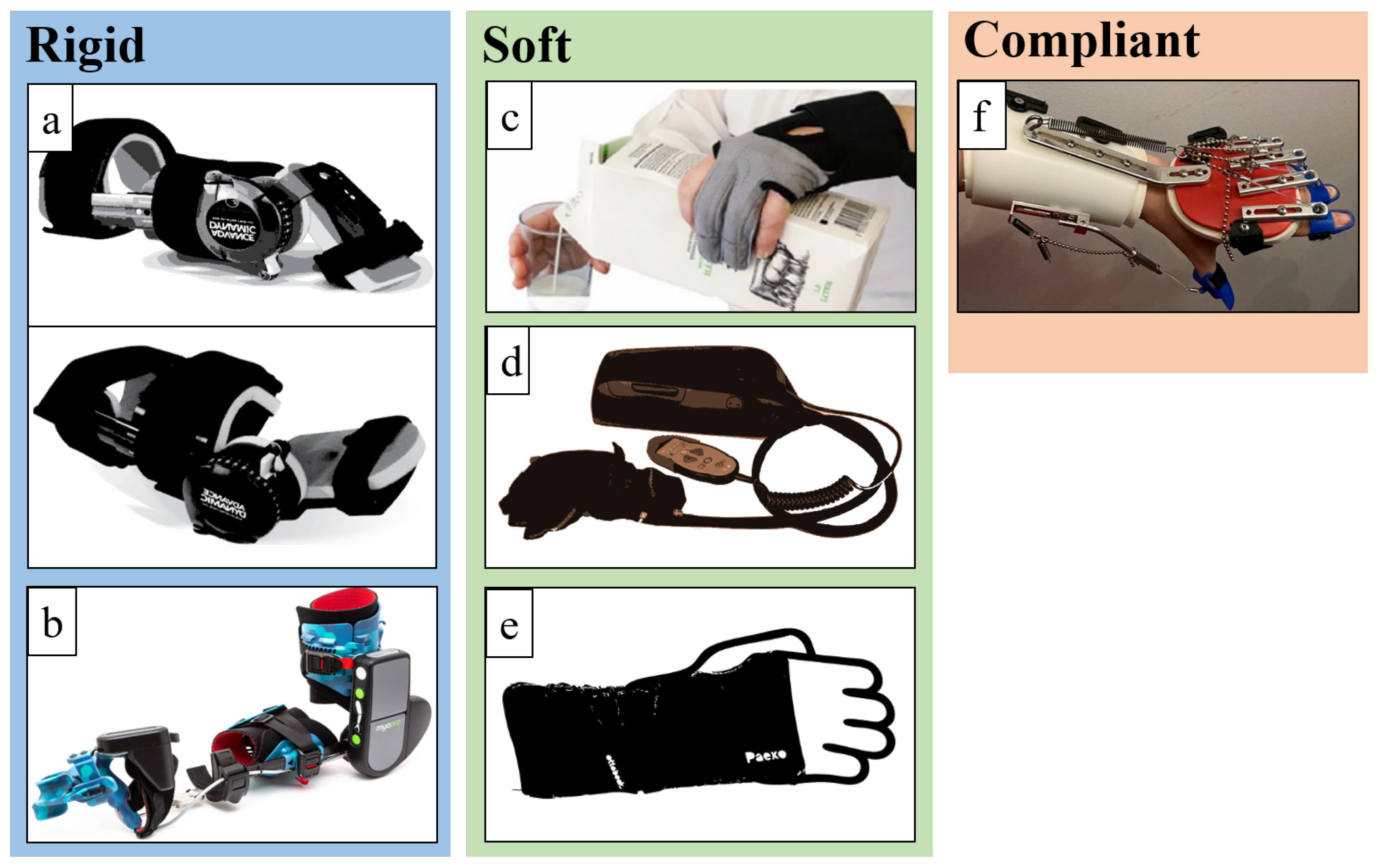
3.1. Rigid Devices
3.1.1. Development of a Portable Wrist Exoskeleton (PWE)
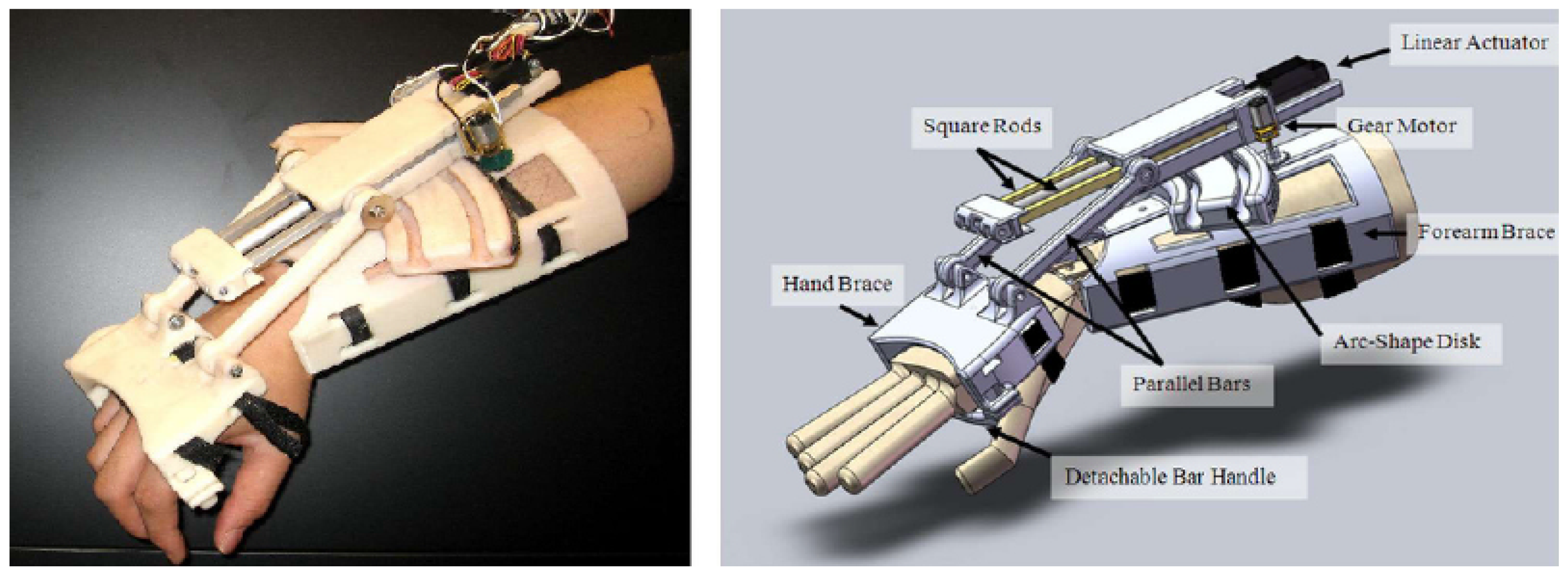
3.1.2. The eWrist—A Wearable Wrist Exoskeleton
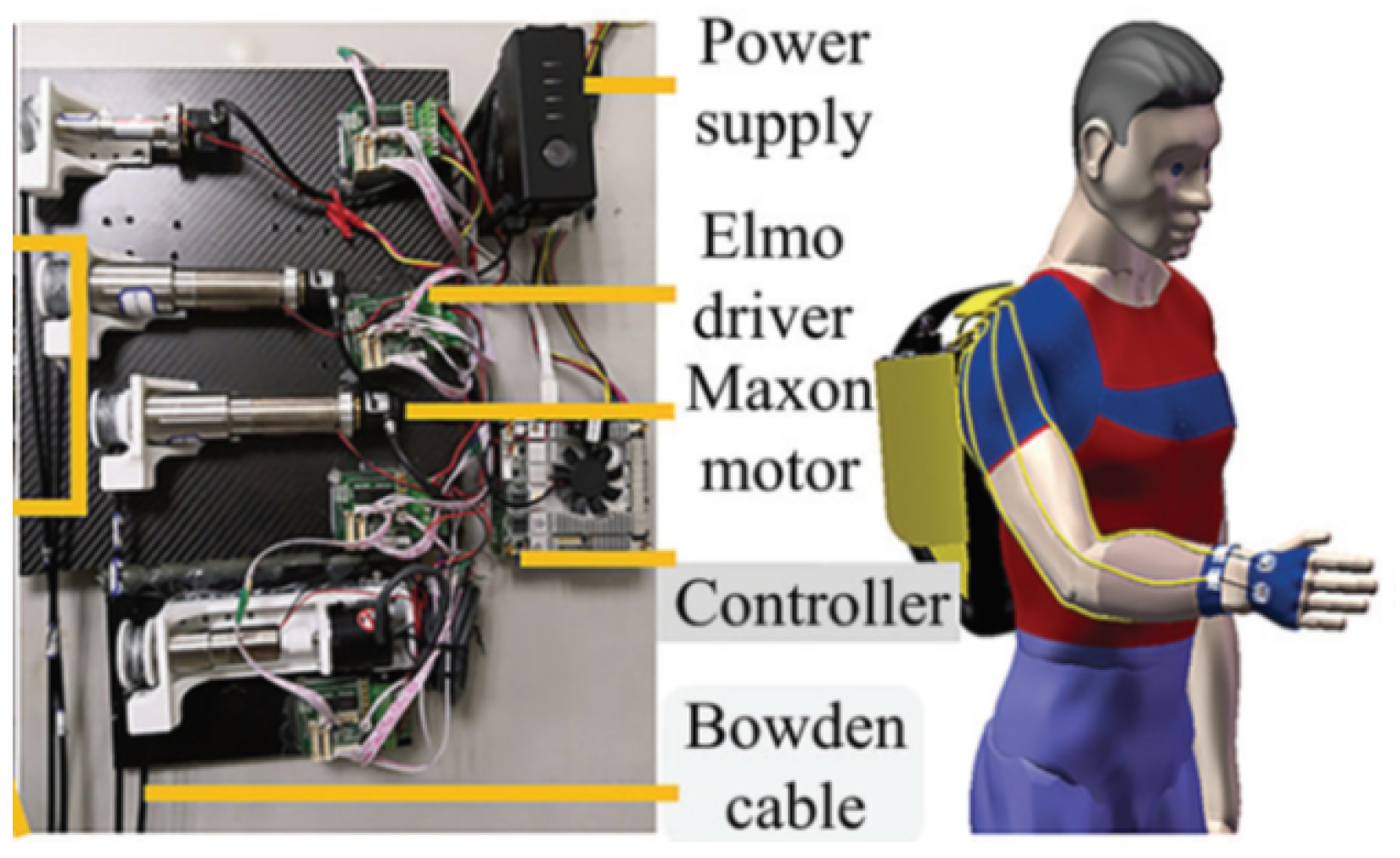
3.1.3. Robotic Orthosis for Wrist Assistance
3.2. Soft Devices
3.2.1. Soft Wrist Exosuit
3.2.2. Exo-Wrist—A Soft Tendon-Driven Wrist Wearable Robot for a Dart-Throwing Motion
3.2.3. A Soft Robotic Orthosis for Wrist Rehabilitation
3.2.4. Active Support Splint Driven by a Pneumatic Soft Actuator (ASSIST)
3.2.5. A Soft Robotic Wrist Brace with Origami Actuators

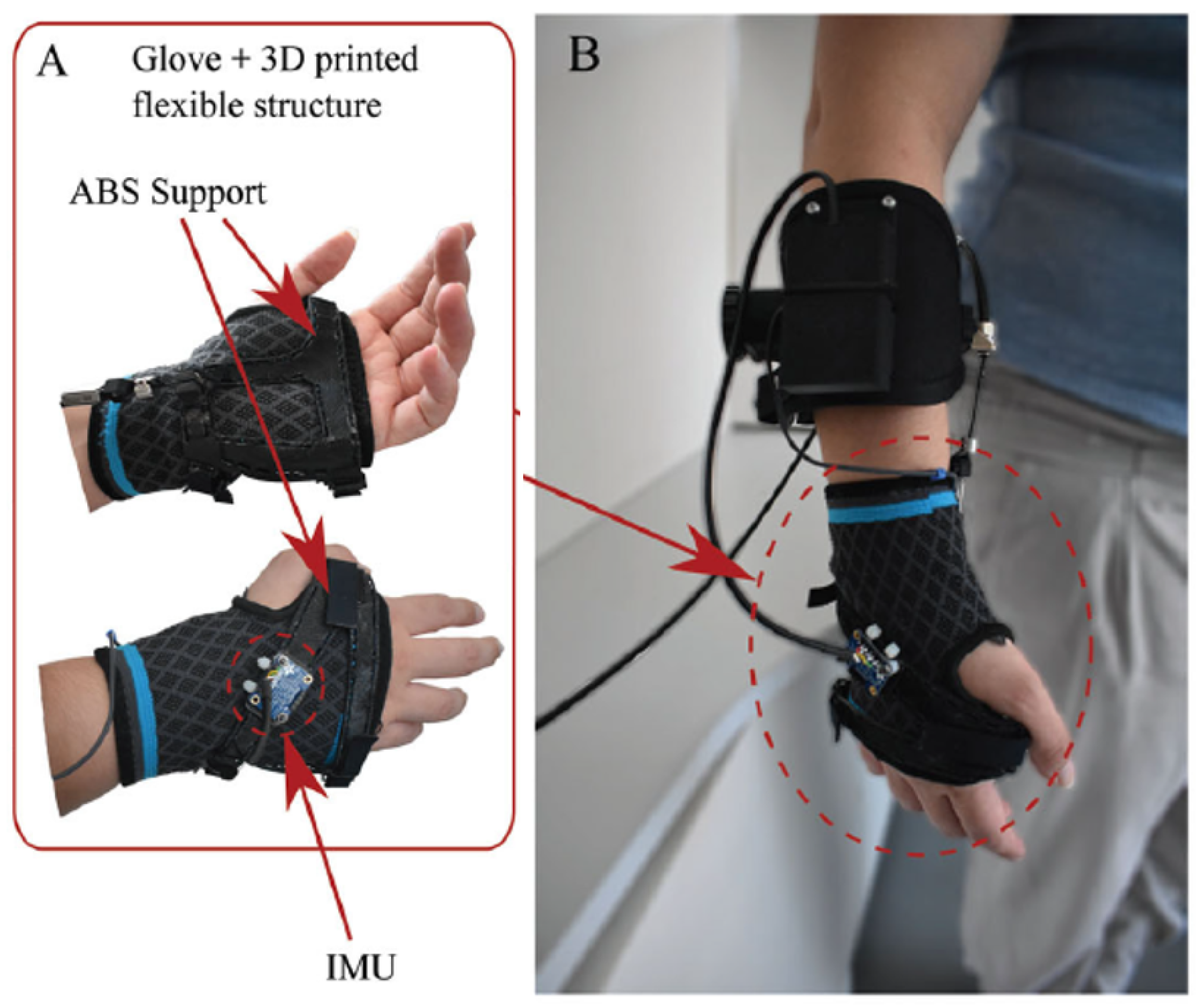
3.2.6. Bioinspired Musculoskeletal Model-Based Soft Wrist Exoskeleton
3.2.7. Exo-Wrist: A Wrist Exoskeleton Actuated by Pneumatic Muscle Actuators
3.2.8. Carpal Tunnel Syndrome Soft Relief Device
3.2.9. Wrist-Assisting Soft Wearable Robot with Integrated SMA Muscle
3.2.10. Wearable SMA-Based Wrist and Forearm Exoskeleton
3.2.11. ASR: A Wearable Glove for Hand Grasping
3.3. Compliant Devices
3.3.1. SMA-Based Wrist Exoskeleton
3.3.2. Script: A Passive Orthosis
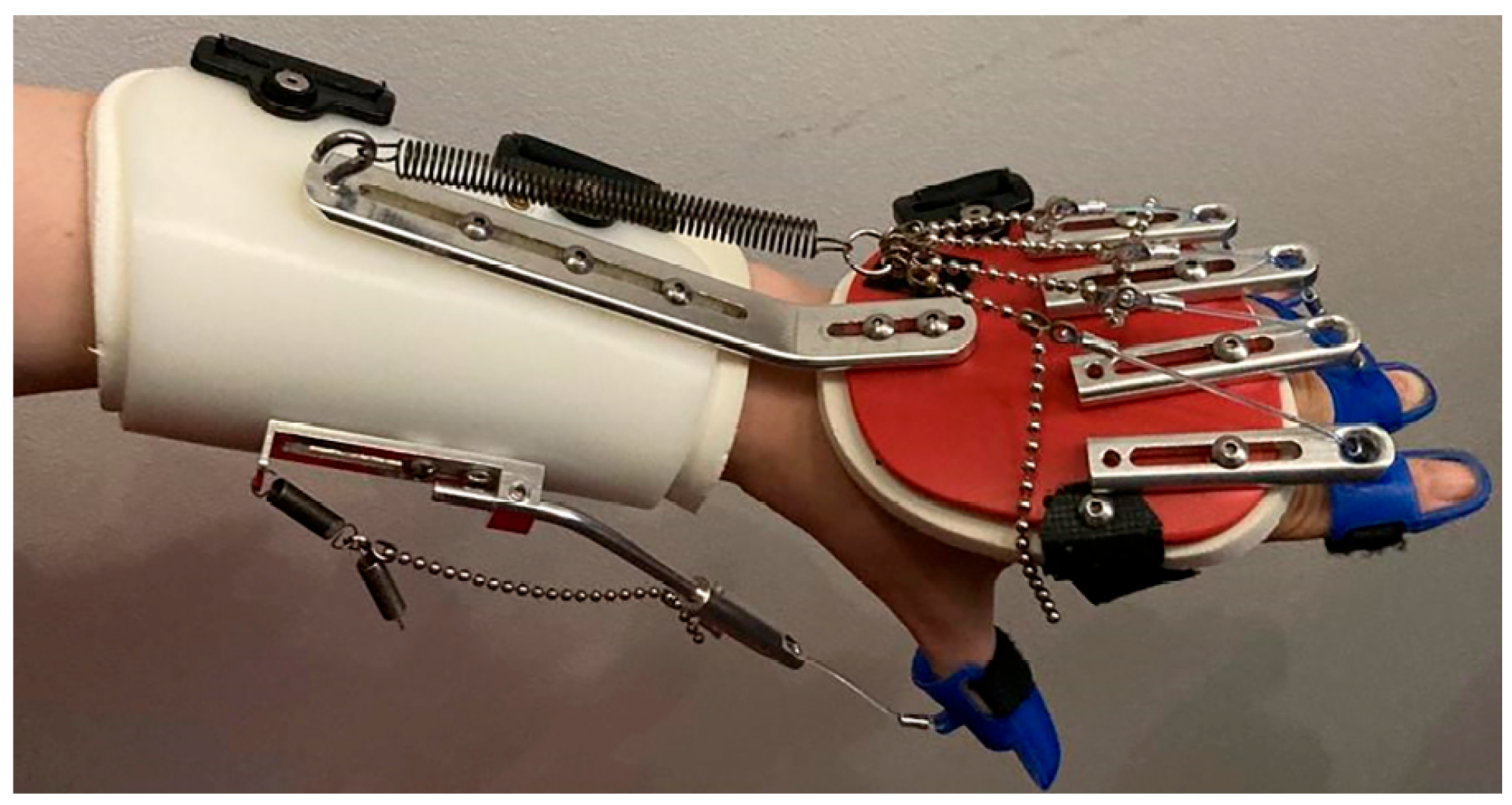
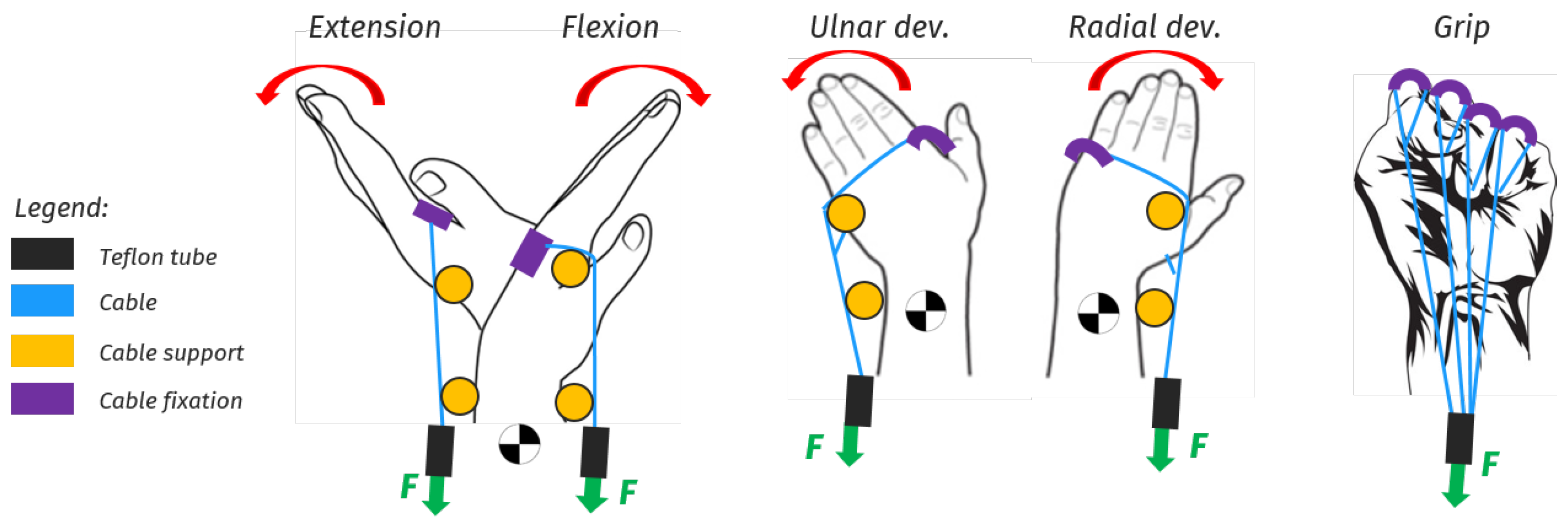
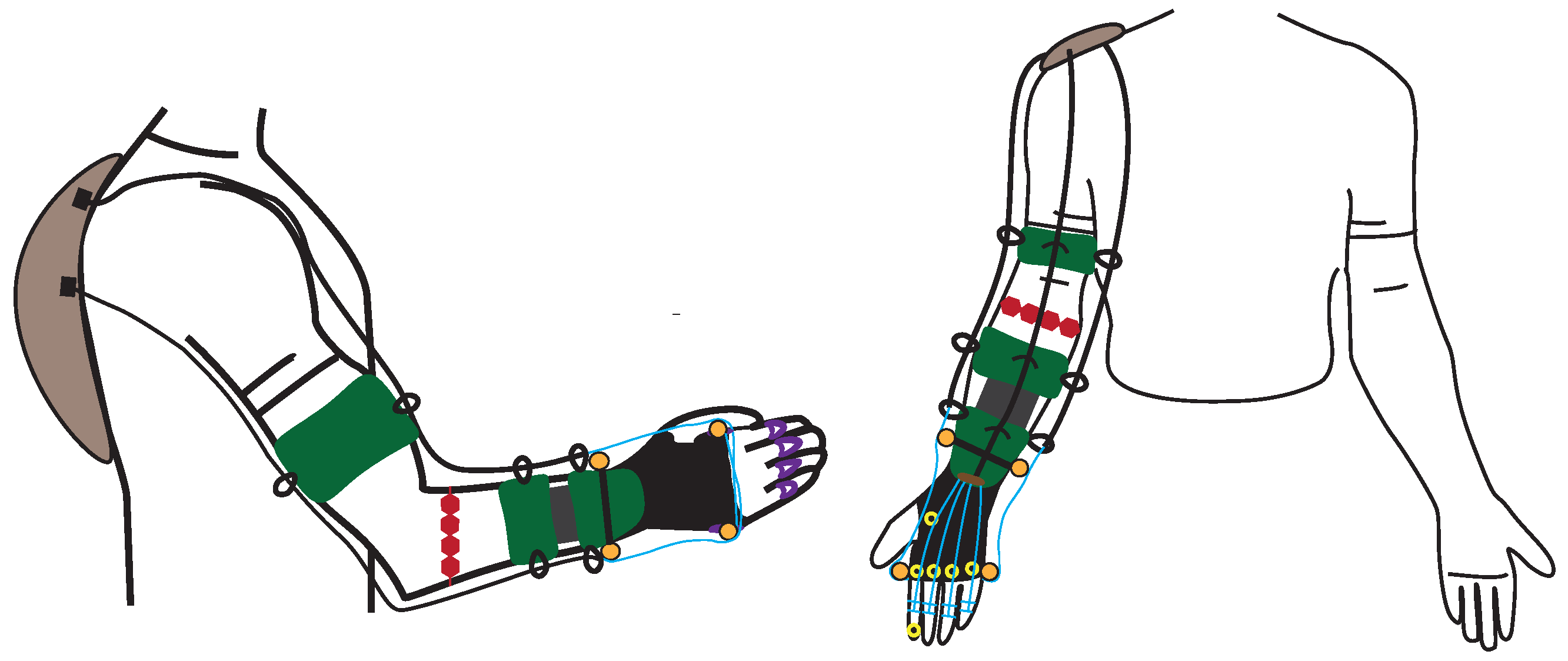
3.3.3. Hand and Wrist Actuated Exoskeleton for Rehabilitation and Training
3.3.4. Low-Profile Two-DoF Wrist Exoskeleton
4. Commercial Devices
4.1. Rigid Devices
4.1.1. JAS Wrists
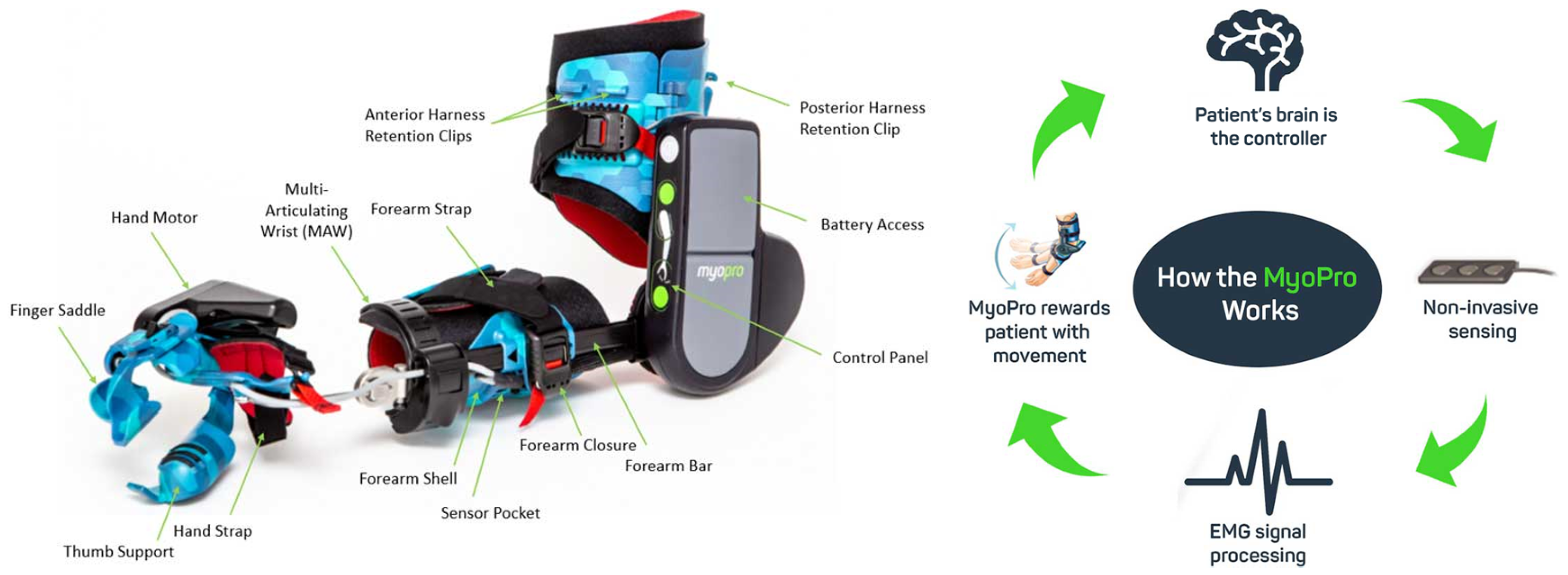
4.1.2. MyoPro Orthosis
4.2. Soft Devices
4.2.1. Carbonhand®
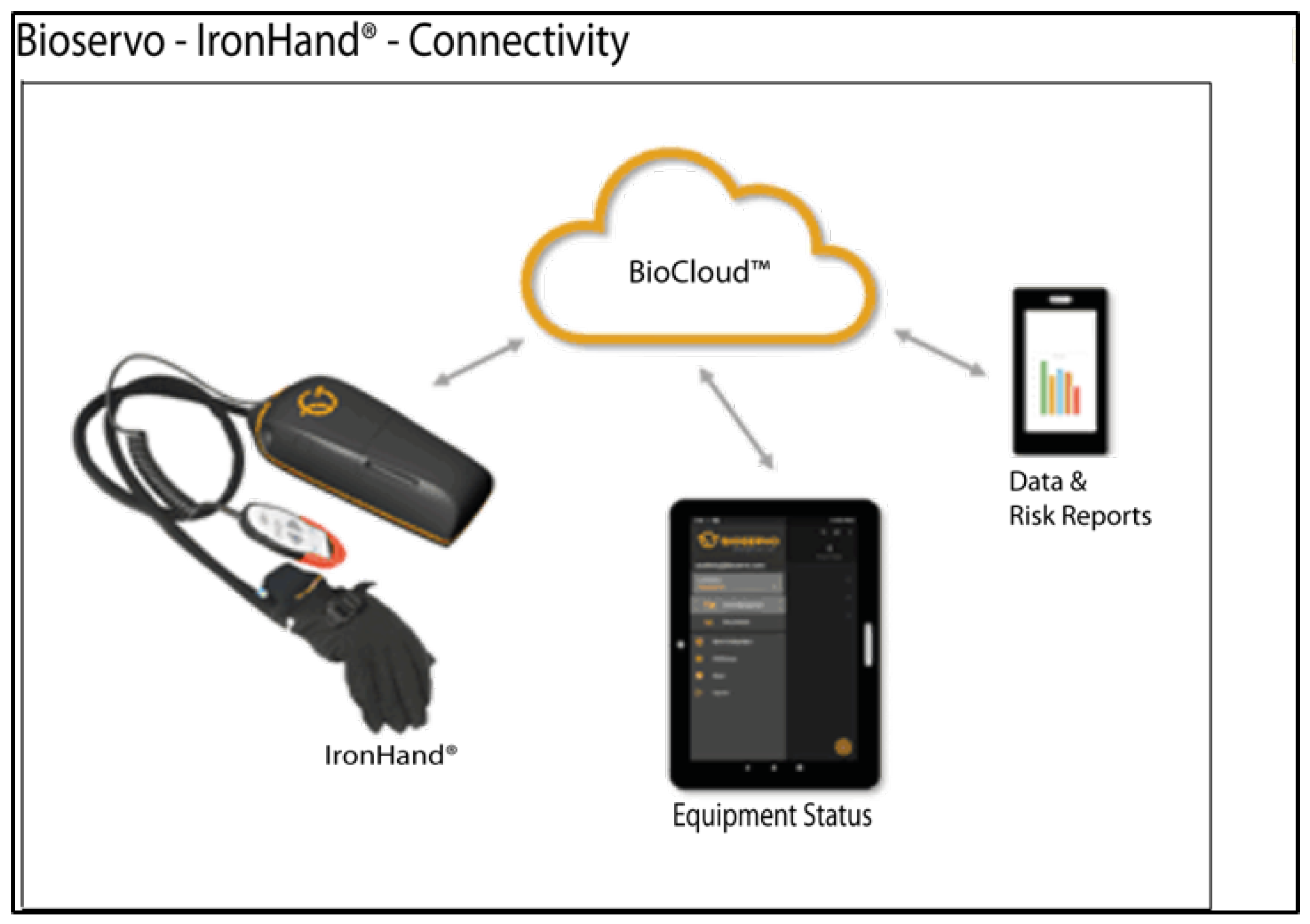
4.2.2. Ironhand®
4.2.3. Paexo Wrist®
4.3. Compliant Devices
SaeboFlex
5. Design Proposal for a Portable Wrist Exoskeleton
6. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSD | musculoskeletal disorder |
| WRMSD | work-related musculoskeletal disorder |
| WHO | World Health Organization |
| INAIL | Italian Workers Compensatory Authority |
| DARPA | Defense Advanced Research Projects Agency |
| OE | occupational exoskeleton |
| CTS | carpal tunnel syndrome |
| DoF | degree of freedom |
| RoM | range of motion |
| CoR | center of rotation |
| ADL | activities of daily living |
| DTM | dart-throwing motion |
| TRL | technology readiness level |
| ICR | instantaneous center of rotation |
| OA | hand osteoarthritis |
| HAL | hand activity level |
| IMU | inertial magnetic unit |
| FSR | force resistive sensor |
| sEMG | surface electromyography signal |
| CPM | continuous passive motion |
| AAN | assistance-as-needed |
| ML | machine learning |
| PRISMA | preferred reporting items for systematic reviews and meta-analysis |
| WearRA | Wearable Robotics Association |
| SEM | soft extra muscle |
| SCI | spinal cord injury |
| JAS | Joint Active Systems, Inc. |
| PWE | portable wrist exoskeleton |
| NN | neural network |
| SVM | support vector machine |
| FMG | force myography signal |
| CIMT | constraint-induced movement therapy |
| MVC | maximum voluntary contraction |
| SOA | soft origami actuator |
| PMA | pneumatic muscle actuator |
| SMA | shape memory alloy |
| SWA | soft wrist assist |
| RMSE | root mean squared error |
| SPS | static progressive stretch |
| TBI | traumatic brain injury |
| OT | occupational therapy |
Appendix A
| Image | Reference | Device Name | Application Field | Actuation Type | Power Source | Power Transmission | Force/Torque Output [N][Nm] | Active DoF | Assisted Motion | RoM | Sensing | Control | TRL | Weight | Price ($) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | [16] | PWE | Rehab/Assistance | Active | DC motor | Links, gears | 2.3 Nm | 2 | Flex/Extension | ±60° | Potentiometer | CPM | ** 4 | 0.36 kg | - |
| Occupational | 2.5 Nm | Rad/Ulnar dev. | ±25° | sEMG | M.L. | ||||||||||
 | [13,17] | eWrist | Rehab/Assistance | Active | DC motor | Links, gears | up to 3.7 Nm | 1 | Flex/Extension | range 154° | sEMG, load cell, | AAN | ** 4 | 0.56 kg | - |
| encoder | |||||||||||||||
 | [18] | * - | Rehabilitation | Passive | DC motor | Links, gears | up to 1.12 Nm | 1 | Flex/Extension | range 120° | Triggered | CPM | ** 4 | 0.33 kg | * - |
| Active | Force (FSR) | M.L. | |||||||||||||
 | [11] | Wrist exosuit | Occupational | Active | Brushless motor | Bowden cables | 3 Nm | 1 | Flex/Extension | 70°/80° | Load cell, IMU | AAN | ** 4 | 0.3 kg (hand) | - |
 | [19] | Exo-Wrist | Rehabilitation | Active | DC motor | Bowden cables | ≥0.5 Nm | 1 | Extension | ≥50° | sEMG | AAN | ** 4 | 1 kg | - |
 | [20] | * - | Rehabilitation | Active | Pneumatic | Artificial muscles | 120 N | 3 | Flex/Extension | 91° | Pressure | Triggered | ** 4 | 2.26 kg | - |
| Rad/Ulnar dev. | 32° | ||||||||||||||
| Pron/supination | 78° | ||||||||||||||
 | [21] | ASSIST | Rehabilitation | Active | Pneumatic | Artificial muscles | up to 1 Nm | 1 | Flex/Extension | 80° | Flex sensor | AAN | ** 4 | 0.39 kg | - |
| Pressure | |||||||||||||||
 | [22] | SOA-wrist | Rehab/Assistance | Active | Pneumatic | Flexible joint-less | up to 0.76 Nm | 2 | Flex/Extension | 31°/30° | Pressure, | AAN | ** 4 | 1.76 kg | - |
| structures | Rad/Ulnar dev. | 33°/22° | IMU | ||||||||||||
 | [23] | * - | Rehab/Assistance | Active | Brushless motors | Bowden cables | ** ≥5 Nm | 2 | Flex/Extension | range 115° | IMU | CPM | ** 4 | 0.65 kg | - |
| Rad/Ulnar dev. | range 70° | ||||||||||||||
 | [37] | Exo-Wrist | Rehabilitation | Active | Pneumatic | Artificial muscles | 630 N | 2 | Flex/Extension | ±30° | IMU | CPM | ** 4 | 0.43 kg | - |
| Rad/Ulnar dev. | ±30° | ||||||||||||||
 | [34] | * - | Occupational | Active | Pneumatic | Flexible joint-less | * - | 1 | Flex/Extension | ±65° | IMU | AAN | ** 3 | * - | - |
| structure | Pressure | ||||||||||||||
 | [24,25] | SWA | Rehab/Assistance | Active | Thermal SMA | Compliant | up to 1.32 Nm | 2 | Flex/Extension | 38°/50° | Encoder | AAN | ** 4 | 1.9 kg | - |
| ≥0.6 Nm | Rad/Ulnar dev. | 34°/35° | Load cell | ||||||||||||
 | [26] | * - | Rehabilitation | Active | Thermal SMA | Pulley-tendons | * - | 3 | Flex/Extension | ** up to 50°/48° | Potentiometer | CPM | ** 3 | 0.95 kg | * - |
| Rad/Ulnar dev. | ** up to 16°/29° | Load cell | |||||||||||||
| Pron/Supination | ** up to 52° | ||||||||||||||
 | [38] | ASR | Rehab/Assistance | Active | Thermal SMA | Tendons | 40 N | 1 | Gripping | - | Load cell | Triggered | ** 3 | 300 g | - |
 | [27] | SMA-wrist exo | Rehabilitation | Active | Thermal SMA | Linkages | ≥0.5 Nm | 2 | Flex/Extension | range 50° | Potentiometer | CPM | ** 4 | 1 kg | 1060 |
| Rad/Ulnar dev. | range 40° | ||||||||||||||
 | [28] | SCRIPT | Rehabilitation | Passive | Springs | Links, tendons | 0.5–2 Nm | 1 | Flex/Extension | 45°/30° | Potentiometer | - | ** 4 | 0.65 kg | - |
 | [14,29] | * - | Rehabilitation | Active | DC motor | Tendons | 0.4 Nm | 2 | Flex/Extension | All | IMU | CPM | 4 | 0.3 kg | 160 |
| Rad/Ulnar dev. | |||||||||||||||
 | [30] | * - | Rehabilitation | Active | Linear DC motor | Leaf springs | 0.26 to 2.47 Nm | 2 | Flex/Extension | 57°/68 | Potentiometer | CPM | ** 4 | 0.51 kg | - |
| 0.65 Nm | Rad/Ulnar dev. | 40°/14° | |||||||||||||
 | [31] | JAS wrist | Rehabilitation | Passive | ** Springs | Links | - | 1 | Flex/Extension | up to 95° | - | - | 9 | * - | * - |
 | [32] | MyoPro orthosis | Rehab/Assistance | Active | DC motor | Linkages | 10 to 20 N | 2 | Flex/Extension | improve up to 35° | sEMG | AAN | 9 | 1.8 kg | 10,000 |
| Rad/Ulnar dev. | |||||||||||||||
 | [35] | Carbonhand | Assistance | Active | DC motor | Bowden cables | ** 60 N | 1 | Gripping | All | Force (FSR) | AAN | 9 | 0.7 kg | 7000 |
 | [35] | Ironhand | Occupational | Active | DC motor | Bowden cables | 80 N | 1 | Gripping | All | Force (FSR) | AAN | 9 | 2.5 kg | 6500 |
 | [36] | Paexo Wrist | Occupational | Passive | Leaf spring | Elastic | - | * 1 | Holding | All | - | - | 9 | * - | 160 |
 | [33] | SaeboFlex | Rehabilitation | Passive | Springs | Links, elastics | - | 1 | Flex/Extension | improve up to 6° | - | - | 9 | 1.6 kg | 600 |
| Experimental Tests Conducted on the Device in a Real Environment or Laboratory | ||||||
|---|---|---|---|---|---|---|
| Reference | Subjects | Age | Gender | Clinical Condition | Setting | Testing Period |
| Research prototypes | ||||||
| [16] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [13,17] | 15 | 26 ± 3.4 | 7 M, 8 F | Healthy | Lab. | n.d. |
| 2 | 60 ± 8 | M | Stroke | Lab. | n.d. | |
| [18] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [11] | 4 | 28 ± 4.0 | M | Healthy | Lab. | n.d. |
| [19] | 3 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [20] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| [21] | 5 | n.d. | M | Healthy | Lab. | n.d. |
| [22] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [23] | 1 | n.d. | n.d. | Healthy | Lab. | n.d. |
| 3 | 47 ± 10.0 | M | Stroke | Lab. | ||
| [37] | 1 | n.d. | M | Healthy | Lab. | n.d. |
| [34] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [24,25] | 5 | n.d. | 3 M, 2 F | Healthy | Lab. | n.d. |
| [26] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| [38] | 1 | n.d. | M | Healthy | Lab. | n.d. |
| [27] | 3 | n.d. | M | Healthy | Lab. | n.d. |
| [28] | 33 | n.d. | n.d. | Stroke | Lab. | n.d. |
| [14,29] | 1 | n.d. | M | Impaired Hand | Lab. | 60 trials |
| [30] | - | n.d. | n.d. | n.d. | Lab. | n.d. |
| Commercial devices | ||||||
| [31,59,60,61] | 133 | 53 ± 17.6 | 55 M, 78 F | Radius fractures | Clinic | 3–20 weeks |
| [32,62,63,64,65,66,67,68] | 1 | 62 | M | Stroke | Clinic/Home | 3 years |
| 2 | 53 ± 22 | M | SCI | Clinic | 6 weeks | |
| 18 | 55.5 ± 21.5 | 11 M, 7 F | Stroke | Clinic | A day | |
| 13 | 51 ± 19.9 | 5 M, 8 F | 7 Stroke, 6 TBI | Clinic/Home | 18 weeks | |
| 18 | 52.5 ± 14.7 | 13 M, 5 F | Stroke | Home | 3 months | |
| [35,70,71] | 15 | 41.5 ± 23.5 | n.d. | SCI | Home | 12 weeks |
| 63 | 54 ± 36 | n.d. | Impaired Hand | Home | 6 weeks | |
| [35,72] | 8 | n.d. | 4 M, 4 F | Healthy | Industry | 2 weeks |
| [36] | - | n.d. | n.d. | n.d. | Industry | n.d. |
| [33,56,74,75,76,77,78] | 10 | n.d. | 7 M, 3 F | Stroke | Clinic | 4 weeks |
| 8 | 70 ± 15 | 4 M, 4 F | Stroke | Clinic | 12 weeks | |
| 11 | 60.2 ± 7.89 | 7 M, 5 F | Stroke | Clinic | n.d. | |
| 1 | 43 | F | Stroke | Clinic | 16 weeks | |
References
- Gonçalves, H.; Cardoso, A.; Mattos, D.; Deola Borges, G.; Anacleto, P.; Colim, A.; Carneiro, P.; Arezes, P.M. Assessment of Work-Related Musculoskeletal Disorders by Observational Methods in Repetitive Tasks—A Systematic Review. In Occupational and Environmental Safety and Health III; Springer: Cham, Switzerland, 2022; pp. 455–463. [Google Scholar] [CrossRef]
- Ramazzini, B. De morbis artificum diatriba [diseases of workers]. Am. J. Public Health 2001, 91, 1380–1382. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.J.; Fine, L.J.; Goldstein, S.A.; Lifshitz, Y.R.; Silverstein, B.A. Ergonomics considerations in hand and wrist tendinitis. J. Hand Surg. 1987, 12, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, J.K.; Rest, K.M.; Frings-Dresen, M.H. Criteria document for evaluating the work-relatedness of upper-extremity musculoskeletal disorders. Scand. J. Work Environ. Health 2001, 27, 1–102. Available online: http://www.jstor.org/stable/40967163 (accessed on 31 March 2022). [CrossRef] [PubMed]
- Franco, G.; Franco, F. Bernardino Ramazzini: The father of occupational medicine. Am. J. Public Health 2001, 91, 1382. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Musculoskeletal Conditions, Key Facts. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 14 July 2022).
- INAIL-Italian Workers’ Compensatory Authority. Occupational Exoskeletons: Wearable Robotic Devices to Prevent Work Related Musculoskeletal Disorders in the Workplace of the Future. 2020. Available online: http://www.osha.europa.eu (accessed on 1 November 2021).
- European Agency for Safety and Health at Work (EU-OSHA). Work-Related Musculoskeletal Disorders—Facts and Figures; Standard, European Agency for Safety and Health at Work (EU-OSHA); Publications Office of the European Union: Luxembourg, 2020. [CrossRef]
- Pons, J.L. Wearable Robots: Biomechatronic Exoskeletons; John Wiley & Sons Ltd.: Chichester, UK, 2008; pp. 4, 11, 219, 332. ISBN 9780470512944. [Google Scholar]
- du Plessis, T.; Djouani, K.; Oosthuizen, C. Review of Active Hand Exoskeletons for Rehabilitation and Assistance. Robotics 2021, 10, 40. [Google Scholar] [CrossRef]
- Chiaradia, D.; Tiseni, L.; Xiloyannis, M.; Solazzi, M.; Masia, L.; Frisoli, A. An assistive soft wrist exosuit for flexion movements with an ergonomic reinforced glove. Front. Robot. AI 2021, 7, 595862. [Google Scholar] [CrossRef] [PubMed]
- Crea, S.; Beckerle, P.; De Looze, M.; De Pauw, K.; Grazi, L.; Kermavnar, T.; Masood, J.; O’Sullivan, L.W.; Pacifico, I.; Rodriguez-Guerrero, C.; et al. Occupational exoskeletons: A roadmap toward large-scale adoption. Methodology and challenges of bringing exoskeletons to workplaces. Wearable Technol. 2021, 2, e11. [Google Scholar] [CrossRef]
- Lambelet, C.; Temiraliuly, D.; Siegenthaler, M.; Wirth, M.; Woolley, D.G.; Lambercy, O.; Gassert, R.; Wenderoth, N. Characterization and wearability evaluation of a fully portable wrist exoskeleton for unsupervised training after stroke. J. Neuro Eng. Rehabil. 2020, 17, 132. [Google Scholar] [CrossRef]
- Dragusanu, M.; Iqbal, M.Z.; Baldi, T.L.; Prattichizzo, D.; Malvezzi, M. Design, Development, and Control of a Hand/Wrist Exoskeleton for Rehabilitation and Training. IEEE Trans. Robot. 2022, 38, 1472–1488. [Google Scholar] [CrossRef]
- Pitzalis, R.F.; Park, D.; Caldwell, D.G.; Berselli, G.; Ortiz, J. State of the Art in Wearable Wrist Exoskeletons Part I: Background Needs and Design Requirements. Machines 2023, 11, 458. [Google Scholar] [CrossRef]
- Xiao, Z.G.; Menon, C. Towards the development of a portable wrist exoskeleton. In Proceedings of the 2011 IEEE International Conference on Robotics and Biomimetics, Karon Beach, Thailand, 7–11 December 2011; pp. 1884–1889. [Google Scholar] [CrossRef]
- Lambelet, C.; Lyu, M.; Woolley, D.; Gassert, R.; Wenderoth, N. The eWrist—A wearable wrist exoskeleton with sEMG-based force control for stroke rehabilitation. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 726–733. [Google Scholar] [CrossRef]
- Sangha, S.; Elnady, A.M.; Menon, C. A compact robotic orthosis for wrist assistance. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 1080–1085. [Google Scholar] [CrossRef]
- Choi, H.; Kang, B.B.; Jung, B.K.; Cho, K.J. Exo-wrist: A soft tendon-driven wrist-wearable robot with active anchor for dart-throwing motion in hemiplegic patients. IEEE Robot. Autom. Lett. 2019, 4, 4499–4506. [Google Scholar] [CrossRef]
- Bartlett, N.W.; Lyau, V.; Raiford, W.A.; Holland, D.; Gafford, J.B.; Ellis, T.D.; Walsh, C.J. A soft robotic orthosis for wrist rehabilitation. J. Med. Devices 2015, 9, 030918. [Google Scholar] [CrossRef]
- Sasaki, D.; Noritsugu, T.; Takaiwa, M. Development of active support splint driven by pneumatic soft actuator (ASSIST). In Proceedings of the 2005 IEEE international Conference on Robotics and Automation, Barcelona, Spain, 18–22 April 2005; pp. 520–525. [Google Scholar] [CrossRef]
- Liu, S.; Fang, Z.; Liu, J.; Tang, K.; Luo, J.; Yi, J.; Hu, X.; Wang, Z. A compact soft robotic wrist brace with origami actuators. Front. Robot. AI 2021, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, T.; Yang, Y.; Yu, P.; Xue, X.; Zhao, X.; Song, G.; Elhajj, I.H.; Wang, W.; Xi, N.; et al. Bioinspired musculoskeletal model-based soft wrist exoskeleton for stroke rehabilitation. J. Bionic Eng. 2020, 17, 1163–1174. [Google Scholar] [CrossRef]
- Jeong, J.; Yasir, I.B.; Han, J.; Park, C.H.; Bok, S.K.; Kyung, K.U. Design of shape memory alloy-based soft wearable robot for assisting wrist motion. Appl. Sci. 2019, 9, 4025. [Google Scholar] [CrossRef]
- Jeong, J.; Hyeon, K.; Han, J.; Park, C.H.; Ahn, S.Y.; Bok, S.K.; Kyung, K.U. Wrist assisting soft wearable robot with stretchable coolant vessel integrated SMA muscle. IEEE/ASME Trans. Mechatronics 2021, 27, 1046–1058. [Google Scholar] [CrossRef]
- Hope, J.; McDaid, A. Development of wearable wrist and forearm exoskeleton with shape memory alloy actuators. J. Intell. Robot. Syst. 2017, 86, 397. [Google Scholar] [CrossRef]
- Serrano, D.; Copaci, D.S.; Moreno, L.; Blanco, D. SMA based wrist exoskeleton for rehabilitation therapy. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 2318–2323. [Google Scholar] [CrossRef]
- Ates, S.; Haarman, C.J.; Stienen, A.H. SCRIPT passive orthosis: Design of interactive hand and wrist exoskeleton for rehabilitation at home after stroke. Auton. Robot. 2017, 41, 711–723. [Google Scholar] [CrossRef]
- Dragusanu, M.; Baldi, T.L.; Iqbal, Z.; Prattichizzo, D.; Malvezzi, M. Design, development, and control of a tendon-actuated exoskeleton for wrist rehabilitation and training. In Proceedings of the 2020 IEEE International Conference on Robotics and Automation (ICRA), Paris, France, 31 May–31 August 2020; pp. 1749–1754. [Google Scholar] [CrossRef]
- Higuma, T.; Kiguchi, K.; Arata, J. Low-profile two-degree-of-freedom wrist exoskeleton device using multiple spring blades. IEEE Robot. Autom. Lett. 2017, 3, 305–311. [Google Scholar] [CrossRef]
- JAS-Joint Active Systems. JAS Wrist. 2017. Available online: https://www.jointactivesystems.com/products/areas/wrist (accessed on 5 August 2022).
- Myomo, Inc. MyoPro Orthosis. 2006. Available online: https://myomo.com/what-is-a-myopro-orthosis/ (accessed on 8 August 2022).
- Saebo Inc. SaeboFlex. Available online: https://www.saebo.com/shop/saeboflex/ (accessed on 30 November 2022).
- Zhu, M.; Adams, W.; Polygerinos, P. Carpal tunnel syndrome soft relief device for typing applications. In Proceedings of the 2017 Design of Medical Devices Conference, Minneapolis, MN, USA, 10–13 April 2017; American Society of Mechanical Engineers: New York, NY, USA, 2017; Volume 40672, p. V001T03A003. [Google Scholar] [CrossRef]
- Bioservo Technologies. Bioservo Strength for Life—Professional and Healthcare. Available online: https://www.bioservo.com/ (accessed on 7 September 2022).
- Ottobock. Paexo Wrist Exoskeleton. Available online: https://paexo.com/paexo-wrist/?lang=it (accessed on 5 August 2022).
- Andrikopoulos, G.; Nikolakopoulos, G.; Manesis, S. Motion control of a novel robotic wrist exoskeleton via pneumatic muscle actuators. In Proceedings of the 2015 IEEE 20th Conference on Emerging Technologies & Factory Automation (ETFA), Luxembourg, 8–11 September 2015; pp. 1–8. [Google Scholar] [CrossRef]
- Hadi, A.; Alipour, K.; Kazeminasab, S.; Elahinia, M. ASR glove: A wearable glove for hand assistance and rehabilitation using shape memory alloys. J. Intell. Mater. Syst. Struct. 2018, 29, 1575–1585. [Google Scholar] [CrossRef]
- Ferguson, R.; Riley, N.D.; Wijendra, A.; Thurley, N.; Carr, A.J.; Dean, B.J.F. Wrist pain: A systematic review of prevalence and risk factors–what is the role of occupation and activity? BMC Musculoskelet. Disord. 2019, 20, 542. [Google Scholar] [CrossRef] [PubMed]
- Noronha, B.; Accoto, D. Exoskeletal devices for hand assistance and rehabilitation: A comprehensive analysis of state-of-the-art technologies. IEEE Trans. Med. Robot. Bionics 2021, 3, 525–538. [Google Scholar] [CrossRef]
- Gopura, R.; Kiguchi, K. Mechanical designs of active upper-limb exoskeleton robots: State-of-the-art and design difficulties. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, Kyoto, Japan, 23–26 June 2009; pp. 178–187. [Google Scholar] [CrossRef]
- Gopura, R.; Bandara, D.; Kiguchi, K.; Mann, G.K. Developments in hardware systems of active upper-limb exoskeleton robots: A review. Robot. Auton. Syst. 2016, 75, 203–220. [Google Scholar] [CrossRef]
- Nikhil, G.; Yedukondalu, G.; Rao, S. Robotic exoskeletons: A review on development. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 529–542. [Google Scholar]
- Tiboni, M.; Borboni, A.; Vérité, F.; Bregoli, C.; Amici, C. Sensors and actuation technologies in exoskeletons: A review. Sensors 2022, 22, 884. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Jamwal, P.K.; Van Vliet, P.; Ghayesh, M.H. State-of-the-art robotic devices for wrist rehabilitation: Design and control aspects. IEEE Trans. Hum.-Mach. Syst. 2020, 50, 361–372. [Google Scholar] [CrossRef]
- Cornejo, J.; Huamanchahua, D.; Huamán-Vizconde, S.; Terrazas-Rodas, D.; Sierra-Huertas, J.; Janampa-Espinoza, A.; Gonzáles, J.; Cardona, M. Mechatronic exoskeleton systems for supporting the biomechanics of shoulder-elbow-wrist: An innovative review. In Proceedings of the 2021 IEEE International IOT, Electronics and Mechatronics Conference (IEMTRONICS), Toronto, ON, Canada, 21–24 April 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Khokhar, Z.O.; Xiao, Z.G.; Menon, C. Surface EMG pattern recognition for real-time control of a wrist exoskeleton. Biomed. Eng. Online 2010, 9, 41. [Google Scholar] [CrossRef]
- Vardakastani, V.; Bell, H.; Mee, S.; Brigstocke, G.; Kedgley, A.E. Clinical measurement of the dart throwing motion of the wrist: Variability, accuracy and correction. J. Hand Surg. Eur. Vol. 2018, 43, 723–731. [Google Scholar] [CrossRef]
- Braidotti, F.; Atzei, A.; Fairplay, T. Dart-Splint: An innovative orthosis that can be integrated into a scapho-lunate and palmar midcarpal instability re-education protocol. J. Hand Ther. 2015, 28, 329–335. [Google Scholar] [CrossRef]
- Kaufman-Cohen, Y.; Friedman, J.; Levanon, Y.; Jacobi, G.; Doron, N.; Portnoy, S. Wrist plane of motion and range during daily activities. Am. J. Occup. Ther. 2018, 72, 7206205080p1–7206205080p10. [Google Scholar] [CrossRef]
- Brigstocke, G.; Hearnden, A.; Holt, C.; Whatling, G. In-vivo confirmation of the use of the dart thrower’s motion during activities of daily living. J. Hand Surg. Eur. Vol. 2014, 39, 373–378. [Google Scholar] [CrossRef] [PubMed]
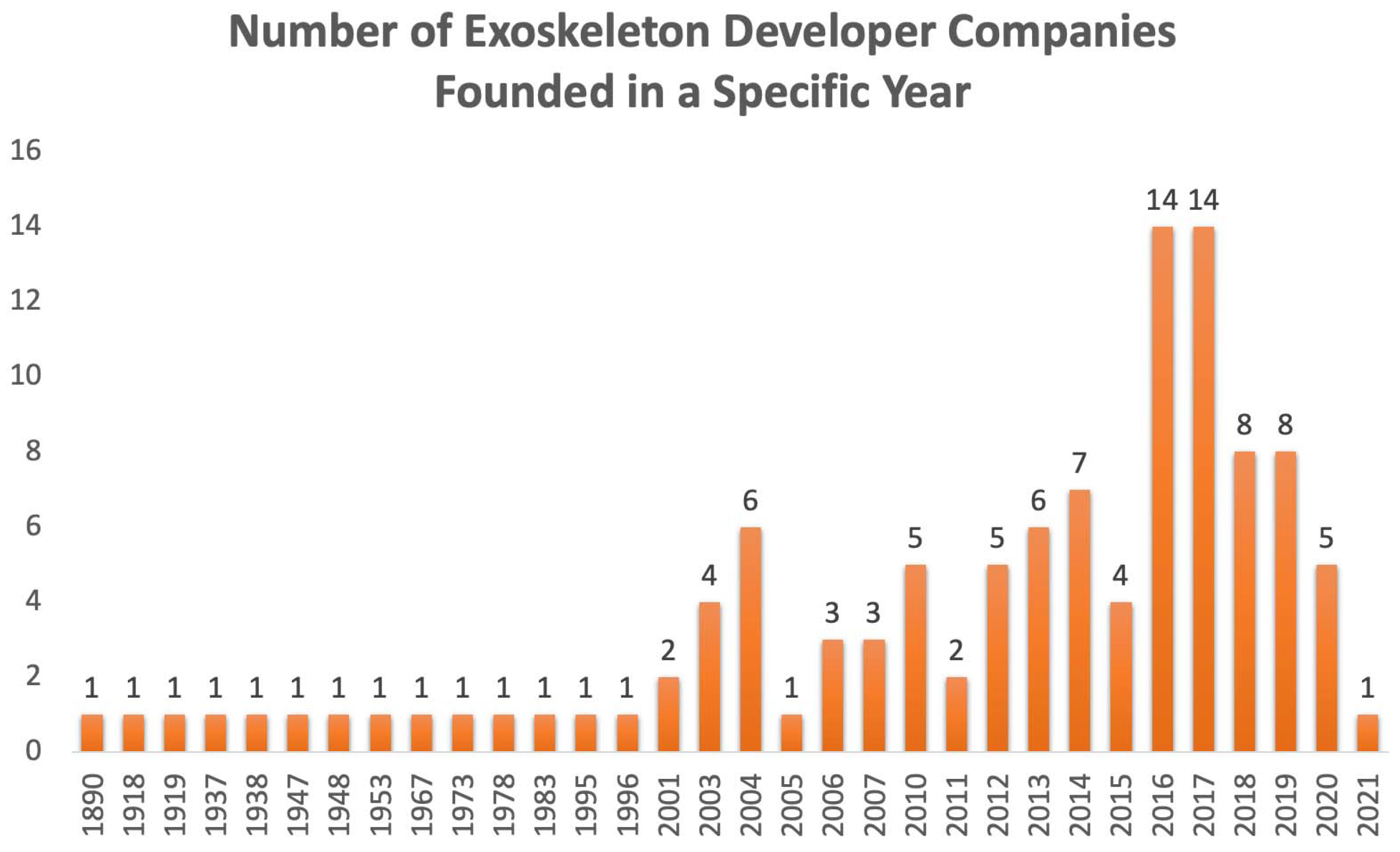
- Borislav, M. The Number of Companies Making Industrial Exoskeletons Has Been Quietly Increasing for the Past Five Years. 2020. Available online: https://www.forbes.com/sites/borislavmarinov/2020/09/24/the-number-of-companies-making-industrial-exoskeletons-has-been-quietly-increasing-for-the-past-five-years/?sh=231b1c067bf4 (accessed on 5 August 2022).
- Borislav, M. Updated Directory of Exoskeleton Companies and Industry Statistics. 2021. Available online: https://exoskeletonreport.com/2021/12/updated-directory-of-exoskeleton-companies-and-industry-statistics/ (accessed on 5 August 2022).
- Radder, B.; Prange-Lasonder, G.B.; Kottink, A.I.; Holmberg, J.; Sletta, K.; van Dijk, M.; Meyer, T.; Melendez-Calderon, A.; Buurke, J.H.; Rietman, J.S. Home rehabilitation supported by a wearable soft-robotic device for improving hand function in older adults: A pilot randomized controlled trial. PLoS ONE 2019, 14, e0220544. [Google Scholar] [CrossRef] [PubMed]
- Hyttinge, M.; Einarsson, F. Bioservo Technologies. Technical Report, Redeye, Equity Research and Investment Banking Company, 7 September 2021, Redeye, Mäster Samuelsgatan 42, 10tr, Box 7141, 103 87 Stockholm. Available online: https://www.redeye.se/company/bioservo-technologies (accessed on 1 November 2022).
- Moskiewicz, D.; Mraz, M.; Chamela-Bilińska, D. Botulinum Toxin and Dynamic Splint Restore Grasping Function after Stroke: A Case Report. Int. J. Environ. Res. Public Health 2023, 20, 4873. [Google Scholar] [CrossRef] [PubMed]
- Lucado, A.M.; Li, Z.; Russell, G.B.; Papadonikolakis, A.; Ruch, D.S. Changes in impairment and function after static progressive splinting for stiffness after distal radius fracture. J. Hand Ther. 2008, 21, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lucado, A.M.; Li, Z. Static progressive splinting to improve wrist stiffness after distal radius fracture: A prospective, case series study. Physiother. Theory Pract. 2009, 25, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Berner, S.H.; Willis, F.B. Dynamic splinting in wrist extension following distal radius fractures. J. Orthop. Surg. Res. 2010, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Static progressive orthoses for the upper extremity: A comprehensive literature review. Hand 2012, 7, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, N.; Yao, B.; Anis, H.K.; Khlopas, A.; Sultan, A.A.; Newman, J.M.; Mont, M.A. Patient satisfaction and outcomes of static progressive stretch bracing: A 10-year prospective analysis. Ann. Transl. Med. 2019, 7, 67. [Google Scholar] [CrossRef]
- Dunaway, S.; Dezsi, D.B.; Perkins, J.; Tran, D.; Naft, J. Case report on the use of a custom myoelectric elbow–wrist–hand orthosis for the remediation of upper extremity paresis and loss of function in chronic stroke. Mil. Med. 2017, 182, e1963–e1968. [Google Scholar] [CrossRef]
- Androwis, G.J.; Engler, A.; Rana, S.; Kirshblum, S.; Yue, G.H. The Rehabilitation Effects of Myoelectric Powered Wearable Orthotics on Improving Upper Extremity Function in Persons with SCI. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 4944–4948. [Google Scholar]
- Androwis, G.J.; Engler, A.; Rana, S.; Kirshblum, S.; Yue, G. Upper Extremity Functional Improvements in Persons with SCI Resulted from Daily Utilization of Myoelectric Powered Wearable Orthotics. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Virtual, 1–5 November 2021; pp. 4949–4952. [Google Scholar]
- Hoppe-Ludwig, S.; Armitage, J.; Turner, K.L.; O’Brien, M.K.; Mummidisetty, C.K.; Koch, L.M.; Kocherginsky, M.; Jayaraman, A. Usability, functionality, and efficacy of a custom myoelectric elbow-wrist-hand orthosis to assist elbow function in individuals with stroke. J. Rehabil. Assist. Technol. Eng. 2021, 8, 20556683211035057. [Google Scholar] [CrossRef]
- Pundik, S.; McCabe, J.; Skelly, M.; Salameh, A.; Naft, J.; Chen, Z.; Tatsuoka, C.; Fatone, S. Myoelectric Arm Orthosis in Motor Learning-Based Therapy for Chronic Deficits After Stroke and Traumatic Brain Injury. Front. Neurol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.R.; Hofland, N.; Chen, Z.; Tatsuoka, C.; Richards, L.G.; Bruestle, M.; Kovelman, H.; Naft, J. Myoelectric Arm Orthosis Assists Functional Activities: A 3-Month Home Use Outcome Report. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100279. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.P.; Henniger, D.; Perkins, J.; Skelly, M.; Tatsuoka, C.; Pundik, S. Feasibility and clinical experience of implementing a myoelectric upper limb orthosis in the rehabilitation of chronic stroke patients: A clinical case series report. PLoS ONE 2019, 14, e0215311. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Ingvast, J.; Wikander, J.; von Holst, H. The Soft Extra Muscle system for improving the grasping capability in neurological rehabilitation. In Proceedings of the 2012 IEEE-EMBS Conference on Biomedical Engineering and Sciences, Langkawi, Malaysia, 17–19 December 2012; pp. 412–417. [Google Scholar] [CrossRef]
- Osuagwu, B.A.; Timms, S.; Peachment, R.; Dowie, S.; Thrussell, H.; Cross, S.; Heywood, T.; Shirley, R.; Taylor, J. Clinical Trial of the Soft Extra Muscle Glove to Assess Orthotic and Long-Term Functional Gain Following Chronic Incomplete Tetraplegia: Preliminary Functional Results. In Proceedings of the International Conference on NeuroRehabilitation, Pisa, Italy, 16–20 October 2018; Springer: Cham, Switzerland, 2018; pp. 385–389. [Google Scholar] [CrossRef]
- Kottink, A.I.R.; Nikamp, C.D.; Bos, F.P.; van der Sluis, C.K.; van den Broek, M.; Onneweer, B.; Stolwijk-Swüste, J.M.; Brink, S.M.; Voet, N.B.; Buurke, J.B.; et al. Therapeutic Effect of a Soft Robotic Glove for Activities of Daily Living In People with Impaired Hand Strength: Protocol for a Multicenter Clinical Trial (iHand). JMIR Res. Protoc. 2022, 11, e34200. [Google Scholar] [CrossRef] [PubMed]
- Cousins, D.; Porto, R.; Bigelo, A.; Fox, R.; Libs, B.; Holmes, M.; Cort, J. Effects of the Ironhand® Soft Exoskeleton on Forearm Muscle Activity During in Field Automotive Assembly Tasks. Available online: https://ssrn.com/abstract=4559929 (accessed on 2 September 2023).
- Hoffman, H.B.; Blakey, G.L. New design of dynamic orthoses for neurological conditions. NeuroRehabilitation 2011, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-S.; Woo, Y.-K.; Yi, C.-H.; Kwon, O.-Y.; Jung, M.-Y.; Lee, Y.-H.; Hwang, S.; Choi, B.-R. Effect of intensive training with a pring-assisted hand orthosis on movement smoothness in upper extremity following stroke: A pilot clinical trial. Top. Stroke Rehabil. 2012, 19, 320–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stuck, R.A.; Marshall, L.M.; Sivakumar, R. Feasibility of SaeboFlex upper-limb training in acute stroke rehabilitation: A clinical case series. Occup. Ther. Int. 2014, 21, 108–114. [Google Scholar] [CrossRef]
- Andriske, L.; Verikios, D.; Hitch, D. Patient and therapist experiences of the SaeboFlex: A pilot study. Occup. Ther. Int. 2017, 2017, 5462078. [Google Scholar] [CrossRef]
- Barry, J.; Saabye, S.; Baker, D.; Fogarty, J.; Beck, K. Effects of the saeboflex® orthosis and a home exercise program on upper extremity recovery in individuals with chronic. J. Neurol. Phys. Ther. 2006, 30, 207. [Google Scholar] [CrossRef]
- Lannin, N.A.; Cusick, A.; Hills, C.; Kinnear, B.; Vogel, K.; Matthews, K.; Bowring, G. Upper limb motor training using a Saebo™ orthosis is feasible for increasing task-specific practice in hospital after stroke. Aust. Occup. Ther. J. 2016, 63, 364–372. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, J.; Li, Z.; Su, C.Y. Adaptive impedance control of robotic exoskeletons using reinforcement learning. In Proceedings of the 2016 International Conference on Advanced Robotics and Mechatronics (ICARM), Macau, China, 18–20 August 2016; pp. 243–248. [Google Scholar] [CrossRef]
- Ren, J.L.; Chien, Y.H.; Chia, E.Y.; Fu, L.C.; Lai, J.S. Deep learning based motion prediction for exoskeleton robot control in upper limb rehabilitation. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 5076–5082. [Google Scholar] [CrossRef]
- Rose, L.; Bazzocchi, M.C.; Nejat, G. A model-free deep reinforcement learning approach for control of exoskeleton gait patterns. Robotica 2022, 40, 2189–2214. [Google Scholar] [CrossRef]
- Côté-Allard, U.; Fall, C.L.; Drouin, A.; Campeau-Lecours, A.; Gosselin, C.; Glette, K.; Laviolette, F.; Gosselin, B. Deep learning for electromyographic hand gesture signal classification using transfer learning. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Theurel, J.; Desbrosses, K. Occupational exoskeletons: Overview of their benefits and limitations in preventing work-related musculoskeletal disorders. IISE Trans. Occup. Ergon. Hum. Factors 2019, 7, 264–280. [Google Scholar] [CrossRef]
- Elprama, S.A.; Vanderborght, B.; Jacobs, A. An industrial exoskeleton user acceptance framework based on a literature review of empirical studies. Appl. Ergon. 2022, 100, 103615. [Google Scholar] [CrossRef] [PubMed]










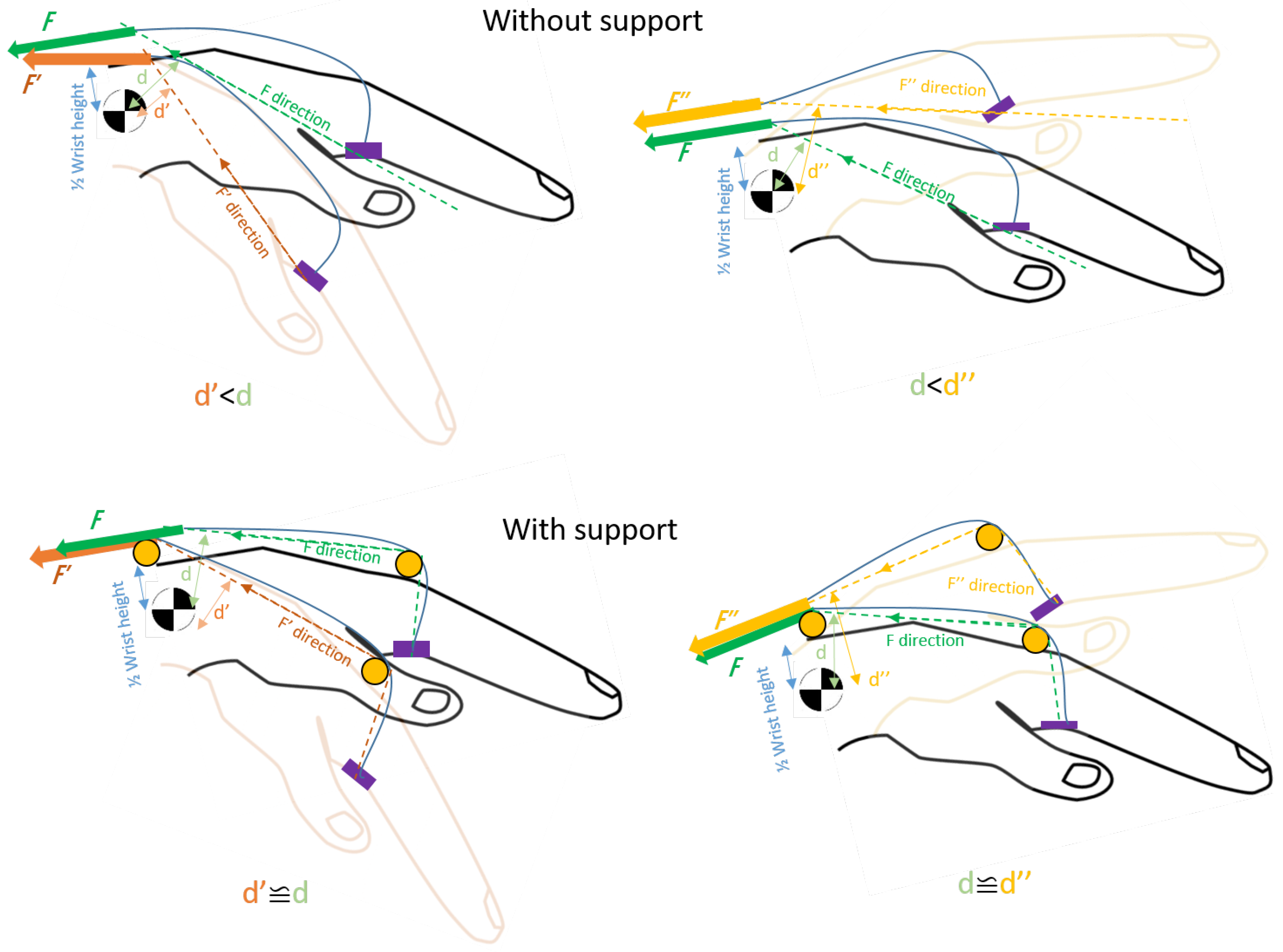
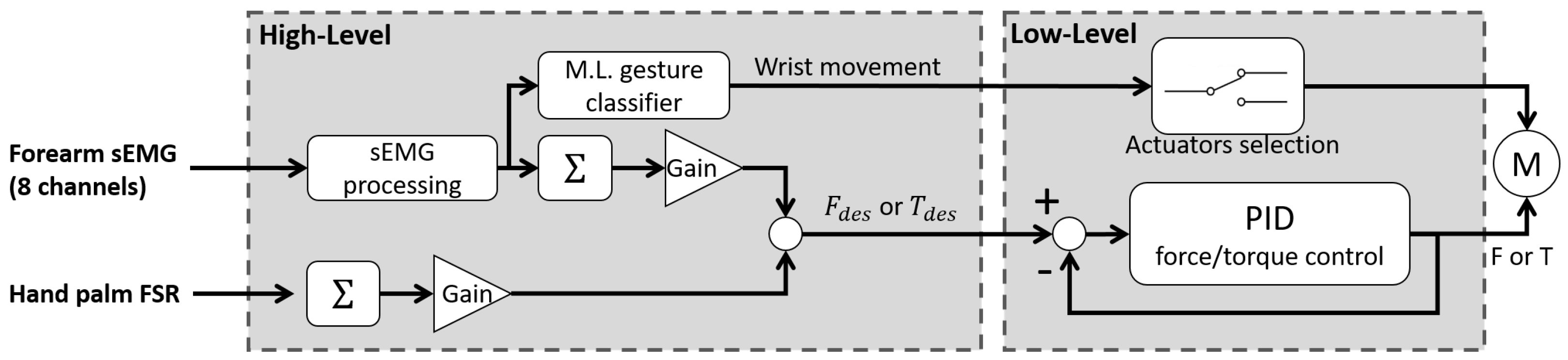
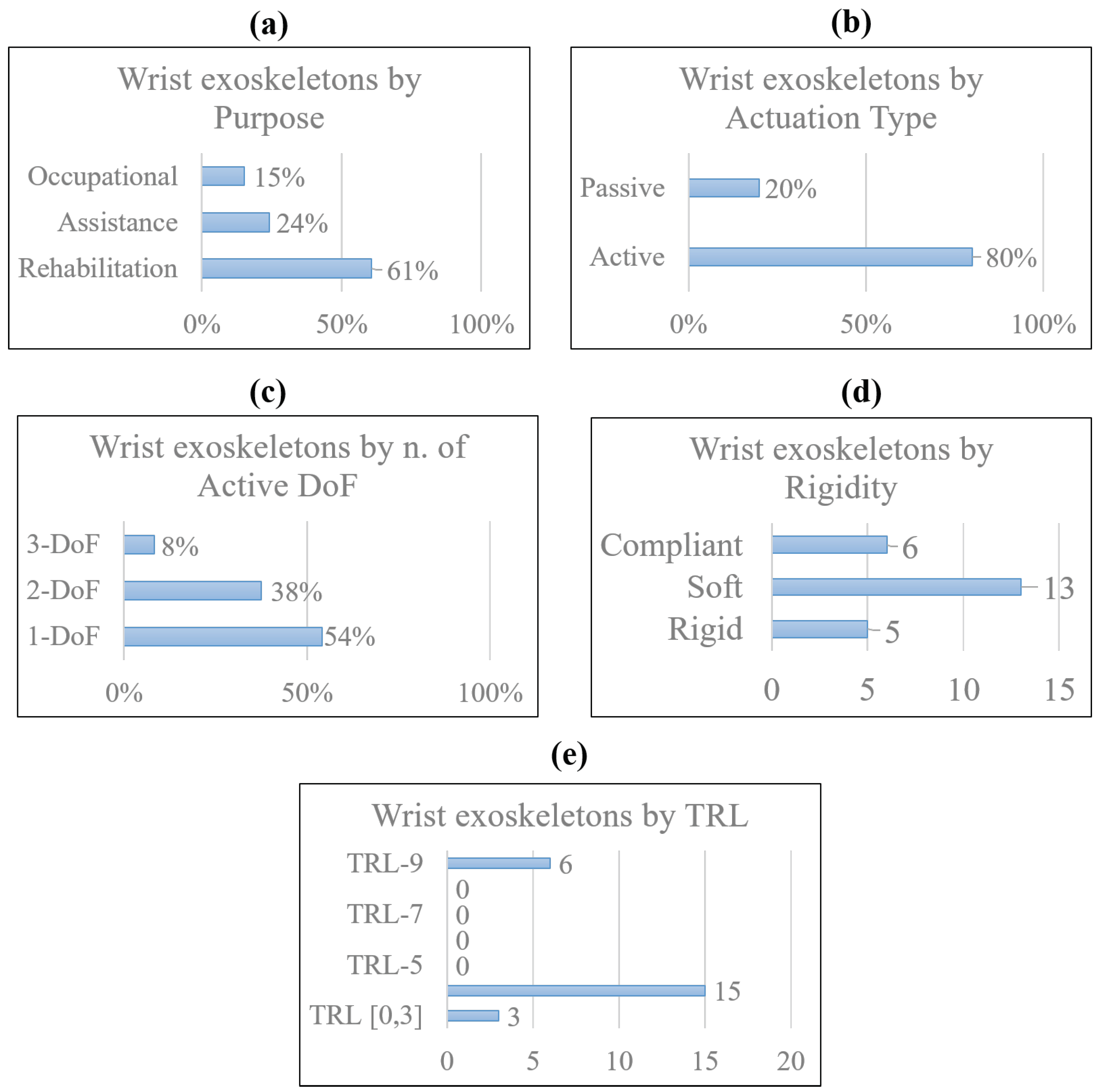

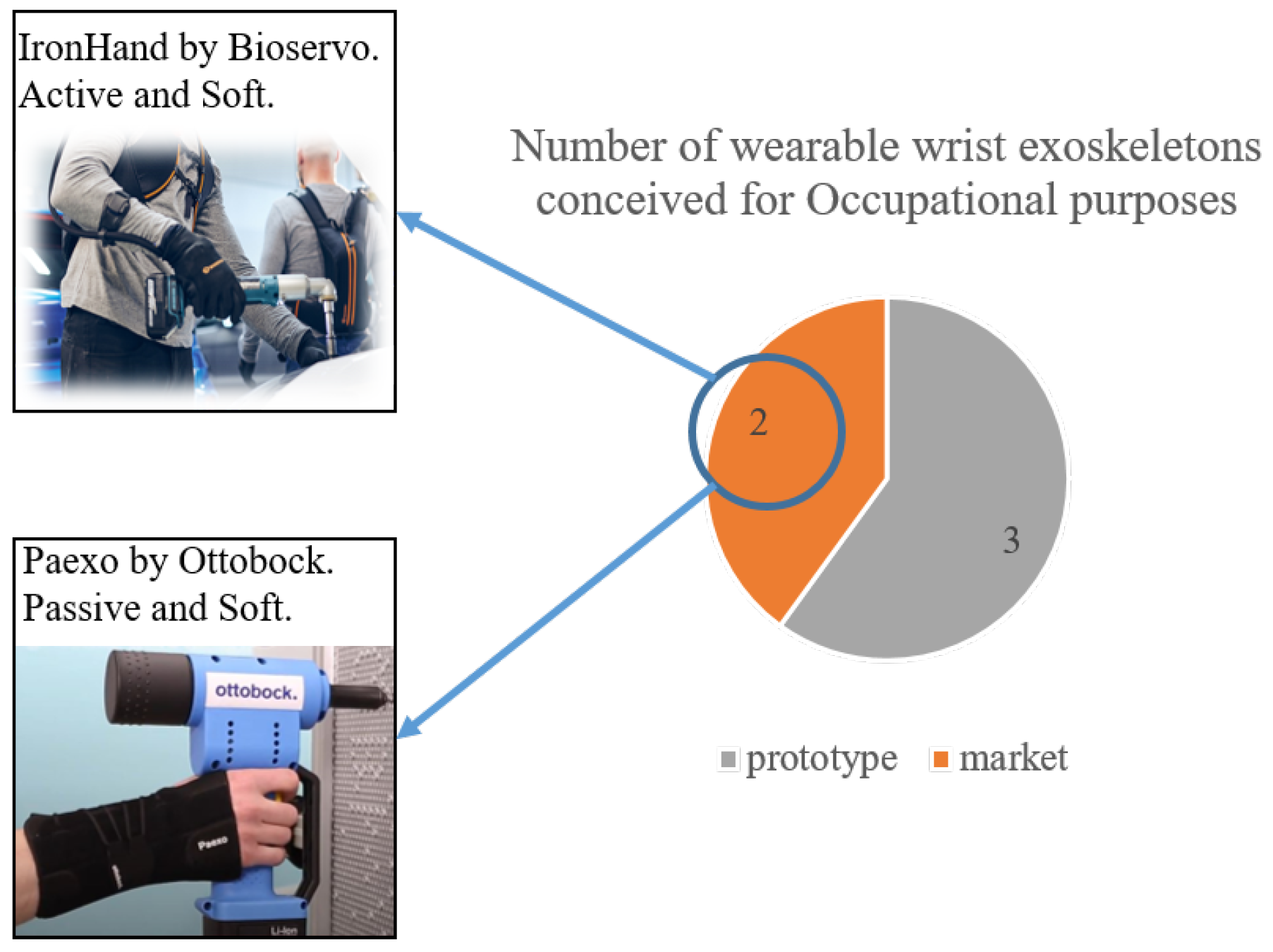
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitzalis, R.F.; Park, D.; Caldwell, D.G.; Berselli, G.; Ortiz, J. State of the Art in Wearable Wrist Exoskeletons Part II: A Review of Commercial and Research Devices. Machines 2024, 12, 21. https://doi.org/10.3390/machines12010021
Pitzalis RF, Park D, Caldwell DG, Berselli G, Ortiz J. State of the Art in Wearable Wrist Exoskeletons Part II: A Review of Commercial and Research Devices. Machines. 2024; 12(1):21. https://doi.org/10.3390/machines12010021
Chicago/Turabian StylePitzalis, Roberto Francesco, Daegeun Park, Darwin G. Caldwell, Giovanni Berselli, and Jesús Ortiz. 2024. "State of the Art in Wearable Wrist Exoskeletons Part II: A Review of Commercial and Research Devices" Machines 12, no. 1: 21. https://doi.org/10.3390/machines12010021
APA StylePitzalis, R. F., Park, D., Caldwell, D. G., Berselli, G., & Ortiz, J. (2024). State of the Art in Wearable Wrist Exoskeletons Part II: A Review of Commercial and Research Devices. Machines, 12(1), 21. https://doi.org/10.3390/machines12010021