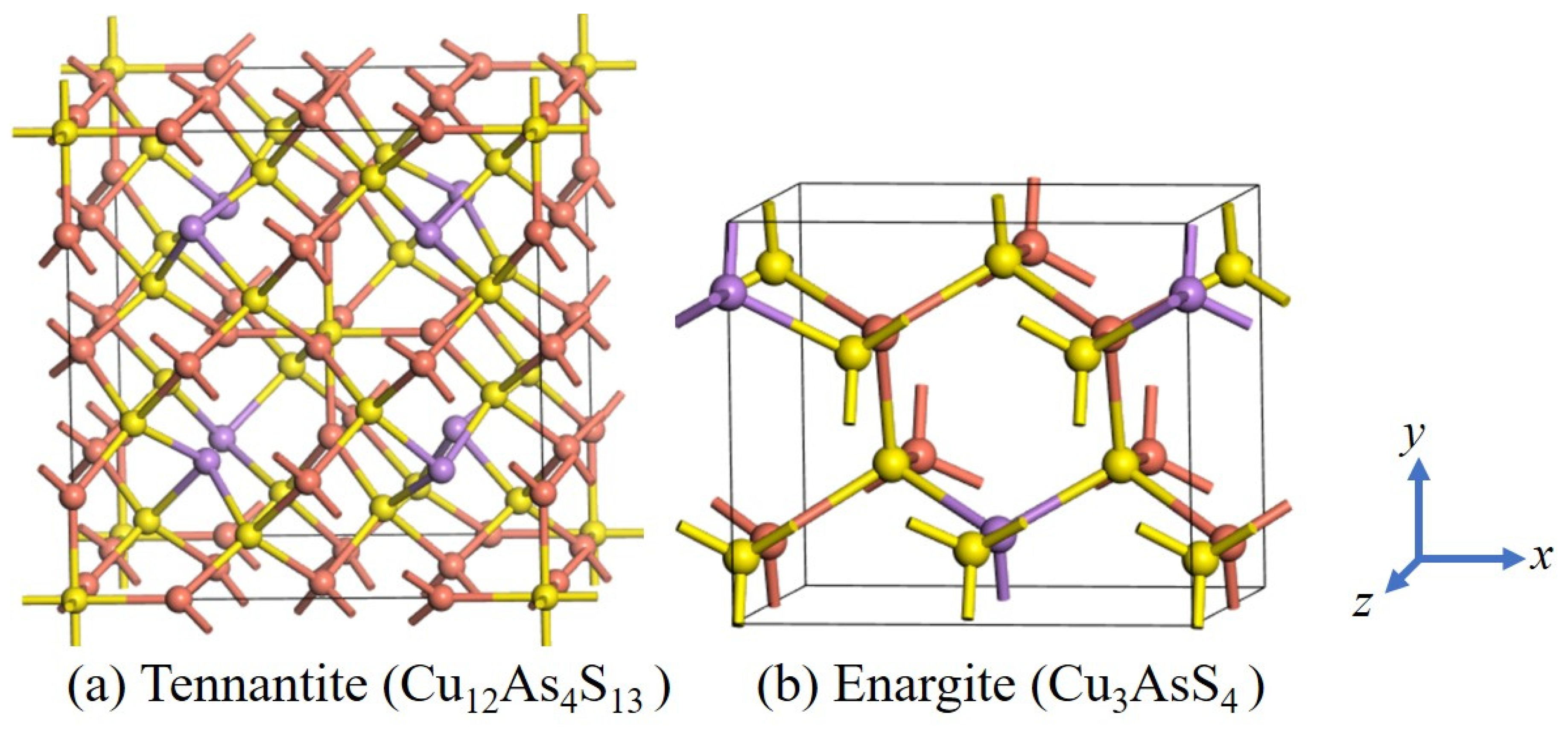
3.1. Crystal Structures of Tennantite and Enargite
The chemical formulas of tennantite and enargite are Cu
12As
4S
13 and Cu
3AsS
4, respectively. The former belongs to the cubic crystal system, while the latter belongs to the orthorhombic crystal system. Their structural models are shown in
Figure 1. The calculated lattice constant for tennantite is
a =
b =
c = 10.254 Å, which is close to the experimental value of
a =
b =
c = 10.190 Å [
18], with a minor error of only 0.68%. For enargite, the calculated lattice constants are
a = 7.448 Å,
b = 6.441 Å, and
c = 6.162 Å, also close to the experimental values (
a = 7.426 Å,
b = 6.452 Å,
c = 6.163 Å) [
19], with errors within a very small margin of 0.3%. These results confirm the reliability of the calculations.
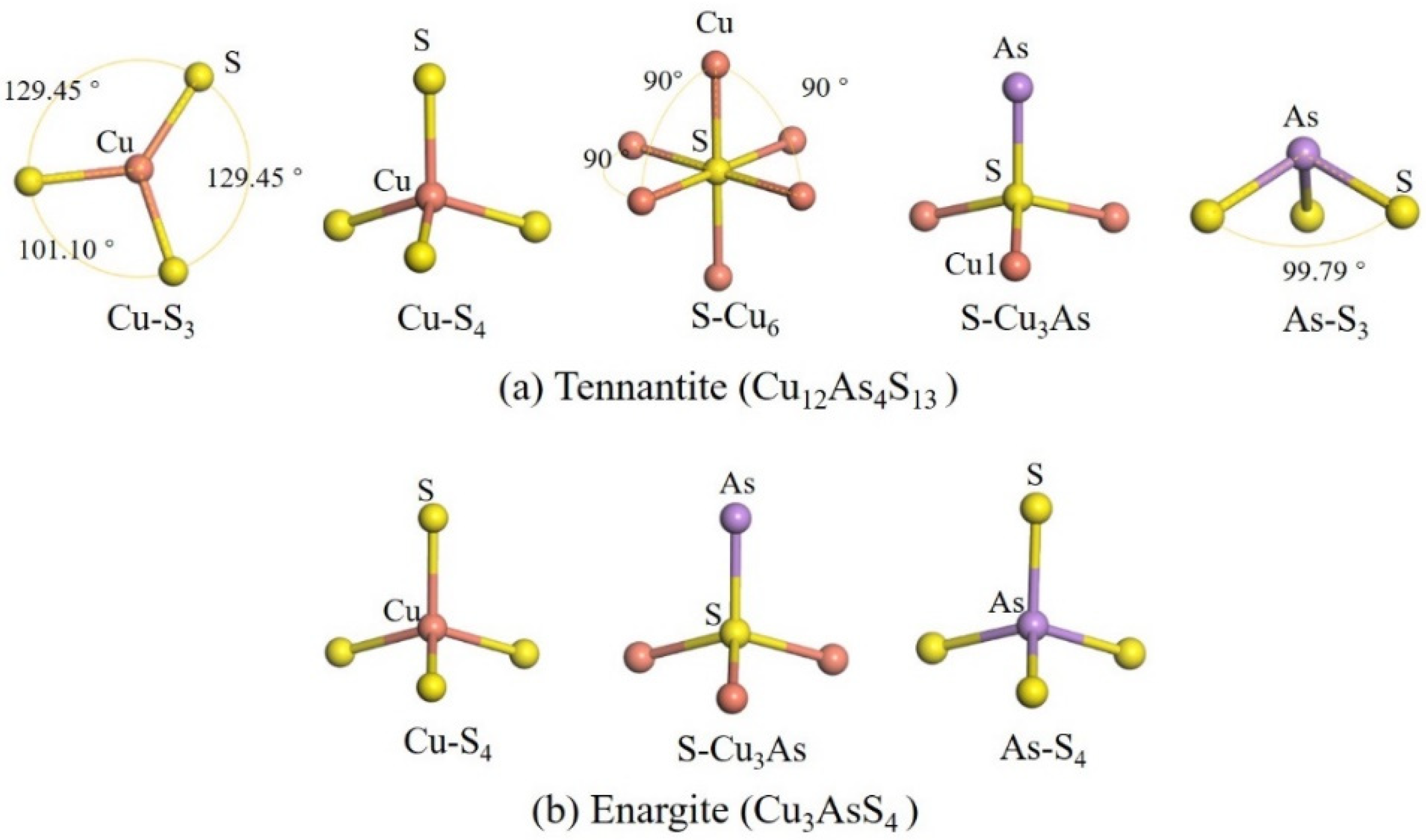
Figure 2 shows the coordination environments of the atoms. In tennantite, Cu atoms exhibit both 3-fold and 4-fold coordination, present in equal amounts [
4], forming planar triangular and tetrahedral structures, respectively. As atoms are 3-coordinated, resembling the ammonia molecule structure. S atoms show both 4-fold and 6-fold coordination, forming tetrahedral and octahedral structures. In enargite, all Cu, As, and S atoms are 4-coordinated.
Regarding bonding, in both minerals, Cu atoms bond only with S atoms and not directly with As atoms, while S atoms bond with both Cu and As atoms. However, in tennantite, S atoms form a 6-coordinated structure only when bonded exclusively to Cu atoms; when bonded to both Cu and As atoms, they form a 4-coordinated structure. The ratio of these two S coordination types is 1:12, meaning the 4-coordinated S atoms constitute the majority.
It can be observed that the coordination environments of Cu and As differ significantly between the two minerals, while the overall S environments are relatively similar (with the 6-coordinated S in tennantite being very rare). In tennantite, 3-coordinated (planar triangle) and 4-coordinated (tetrahedral) Cu atoms each account for half of the total Cu content, whereas in enargite, all Cu atoms are 4-coordinated (tetrahedral). This structural difference may influence their reactivity. The planar triangle structure represents a coordinatively unsaturated system for Cu, with potential coordination sites above and below the copper center that can interact with molecules without requiring the dissociation of an existing ligand to create space. This could lead to a lower energy barrier for molecular interactions. These factors suggest that the reactivity of these sites may be higher.
For the As in tennantite, which has a structure analogous to the arsenic hydride molecule (AsH3). In the presence of oxygen, this might also make it more susceptible to oxidation. These aspects require further surface studies.
Mulliken bond lengths and populations in the crystals were calculated, as shown in
Table 1. Bond population shows how the electron density is distributed between atoms. A value greater than zero indicates a bonding interaction, with higher values signifying stronger covalency. Values close to or less than zero may suggest ionic bonding, non-bonding, or anti-bonding interactions.
It can be seen that all bond population values are positive, indicating bonding interactions exist between the atoms. Since Cu does not bond directly with As, only Cu-S and As-S bonds are displayed. The population values for As-S bonds are significantly lower than those for Cu-S bonds, indicating their covalent character is weaker compared to Cu-S bonds.
In tennantite, the 3-coordinated Cu-S bonds are of two types. One has a bond length of 2.236 Å and a population value of 0.61, indicating very strong covalency. The other has a bond length of 2.270 Å and a population value of 0.35, representing the weakest covalency, even weaker than the longer 4-coordinated Cu-S bond (2.314 Å, population value 0.47). Further observation reveals that the S atoms in the weakest covalent Cu-S bond are in S-Cu6 configurations (i.e., S coordinated to six Cu atoms forming an octahedron), whereas the S atoms in the stronger covalent Cu-S bonds (population values 0.61 and 0.47) are in S-Cu3As configurations (i.e., S coordinated to three Cu atoms and one As atom forming a tetrahedron). This suggests that the arsenic environment enhances the covalency of the Cu-S bonds, potentially leading to increased hydrophobicity of the mineral.
Unlike tennantite, the Cu-S4 coordination in enargite is a distorted tetrahedron, with bond lengths ranging from 2.027 Å to 2.332 Å and population values between 0.38 and 0.56. The maximum bond length is longer than that in tennantite (2.314 Å), and the maximum population value is lower than that in tennantite (0.61). However, the minimum population value is higher than that in tennantite (0.35). Furthermore, the As-S bonds in enargite are also non-uniform, with lengths ranging from 2.252 Å to 2.265 Å and population values between 0.04 and 0.26. Overall, it is thought that tennantite exhibits stronger covalency, suggesting better hydrophobicity.
It is worth noting that the maximum Cu-S bonds in tennantite and enargite are larger than those in chalcopyrite, while the minimum Cu-S bonds are smaller than those in chalcopyrite [
20]. Additionally, the As-S bond lengths are much shorter than those in the arsenopyrite crystal [
21]. We have conducted research on chalcopyrite and arsenopyrite and found that the covalent property of Cu-S in tennantite and enargite is stronger than that in chalcopyrite, while that of As-S is weaker than that in arsenopyrite [
22,
23]. It is once again confirmed that the presence of arsenic can increase the covalent property of crystals.
3.2. Electronic Properties of Atoms
The Hirshfeld charges of the atoms were analyzed and shown in
Table 2. In Tennantite (Cu
12As
4S
13), both the 3-coordinated and 4-coordinated Cu atoms have a charge of +0.14 e. The 3-coordinated As has a charge of +0.17 e. The 4-coordinated S (S-Cu
3As) has a charge of −0.17 e, while the 6-coordinated S (S-Cu
6) has a charge of −0.27 e. In Enargite (Cu
3AsS
4), the 4-coordinated Cu and As have charges of +0.13 e and +0.26 e, respectively. The charges of the 4-coordinated S atoms range from −0.15 e to −0.17 e. It can be observed that the charge differences for Cu and S atoms between the two crystals are relatively small (although the charge of the 6-coordinated S in tennantite differs more significantly from the 4-coordinated S, it constitutes a very small fraction and can thus be neglected). However, there is a considerable difference in the charge of the As atoms.
The charge differences alone cannot determine the variation in copper valence states; therefore, the issue of copper valence will be discussed in more detail later. The following section will discuss the oxidation state of arsenic. The As in tennantite has a lower positive charge (+0.17 e), indicating a lower oxidation state compared to the As in enargite (charge of +0.26 e). Previous bond length calculation results showed that the As-S bond length in 3-coordinated As in tennantite (2.283 Å) is significantly longer than the As-S bond lengths in 4-coordinated As in enargite (ranging from 2.252 Å to 2.265 Å). This suggests that the As ionic radius in tennantite is larger, corresponding to a lower valence state. These results are consistent with ore-forming practices: enargite forms in high-sulfur and oxidizing environments where As is considered to have a +5 valence state, whereas tennantite forms in reducing environments where the As valence state is generally considered to be +3.
The As atom in tennantite exhibits a 3-coordinated structure analogous to the arsenic hydride molecule (AsH
3). The As in AsH
3 has a pair of lone electrons and is prone to oxidation. To investigate whether there are also lone pairs of electrons in the As of AsS
3, the differential charge density of arsenic was analyzed, as shown in
Figure 3. It can be observed that in Tennantite, on the side opposite to the three As-S bonds, there is an electron-rich region (red area), confirming the existence of lone pair electrons on the As atom.
Combining this with the previously analyzed Hirshfeld charge indicates a lower oxidation state. Due to its stronger reducibility, if oxygen or oxidizing agents are present, it is more prone to lose electrons and undergo oxidation, leading to increased hydrophilicity.
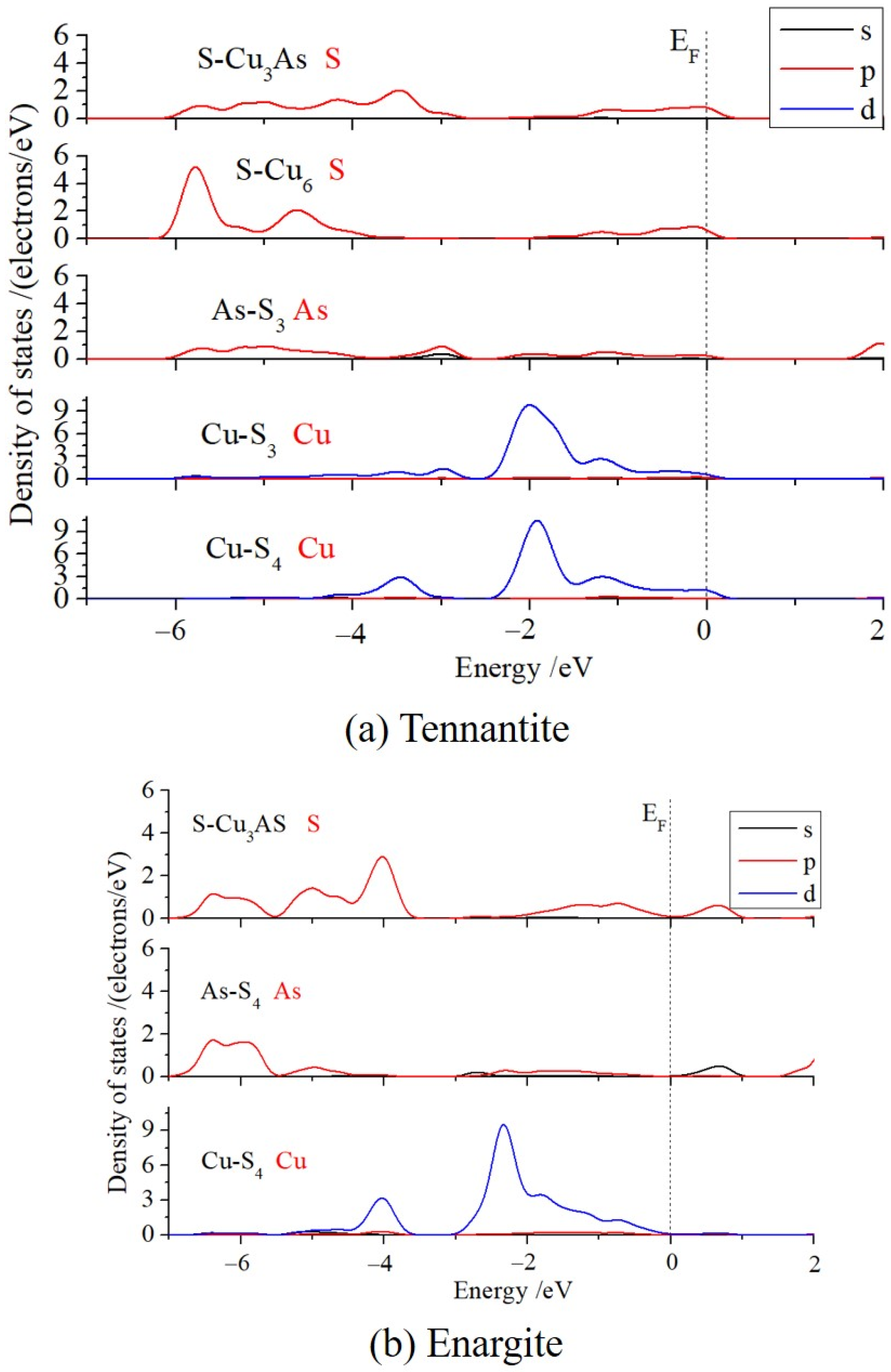
The density of states (DOS) near the Fermi level was calculated (as shown in
Figure 4) to analyze the bonding between atoms and the characteristics of the reactive orbitals. The comparative results for the two types of S and the two types of Cu in tennantite were discussed below. The S 3p states of S-Cu
6 and S-Cu
3As are quite similar near the Fermi level. However, the S 3p of S-Cu
6 exhibits a distinct sharp peak in the energy range of −6 eV to −5 eV, whereas the DOS of S 3p for S-Cu
3As is flat and has a lower intensity. Similarly, the Cu 3d of Cu-S
4 also shows a pronounced DOS peak in the range of −4 eV to −3 eV, while the Cu 3d DOS of Cu-S
3 is very small in this region, and the entire distribution is shifted towards higher energies. These results indicate that the 4-coordinated S and 3-coordinated Cu in tennantite are less stable and have higher reactivity compared to the 6-coordinated S and 4-coordinated Cu.
The Cu 3d states of the 4-coordinated Cu in enargite are very similar to those of the 4-coordinated Cu in tennantite, but the overall DOS of the former is located at lower energies and lacks electronic states at the Fermi level. This suggests that the copper structure in enargite is more stable, whereas the Cu 3d electrons in tennantite are more active.
Previous charge calculations showed that the Cu charges in both crystals are almost identical, making it impossible to determine their valence states from this alone. However, analysis of the DOS can provide insight. In enargite, the Cu 3d band is fully occupied, lying entirely below the Fermi level (EF), indicating a d10 configuration, i.e., Cu tends to be in the +1 oxidation state. This valence state is consistent with its formula, Cu3+1As+5S4−2. In tennantite, a very small portion of the Cu 3d states crosses the Fermi level, and the 3d band is partially occupied, suggesting the possible presence of a d9 component, i.e., Cu in the +2 oxidation state. However, according to the chemical formula Cu12As4+3S13−2, the average valence of Cu should be +1.17, closer to +1. Therefore, further analysis of the copper valence state is needed. Combining this with the DOS, it can be seen that near the Fermi level, the Cu 3d and S 3p orbitals form strong p-d orbital hybridization, with electrons highly shared between Cu and S, resulting in strong covalent interactions and consequently metallic character. This aligns with the observed metallic conductivity and luster of tennantite. This indicates that strong covalent interactions lead to the delocalization of d electrons, causing the d orbitals to cross the Fermi level. In summary, it can be concluded that the Cu in tennantite is not purely +1 or +2, but rather exists in a mixed valence state dominated by +1, with some +2 character.
The DOS of As 4p in tennantite is low and flat in the range of −6 eV to −4 eV, whereas the As 4p DOS in enargite shows a higher and more concentrated peak at lower energies, between −7 eV and −5.5 eV. This suggests that the arsenic in enargite is more stable, while the arsenic in tennantite is more reactive and potentially more easily oxidized.
Finally, a bonding analysis was performed. It can be observed that the S 3p in S-Cu3As and the Cu 3d in Cu-S4 exhibit strong hybridization in the ranges of −4.5 eV to −3 eV and −1.5 eV to 0 eV, whereas the S 3p in S-Cu6 and the Cu 3d in Cu-S3 show only moderate hybridization within the −1.5 eV to 0 eV range. This indicates stronger covalency in the bonding between the former pair, i.e., the Cu-S bond within the arsenic environment has greater covalent character, which is consistent with the Mulliken bond population analysis.
In enargite, both Cu (Cu-S4) and As (As-S4) bond only with S, while S (S-Cu3As) bonds with both Cu and As. A strong hybridization peak between Cu 3d and S 3p is observed in the range of −5.5 eV to −3.5 eV, with weaker hybridization occurring between −2 eV and 0 eV.
In tennantite, the S 3p in S-Cu3As and the As 4p in As-S3 show weak hybridization from −6 eV to −2.8 eV, and also weak hybridization from −1.5 eV to 0 eV. In contrast, the S 3p in S-Cu3As and the As 4p in As-S4 in enargite exhibit relatively strong hybridization in the range of −7 eV to −5.5 eV, forming a distinct hybridization peak. This indicates that the As-S orbital hybridization in tennantite is weaker but spans a broader energy range, whereas the As-S hybridization in enargite is slightly stronger but confined to a narrower range. Combined with the earlier bond population analysis, the covalent character of the As-S bonds in both crystals is similar.
Compared with the Cu and As in chalcopyrite [
22] and arsenopyrite [
23], it is shown that the Cu 3d electronic structure of tennantite or enargite near the Fermi level is relatively similar to that of chalcopyrite, but it moves towards the lower energy level As a whole, while As 4p in arsenopyrite is distributed near the Fermi level. These results indicate that the Cu 3d electrons in chalcopyrite and As 4p electrons are more active than in tennantite or enargite.
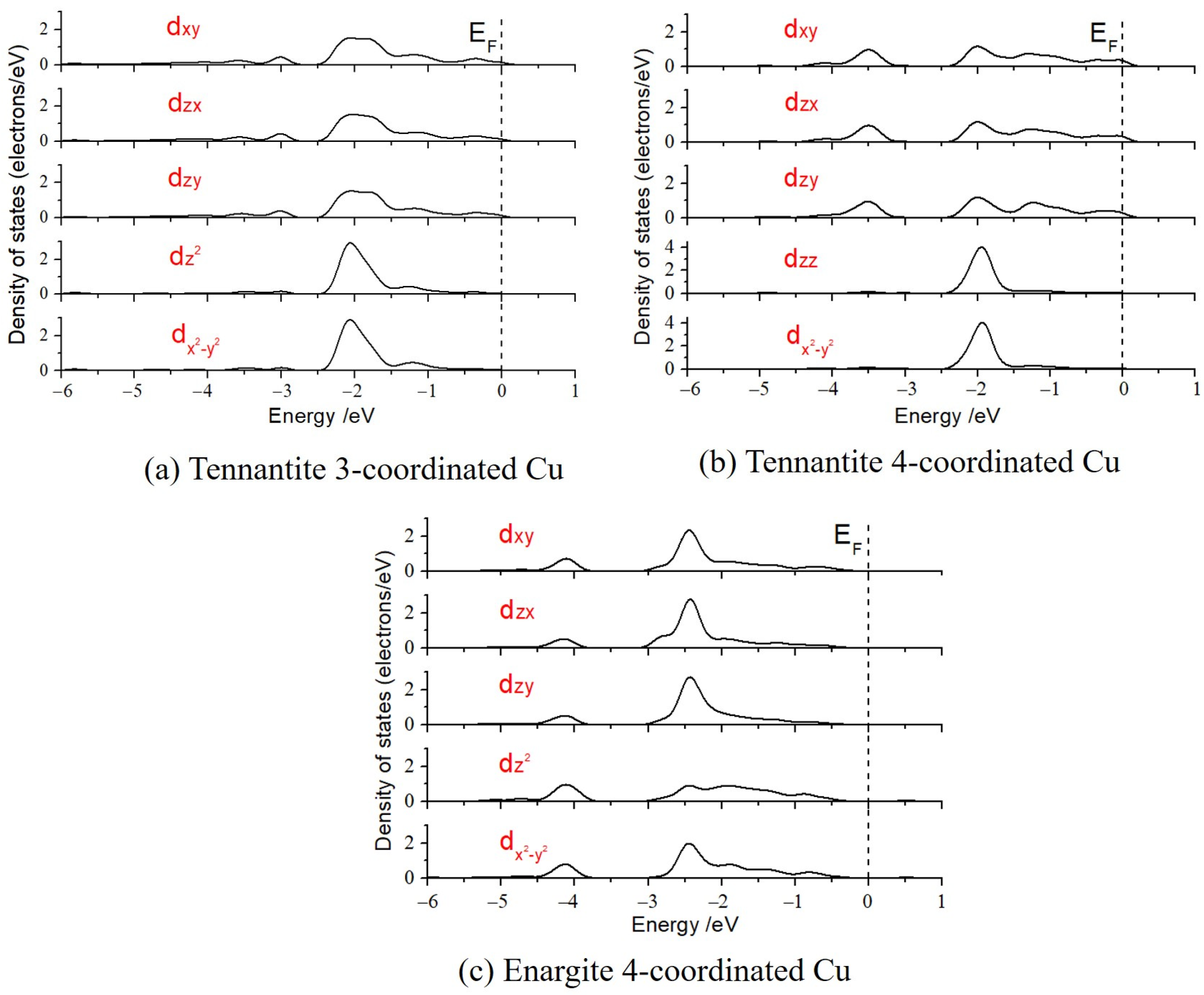
3.3. Splitting of Cu 3d Orbitals in the Crystal Field
The five split d-orbitals were calculated (as shown in
Figure 5), and the energy levels of these five d-orbitals were determined using the weight center method, establishing the energy level ordering for the different orbitals, as listed in
Table 3. From the perspective of orbital splitting, the d-orbitals of the planar triangle and tetrahedral copper in tennantite can be divided into two groups: d
x2-y2 and d
z2 form one group, while d
xy, d
zx, and d
yz form another group, with the latter group having lower energy levels. The energy distinction among the d-orbitals of the tetrahedral copper in enargite is less pronounced but can still be categorized into four groups: d
z2 has the lowest energy level, followed by d
xy, then d
x2-y2 and d
zx, which are close in energy, and finally d
yz with the highest energy level. It is important to note that the energy differences between the d-orbitals for the various copper types are very small, indicating strong electron delocalization.
It can be observed that in tennantite, the energies of the five d-orbitals for the 4-coordinated tetrahedral Cu are similar to those of the 3-coordinated planar triangle copper. In contrast, the d-orbital energies of the 4-coordinated Cu in enargite are significantly lower than those in tennantite (approximately 0.4 eV higher in tennantite).
The strong d-electron delocalization results in relatively low splitting energies (the energy difference between the highest and lowest energy orbitals) of the Cu d-orbitals in both copper-arsenic minerals. Particularly, the planar triangular Cu exhibits the smallest splitting energy (0.029 eV), while the splitting energy of tetrahedral Cu in tennantite (0.066 eV) is greater than that of Cu in enargite (0.034 eV). The above studies indicated that the oxidation state of copper in tennantite is slightly higher than that in enargite, and Mulliken charge calculations also show that Cu in enargite carries +0.13 e, while Cu in tennantite carries +0.14 e. Under the same ligands and coordination number, a lower oxidation state leads to a smaller splitting energy, resulting in a less stable structure and higher reactivity. Thus, it can be concluded that the 4-coordinated copper structure in enargite is less stable. This aligns with the findings of Sasaki et al. [
2], where the dissolved Cu content from enargite was much higher than that from tennantite in pH 5 and pH 11 solutions.