A Strategic Pre-Mechanical Activation Approach for Reducing Acid Consumption and Ion Release on Acid Leaching of Lithium-Bearing Clays
Abstract
1. Introduction
2. Materials and Methods
Material
3. Method
Characterization of Active Powders
4. Results and Discussion
4.1. Effect of Leach Parameters on Li Recovery
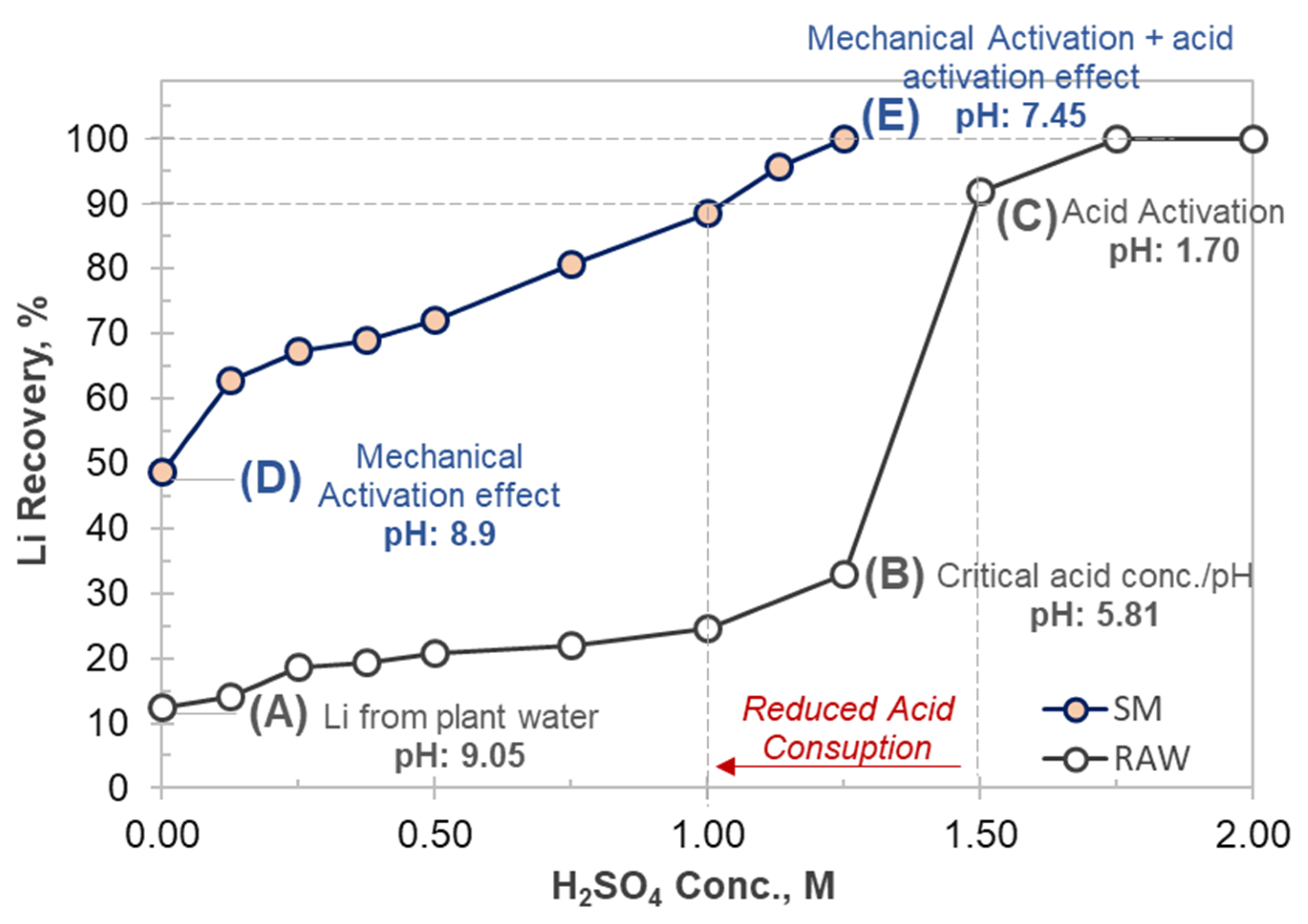
4.1.1. Effect of H2SO4 Concentration
4.1.2. Effect of Leach Time
4.1.3. Effect of Leach Temperature
4.1.4. Effect of Liquid to Solid Ratio
4.2. Discussion
4.3. Conceptual Leaching Mechanism of Mechanical Activation-Assisted Lithium Recovery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Büyükburç, A.; Maraşlıoğlu, D.; Bilici, M.S.U.; Köksal, G. Extraction of lithium from boron clays by using natural and waste materials and statistical modelling to achieve cost reduction. Miner. Eng. 2006, 19, 515517. [Google Scholar] [CrossRef]
- Seredin, V.V.; Tomson, I.N. The West Primorye noble-rare metal zone: A new Cenozoic metallogenic taxon in the Russian Far East. Dokl. Earth Sci. 2008, 421, 745–750. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Hoeck, M.; Bertau, M. Lithium market research-global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- King, H.E.; Salisbury, A.; Huijsmans, J.; Dzade, N.Y.; Plümper, O. Influence of Inorganic Solution Components on Lithium Carbonate Crystal Growth. Cryst. Growth Des. 2019, 19, 6994–7006. [Google Scholar] [CrossRef]
- Peiró, L.T.; Méndez, G.V.; Ayres, R.U. Lithium: Sources, production, uses, and recovery outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Asif, M.B.; Kentish, S.; Nghiem, L.D.; Hai, F.I. Lithium enrichment from a simulated Salt Lake brine using an integrated nanofiltration-membrane distillation process. J. Environ. Chem. Eng. 2019, 7, 103395. [Google Scholar] [CrossRef]
- Battaglia, G.; Berkemeyer, L.; Cipollina, A.; Cortina, J.L.; de Labastida, M.F.; Rodriguez, J.L.; Winter, D. Recovery of Lithium Carbonate from Dilute Li-Rich Brine via Homogenous and Heterogeneous Precipitation. Ind. Eng. Chem. Res. 2022, 61, 13589–13602. [Google Scholar] [CrossRef]
- Bonin, L.; Deduytsche, D.; Wolthers, M.; Flexer, V.; Rabaey, K. Boron extraction using selective ion exchange resins enables effective magnesium recovery from lithium rich brines with minimal lithium loss. Sep. Purif. Technol. 2021, 275, 119177. [Google Scholar] [CrossRef]
- Guo, Z.; Ji, Z.; Chen, Q.; Liu, J.; Zhao, Y.; Li, F.; Liu, Z.; Yuan, J. Prefractionation of LiCl from Concentrated seawater/Salt Lake brines by electrodialysis with monovalent selective ion Exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Balaz, P. Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Sun, S.; Ye, F.; Song, F.; Li, Y.; Wang, J.; Yu, J. Extraction of Lithium from Salt Lake Brine and Mechanism Research. Chin. J. Inorg. Chem. 2011, 27, 439–444. [Google Scholar]
- Ushak, S.; Gutierrez, A.; Galazutdinova, Y.; Barreneche, C.; Cabeza, L.F. Grágeda, Influence of alkaline chlorides on thermal energy storage properties of bischofite. Int. J. Energy Res. 2016, 40, 1556–1563. [Google Scholar] [CrossRef]
- Tesla Inc. Selective Extraction of Lithium from Clay Minerals. Patent US20210207243A1, 8 July 2021. [Google Scholar]
- Mordoğan, H.; Helvacı, C.; Malayoğlu, U. Lithium Occurrence in Borate Deposit Clays and Modern Lakes: Evaluation Possibilities. In Proceedings of the Industrial Raw Materials Symposium, İzmir, Turkey, 21 April 1995. [Google Scholar]
- Akyıldız, S. Evaluation of the Clays and Processing Wastes of the Kırka Borax Mine in Terms of Lithium Content. Master’s Thesis, Graduate School of Natural and Applied Sciences, Dokuz Eylul University, İzmir, Turkey, 2015. [Google Scholar]
- Celep, O.; Yazıcı, E.Y.; Deveci, H. Recovery of lithium from ores and brines. Mining 2022, 61, 105–120. [Google Scholar] [CrossRef]
- Ulusoy, M. Is Lithium the Oil of the Future? Metalurji 2016, 178, 45–48. [Google Scholar]
- Yörükoğlu, A.; Akkurt, F.; Karakaş, S.; Özkasapoğlu, S. Recovery of lithium from boron wastes and its economical evaluation. In Proceedings of the IMPC Eurasia Conference, Antalya, Turkey, 31 October 2019. [Google Scholar]
- Yücel, A.; Sarıkaya, M.; Yılmaz, H.C.; Depci, T. Extraction of lithium from boron ore wastes and precipitation as lithium carbonate. Can. Metall. Q. 2025, 64, 2437–2452. [Google Scholar] [CrossRef]
- Obut, A.; Ehsani, İ.; Aktosun, Z.; Yörükoğlu, A.; Girgin, İ.; Temel, A.; Deveci, H. Leaching behavior of lithium, cesium and rubidium from a clay sample of Kırka borate deposit in sulfuric acid solutions. J. Boron 2020, 5, 170–175. [Google Scholar]
- Yao, G.; Zang, H.; Wang, J.; Wu, P.; Qiu, J.; Lyu, X. Effect of mechanical activation on the pozzolanic activity of muscovite. Clays Clay Miner. 2019, 67, 209–216. [Google Scholar] [CrossRef]
- Manosa, J.; Calvo-de la Rosa, J.; Silvello, A.; Maldonado-Alameda Chimenos, A.J.M. Kaolinite structural modifications induced by mechanical activation. Appl. Clay Sci. 2023, 238, 106918. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemistry and mechanical activation of solids. Russ. Chem. Rev. 2006, 75, 177–189. [Google Scholar] [CrossRef]
- Mucsi, G. A Review on mechanical activation and mechanical alloying in stirred media mill. Chem. Eng. Res. Des. 2019, 148, 460–474. [Google Scholar] [CrossRef]
- Aladağ, M.; Erdem, M. Selective lithium leaching from dolomite-hosted clay-based boron extraction waste with oxalic acid to obtain a pregnant solution with a low Mg/Li ratio. Sep. Purif. Technol. 2025, 377, 134428. [Google Scholar] [CrossRef]
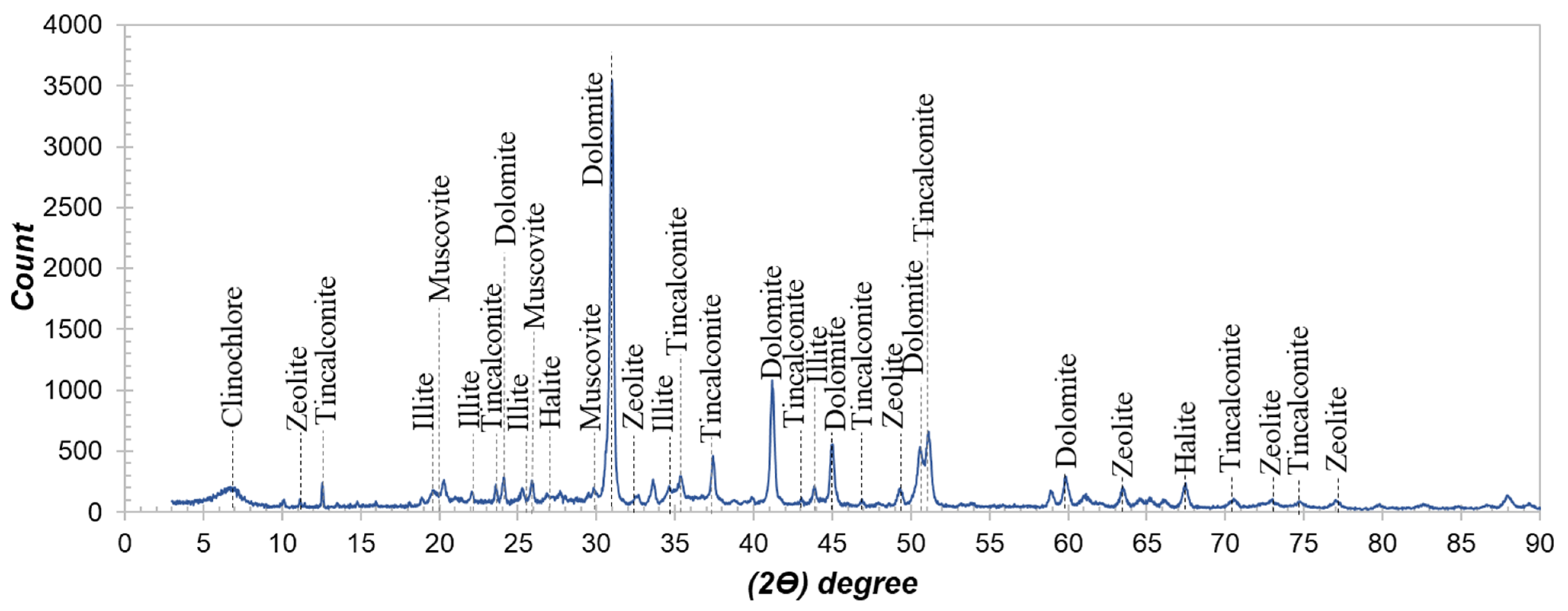

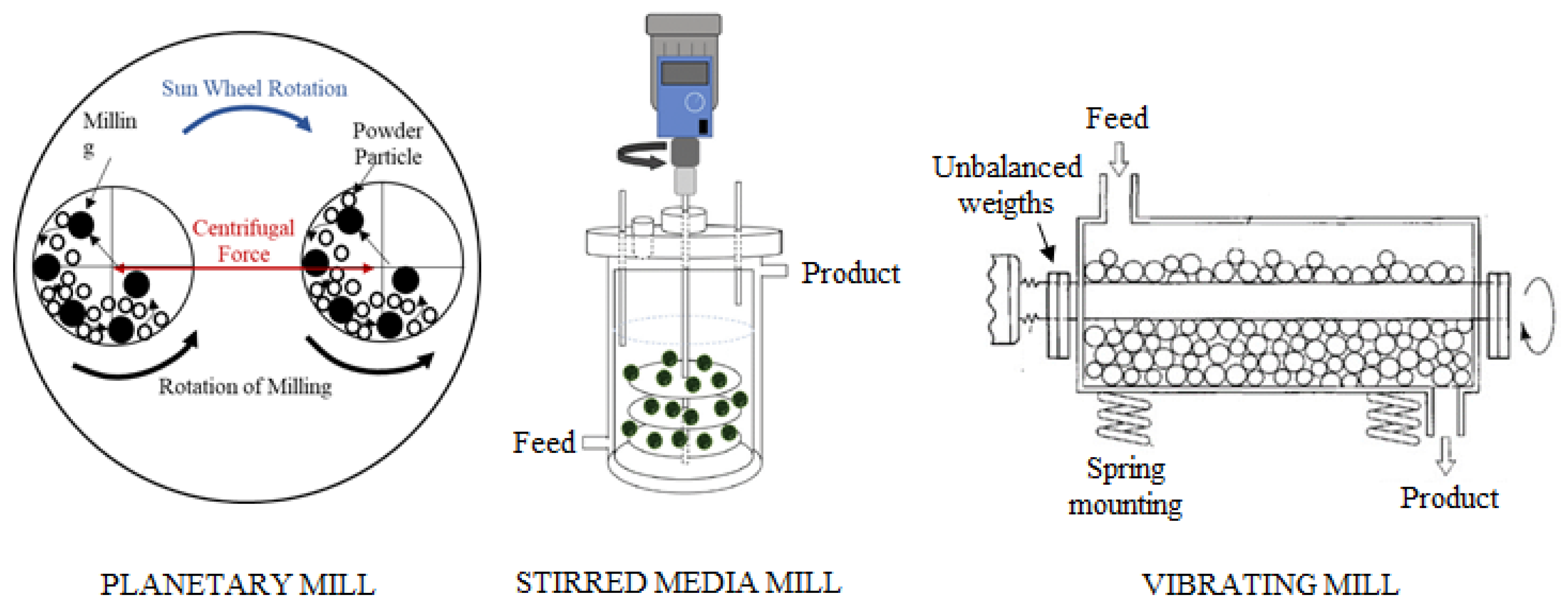

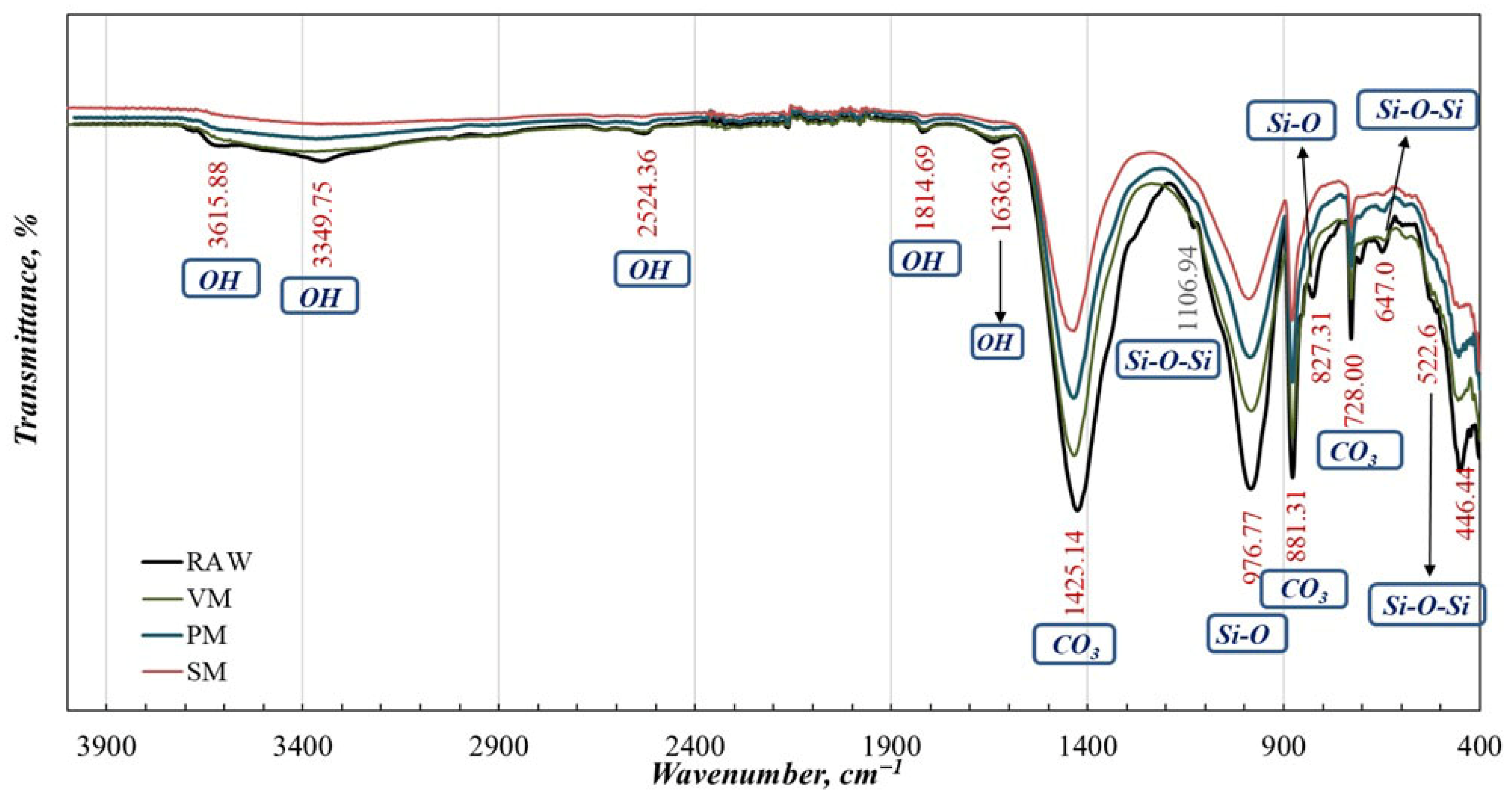


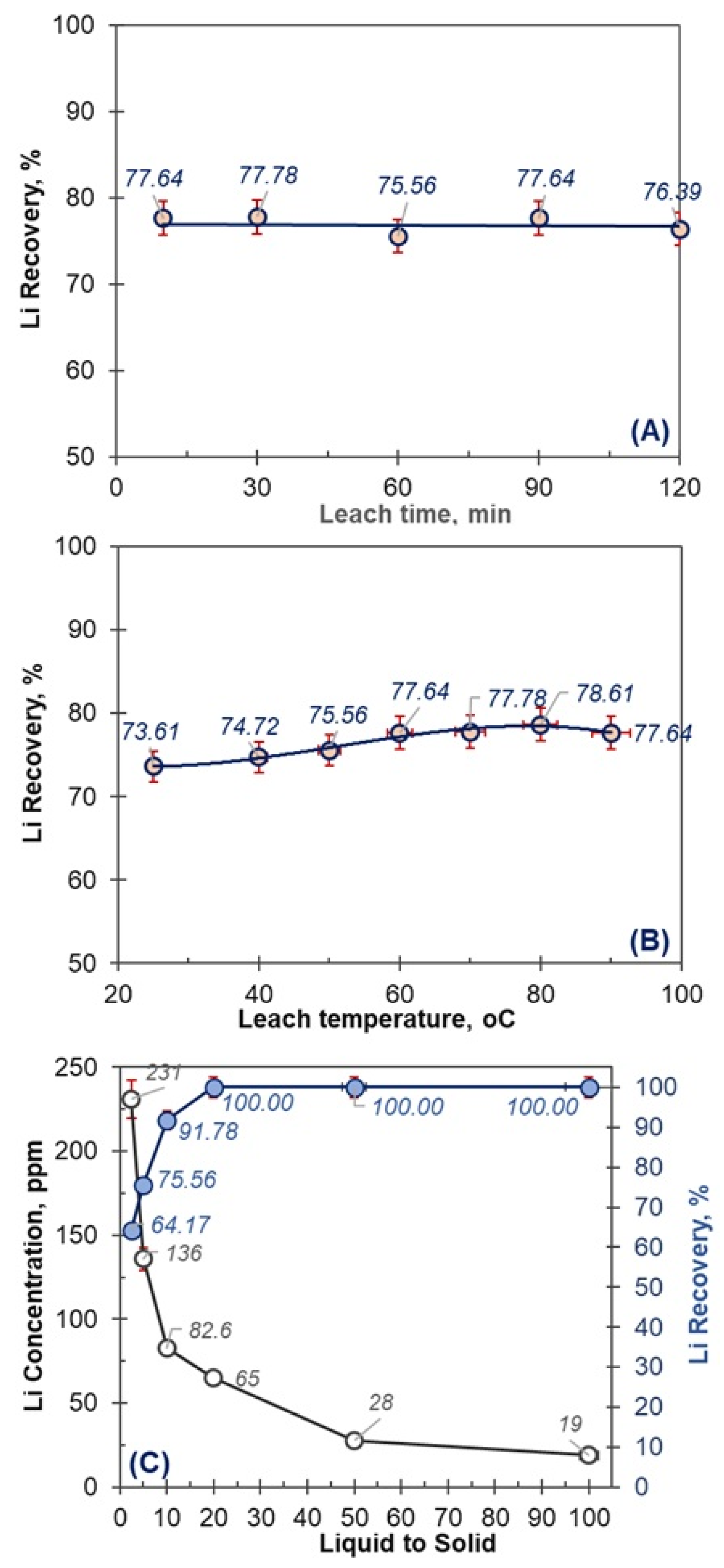
| Element | Li (ppm) | K (%) | Na (%) | Ca (%) | Mg (%) | Al (%) | Fe (%) | B2O3 (%) |
|---|---|---|---|---|---|---|---|---|
| Value | 900 | 0.62 | 2.15 | 11.16 | 10.16 | 0.69 | 0.25 | 10–12 |
| Mechanical Activation Device | Variables | Operating Ranges | Cell/Ball Properties |
|---|---|---|---|
| PM | BPR | ~20:1 | Monosize (8 mm) Zirconia Balls Cell interior coated with alumina Cell volume: 300 mL |
| Mixing time | 150 min | ||
| Mixing speed | 1500 rpm | ||
| SM | BPR | ~70:1 | Monosize (3 mm) Alumina Balls Cell interior coated with alumina Cell volume: 750 mL |
| Mixing time | 150 min | ||
| Mixing speed | 550 rpm | ||
| VM | BPR | ~140:1 | Monosize (10 mm) Steel Balls Cell interior coated with steel Cell volume: 750 mL |
| Mixing time | 150 min | ||
| Mixing speed | NA |
| Mech. Act. Device | Sample gr | Ball gr | Temp. at Act. * | Size, Micron | DoCr., % ** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D90 | D50 | D10 | D | T | Cl | I | M | Z | ||||
| Raw | - | - | - | 102.5 | 14.6 | 0.90 | 31.5 | 10.4 | 1.7 | 7.2 | 5.8 | 4.4 |
| PM | 15.0 | 280 | ~50–60 | 103.9 | 11.7 | 0.97 | 36.4 | 10.6 | 0.7 | 5.9 | 2.9 | 3.9 |
| SM | 15.0 | 1000 | ~30–40 | 81.0 | 11.6 | 0.94 | 32.2 | 10.2 | 1.1 | 5.5 | 3.3 | 3.7 |
| VM | 15.0 | 2000 | ~25–35 | 62.3 | 13.8 | 1.24 | 32.4 | 10.3 | 1.4 | 6.2 | 4.2 | 4.2 |
| Sample | H2SO4 (M) | Leach Temp. (°C) | Leach Time (min) | Ion Concentration in Leachate, ppm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Al3+ | Fe3+ | B2O3− | ||||
| Raw | 0 * | 80 | 20 | 56.0 | 9759 | 2.5 | 9.8 | 0.061 | 0.10 | 14,612 |
| 0.25 | 50 | 60 | 54.2 | 5102 | 531.4 | 1407 | 0.00 | 0.05 | 12,510 | |
| 0.50 | 60.7 | 5460 | 558.7 | 4300 | 0.00 | 0.05 | 12,000 | |||
| 0.75 | 68.1 | 5402 | 561.5 | 6760 | 0.00 | 0.05 | 12,630 | |||
| 1.00 | 72.4 | 5317 | 564.5 | 9422 | 0.00 | 0.05 | 12,285 | |||
| 1.25 | 72.5 | 4984 | 541.0 | 13,018 | 0.00 | 1.82 | 11,690 | |||
| 1.50 | 125.8 | 5512 | 564.1 | 17,599 | 201.6 | 72.74 | 13,160 | |||
| 1.75 | 192.4 | 5700 | 548.3 | 21,060 | 341.7 | 117.6 | 7890 | |||
| SMill Active powder | 0.125 | 50 | 60 | 156.9 | 4588 | 382.3 | 1204 | 0.00 | 0.02 | 6900 |
| 0.250 | 184.5 | 4846 | 826.1 | 2603 | 0.00 | 0.03 | 8080 | |||
| 0.375 | 186.1 | 5107 | 619.7 | 3840 | 0.00 | 0.00 | 9890 | |||
| 0.500 | 203.3 | 4669 | 583.7 | 4833 | 0.00 | 0.05 | 10,155 | |||
| 0.75 | 212.3 | 5087 | 599.9 | 8233 | 0.00 | 0.05 | 10,185 | |||
| 1.00 | 230.6 | 4888 | 576.6 | 10,603 | 0.00 | 0.03 | 10,830 | |||
| 1.25 | 262.8 | 5058 | 442.6 | 15,113 | 0.00 | 1.65 | 11,775 | |||
| Process | Conditions | Li Rec, % | Source |
|---|---|---|---|
| Acid Leach | 160 g/L H2SO4 (~1.63 M H2SO4) It was stated that the Mg conc. in leachate were in the levels of 20,000 ppm | 99.0 | [17] |
| 2 M H2SO4, @90 °C | 97.2 | [23] | |
| 0.5 M Oxalic Acid for 60–120 min leach time at over 75 °C It was reported that the dissolution of Mg ions was prevented by the use of oxalic acid. | >95 | [28] | |
| 1.50–1.75 M H2SO4 5–30 min leach time at 60 °C | >90 | This Study | |
| 1.75–2.00 M H2SO4 5–30 min leach time at 60 °C | ~100.0 | ||
| Roasting + Water Leach | @ 900 °C (10% Solids) | 77.0 | [17] |
| Mixture of clay + limestone + gypsum | 82.0 | [20] | |
| Mixture of clay + limestone + gypsum (@ 950 °C for 60 min) | 89.4 | [21] | |
| Mixture of clay + CaS/CaC: 5/2/2 (@ 900 °C for 60 min) | 97.2 | [22] | |
| Mechanical Activation + Acid Leach | Stirred mill activation 1.0 M H2SO4 5–30 min leach time at 60 °C | >90 | This Study |
| Stirred mill activation 1.25 M H2SO4 5–30 min leach time at 60 °C | ~100.0 | ||
| Planetary mill activation 1.0 M H2SO4 5–30 min leach time at 60 °C | >90 | ||
| Planetary mill activation 1.25 M H2SO4 5–30 min leach time at 60 °C | ~100.0 | ||
| Vibrating mill activation 1.0 M H2SO4 5–30 min leach time at 60 °C | >90 | ||
| Vibrating mill activation 1.25 M H2SO4 5–30 min leach time at 60 °C | ~100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Boylu, F.; Obuz Teker, G.; Ersever Angur, G.; Özdemir, O.; Güven, O.; Celik, M.S. A Strategic Pre-Mechanical Activation Approach for Reducing Acid Consumption and Ion Release on Acid Leaching of Lithium-Bearing Clays. Minerals 2026, 16, 3. https://doi.org/10.3390/min16010003
Boylu F, Obuz Teker G, Ersever Angur G, Özdemir O, Güven O, Celik MS. A Strategic Pre-Mechanical Activation Approach for Reducing Acid Consumption and Ion Release on Acid Leaching of Lithium-Bearing Clays. Minerals. 2026; 16(1):3. https://doi.org/10.3390/min16010003
Chicago/Turabian StyleBoylu, Feridun, Gülsen Obuz Teker, Gafure Ersever Angur, Orhan Özdemir, Onur Güven, and Mehmet S. Celik. 2026. "A Strategic Pre-Mechanical Activation Approach for Reducing Acid Consumption and Ion Release on Acid Leaching of Lithium-Bearing Clays" Minerals 16, no. 1: 3. https://doi.org/10.3390/min16010003
APA StyleBoylu, F., Obuz Teker, G., Ersever Angur, G., Özdemir, O., Güven, O., & Celik, M. S. (2026). A Strategic Pre-Mechanical Activation Approach for Reducing Acid Consumption and Ion Release on Acid Leaching of Lithium-Bearing Clays. Minerals, 16(1), 3. https://doi.org/10.3390/min16010003









