Numerical Analysis of The Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production
Abstract
:1. Introduction
2. Numerical Model
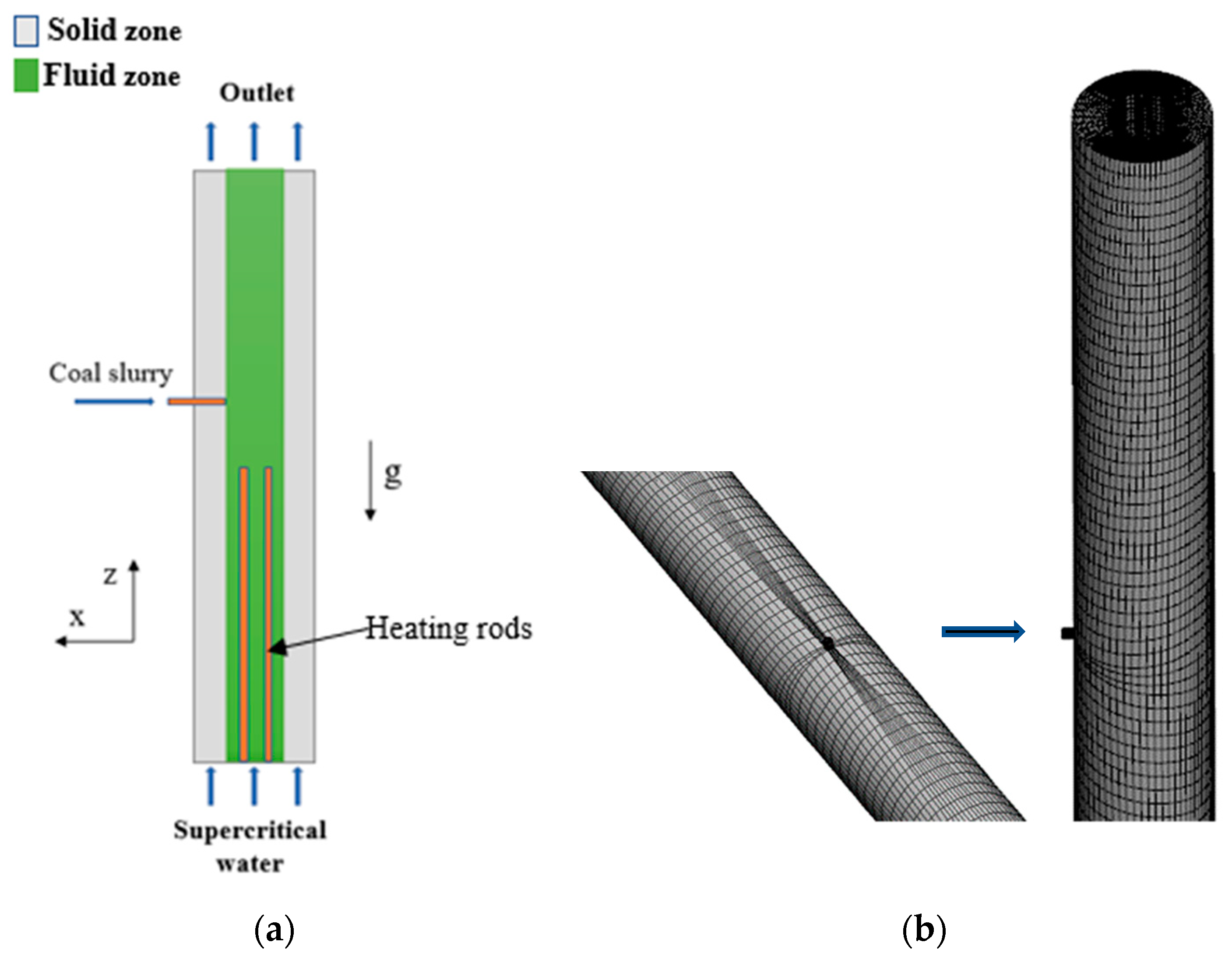
2.1. Physical Model
2.2. Governing Equations
2.3. Reaction Kinetics
2.4. Numerical Solution Method and Boundary Conditions
3. Model Validation
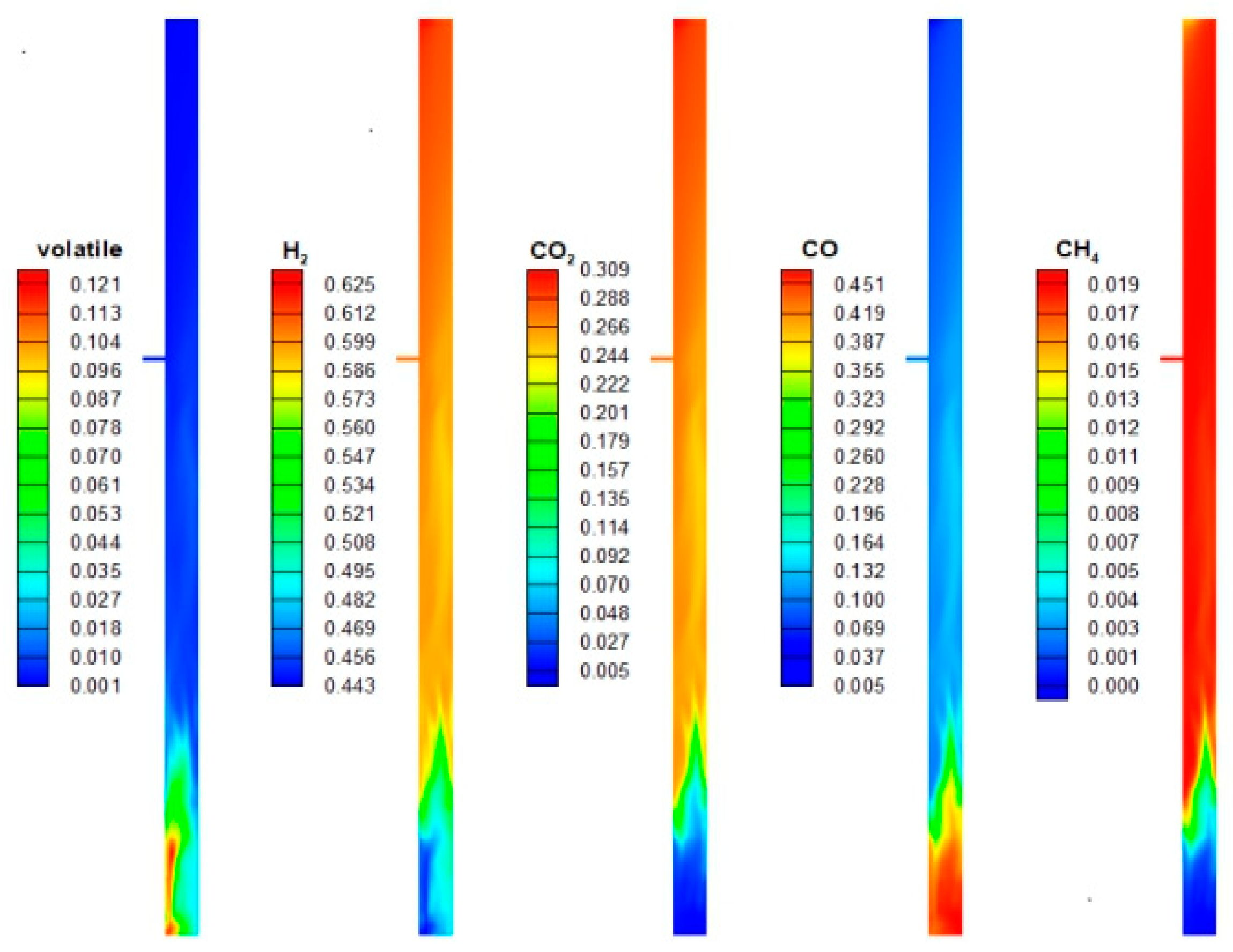
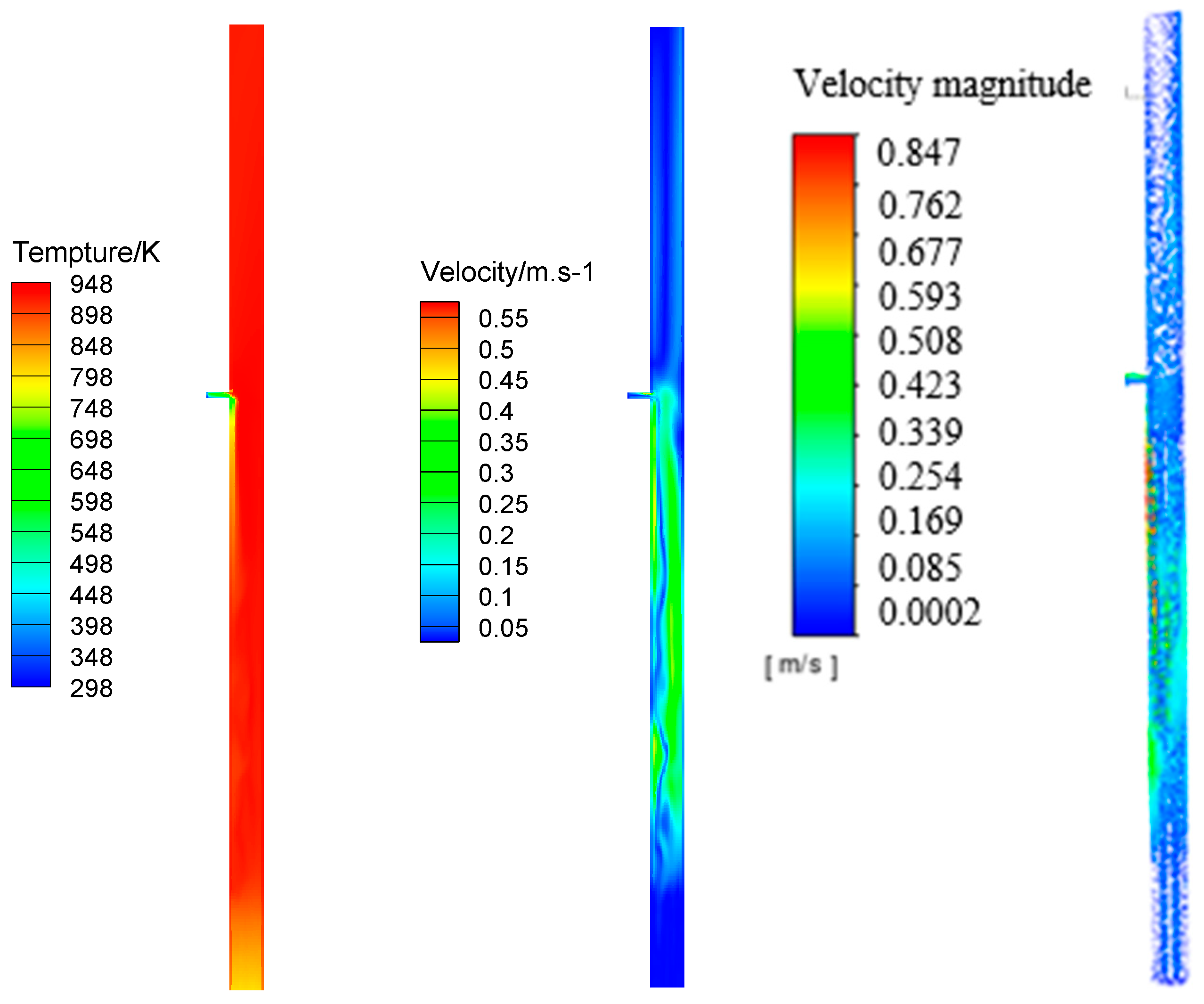
3.1. Distribution of Components
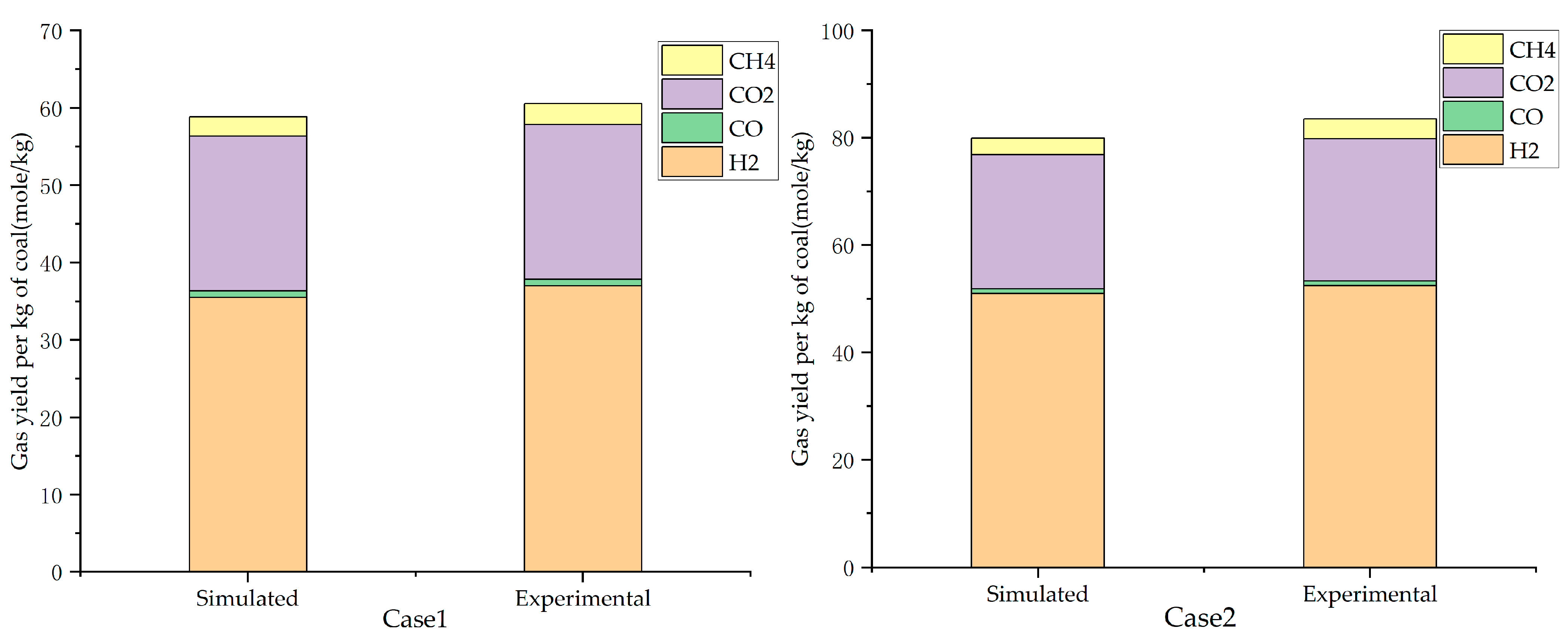
3.2. Simulated and Experimental Values
4. Temperature Characteristics
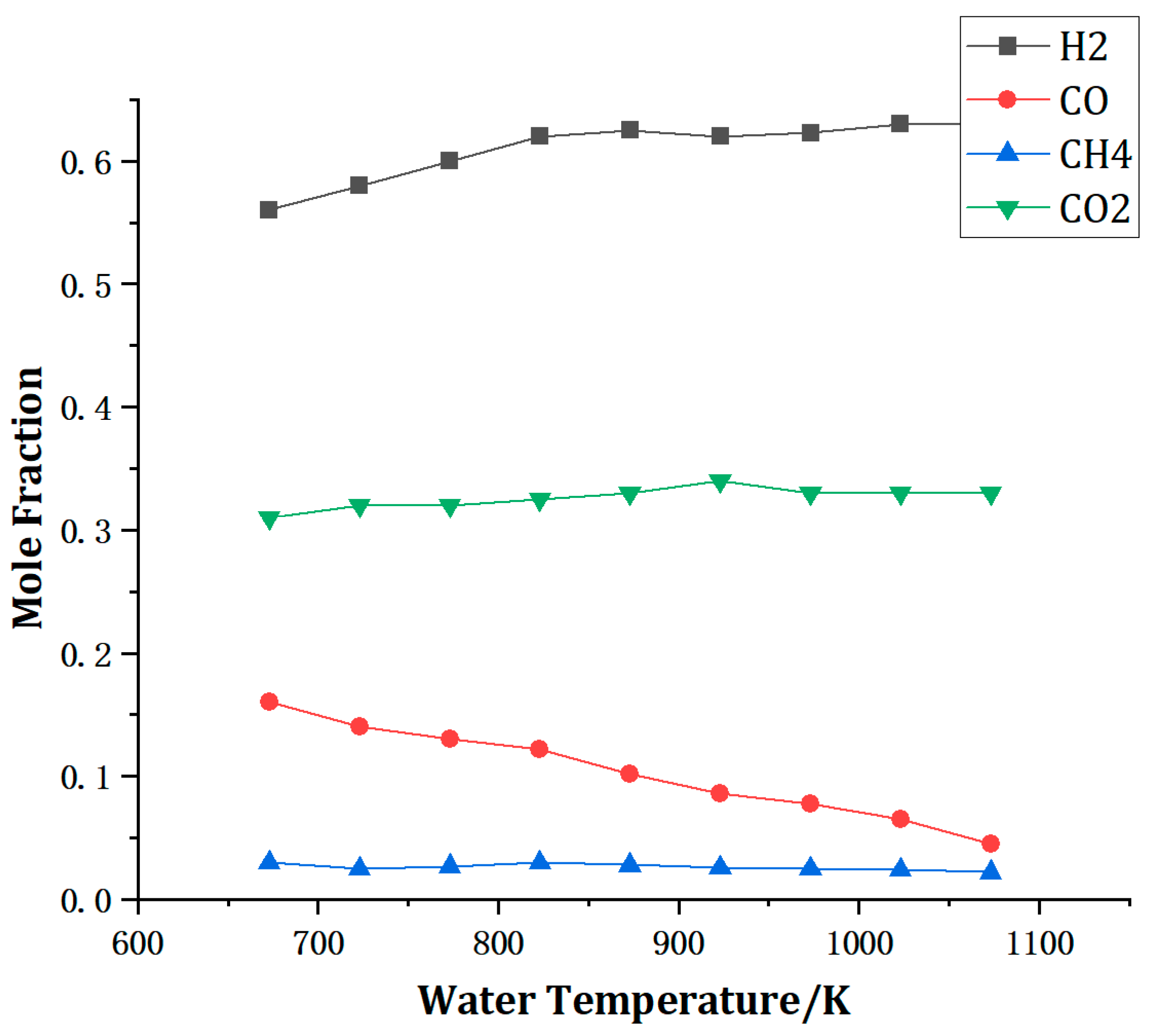
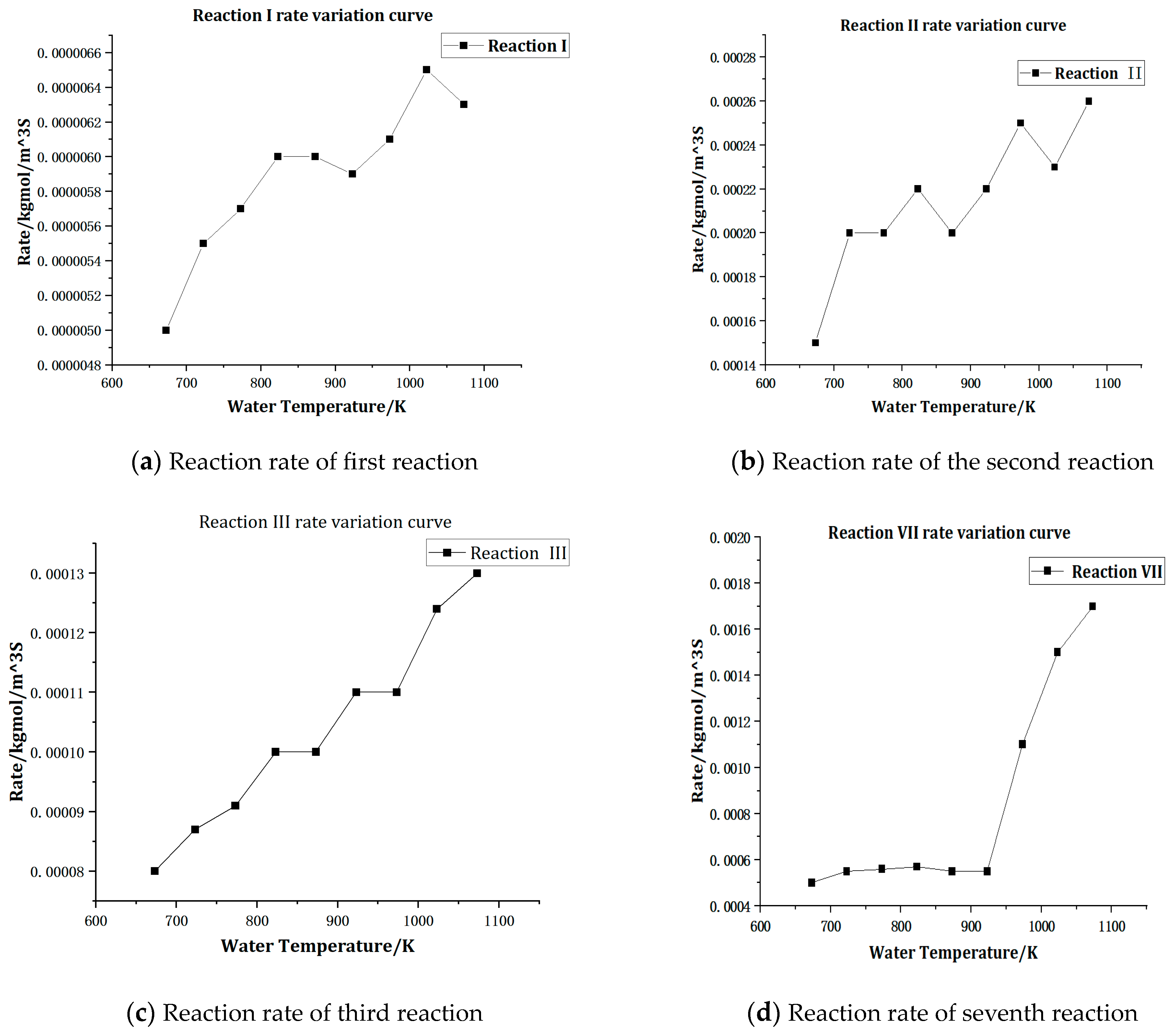
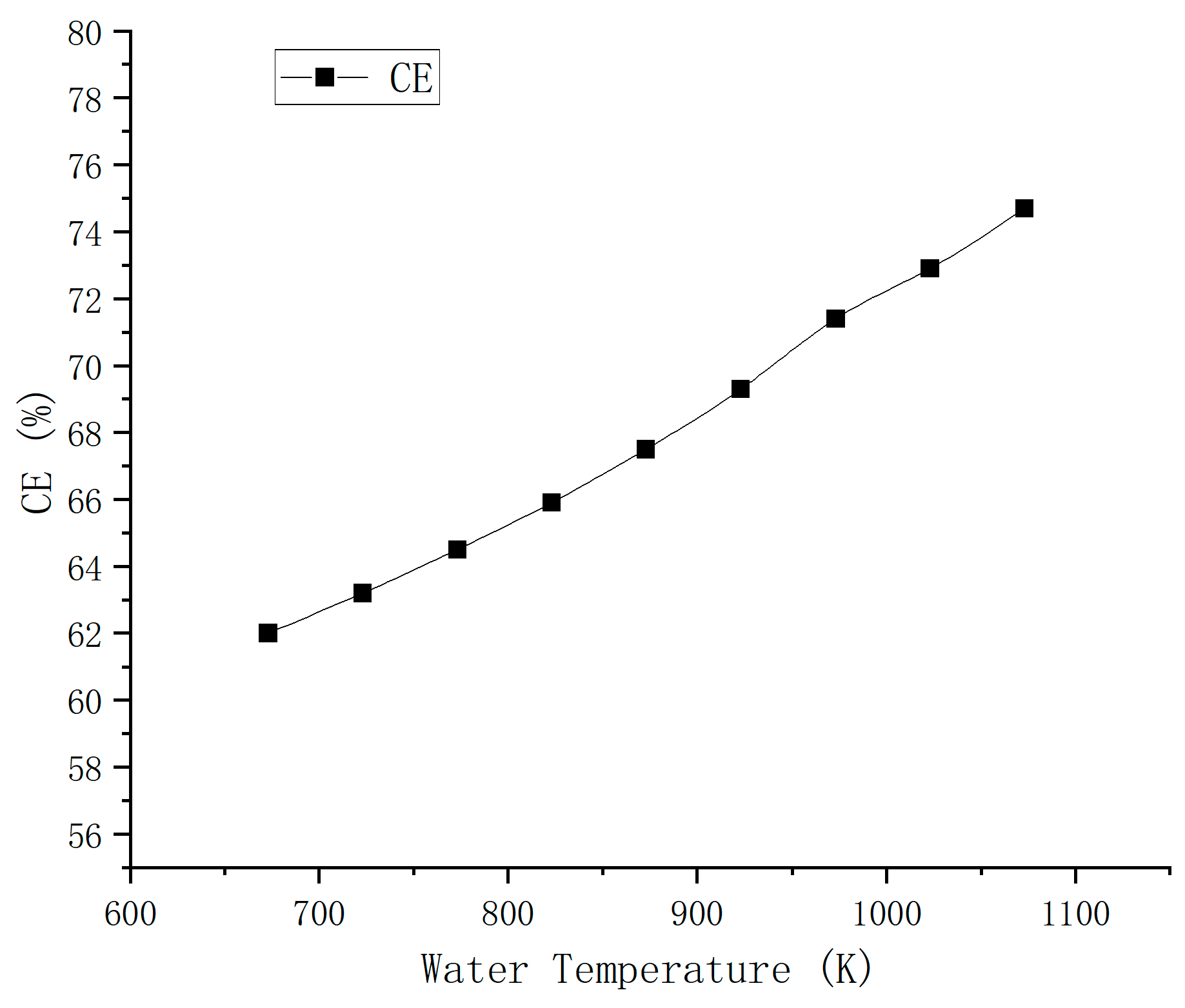
4.1. Effect of Supercritical Water Temperature on Yield and Reaction Rate
4.2. Effect of Coal Slurry Temperature on the Reactor
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCWG | Supercritical water gasification |
| CFD | Computational fluid dynamics |
| SCWB | Supercritical water-fluidized bed |
| UDF | User designed function |
| ISWR | Integrated supercritical water reactor |
| LBL | Line by line |
| D.O. | Discrete ordinate |
| DDPM | Dense discrete phase model |
| SCW | Supercritical water |
| VOL | Volatile carbon |
References
- Shi, Z.; Shi, X. Efforts to Promote the Achievement of Carbon Neutrality. Red Flag Manuscr. 2021, 4. [Google Scholar]
- Liu, R.H.; Wang, G.; Huang, N.; Ding, M.L. Research on the path of China’s science and technology innovation to support carbon peaking and carbon neutrality. Guangxi Soc. Sci. 2021, 8, 1–7. [Google Scholar]
- Xu, J.; Rao, L. Analysis of the current situation and prospect of hydrogen energy application. Stand. Qual. Mach. Ind. 2021, 4, 39–42. [Google Scholar]
- Xu, S.; Yu, B. Current status and future prospects of hydrogen energy technology development in China. J. Beijing Univ. Technol. 2021, 23, 1–12. [Google Scholar]
- Jin, H.; Fan, C.; Guo, L.; Liu, S.; Cao, C.; Wang, R. Experimental study on hydrogen production by lignite gasification in supercritical water fluidized bed reactor using external recycle of liquid residual. Energy Convers. Manag. 2017, 145, 214–219. [Google Scholar] [CrossRef]
- Lan, R.; Jin, H.; Guo, L.; Ge, Z.; Guo, S.; Zhang, X. Hydrogen Production by Catalytic Gasification of Coal in Supercritical Water. Energy Fuels 2014, 28, 6911–6917. [Google Scholar] [CrossRef]
- Fan, C.; Guo, S.; Jin, H. Numerical study on coal gasification in supercritical water fluidized bed and exploration of complete gasification under mild temperature conditions. Chem. Eng. Sci. 2019, 206, 134–145. [Google Scholar] [CrossRef]
- Guo, L.; Jin, H. Boiling coal in water: Hydrogen production and power generation system with zero net CO2 emission based on coal and supercritical water gasification. Int. J. Hydrogen Energy 2013, 38, 12953–12967. [Google Scholar] [CrossRef]
- Wang, R.; Guo, L.; Jin, H.; Lu, L.; Yi, L.; Zhang, D.; Chen, J. DFT study of the enhancement on hydrogen production by alkaline catalyzed water gas shift reaction in supercritical water. Int. J. Hydrogen Energy 2018, 43, 13879–13886. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, X.; Guo, L.; Zhu, C.; Cao, C.; Wu, Z. Experimental investigation on methanation reaction based on coal gasification in supercritical water. Int. J. Hydrogen Energy 2017, 42, 4636–4641. [Google Scholar] [CrossRef]
- Jin, H.; Wang, C.; Fan, C.; Guo, L.; Cao, C.; Cao, W. Experimental investigation on the influence of the pyrolysis operating parameters upon the char reaction activity in supercritical water gasification. Int. J. Hydrogen Energy 2018, 43, 13887–13895. [Google Scholar] [CrossRef]
- Ren, Z.; Jin, F.; Liu, S.; Ou, Z. Numerical analysis of particle flow and heat transfer characteristics in a supercritical water fluidized bed hydrogen production reactor for coal. J. Eng. Thermophys. 2020, 41, 154–160. [Google Scholar]
- Ou, Z.; Jin, H.; Ren, Z.; Zhu, S.; Song, M.; Guo, L. Mathematical model for coal conversion in supercritical water: Reacting multiphase flow with conjugate heat transfer. Int. J. Hydrogen Energy 2019, 44, 15746–15757. [Google Scholar] [CrossRef]
- Ge, Z.; Guo, L.; Jin, H. Hydrogen production by non-catalytic partial oxidation of coal in supercritical water: The study on reaction kinetics. Int. J. Hydrogen Energy 2017, 42, 9660–9666. [Google Scholar] [CrossRef]
- Liu, S.; Guo, L.; Jin, H.; Li, L. Hydrogen production by supercritical water gasification of coal: A reaction kinetic model including nitrogen and sulfur elements. Int. J. Hydrogen Energy 2020, 45, 31732–31744. [Google Scholar] [CrossRef]
- Su, X.; Guo, L.; Jin, H. Mathematical Modeling for Coal Gasification Kinetics in Supercritical Water. Energy Fuels 2016, 30, 9028–9035. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.J. Numerical simulation of two-phase flow characteristics of supercritical water circulating fluidized bed. J. Eng. Thermophys. 2018, 39, 127–132. [Google Scholar]
- Zhao, P.; Liu, H.; Xie, X.; Wang, S.; Liu, J.; Wang, X.; Xie, R.; Zuo, S. Efficient Surrogate-Assisted Parameter Analysis for Coal-Supercritical Water Fluidized Bed Reactor with Adaptive Sampling. Machines 2023, 11, 295. [Google Scholar] [CrossRef]
- Askarishahi, M.; Salehi, M.-S.; Radl, S. Challenges in the Simulation of Drying in Fluid Bed Granulation. Processes 2023, 11, 569. [Google Scholar] [CrossRef]
- Lv, Y.; Jin, H.; Li, G.; Wang, H. Research progress on coal utilization technology based on supercritical water gasification for hydrogen production. J. Coal 2022, 47, 3870–3885. [Google Scholar] [CrossRef]
- Vostrikov, A.A.; Psarov, S.A.; Dubov, D.Y.; Fedyaeva, O.N.; Sokol, M.Y. Kinetics of Coal Conversion in Supercritical Water. Energy Fuels 2007, 21, 2840–2845. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Y. Modeling of Wall-to-Bed Heat Transfer in a Supercritical Water Fluidized Bed by the Packet Approach. Ind. Eng. Chem. Res. 2020, 59, 22640–22655. [Google Scholar] [CrossRef]
- Yao, L.; Lu, Y. Supercritical water gasification of glucose in fluidized bed reactor: A numerical study. Int. J. Hydrogen Energy 2017, 42, 7857–7865. [Google Scholar] [CrossRef]
- Adnan, M.; Sun, J.; Ahmad, N.; Wei, J.J. Comparative CFD modeling of a bubbling bed using a Eulerian–Eulerian two-fluid model (TFM) and a Eulerian-Lagrangian dense discrete phase model (DDPM). Powder Technol. 2021, 383, 418–442. [Google Scholar] [CrossRef]
- Ding, J.; Gidaspow, D. A bubbling fluidization model using kinetic theory of granular flow. Aiche J. 2010, 36, 523–538. [Google Scholar] [CrossRef]



| Min. Diameter (mm) | Max. Diameter (mm) | Mean Diameter (mm) | Spread Parameter |
|---|---|---|---|
| 0.075 | 0.3 | 0.2264 | 3.3185 |
| Scattering Factor | Emissivity | Density (kg/m3) | Specific Heat (J/kg·K) |
| 0.6 | 0.9 | 1300 | 1680 |
| Parameters | Yimin Coal (wt%) |
|---|---|
| Proximate analysis (air dried) | |
| Moisture | 18.42 |
| Fixed carbon | 33.73 |
| Volatile matter | 32.21 |
| Ash | 15.46 |
| Ultimate analysis (dry base) | |
| C | 40.5 |
| H | 3.25 |
| N | 0.57 |
| S | 0.19 |
| O | 21.43 |
| Boundary Conditions | |
|---|---|
| Coal slurry | Mass flow inlet |
| Supercritical water | Mass flow inlet |
| Outlet | Pressure outlet |
| Outer wall | Constant temperature wall |
| Interface | Coupled wall |
| Heating rod | Constant heat flux |
| Operating Conditions | Case1 | Case2 |
|---|---|---|
| Mass flow rate of the coal slurry (g/s) | 0.3 | 0.3 |
| Temperature of coal slurry (K) | 298 | 298 |
| Coal slurry concentration (wt%) | 30 | 30 |
| Mass flow rate of SCW (g/s) | 2.7 | 2.7 |
| Temperature of SCW (K) | 773 | 823 |
| Temperature of outer wall (K) | 923 | 923 |
| Total power of heating roads (w) | 2000 | 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xie, R.; Liu, J.; Zhao, P.; Liu, H.; Wang, X. Numerical Analysis of The Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production. Machines 2023, 11, 546. https://doi.org/10.3390/machines11050546
Wang S, Xie R, Liu J, Zhao P, Liu H, Wang X. Numerical Analysis of The Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production. Machines. 2023; 11(5):546. https://doi.org/10.3390/machines11050546
Chicago/Turabian StyleWang, Shiqi, Rong Xie, Jiali Liu, Pu Zhao, Haitao Liu, and Xiaofang Wang. 2023. "Numerical Analysis of The Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production" Machines 11, no. 5: 546. https://doi.org/10.3390/machines11050546
APA StyleWang, S., Xie, R., Liu, J., Zhao, P., Liu, H., & Wang, X. (2023). Numerical Analysis of The Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production. Machines, 11(5), 546. https://doi.org/10.3390/machines11050546







