Review on Wearable System for Positioning Ultrasound Scanner
Abstract
1. Introduction
2. The US Principles
2.1. Propagation Speed of Sound Waves
2.2. Sound Waves Interactions
2.3. Image Resolution
2.4. Transducers
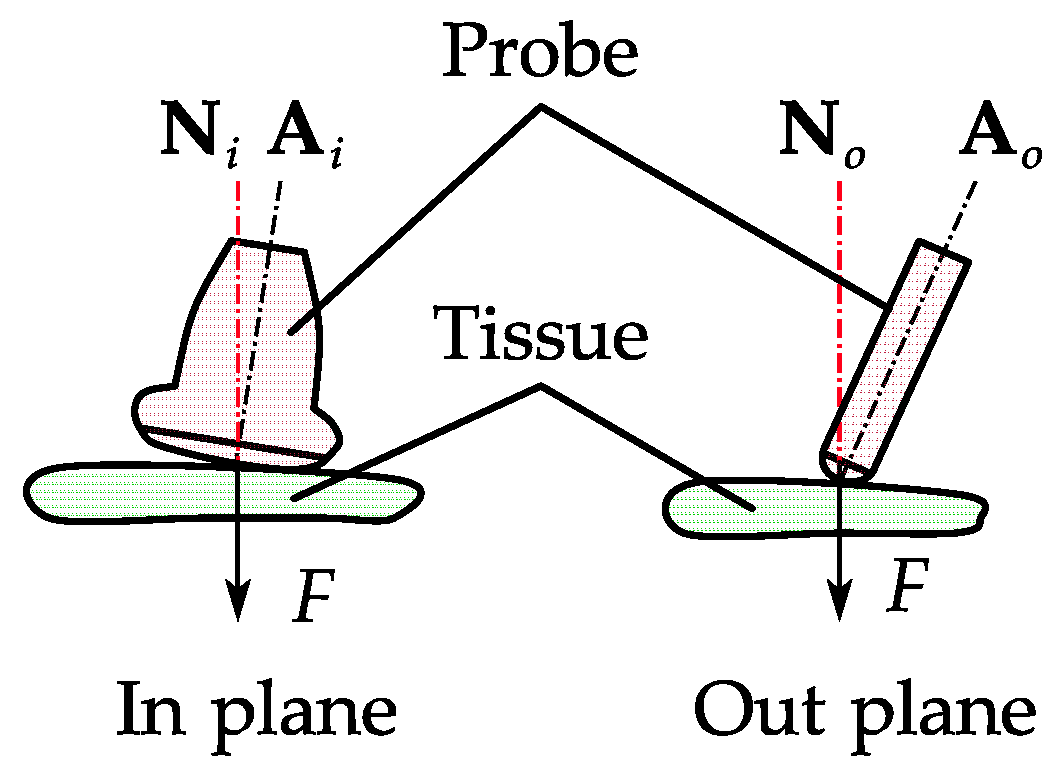
2.5. Probe Positioning
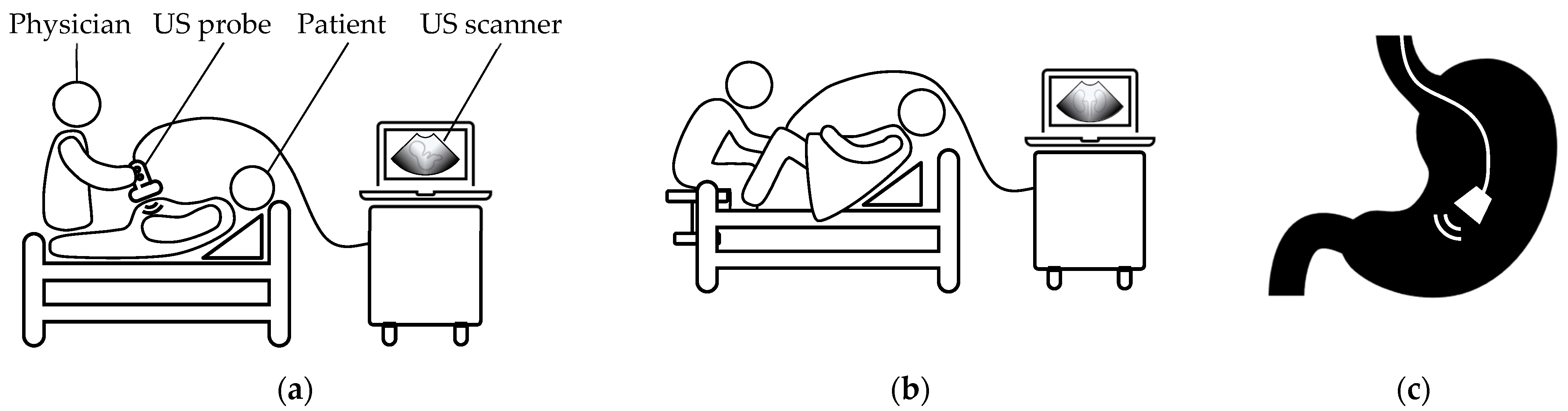
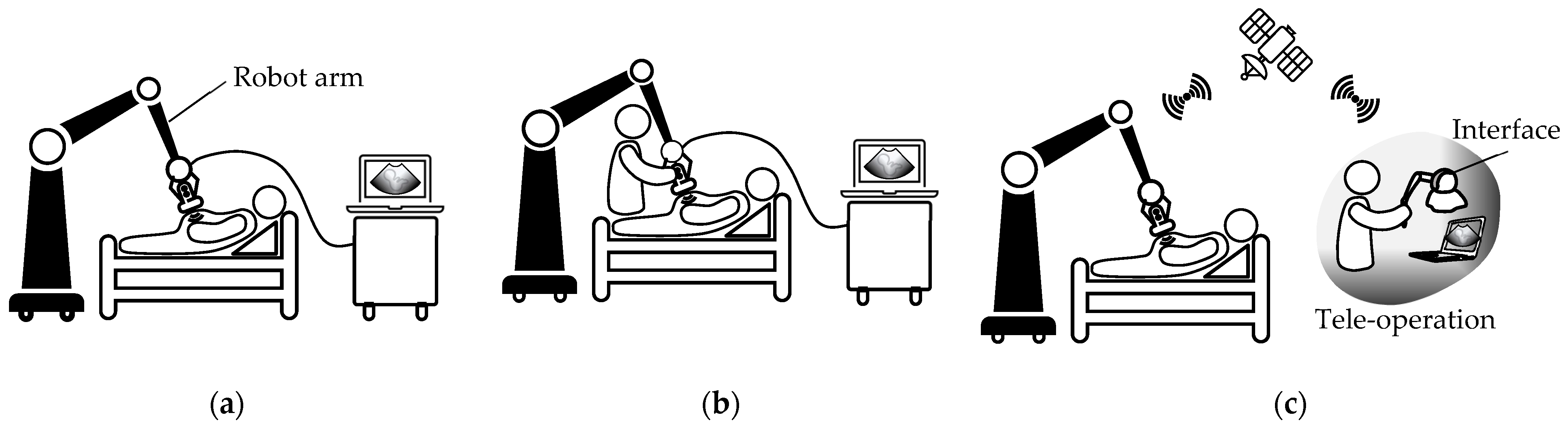
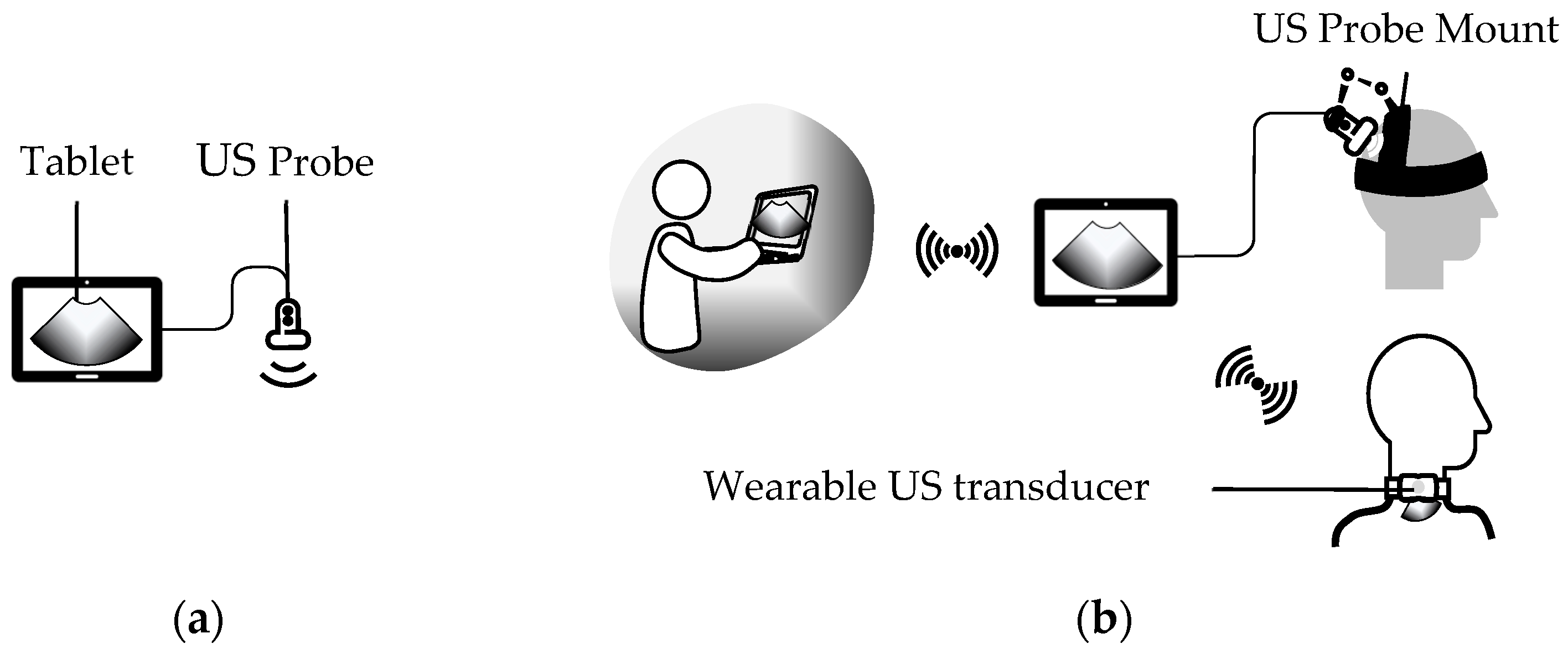
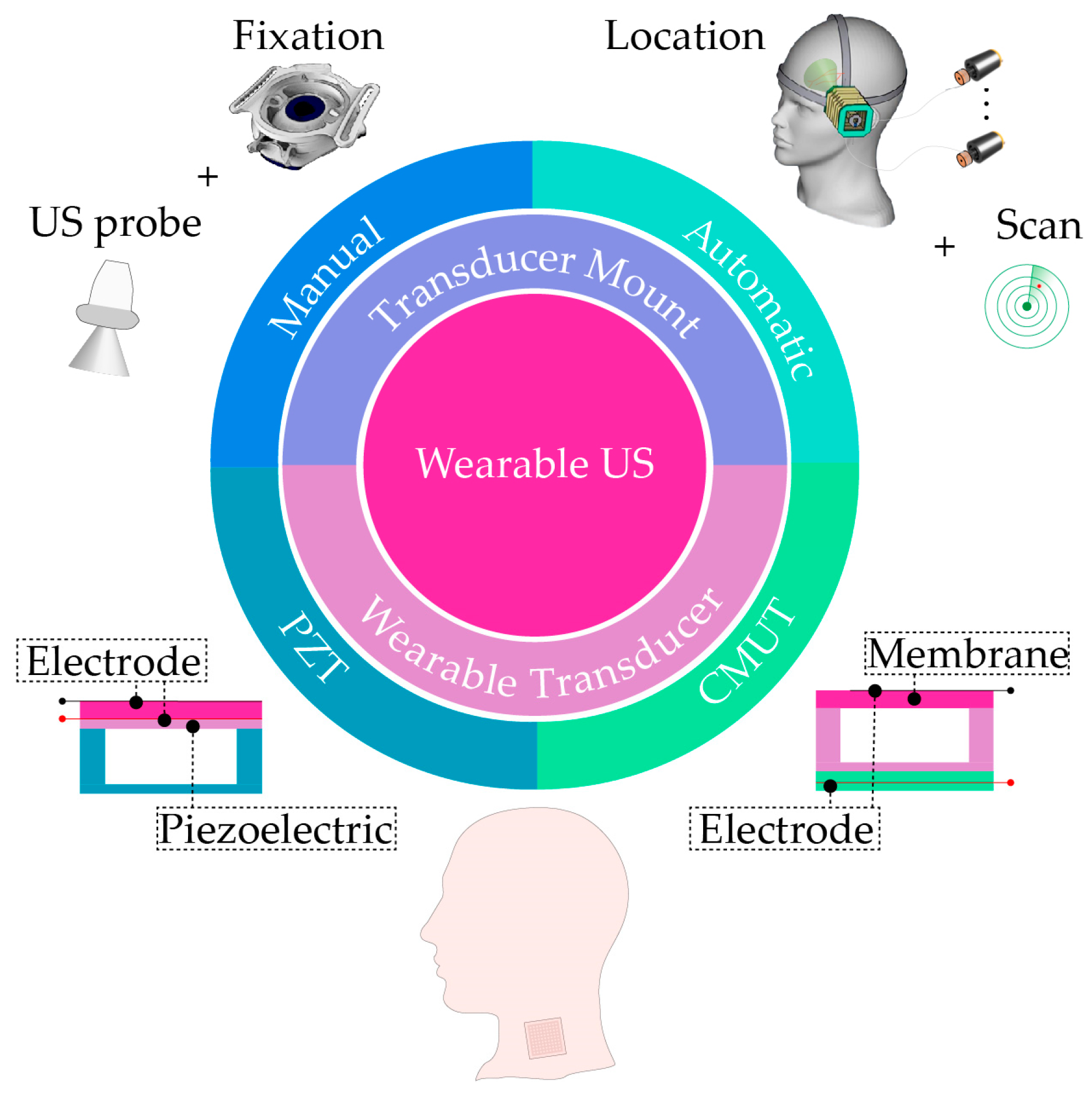
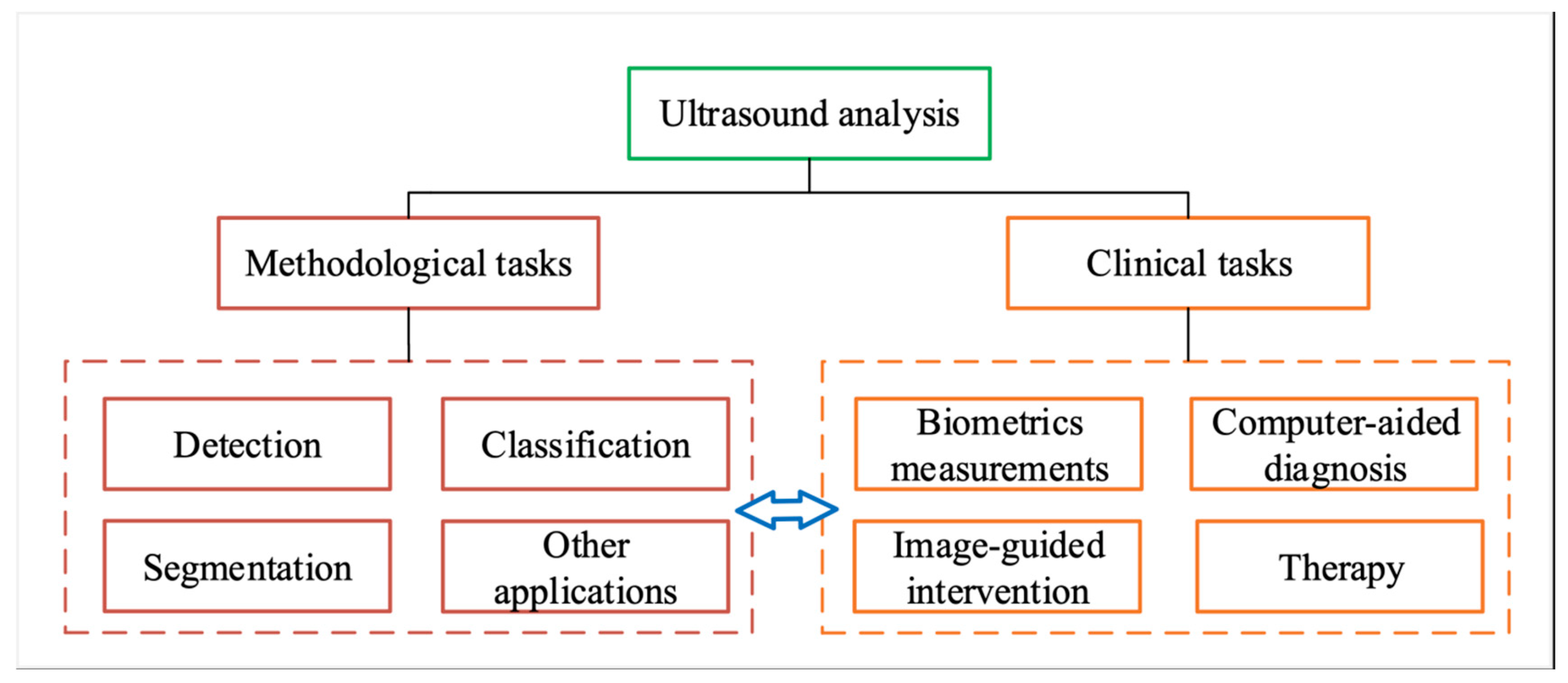
3. Wearable External US Scanner
3.1. Wearable External US Transducer Mounts
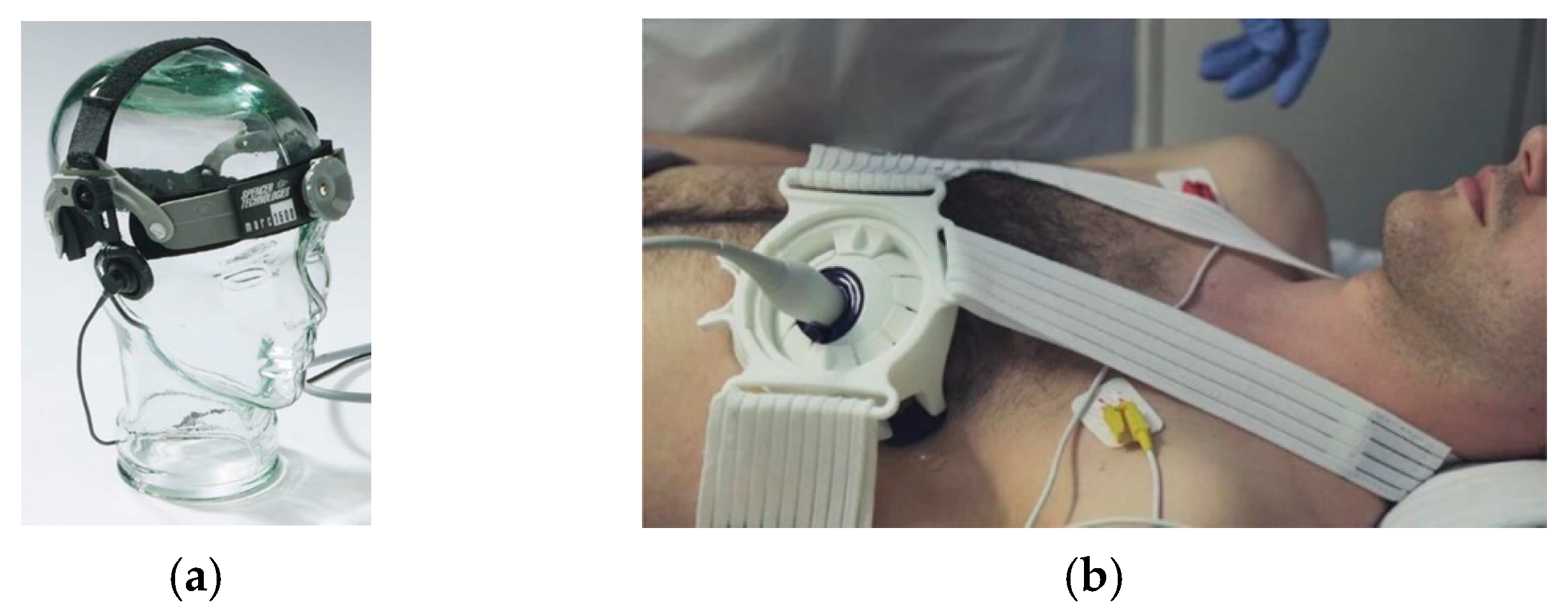
- Manual External US Transducer Mounts
| Name | Solution | Body Part | Application | Level | Ref |
|---|---|---|---|---|---|
| Giller et al., 1997 | Fixation, Adhesion | Head | TCD | Custom | [22] |
| Marc 600 | Fixation | Head | TCD | Commercial | [23,24,25] |
| DiaMon LAM rack, Adhesive set, Elastic headband, | Fixation, Strap and Adhesion | Head | TCD | Commercial | [26] |
| Yangmo Yoo et al. 2019 | Neckband | Neck | CCA | Custom | [28] |
| Chih-Chung Huang et al., 2019 | Low profile Transducer | General | General | Custom | [33] |
| Honghai Liu et al., 2015 | Band | Wrist | Gesture Prediction | Custom | [29,30,31,32] |
| Davinia et al., 2021 | Fixation, Strap | Intercostal Space | RUSI | Custom | [34] |
| Mitsuhiko et al., 2017 | Fixation, Strap | Wrist | CTR assessment | Custom | [35,36] |
| ProbeFix series | Fixation, Strap | Cardiac, Limb | Muscle scan, Echocardiography | Commercial | [27,37,40] |
| Andrew et al., 2004 | Knee brace | Knee | Patellar position | Custom | [38] |
| Prue et al., 2019 | Fixation, Strap | Low limb | Achilles strain | Custom | [39] |
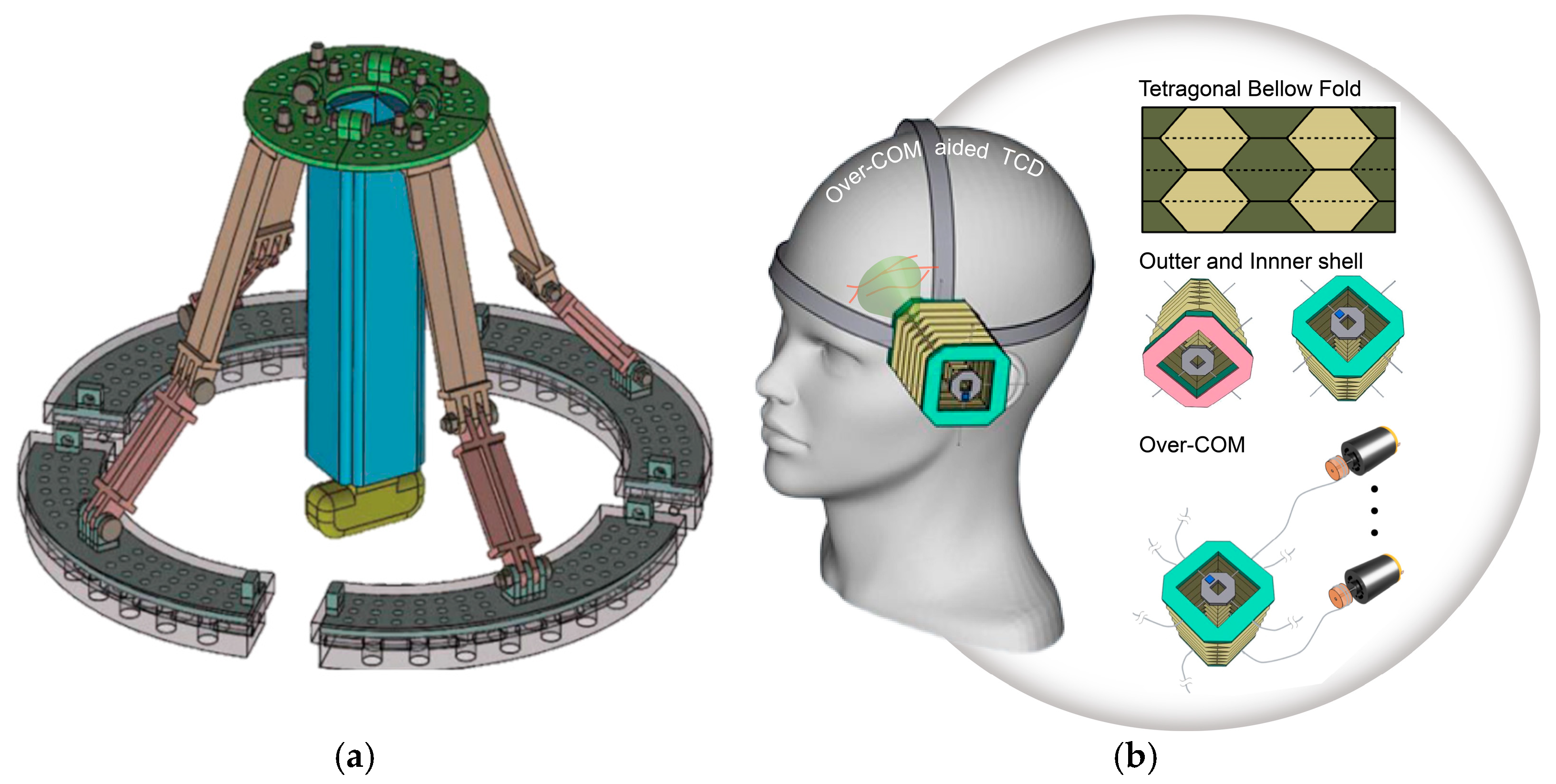
- Autonomous External US Transducer Mounts

| Name | Solution | DOF | Dimensions | Weight | Body Part | Application | Level | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sungon Lee et al., 2016 | Linkage | 5 | - | - | Head | TCD | Custom | [41] |
| Hongliang Ren et al., 2016 | Soft actuator | Linear motion Single plane Semi-circle | - | - | General | General | Custom | [42] |
| Hongliang Ren et al., 2023 | Origami | 5 | Head | TCD | Custom | [43] | ||
| Robotic Probe | Headframe | - | 10.5 × 6 × 2 cm | 55 g | Head | TCD | Commercial | [44,45,46] |
| Dolphin/XF | Headframe | - | 8.5 × 7.5 × 3.5 cm | 126 g | Head | TCD | Commercial | [47] |
| TCD-X | Headset | - | - | - | Head | TCD | Commercial | [48,49] |
| Lucid M1 with headset | Headframe | - | - | - | Head | TCD | Commercial | [50] |
| ATUSA | Strap | - | - | - | Breast | Breast US | Commercial | [51] |
| SENS-U | Adhesion | - | - | - | Abdomen | Bladder US | Commercial | [52,53,54] |
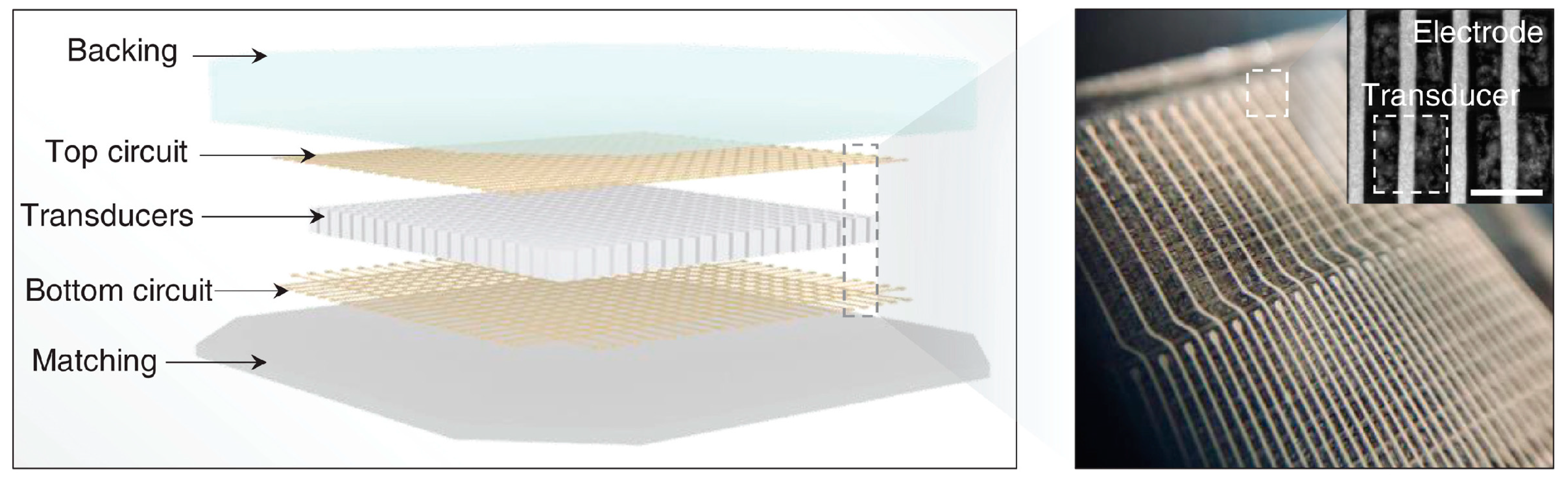
3.2. Wearable External US Transducers
| Name | Solution | Penetration Depth | Operating Frequency | Application | Level | Ref. |
|---|---|---|---|---|---|---|
| Haruhiko et al., 1999 | PZT-based | 14 mm | 4 and 8.1 MHz | CCA | Custom | [56,57] |
| Yuu Ono et al., 2013 | PZT-based | 23 mm | 2.2 MHz | General | Custom | [58,59] |
| Dawei Wu et al., 2021 | Flexible PMUT | 140 mm | 2 MHz | General | Custom | [60] |
| Sheng Xu et al., 2018 | PZT-based | 140 mm | 2 MHz | General | Custom | [61,62,63,64] |
| Steve et al., 2019 | PZT-based | 20 mm | 5 MHz | General | Custom | [65] |
| Xuanhe Zhao et al., 2022 | PZT-based | 60 mm | 3, 7, and 10 MHz | General | Custom | [66] |
| Anshuman et al., 2011 | CMUT | 50 mm | 5 MHz | General | Custom | [68] |
| FloPatch | Continuous Wave Transducer | 40 mm | 4 MHz | CCA | Commercial | [69,70,71,72] |
| Carlos et al., 2018 | CMUT | 85 mm | 5.8 MHz | General | Commercial | [73,74] |
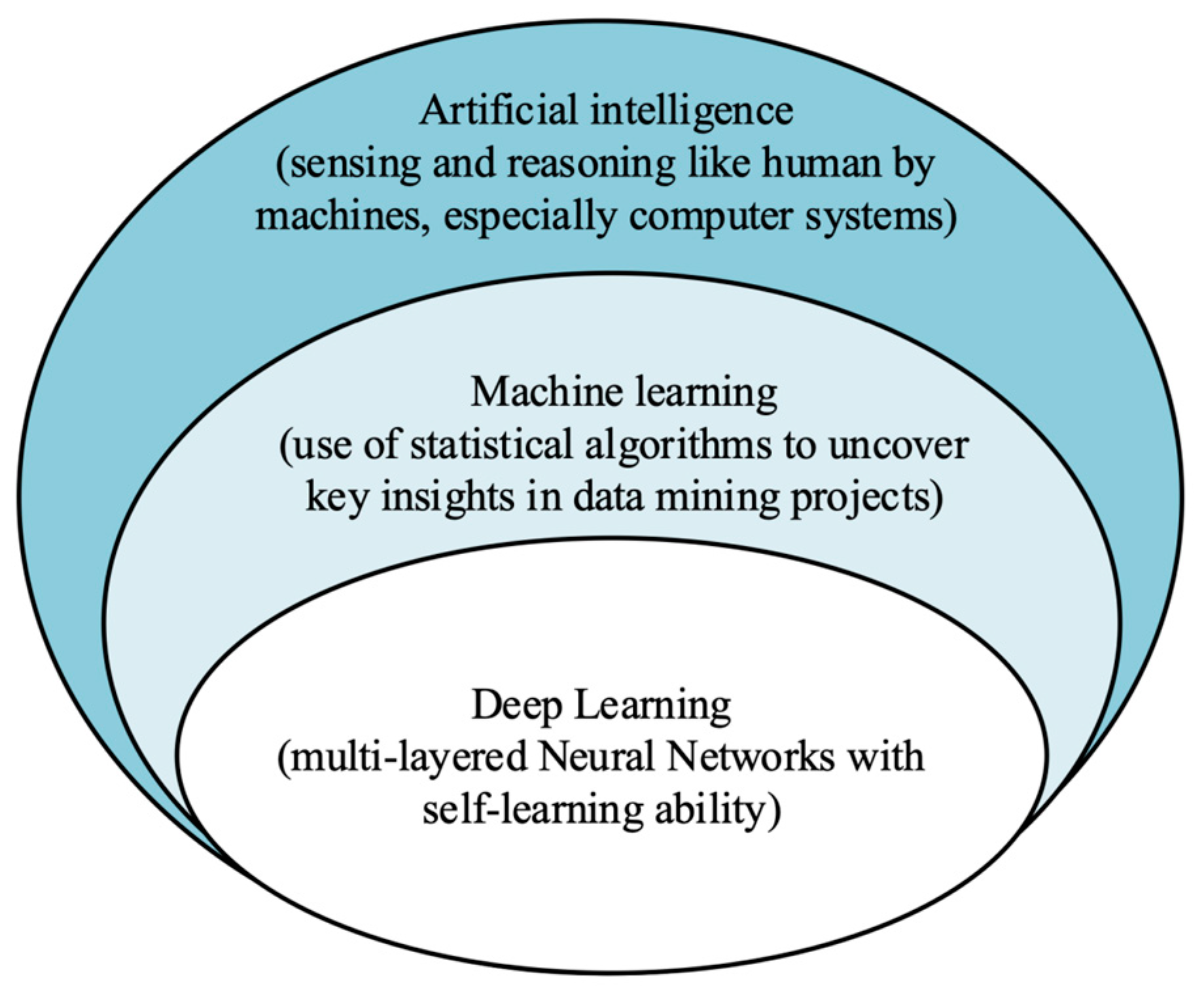
4. Applications of Artificial Intelligence in Wearable US
4.1. Supervised Learning
4.2. Supervised Learning
4.3. Semi-Supervised Learning
4.4. Reinforcement Learning
4.5. Deep Learning
5. Future Directions of Wearable External US Scan
- Issues with existing apparatuses
- Future Trends in Hardware
- Future Trends in Software
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shampo, M.A.; Kyle, R.A. Karl Theodore Dussik—Pioneer in ultrasound. Mayo Clin. Proc. 1995, 70, 1136. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N.; Liang, H.-D. Medical ultrasound: Imaging of soft tissue strain and elasticity. J. R. Soc. Interface 2011, 8, 1521–1549. [Google Scholar] [CrossRef] [PubMed]
- Fenster, A.; Downey, D.B.; Cardinal, H.N. Three-dimensional ultrasound imaging. Phys. Med. Biol. 2001, 46, R67. [Google Scholar] [CrossRef] [PubMed]
- Wells, P.N. Ultrasound imaging. Phys. Med. Biol. 2006, 51, R83. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of ultrasound in medicine. Acta Inform. Med. 2011, 19, 168–171. [Google Scholar] [CrossRef]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Academic Press: Cambridge, MA, USA, 2004. [Google Scholar]
- Chan, V.; Perlas, A. Basics of ultrasound imaging. In Atlas of Ultrasound-Guided Procedures in Interventional Pain Management; Springer: Berlin/Heidelberg, Germany, 2011; pp. 13–19. [Google Scholar]
- Ye, D.; Xue, J.; Yuan, S.; Zhang, F.; Song, S.; Wang, J.; Meng, M.Q.-H. Design and control of a magnetically-actuated capsule robot with biopsy function. IEEE Trans. Biomed. Eng. 2022, 69, 2905–2915. [Google Scholar] [CrossRef]
- Yuan, S.; Wan, Y.; Song, S. RectMag3D: A magnetic actuation system for steering milli/microrobots based on rectangular electromagnetic coils. Appl. Sci. 2020, 10, 2677. [Google Scholar] [CrossRef]
- Llamas-Alvarez, A.M.; Tenza-Lozano, E.M.; Latour-Perez, J. Accuracy of lung ultrasonography in the diagnosis of pneumonia in adults: Systematic review and meta-analysis. Chest 2017, 151, 374–382. [Google Scholar] [CrossRef]
- Powles, A.E.; Martin, D.J.; Wells, I.T.; Goodwin, C.R. Physics of ultrasound. Anaesth. Intensive Care Med. 2018, 19, 202–205. [Google Scholar] [CrossRef]
- von Haxthausen, F.; Böttger, S.; Wulff, D.; Hagenah, J.; García-Vázquez, V.; Ipsen, S. Medical robotics for ultrasound imaging: Current systems and future trends. Curr. Robot. Rep. 2021, 2, 55–71. [Google Scholar] [CrossRef]
- Monfaredi, R.; Wilson, E.; Azizi koutenaei, B.; Labrecque, B.; Leroy, K.; Goldie, J.; Louis, E.; Swerdlow, D.; Cleary, K. Robot-assisted ultrasound imaging: Overview and development of a parallel telerobotic system. Minim. Invasive Ther. Allied Technol. 2015, 24, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Baribeau, Y.; Sharkey, A.; Chaudhary, O.; Krumm, S.; Fatima, H.; Mahmood, F.; Matyal, R. Handheld point-of-care ultrasound probes: The new generation of POCUS. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.E. Basic physics of ultrasound imaging. Crit. Care Med. 2007, 35, S131–S137. [Google Scholar] [CrossRef]
- Sigrist, R.M.; Liau, J.; El Kaffas, A.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Cootney, R.W. Ultrasound imaging: Principles and applications in rodent research. Ilar J. 2001, 42, 233–247. [Google Scholar] [CrossRef]
- Urone, P.; Hinrichs, R. College Physics; OpenStax: Houston, TX, USA, 2012. [Google Scholar]
- Abbara, S.; Achenbach, S. CT and MR in Cardiology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wang, J.; Zheng, Z.; Chan, J.; Yeow, J.T. Capacitive micromachined ultrasound transducers for intravascular ultrasound imaging. Microsyst. Nanoeng. 2020, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Grimm, M.; Zhou, M.; Esteban, J.; Simson, W.; Zahnd, G.; Navab, N. Automatic normal positioning of robotic ultrasound probe based only on confidence map optimization and force measurement. IEEE Robot. Autom. Lett. 2020, 5, 1342–1349. [Google Scholar] [CrossRef]
- Giller, C.A.; Giller, A.M. A new method for fixation of probes for transcranial Doppler ultrasound. J. Neuroimaging 1997, 7, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Kaczynski, J.; Home, R.; Shields, K.; Walters, M.; Whiteley, W.; Wardlaw, J.; Newby, D.E. Reproducibility of Transcranial Doppler ultrasound in the middle cerebral artery. Cardiovasc. Ultrasound 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Garami, Z.F.; Bismuth, J.; Charlton-Ouw, K.M.; Davies, M.G.; Peden, E.K.; Lumsden, A.B. Feasibility of simultaneous pre-and postfilter transcranial Doppler monitoring during carotid artery stenting. J. Vasc. Surg. 2009, 49, 340–345.e342. [Google Scholar] [CrossRef]
- Lao, A.; Sharma, V.; Tsivgoulis, G.; Malkoff, M.; Alexandrov, A.; Frey, J. Effect of body positioning during transcranial Doppler detection of right-to-left shunts. Eur. J. Neurol. 2007, 14, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Kho, E.; Sperna Weiland, N.H.; Vlaar, A.P.; Veelo, D.P.; van der Ster, B.J.; Corsmit, O.T.; Koolbergen, D.R.; Dilai, J.; Immink, R.V. Cerebral hemodynamics during sustained intra-operative hypotension. J. Appl. Physiol. 2022, 132, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Blans, M.; Bosch, F.; van der Hoeven, J. The use of an external ultrasound fixator (Probefix) on intensive care patients: A feasibility study. Ultrasound J. 2019, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Song, I.; Yoon, J.; Kang, J.; Kim, M.; Jang, W.S.; Shin, N.-Y.; Yoo, Y. Design and implementation of a new wireless carotid neckband Doppler system with wearable ultrasound sensors: Preliminary results. Appl. Sci. 2019, 9, 2202. [Google Scholar] [CrossRef]
- Hettiarachchi, N.; Ju, Z.; Liu, H. A new wearable ultrasound muscle activity sensing system for dexterous prosthetic control. In Proceedings of the 2015 IEEE International Conference on Systems, Man, and Cybernetics, Kowloon Tong, Hong Kong, 9–12 October 2015; pp. 1415–1420. [Google Scholar]
- Yang, X.; Chen, Z.; Hettiarachchi, N.; Yan, J.; Liu, H. A wearable ultrasound system for sensing muscular morphological deformations. IEEE Trans. Syst. Man Cybern. Syst. 2019, 51, 3370–3379. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhou, D.; Li, Y.; Liu, H. Towards wearable A-mode ultrasound sensing for real-time finger motion recognition. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1199–1208. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Fang, Y.; Zhou, D.; Liu, H. Simultaneous prediction of wrist/hand motion via wearable ultrasound sensing. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 970–977. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lee, P.-Y.; Chen, P.-Y.; Liu, T.-Y. Design and implementation of a smartphone-based portable ultrasound pulsed-wave Doppler device for blood flow measurement. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 182–188. [Google Scholar] [CrossRef]
- Marugán-Rubio, D.; Chicharro, J.L.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Rodríguez-Sanz, D.; Vicente-Campos, D.; Dávila-Sánchez, G.J.; Calvo-Lobo, C. Concurrent Validity and Reliability of Manual Versus Specific Device Transcostal Measurements for Breathing Diaphragm Thickness by Ultrasonography in Lumbopelvic Pain Athletes. Sensors 2021, 21, 4329. [Google Scholar] [CrossRef]
- Nanno, M.; Sawaizumi, T.; Kodera, N.; Tomori, Y.; Takai, S. Ultrasound evaluation of the transverse movement of the flexor pollicis longus tendon on the distal radius during wrist and finger motion in healthy volunteers. J. Nippon. Med. Sch. 2015, 82, 220–228. [Google Scholar] [CrossRef]
- Nanno, M.; Kodera, N.; Tomori, Y.; Hagiwara, Y.; Takai, S. Median nerve movement in the carpal tunnel before and after carpal tunnel release using transverse ultrasound. J. Orthop. Surg. 2017, 25, 2309499017730422. [Google Scholar] [CrossRef]
- Heres, H.M.; Sjoerdsma, M.; Schoots, T.; Rutten, M.; van de Vosse, F.N.; Lopata, R.G. Image acquisition stability of fixated musculoskeletal sonography in an exercise setting: A quantitative analysis and comparison with freehand acquisition. J. Med. Ultrason. 2020, 47, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-F.; Bull, A.M.; McGregor, A.H.; Amis, A.A. Active patellar tracking measurement: A novel device using ultrasound. Am. J. Sport. Med. 2004, 32, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P.; Ellis, R.F.; Carroll, M. Reliability of a two-probe ultrasound imaging procedure to measure strain in the Achilles tendon. J. Foot Ankle Res. 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sjoerdsma, M.; Caresio, C.; Tchang, B.; Meeder, A.; van de Vosse, F.; Lopata, R. The feasibility of dynamic musculoskeletal function analysis of the vastus lateralis in endurance runners using continuous, hands-free ultrasound. Appl. Sci. 2021, 11, 1534. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S. Development of a wearable robotic positioning system for noninvasive transcranial focused ultrasound stimulation. IEEE/ASME Trans. Mechatron. 2016, 21, 2284–2293. [Google Scholar] [CrossRef]
- Ren, H.; Gu, X.; Tan, K.L. Human-compliant body-attached soft robots towards automatic cooperative ultrasound imaging. In Proceedings of the 2016 IEEE 20th International Conference on Computer Supported Cooperative Work in Design (CSCWD), Nanchang, China, 4–6 May 2016; pp. 653–658. [Google Scholar]
- Li, L.; Long, F.L.J.; Lim, I.; Sun, T.; Ren, H. An Overhead Collapsible Origami-Based Mount for Medical Applications. Robotics 2023, 12, 21. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Czosnyka, M.; Smielewski, P. Optimal cerebral perfusion pressure via transcranial Doppler in TBI: Application of robotic technology. Acta Neurochir. 2018, 160, 2149–2157. [Google Scholar] [CrossRef]
- Zeiler, F.; Smielewski, P. Application of robotic transcranial Doppler for extended duration recording in moderate/severe traumatic brain injury: First experiences. Crit. Ultrasound J. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Khan, D.Z.; Placek, M.M.; Smielewski, P.; Budohoski, K.P.; Anwar, F.; Hutchinson, P.J.; Bance, M.; Czosnyka, M.; Helmy, A. Robotic semi-automated transcranial doppler assessment of cerebrovascular autoregulation in post-concussion syndrome: Methodological considerations. Neurotrauma Rep. 2020, 1, 218–231. [Google Scholar] [CrossRef]
- Krakauskaite, S.; Kumpaitiene, B.; Svagzdiene, M.; Sirvinskas, E.; Petkus, V.; Chaleckas, E.; Kasputyte, G.; Gailiusas, M.; Benetis, R.; Ragauskas, A. Non-invasive Intracranial Pressure Dynamics During Cardiac Bypass Surgery: Prospective Study. In Proceedings of the 12th International Conference on Biomedical Engineering and Technology, Tokyo, Japan, 15–18 June 2023; pp. 175–179. [Google Scholar]
- Pizzarelli, G.; Gennai, S.; Leone, N.; Covic, T.; Moratto, R.; Silingardi, R. Transcranial Doppler detects micro emboli in patients with asymptomatic carotid stenoses undergoing endarterectomy. J. Vasc. Surg. 2022, 77, 811–817. [Google Scholar] [CrossRef]
- Aarli, S.J.; Novotny, V.; Thomassen, L.; Kvistad, C.E.; Logallo, N.; Fromm, A. Persistent microembolic signals in the cerebral circulation on transcranial Doppler after intravenous sulfur hexafluoride microbubble infusion. J. Neuroimaging 2020, 30, 146–149. [Google Scholar] [CrossRef]
- Thorpe, S.G.; Thibeault, C.M.; Wilk, S.J.; O’Brien, M.; Canac, N.; Ranjbaran, M.; Devlin, C.; Devlin, T.; Hamilton, R.B. Velocity curvature index: A novel diagnostic biomarker for large vessel occlusion. Transl. Stroke Res. 2019, 10, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Overman, D. FDA Clears iSono Health’s ATUSA, an Automated and Wearable 3D Breast Ultrasound System. Available online: https://axisimagingnews.com/radiology-products/imaging-equipment/ultrasound/fda-clears-isono-healths-atusa-an-automated-and-wearable-3d-breast-ultrasound-system (accessed on 22 February 2023).
- van Leuteren, P.G.; Klijn, A.J.; de Jong, T.P.; Dik, P. SENS-U: Validation of a wearable ultrasonic bladder monitor in children during urodynamic studies. J. Pediatr. Urol. 2018, 14, 569.e561–569.e566. [Google Scholar] [CrossRef] [PubMed]
- Kwinten, W.; van Leuteren, P.; van Duren–van Iersel, M.; Dik, P.; Jira, P. SENS-U: Continuous home monitoring of natural nocturnal bladder filling in children with nocturnal enuresis–a feasibility study. J. Pediatr. Urol. 2020, 16, 196.e191–196.e196. [Google Scholar] [CrossRef] [PubMed]
- van Leuteren, P.G.; Nieuwhof-Leppink, A.J.; Dik, P. SENS-U: Clinical evaluation of a full-bladder notification–a pilot study. J. Pediatr. Urol. 2019, 15, 381.e381–381.e385. [Google Scholar] [CrossRef] [PubMed]
- La, T.G.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2022, 7, 2100798. [Google Scholar] [CrossRef]
- Awad, E. Design of a Wearable Ultrasound Doppler Sensor to Monitor Blood Flow in the Common Carotid Artery. Ph.D. Dissertation, Massachusetts Institute of Technology, Cambridge, MA, USA, 1999. [Google Scholar]
- Awad, E.; Asada, H. The Doppler necklace: A wearable and noninvasive ultrasound sensor for continuous monitoring of blood flow in the common carotid artery. In Proceedings of the First Joint BMES/EMBS Conference. 1999 IEEE Engineering in Medicine and Biology 21st Annual Conference and the 1999 Annual Fall Meeting of the Biomedical Engineering Society, Atlanta, GA, USA, 13–16 October 1999; Volume 792, p. 795. [Google Scholar]
- AlMohimeed, I.; Turkistani, H.; Ono, Y. Development of wearable and flexible ultrasonic sensor for skeletal muscle monitoring. In Proceedings of the 2013 Ieee International Ultrasonics Symposium (Ius), Prague, Czech Republic, 21–25 July 2013; pp. 1137–1140. [Google Scholar]
- AlMohimeed, I. Development of Wearable Ultrasonic Sensors for Monitoring Muscle Contraction. Master Thesis, Carleton University, Ottawa, ON, Canada, 2013. [Google Scholar]
- Liu, W.; Zhu, C.; Wu, D. Flexible piezoelectric micro ultrasonic transducer array integrated on various flexible substrates. Sens. Actuators A Phys. 2021, 317, 112476. [Google Scholar] [CrossRef]
- Hu, H. Continuous Monitoring of Deep Tissue with a Stretchable Ultrasonic Patch. Ph.D. Dissertation, UC San Diego, La Jolla, CA, USA, 2021. [Google Scholar]
- Hu, H.; Zhu, X.; Wang, C.; Zhang, L.; Li, X.; Lee, S.; Huang, Z.; Chen, R.; Chen, Z.; Wang, C. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 2018, 4, eaar3979. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; Xu, S. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Wang, C.; Qi, B.; Lin, M.; Zhang, Z.; Makihata, M.; Liu, B.; Zhou, S.; Huang, Y.-h.; Hu, H.; Gu, Y. Continuous monitoring of deep-tissue haemodynamics with stretchable ultrasonic phased arrays. Nat. Biomed. Eng. 2021, 5, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, V.; Dehghanzadeh, P.; Enwia, G.; Bayat, M.; Majerus, S.J.; Mandal, S. Flexible body-conformal ultrasound patches for image-guided neuromodulation. IEEE Trans. Biomed. Circuits Syst. 2019, 14, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Wang, L.; Makihata, M.; Liu, H.-C.; Zhou, T.; Zhao, X. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 2022, 377, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Nuckols, R.W.; Lee, S.; Swaminathan, K.; Orzel, D.; Howe, R.D.; Walsh, C.J. Individualization of exosuit assistance based on measured muscle dynamics during versatile walking. Sci. Robot. 2021, 6, eabj1362. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.; Choe, J.W.; Lee, B.C.; Cristman, P.; Oralkan, Ö.; Khuri-Yakub, B.T. Miniaturized, wearable, ultrasound probe for on-demand ultrasound screening. In Proceedings of the 2011 IEEE International Ultrasonics Symposium, Orlando, FL, USA, 18–21 October 2011; pp. 1060–1063. [Google Scholar]
- Kenny, J.-É.S.; Munding, C.E.; Eibl, J.K.; Eibl, A.M.; Long, B.F.; Boyes, A.; Yin, J.; Verrecchia, P.; Parrotta, M.; Gatzke, R. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Kenny, J.-É.S. Functional hemodynamic monitoring with a wireless ultrasound patch. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Munding, C.; Acconcia, C.; Elfarnawany, M.; Eibl, J.; Verrecchia, P.; Leonard, P.; Boyes, A.; Yang, Z.; Atoui, R.; Demore, C. In vitro and clinical demonstration of relative velocity measurements with the Flopatch™: A wearable Doppler ultrasound patch. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar]
- Kenny, J.-É.S.; Clarke, G.; Myers, M.; Elfarnawany, M.; Eibl, A.M.; Eibl, J.K.; Nalla, B.; Atoui, R. A wireless wearable doppler ultrasound detects changing stroke volume: Proof-of-Principle comparison with trans-esophageal echocardiography during coronary bypass surgery. Bioengineering 2021, 8, 203. [Google Scholar] [CrossRef]
- Gerardo, C.D.; Cretu, E.; Rohling, R. Fabrication and testing of polymer-based capacitive micromachined ultrasound transducers for medical imaging. Microsyst. Nanoeng. 2018, 4, 1–12. [Google Scholar] [CrossRef]
- Omidvar, A.; Gerardo, C.D.; Rohling, R.; Cretu, E.; Hodgson, A.J. Flexible polymer-based capacitive micromachined ultrasound transducers (polyCMUTs): Fabrication and characterization. In Proceedings of the 2021 IEEE International Ultrasonics Symposium (IUS), Xi’an, China, 11–16 September 2021; pp. 1–4. [Google Scholar]
- Gerardo, C.D.; Cretu, E.; Rohling, R. Fabrication of circuits on flexible substrates using conductive SU-8 for sensing applications. Sensors 2017, 17, 1420. [Google Scholar] [CrossRef]
- Tuysuzoglu, A.; Tan, J.; Eissa, K.; Kiraly, A.P.; Diallo, M.; Kamen, A. Deep adversarial context-aware landmark detection for ultrasound imaging. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Granada, Spain, 16–20 September 2018; pp. 151–158. [Google Scholar]
- Li, H.; Weng, J.; Shi, Y.; Gu, W.; Mao, Y.; Wang, Y.; Liu, W.; Zhang, J. An improved deep learning approach for detection of thyroid papillary cancer in ultrasound images. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Diamant, A.; Chatterjee, A.; Vallières, M.; Shenouda, G.; Seuntjens, J. Deep learning in head & neck cancer outcome prediction. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Chao, P.C.-P.; Chiang, P.-Y.; Kao, Y.-H.; Tu, T.-Y.; Yang, C.-Y.; Tarng, D.-C.; Wey, C.-L. A portable, wireless photoplethysomography sensor for assessing health of arteriovenous fistula using class-weighted support vector machine. Sensors 2018, 18, 3854. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, X.; Koehl, L.; Tartare, G.; De Jonckheere, J. A wearable system for in-home and long-term assessment of fetal movement. IRBM 2020, 41, 205–211. [Google Scholar] [CrossRef]
- Mesbah, M.; Khlif, M.S.; Layeghy, S.; East, C.E.; Dong, S.; Brodtmann, A.; Colditz, P.B.; Boashash, B. Automatic fetal movement recognition from multi-channel accelerometry data. Comput. Methods Programs Biomed. 2021, 210, 106377. [Google Scholar] [CrossRef]
- Willemink, M.J.; Koszek, W.A.; Hardell, C.; Wu, J.; Fleischmann, D.; Harvey, H.; Folio, L.R.; Summers, R.M.; Rubin, D.L.; Lungren, M.P. Preparing medical imaging data for machine learning. Radiology 2020, 295, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Basak, A.; Ranganathan, V.; Bhunia, S. A wearable ultrasonic assembly for point-of-care autonomous diagnostics of malignant growth. In Proceedings of the 2013 IEEE Point-of-Care Healthcare Technologies (PHT), Bangalore, India, 16–18 January 2013; pp. 128–131. [Google Scholar]
- Hou, D.; Hou, R.; Hou, J. On-device Training for Breast Ultrasound Image Classification. In Proceedings of the 2020 10th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 6–8 January 2020; pp. 0078–0082. [Google Scholar]
- Mnih, V.; Kavukcuoglu, K.; Silver, D.; Rusu, A.A.; Veness, J.; Bellemare, M.G.; Graves, A.; Riedmiller, M.; Fidjeland, A.K.; Ostrovski, G. Human-level control through deep reinforcement learning. Nature 2015, 518, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Everett, M.; Liu, M.; How, J.P. Socially aware motion planning with deep reinforcement learning. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 1343–1350. [Google Scholar]
- Yuan, W.; Stork, J.A.; Kragic, D.; Wang, M.Y.; Hang, K. Rearrangement with nonprehensile manipulation using deep reinforcement learning. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, QLD, Australia, 21–25 May 2018; pp. 270–277. [Google Scholar]
- Burke, M.; Lu, K.; Angelov, D.; Straižys, A.; Innes, C.; Subr, K.; Ramamoorthy, S. Learning rewards for robotic ultrasound scanning using probabilistic temporal ranking. arXiv 2020, arXiv:2002.01240. [Google Scholar]
- Droste, R.; Drukker, L.; Papageorghiou, A.T.; Noble, J.A. Automatic probe movement guidance for freehand obstetric ultrasound. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Lima, Peru, 4–8 October 2020; pp. 583–592. [Google Scholar]
- Jarosik, P.; Lewandowski, M. Automatic ultrasound guidance based on deep reinforcement learning. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 475–478. [Google Scholar]
- Milletari, F.; Birodkar, V.; Sofka, M. Straight to the point: Reinforcement learning for user guidance in ultrasound. In Smart Ultrasound Imaging and Perinatal, Preterm and Paediatric Image Analysis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 3–10. [Google Scholar]
- Sharifrazi, D.; Alizadehsani, R.; Roshanzamir, M.; Joloudari, J.H.; Shoeibi, A.; Jafari, M.; Hussain, S.; Sani, Z.A.; Hasanzadeh, F.; Khozeimeh, F. Fusion of convolution neural network, support vector machine and Sobel filter for accurate detection of COVID-19 patients using X-ray images. Biomed. Signal Process. Control 2021, 68, 102622. [Google Scholar] [CrossRef]
- Oh, S.L.; Jahmunah, V.; Ooi, C.P.; Tan, R.-S.; Ciaccio, E.J.; Yamakawa, T.; Tanabe, M.; Kobayashi, M.; Acharya, U.R. Classification of heart sound signals using a novel deep WaveNet model. Comput. Methods Programs Biomed. 2020, 196, 105604. [Google Scholar] [CrossRef]
- Zhao, L.; Li, K.; Pu, B.; Chen, J.; Li, S.; Liao, X. An ultrasound standard plane detection model of fetal head based on multi-task learning and hybrid knowledge graph. Future Gener. Comput. Syst. 2022, 135, 234–243. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Chang, C.-M.; Liu, K.-T.; Lin, W.-K.; Chiang, H.-Y.; Chung, C.-W.; Ho, M.-R.; Sun, P.-R.; Yang, R.-L.; Chen, K.-T. Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digit. Med. 2019, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Guo, Y.; Wang, Y.; Yu, J.; Li, J.; Zhou, S.; Chang, C. Automatic tumor segmentation in breast ultrasound images using a dilated fully convolutional network combined with an active contour model. Med. Phys. 2019, 46, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, J.; Zhao, X.; Li, J.; Feng, J.; Fan, E. Breast cancer research and treatment reconstruction of unilateral breast structure using three-dimensional ultrasound imaging to assess breast neoplasm. Breast Cancer Res. Treat. 2019, 176, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, Q.; Xiao, Y.; Zheng, H.; Wang, C.; Luo, J. Ultrasound image reconstruction from plane wave radio-frequency data by self-supervised deep neural network. Med. Image Anal. 2021, 70, 102018. [Google Scholar] [CrossRef]
- Gupta, M.; Taneja, H.; Chand, L. Performance enhancement and analysis of filters in ultrasound image denoising. Procedia Comput. Sci. 2018, 132, 643–652. [Google Scholar] [CrossRef]
- Thring, C.; Band, F.; Irving, D.; McAughey, K.; Hughes, D.A. Novel, High Temperature, Low Frequency, Thin Film, NDT Ultrasound Transducers. In Proceedings of the 2020 IEEE International Ultrasonics Symposium (IUS), Las Vegas, NV, USA, 7–11 September 2020; pp. 1–3. [Google Scholar]
- Lammie, C.; Xiang, W.; Azghadi, M.R. Towards memristive deep learning systems for real-time mobile epileptic seizure prediction. In Proceedings of the 2021 IEEE International Symposium on Circuits and Systems (ISCAS), Daegu, Republic of Korea, 22–28 May 2021; pp. 1–5. [Google Scholar]
- Zhu, J.; Zhang, S.; Yu, R.; Liu, Z.; Gao, H.; Yue, B.; Liu, X.; Zheng, X.; Gao, M.; Wei, X. An efficient deep convolutional neural network model for visual localization and automatic diagnosis of thyroid nodules on ultrasound images. Quant. Imaging Med. Surg. 2021, 11, 1368. [Google Scholar] [CrossRef]
- Pesteie, M.; Lessoway, V.; Abolmaesumi, P.; Rohling, R.N. Automatic localization of the needle target for ultrasound-guided epidural injections. IEEE Trans. Med. Imaging 2017, 37, 81–92. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhao, L.; Hassan, R.; Ren, H. Review on Wearable System for Positioning Ultrasound Scanner. Machines 2023, 11, 325. https://doi.org/10.3390/machines11030325
Li L, Zhao L, Hassan R, Ren H. Review on Wearable System for Positioning Ultrasound Scanner. Machines. 2023; 11(3):325. https://doi.org/10.3390/machines11030325
Chicago/Turabian StyleLi, Lailu, Lei Zhao, Rayan Hassan, and Hongliang Ren. 2023. "Review on Wearable System for Positioning Ultrasound Scanner" Machines 11, no. 3: 325. https://doi.org/10.3390/machines11030325
APA StyleLi, L., Zhao, L., Hassan, R., & Ren, H. (2023). Review on Wearable System for Positioning Ultrasound Scanner. Machines, 11(3), 325. https://doi.org/10.3390/machines11030325







