Numerical Modeling of the Major Temporal Arcade Using BUMDA and Jacobi Polynomials
Abstract
1. Introduction
- A modeling strategy addressing both symmetric and asymmetric scenarios is presented to improve the MTA characterization.
- A BUMDA scheme together with Jacobi polynomials with the purpose of improve the modeling of the MTA.
- A set of MTA manual delineations for the benchmark DRIVE dataset has been released for scientific purposes
2. Materials and Methods
2.1. Database of the MTA Images
2.2. Jacobi Polynomials
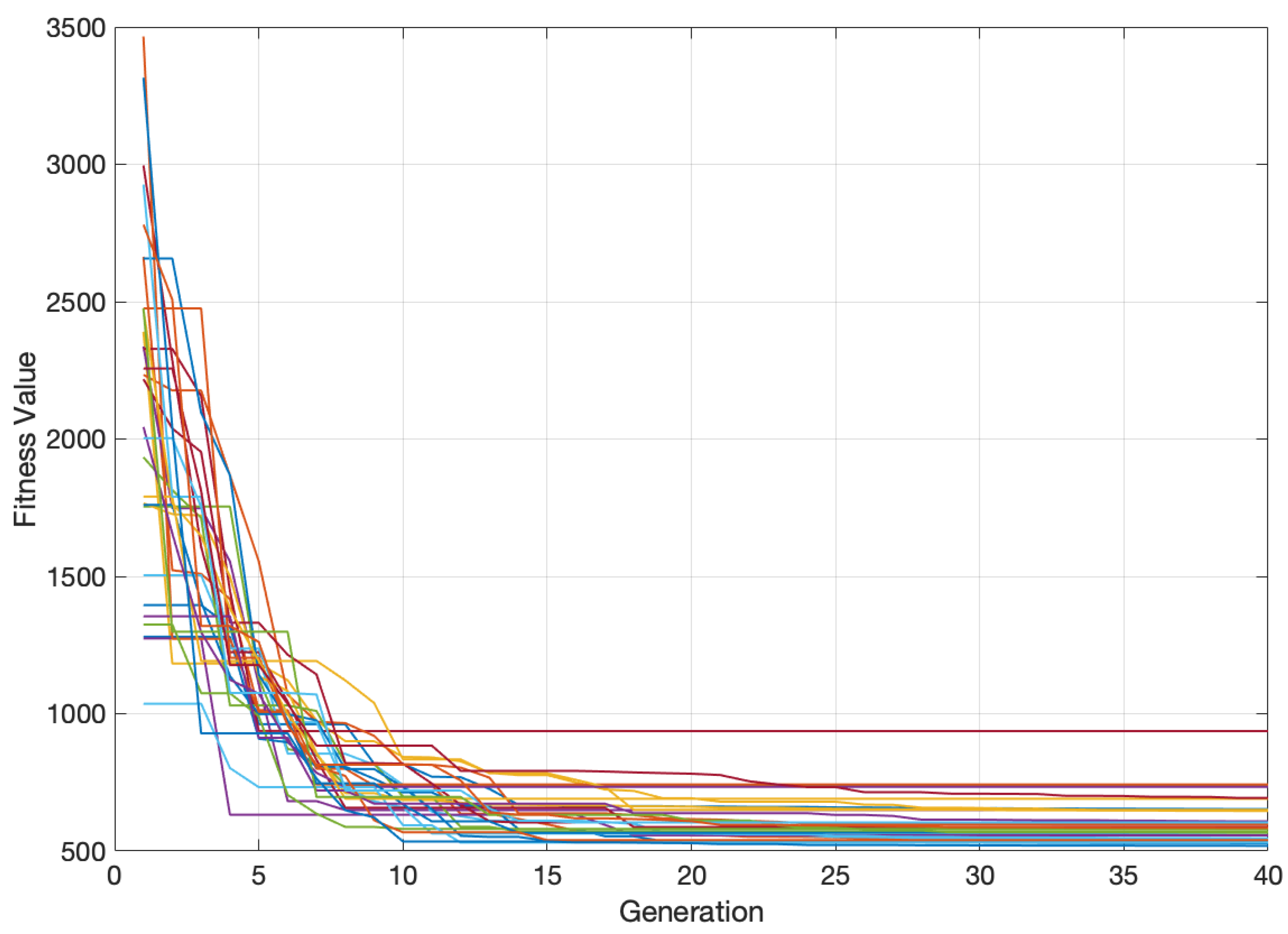
2.3. Boltzmann Univariate Marginal Distribution Algorithm (BUMDA)
| Algorithm 1 MTA numerical modeling by BUMDA |
Input:Population Size, Generations Output:
|
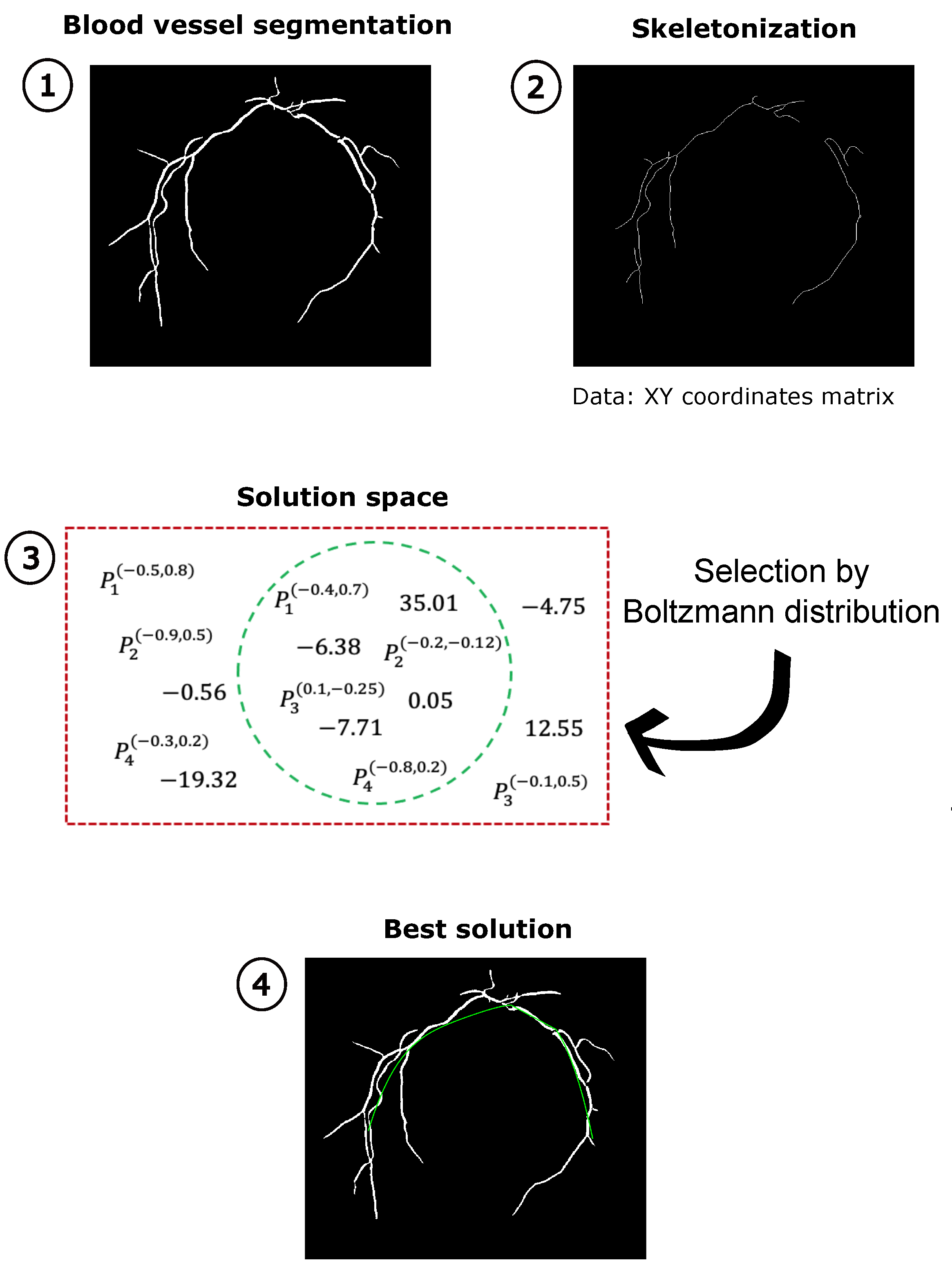
3. Proposed Method for the Numerical Modeling of the MTA
3.1. MTA Segmentation
3.2. BUMDA and Jacobi Polynomials
3.3. Evaluation Measures
| Algorithm 2 Proposed Method |
Input:Fundus Image Output:Best MTA fit
|
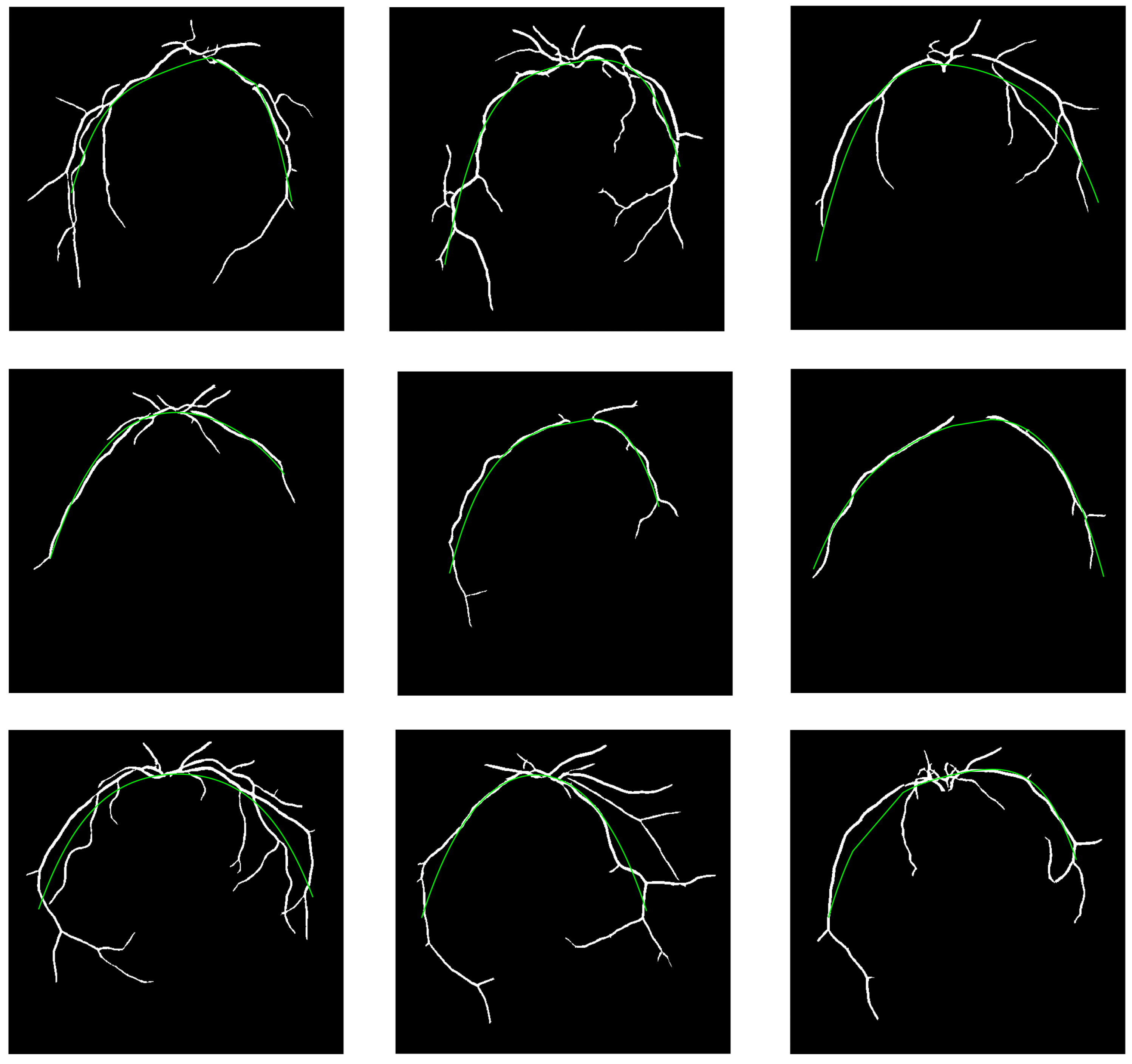
4. Experimental Results and Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MTA | Major Temporal Arcade |
| EDA | Estimation of Distribution Algorithm |
| ROP | Retinopathy of Prematurity |
| MDCP | Mean Distance to the Closest Point |
| RGB | Red, Green, Blue |
| BUMDA | Boltzmann Univariate Marginal Distribution Algorithm |
| Probability Density Function | |
| KL | Kullback–Liebler |
| DCP | Distance to the Closest Point |
References
- Rodríguez-Villalobos, Á.J.; Alvarado-Carrillo, D.E.; Cruz-Aceves, I.; Castellón-Lomelí, C.I.; López-Montero, L.M.; Hernández-González, M.A.; Giacinti, D.J. Estudio de la vida real sobre el modelado numérico de las arcadas temporales superiores e inferiores en imágenes de fondo de retina. Nova Sci. 2022, 14. [Google Scholar] [CrossRef]
- Teus, M.A.; Arranz-Márquez, E.; López-Guajardo, L.; Jiménez-Parras, R. Fondo de ojo. An. PediatrÍA Contin. 2007, 5, 163–166. [Google Scholar]
- Tenorio, G.; Ramírez-Sánchez, V. Retinopatía Diabética; conceptos actuales. Rev. MÉdica Del Hosp. Gen. MÉxico 2010, 73, 193–201. [Google Scholar]
- Oloumi, F.; Rangayyan, R.M.; Ells, A.L. Quantification of the changes in the openness of the major temporal arcade in retinal fundus images of preterm infants with plus disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6728–6735. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Cocker, K.D.; Moseley, M.J.; Paterson, C.; Clay, S.T.; Schulenburg, W.E.; Mills, M.D.; Ells, A.L.; Parker, K.H.; Quinn, G.E.; et al. Computerized analysis of retinal vessel width and tortuosity in premature infants. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Ng, J.; Ells, A.; Fielder, A.R.; Wilson, C.M. The temporal and nasal retinal arteriolar and venular angles in preterm infants. Br. J. Ophthalmol. 2011, 95, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Oloumi, F.; Rangayyan, R.M.; Ells, A.L. Quantitative analysis of the major temporal arcade in retinal fundus images of preterm infants for detection of plus disease. In Proceedings of the IASTED International Conference on Signal and Image Processing, Wuxi, China, 8–10 July 2013; pp. 464–469. [Google Scholar]
- Oloumi, F.; Rangayyan, R.M.; Ells, A.L. Tracking the major temporal arcade in retinal fundus images. In Proceedings of the 2014 IEEE 27th Canadian Conference on Electrical and Computer Engineering (CCECE), Toronto, ON, Canada, 4–7 May 2014; pp. 1–5. [Google Scholar]
- Grewal, J.S.; Fielder, A.R. Sequelae of retinopathy of prematurity. Paediatr. Child Health 2012, 22, 19–24. [Google Scholar] [CrossRef]
- Oloumi, F.; Rangayyan, R.M.; Ells, A.L. Parabolic modeling of the major temporal arcade in retinal fundus images. IEEE Trans. Instrum. Meas. 2012, 61, 1825–1838. [Google Scholar] [CrossRef]
- Oloumi, F.; Rangayyan, R.M.; Ells, A.L. Computer-aided diagnosis of proliferative diabetic retinopathy via modeling of the major temporal arcade in retinal fundus images. J. Digit. Imaging 2013, 26, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H. Generalizing the Hough transform to detect arbitrary shapes. Pattern Recognit. 1981, 13, 111–122. [Google Scholar] [CrossRef]
- Valdez, S.I.; Espinoza-Perez, S.; Cervantes-Sanchez, F.; Cruz-Aceves, I. Hybridization of the Univariate Marginal Distribution Algorithm with Simulated Annealing for Parametric Parabola Detection. In Hybrid Metaheuristics for Image Analysis; Springer: Cham, Switzerland, 2018; pp. 163–186. [Google Scholar]
- Giacinti, D.J.; Cervantes Sánchez, F.; Cruz Aceves, I.; Hernández González, M.A.; López Montero, L.M. Determinación de la parábola de la vasculatura de la retina mediante un algoritmo computacional de segmentación. Nova Sci. 2019, 11. [Google Scholar] [CrossRef]
- Alvarado-Carrillo, D.E.; Cruz-Aceves, I.; Hernández-González, M.A.; López-Montero, L.M. Robust Detection and Modeling of the Major Temporal Arcade in Retinal Fundus Images. Mathematics 2022, 10, 1334. [Google Scholar] [CrossRef]
- Staal, J.; Abràmoff, M.D.; Niemeijer, M.; Viergever, M.A.; Van Ginneken, B. Ridge-based vessel segmentation in color images of the retina. IEEE Trans. Med. Imaging 2004, 23, 501–509. [Google Scholar] [CrossRef]
- Van Assche, W. Ordinary Special Functions. In Encyclopedia of Mathematical Physics; Springer: Berlin, Germany, 2006; pp. 637–645. [Google Scholar]
- Pelikan, M.; Hauschild, M.W.; Lobo, F.G. Estimation of distribution algorithms. In Springer Handbook of Computational Intelligence; Springer: Berlin, Germany, 2015; pp. 899–928. [Google Scholar]
- de Anda-Suárez, J.; Carpio-Valadez, J.M.; Puga-Soberanes, H.J.; Calzada-Ledesma, V.; Rojas-Domínguez, A.; Jeyakumar, S.; Espinal, A. Symmetric-approximation energy-based estimation of distribution (SEED): A continuous optimization algorithm. IEEE Access 2019, 7, 154859–154871. [Google Scholar] [CrossRef]
- Valdez, S.I.; Hernández, A.; Botello, S. A Boltzmann based estimation of distribution algorithm. Inf. Sci. 2013, 236, 126–137. [Google Scholar] [CrossRef]
- Szeg, G. Orthogonal Polynomials; American Mathematical Soc.: Washington, DC, USA, 1939; Volume 23. [Google Scholar]
- Choksi, B.; Venkitaraman, A.; Mali, S. Finding best fit for hand-drawn curves using polynomial regression. Int. J. Comput. Appl. 2017, 975, 8887. [Google Scholar] [CrossRef]
- Tong, Y.; Yu, L.; Li, S.; Liu, J.; Qin, H.; Li, W. Polynomial fitting algorithm based on neural network. ASP Trans. Pattern Recognit. Intell. Syst. 2021, 1, 32–39. [Google Scholar] [CrossRef]
- Ameer, S. Investigating Polynomial Fitting Schemes for Image Compression. 2009. Available online: http://hdl.handle.net/10012/4255 (accessed on 1 December 2022).
- Cruz-Aceves, I.; Cervantes-Sanchez, F.; Avila-Garcia, M. A Novel Multiscale Gaussian-Matched Filter Using Neural Networks for the Segmentation of X-Ray Coronary Angiograms. J. Healthc. Eng. 2018, 11, 5812059. [Google Scholar] [CrossRef] [PubMed]
| MDCP (px.) | Hausdorff (px.) | Time (s) | |
|---|---|---|---|
| Mean | 7.51 | 26.27 | 3.44 |
| Median | 6.17 | 21.29 | 3.43 |
| Variance | 16.30 | 190.91 | 0.001 |
| Maximum | 21.36 | 69.92 | 3.54 |
| Minimum | 4.34 | 14.43 | 3.4 |
| Method | MDCP (px.) | Hausdorff (px.) |
|---|---|---|
| Mean ± Std. | Mean ± Std. | |
| General Hough | ||
| MIPAV | ||
| UMDA+SA | ||
| weigthed-RANSAC | ||
| Proposed Method |
| Method | Execution Time (s) |
|---|---|
| General Hough | (per pixel) |
| MIPAV | 230 |
| UMDA + SA | |
| weighted-RANSAC | |
| Proposed method |
| Method Compared | MDCP (px.) | Hausdorff (px.) |
|---|---|---|
| General Hough | ||
| MIPAV | ||
| UMDA + SA | ||
| weigthed-RANSAC |
| Method Compared | Execution Time Difference (s) |
|---|---|
| General Hough | very high |
| MIPAV | |
| UMDA + SA | |
| weighted-RANSAC |
| Image | Polynomial Serie | |
|---|---|---|
| 01_test | ||
| 03_test | ||
| 06_test | ||
| 07_test | ||
| 09_test | ||
| 12_test | ||
| 14_test | ||
| 16_test | ||
| 17_test |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Álvarez, J.A.; Cruz-Aceves, I.; Hernández-Aguirre, A.; Hernández-González, M.A.; López-Montero, L.M.; Solorio-Meza, S.E. Numerical Modeling of the Major Temporal Arcade Using BUMDA and Jacobi Polynomials. Axioms 2023, 12, 137. https://doi.org/10.3390/axioms12020137
Soto-Álvarez JA, Cruz-Aceves I, Hernández-Aguirre A, Hernández-González MA, López-Montero LM, Solorio-Meza SE. Numerical Modeling of the Major Temporal Arcade Using BUMDA and Jacobi Polynomials. Axioms. 2023; 12(2):137. https://doi.org/10.3390/axioms12020137
Chicago/Turabian StyleSoto-Álvarez, José Alfredo, Iván Cruz-Aceves, Arturo Hernández-Aguirre, Martha Alicia Hernández-González, Luis Miguel López-Montero, and Sergio Eduardo Solorio-Meza. 2023. "Numerical Modeling of the Major Temporal Arcade Using BUMDA and Jacobi Polynomials" Axioms 12, no. 2: 137. https://doi.org/10.3390/axioms12020137
APA StyleSoto-Álvarez, J. A., Cruz-Aceves, I., Hernández-Aguirre, A., Hernández-González, M. A., López-Montero, L. M., & Solorio-Meza, S. E. (2023). Numerical Modeling of the Major Temporal Arcade Using BUMDA and Jacobi Polynomials. Axioms, 12(2), 137. https://doi.org/10.3390/axioms12020137






