Mathematical Analysis of a Fractional COVID-19 Model Applied to Wuhan, Spain and Portugal
Abstract
:1. Introduction
- (i)
- (ii)
2. Preliminaries on Fractional Calculus
- and are continuous for all ;
- for all , where ω and λ are two positive constants.
3. The Considered Fractional-Order COVID-19 Model
- is the rate at which an individual leaves the exposed class by becoming infectious (symptomatic, super-spreaders or asymptomatic);
- is the proportion of progression from exposed class E to symptomatic infectious class I;
- is a relative very low rate at which exposed individuals become super-spreaders;
- is the progression from exposed to asymptomatic class;
- is the average rate at which symptomatic and super-spreaders individuals become hospitalized;
- is the recovery rate without being hospitalized;
- is the recovery rate of hospitalized patients;
- denotes the disease induced death rates due to infected individuals;
- denotes the disease induced death rates due to super-spreaders individuals;
- denotes the disease induced death rates due to hospitalized individuals.
4. Existence and Uniqueness of Positive Solution
5. Stability Analysis
6. Numerical Simulations
6.1. Population Size, Initial Conditions, and Parameters
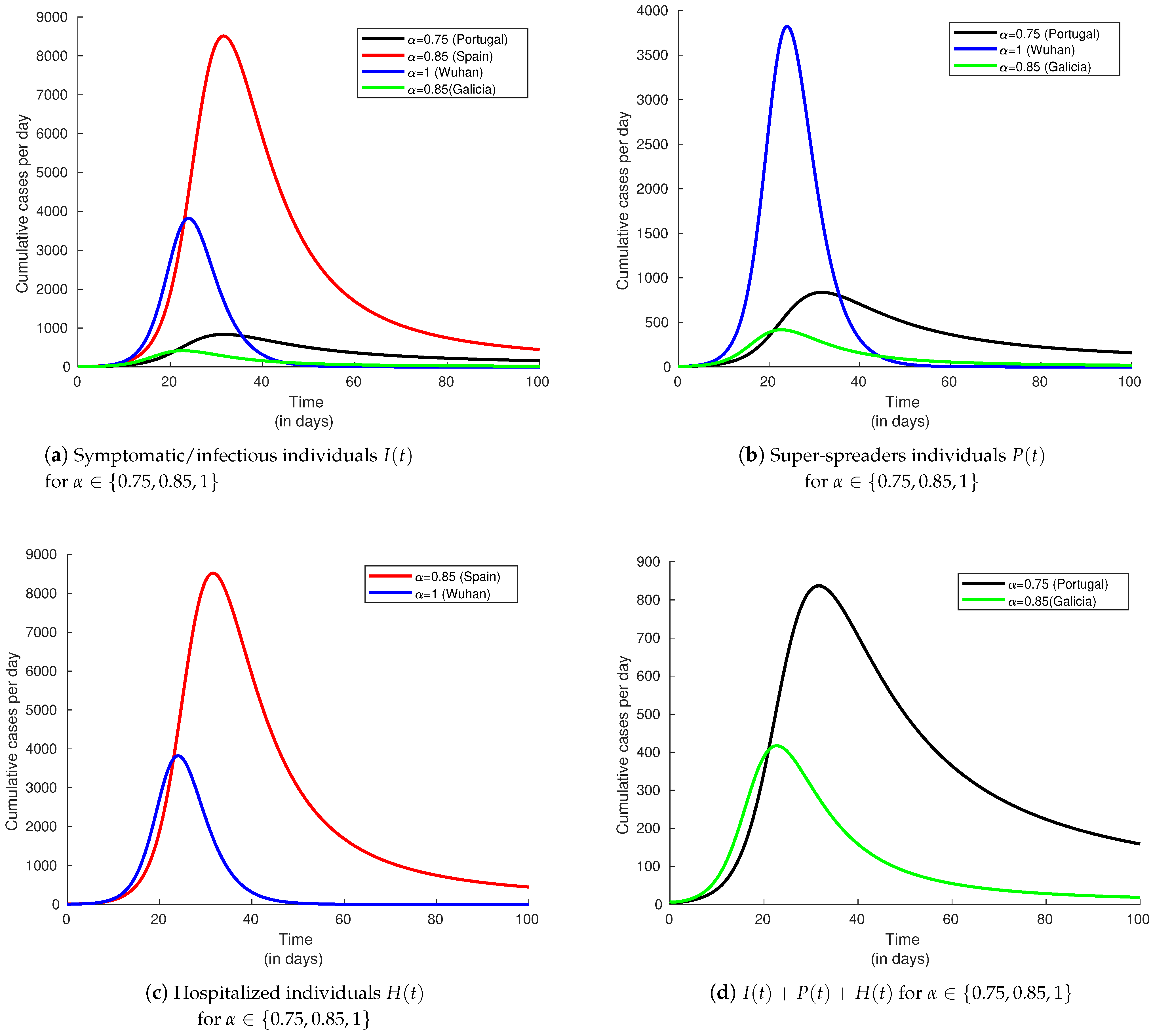
6.2. Index of Memory’s Influence
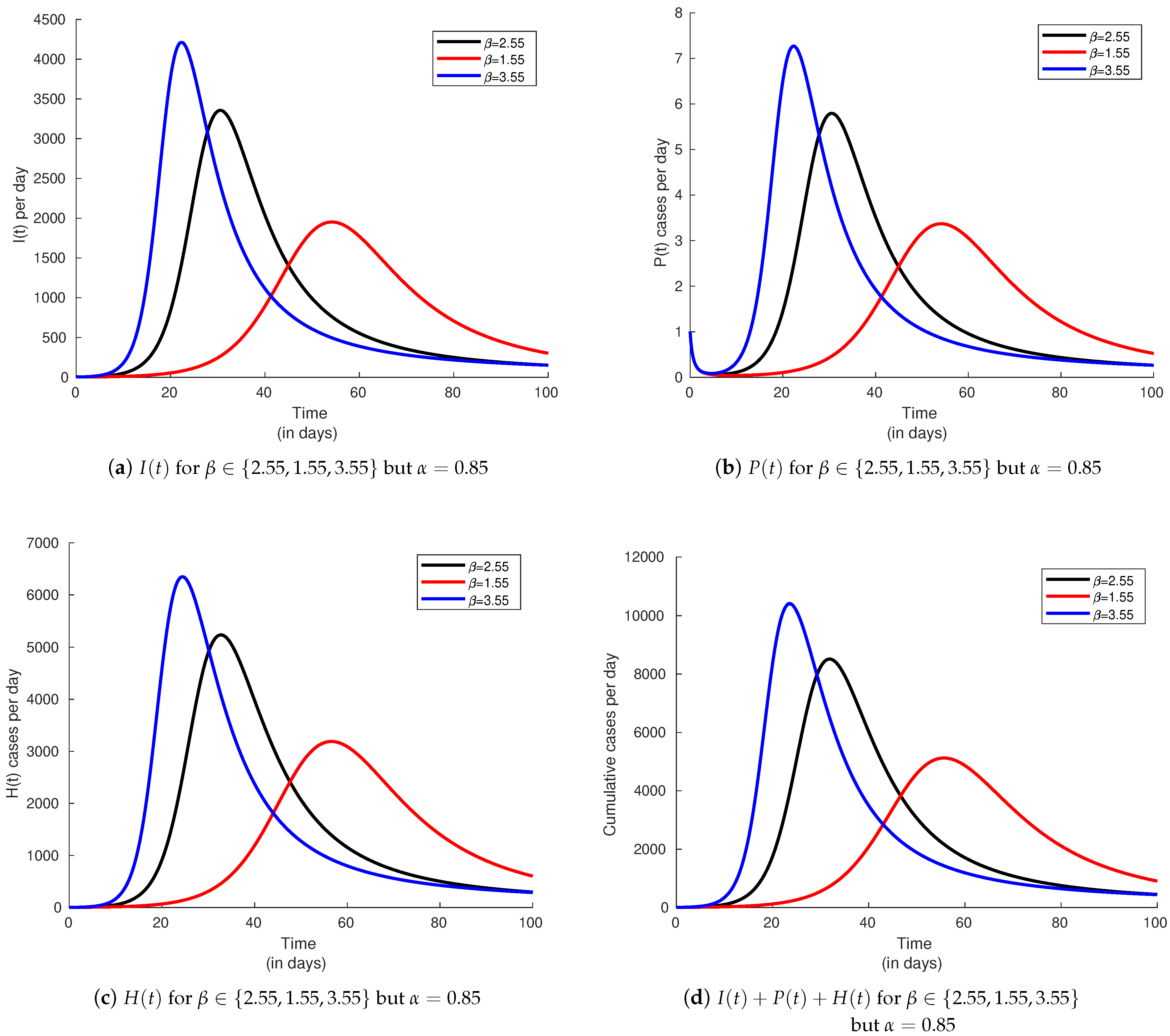
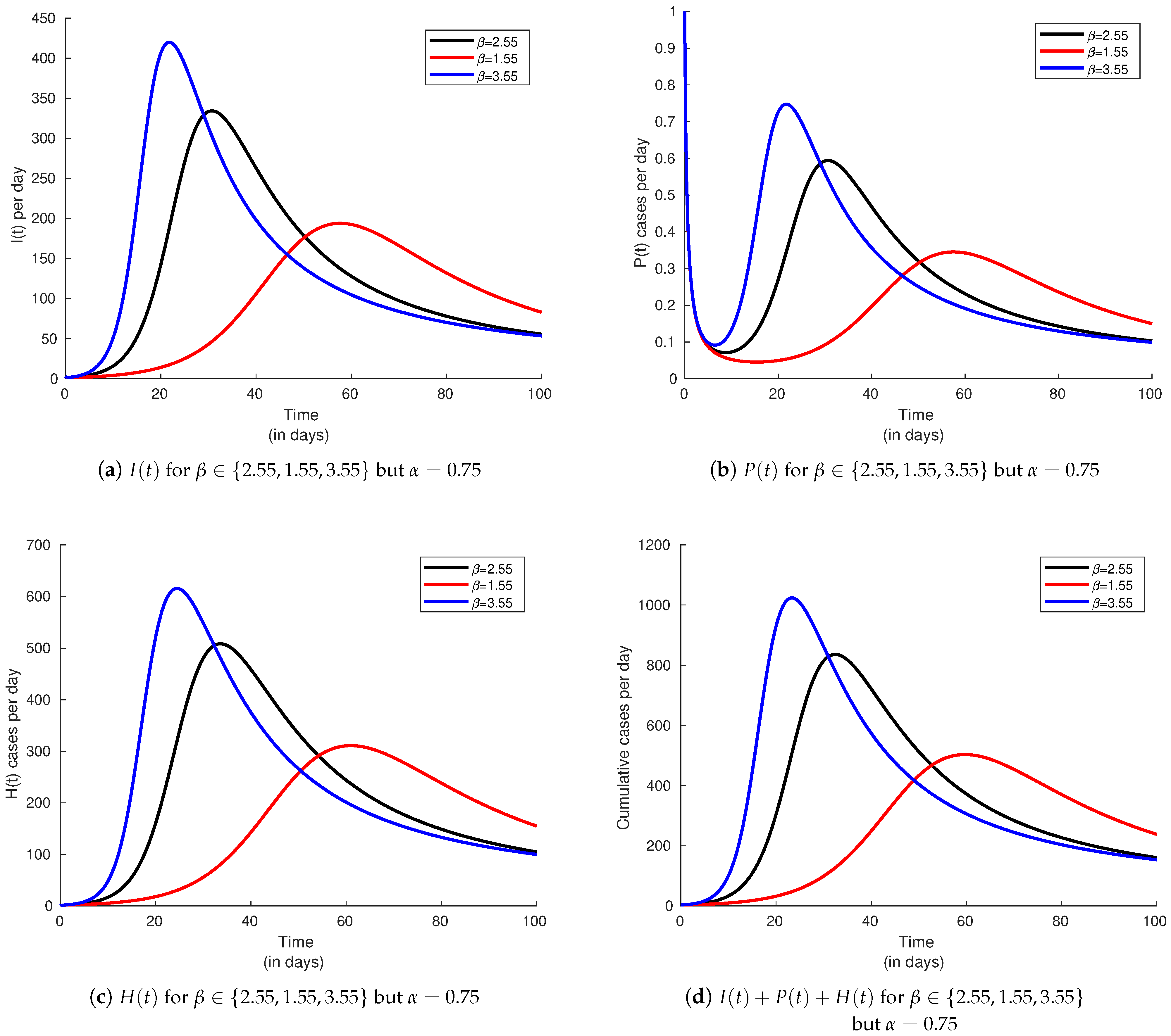
6.3. Infectivity Rate and Effect on the Basic Reproduction Number
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oukouomi Noutchie, S.C.; Atangana, A.; Goufo, E.F.D. On the Mathematical Analysis of Ebola Hemorrhagic Fever: Deathly Infection Disease in West African Countries. BioMed Res. Int. 2014, 2014, 261383. [Google Scholar]
- Yanover, C.; Ngwa, G.A.; Teboh-Ewungkem, M.I. A Mathematical Model with Quarantine States for the Dynamics of Ebola Virus Disease in Human Populations. Comput. Math. Methods Med. 2016, 2016, 9352725. [Google Scholar]
- Yuan, S.; Rachah, A.; Torres, D.F.M. Mathematical Modelling, Simulation, and Optimal Control of the 2014 Ebola Outbreak in West Africa. Discret. Dyn. Nat. Soc. 2015, 2015, 842792. [Google Scholar]
- Djordjevic, J.; Silva, C.J.; Torres, D.F.M. A stochastic SICA epidemic model for HIV transmission. Appl. Math. Lett. 2018, 84, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Duwal, S.; Winkelmann, S.; Schütte, C.; von Kleist, M. Optimal treatment strategies in the context of ’Treatment for Prevention’ against HIV-1 in resource-poor settings. PLoS Comput. Biol. 2015, 11, e1004200. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.J.; Torres, D.F.M. A TB-HIV/AIDS coinfection model and optimal control treatment. Discret. Contin. Dyn. Syst. A 2015, 35, 4639–4663. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, W.; Ruan, S. Analysis of SIR epidemic models with nonlinear incidence rate and treatment. Math. Biosci. 2012, 238, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hethcote, H.W.; van den Driessche, P. Some epidemiological models with nonlinear incidence. J. Math. Biol. 1991, 29, 271–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, V.M.; Espinoza, B.; Bichara, D.; Holechek, S.A.; Castillo-Chavez, C. Role of short-term dispersal on the dynamics of Zika virus in an extreme idealized environment. Infect. Dis. Model. 2017, 2, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Ndaïrou, F.; Area, I.; Nieto, J.J.; Silva, C.J.; Torres, D.F.M. Mathematical modeling of Zika disease in pregnant women and newborns with microcephaly in Brazil. Math. Methods Appl. Sci. 2018, 41, 8929–8941. [Google Scholar] [CrossRef] [Green Version]
- Ndaïrou, F.; Area, I.; Torres, D.F.M. Mathematical Modeling of Japanese Encephalitis under Aquatic Environmental Effects. Mathematics 2020, 8, 1880. [Google Scholar] [CrossRef]
- Rodrigues, H.S.; Monteiro, M.T.T.; Torres, D.F.M. Seasonality effects on dengue: Basic reproduction number, sensitivity analysis and optimal control. Math. Methods Appl. Sci. 2016, 39, 4671–4679. [Google Scholar] [CrossRef] [Green Version]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics I. Proc. R. Soc. Lond. 1927, 115, 700–721. [Google Scholar]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics II. Proc. R. Soc. Lond. 1932, 138, 55–83. [Google Scholar]
- Kermack, W.O.; McKendrick, A.G. Contributions to the mathematical theory of epidemics III. Proc. R. Soc. Lond. 1933, 141, 94–112. [Google Scholar]
- Area, I.; Losada, J.; Ndaïrou, F.; Nieto, J.J.; Tcheutia, D.D. Mathematical modeling of 2014 Ebola outbreak. Math. Methods Appl. Sci. 2017, 40, 6114–6122. [Google Scholar] [CrossRef]
- Jordan, Y.A.; Smith, P. Nonlinear Ordinary Differential Equations. An Introduction to Dynamical Systems; Oxford University Press Inc.: New York, NY, USA, 1999. [Google Scholar]
- Melnik, A.V.; Korobeinikov, A. Lyapunov functions and global stability for SIR and SEIR models with age-dependent susceptibility. Math. Biosci. Eng. 2013, 10, 369–378. [Google Scholar]
- Strogatz, S.H. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering; Addison-Wesley Pub: Reading, MA, USA, 1994. [Google Scholar]
- Teschl, G. Ordinary Differential Equations and Dynamical Systems; American Mathematical Society: Providence, RI, USA, 2012. [Google Scholar]
- Al-Sulami, H.; El-Shahed, M.; Nieto, J.J. On fractional order dengue epidemic model. Math. Probl. Eng. 2014. [Google Scholar] [CrossRef]
- Rosa, S.; Torres, D.F.M. Optimal control and sensitivity analysis of a fractional order TB model. Stat. Optim. Inf. Comput. 2019, 7, 617–625. [Google Scholar] [CrossRef] [Green Version]
- Ndaïrou, F.; Area, I.; Nieto, J.J.; Silva, C.J.; Torres, D.F.M. Fractional model of COVID-19 applied to Galicia, Spain and Portugal. Chaos Solitons Fractals 2021, 144, 110652. [Google Scholar] [CrossRef]
- Du, M.; Wang, Z.; Hu, H. Measuring memory with the order of fractional derivative. Sci. Rep. 2013, 3, 3431. [Google Scholar] [CrossRef] [PubMed]
- Noeiaghdam, S.; Micula, S.; Nieto, J.J. A Novel Technique to Control the Accuracy of a Nonlinear Fractional Order Model of COVID-19: Application of the CESTAC Method and the CADNA Library. Mathematics 2021, 9, 1321. [Google Scholar] [CrossRef]
- Rosa, S.; Torres, D.F.M. Optimal control of a fractional order epidemic model with application to human respiratory syncytial virus infection. Chaos Solitons Fractals 2018, 117, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Boukhouima, A.; Lotfi, E.M.; Mahrouf, M.; Rosa, S.; Torres, D.F.M.; Yousfi, N. Stability analysis and optimal control of a fractional HIV-AIDS epidemic model with memory and general incidence rate. Eur. Phys. J. Plus 2021, 136. [Google Scholar] [CrossRef]
- Samko, S.G.; Kilbas, A.A.; Marichev, O.I. Fractional Integrals and Derivatives; Gordon and Breach Science Publishers: Yverdon, Switzerland, 1993. [Google Scholar]
- Kilbas, A.A.; Srivastava, H.M.; Trujillo, J.J. Theory and Applications of Fractional Differential Equations; North-Holland Mathematics Studies; Elsevier Science B.V.: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Podlubny, I. Fractional Differential Equations; Academic Press, Inc.: San Diego, CA, USA, 1999. [Google Scholar]
- Sidi Ammi, M.R.; Tahiri, M.; Torres, D.F.M. Global Stability of a Caputo Fractional SIRS Model with General Incidence Rate. Math. Comput. Sci. 2021, 15, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Lin, W. Global existence theory and chaos control of fractional differential equations. J. Math. Anal. Appl. 2007, 332, 709–726. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.; Brito da Cruz, A.M.C.; Martins, N.; Monteiro, M.T.T. An epidemiological MSEIR model described by the Caputo fractional derivative. Int. J. Dyn. Control 2019, 7, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Ndaïrou, F.; Area, I.; Nieto, J.J.; Torres, D.F.M. Mathematical modeling of COVID-19 transmission dynamics with a case study of Wuhan. Chaos Solitons Fractals 2020, 135, 109846, reprinted in Chaos Solitons Fractals 2020, 141, 110311. [Google Scholar] [CrossRef]
- Van den Driessche, P.; Watmough, J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002, 180, 29–48. [Google Scholar] [CrossRef]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [PubMed] [Green Version]
- Huo, J.; Zhao, H.; Zhu, L. The effect of vaccines on backward bifurcation in a fractional order HIV model. Nonlinear Anal. Real World Appl. 2015, 26, 289–305. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndaïrou, F.; Torres, D.F.M. Mathematical Analysis of a Fractional COVID-19 Model Applied to Wuhan, Spain and Portugal. Axioms 2021, 10, 135. https://doi.org/10.3390/axioms10030135
Ndaïrou F, Torres DFM. Mathematical Analysis of a Fractional COVID-19 Model Applied to Wuhan, Spain and Portugal. Axioms. 2021; 10(3):135. https://doi.org/10.3390/axioms10030135
Chicago/Turabian StyleNdaïrou, Faïçal, and Delfim F. M. Torres. 2021. "Mathematical Analysis of a Fractional COVID-19 Model Applied to Wuhan, Spain and Portugal" Axioms 10, no. 3: 135. https://doi.org/10.3390/axioms10030135
APA StyleNdaïrou, F., & Torres, D. F. M. (2021). Mathematical Analysis of a Fractional COVID-19 Model Applied to Wuhan, Spain and Portugal. Axioms, 10(3), 135. https://doi.org/10.3390/axioms10030135






