1. Introduction
Pyrite (FeS
2) is the main gangue associated with valuable sulfide ores, such as galena, chalcopyrite, sphalerite, and others [
1,
2,
3,
4,
5]. Pyrite is usually depressed during the flotation of sulfide minerals [
6] by the use of a depressant [
4,
7], pulp-environment management [
8,
9], and grinding-environment changes [
10,
11]. Although these techniques were proven to be effective, the loss of valuable minerals in tailings [
12,
13] and the doping of pyrite in concentrates [
14] still cannot be ignored.
According to the principle of “float less and depress more”, it is more cost-effective to reversely float pyrite while depressing lead concentrates, in a bid to upgrade lead concentrates containing pyrite impurities [
15]. Our previous studies have also confirmed that reverse flotation is an effective method to improve the grade of desired mineral/metal in flotation concentrate, and the development of a new reagents scheme [
16,
17,
18] is the key to achieve this [
19,
20]. Therefore, in this study, we explored an efficient and highly selective galena depressant and a pyrite-removal strategy to upgrade lead concentrates.
Polysaccharide polymers [
21,
22,
23,
24] are a hot topic in mineral processing due to their efficiency and degradability as the depressants for the flotation of sulfide ores [
25,
26,
27]. In recent years, there were many reports on the relations between the depression effect of macromolecular depressants and molecular weight (MW), i.e., low molecular weight (LMW: 1 kDa ≤ MW < 50 kDa), medium molecular weight (MMW: 50 kDa ≤ MW < 500 kDa), and high molecular weight (HMW: 500 kDa ≤ MW < 1000 kDa) [
28,
29,
30,
31]. These studies showed that MMW-macromolecule depressants can selectively depress the mixed-mineral systems through competitive adsorption. However, the selective depression effect of MMW-macromolecule depressants is still not satisfactory, especially for polymetallic sulfide ores.
In this study, the depression effect of chitosan with different molecular weights (ULMW (ultralow molecular weight) = 2−3, 3−6 kDa, LMW = 10 kDa, and MMW = 100 kDa) on galena and pyrite were investigated, and a novel reagent mode was used to evaluate the flotation behavior of the single mineral. The separation performance of this novel reagent mode was testified by the mixed-mineral flotation experiments, and the mechanism of the selective separation was investigated through zeta potential measurements and contact angle tests.
2. Materials and Methods
2.1. Materials and Reagents
The pure pyrite and galena crystal samples were obtained from the Huangshaping Mine, Hunan, China. Firstly, the commonly exposed cleavage surfaces were carefully selected and cleaved to collect samples for contact angle tests. The selected pyrite and galena crystals were respectively embedded in the resin, and the required surfaces remained exposed for further grinding and polishing. In a bid to obtain a flat surface, the desired exposed surfaces of pyrite and galena were successively ground by sandpapers at a roughness of 90, 38, 18, 6.5, and 2.5 µm. The ground surfaces were successively polished with an alumina powder (1.0, 0.5, and 0.05 µm) solution on a polishing cloth [
32].
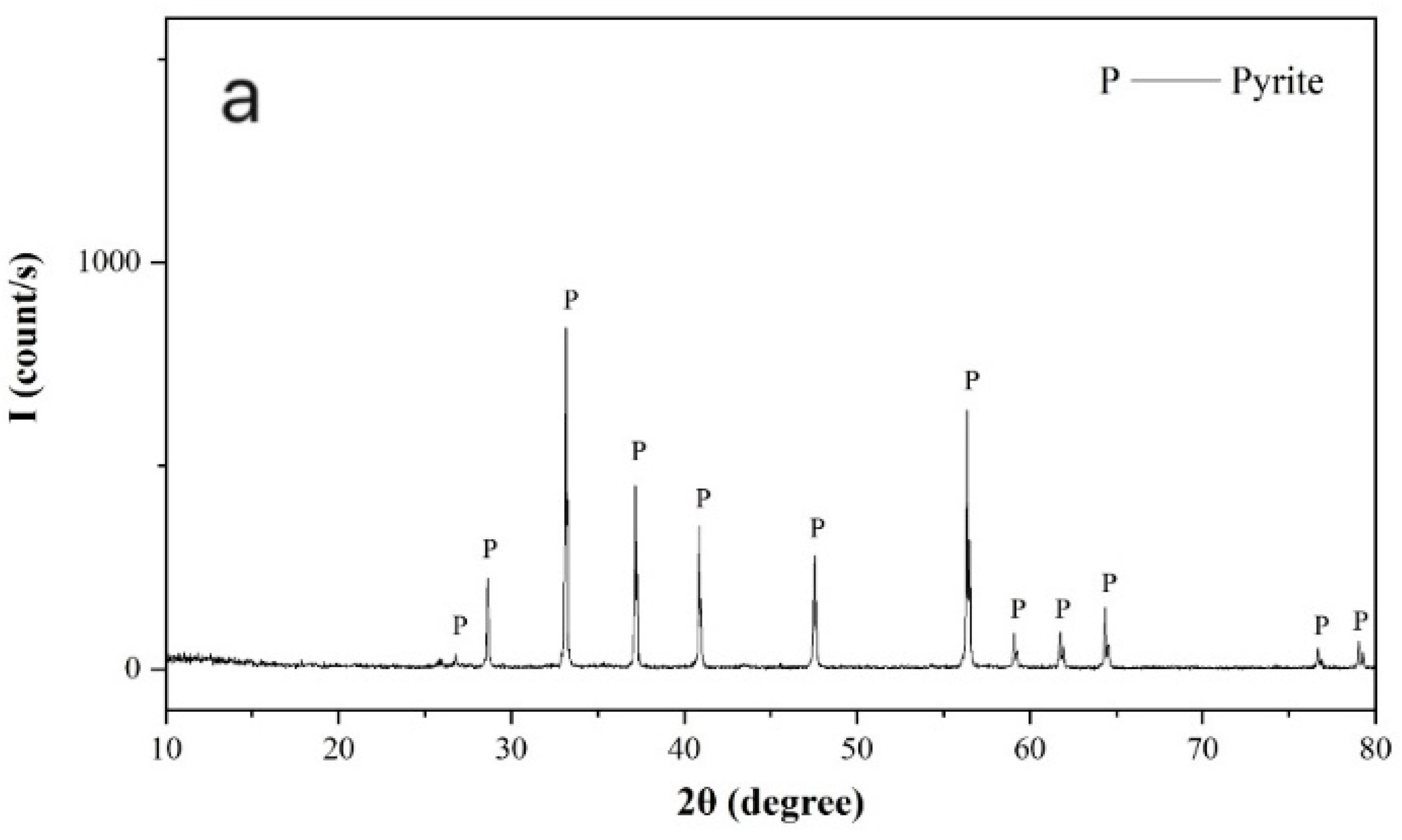
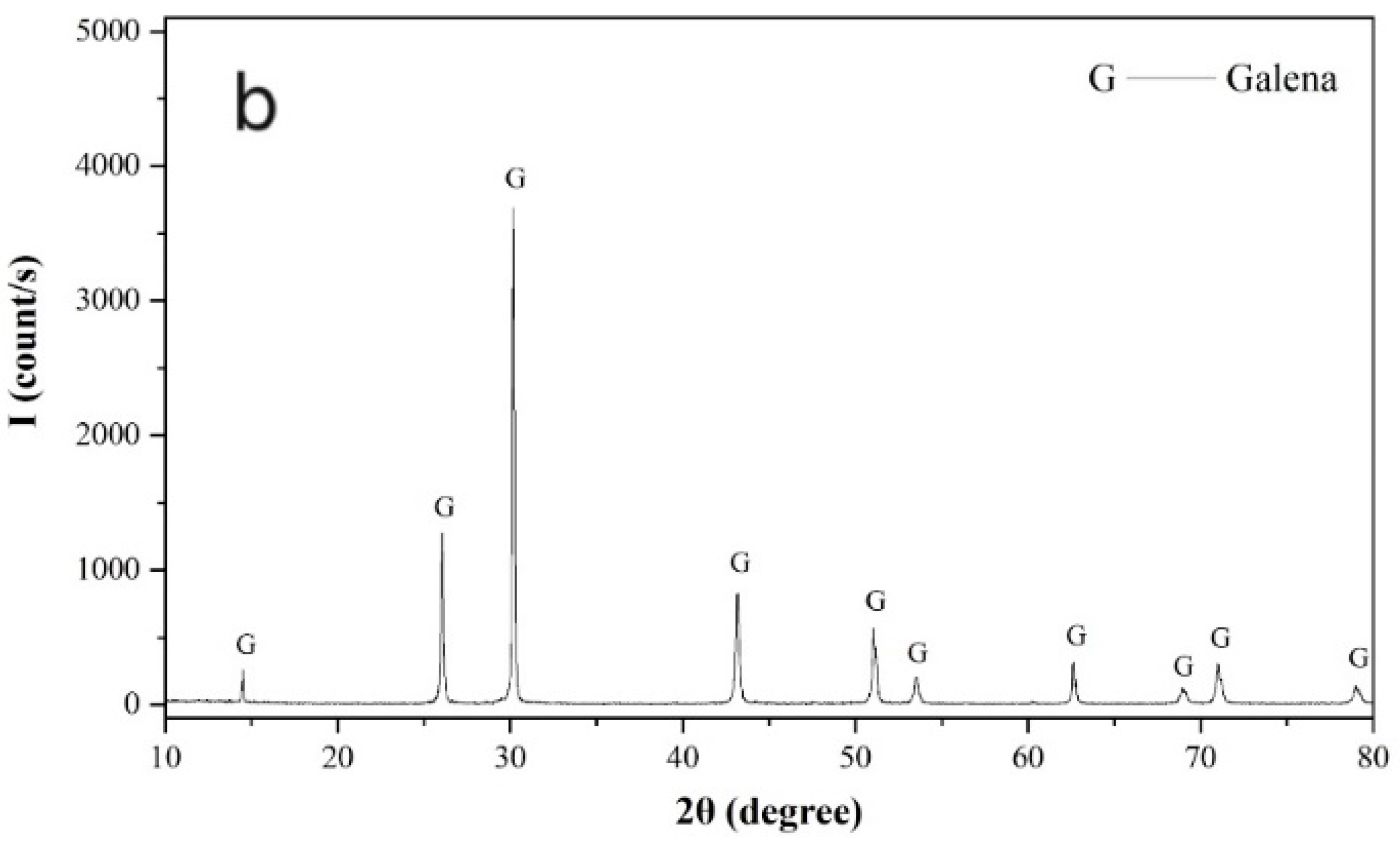
Pure pyrite and galena crystal samples were freshly ground in a ceramic ball mill (Tencan Powder Co., Ltd., Changsha, China) and screened for required flotation samples with a size of −74 + 37 µm. Samples stored within 1–3 days after preparation were further ground to −5 µm in an agate mortar and used for X-ray powder diffraction and zeta potential measurements. As shown in
Figure 1, the X-ray powder diffraction spectra of the pyrite and galena samples confirmed that both the pyrite and galena samples were sufficiently pure.
The ethyl xanthate (90%, Macklin Chemical Reagent Co., Ltd., Changsha, China) was used as a collector [
33], the terpineol (90%, Tianjin Kermil Chemical Reagent Centre, Tianjin, China) as a frother [
34], and the chitosan (Bomei Biotechnology Co., Ltd., Hefei, China) (2–3, 3–6, 10 and 100 kDa, chitosan >99%, water-soluble, deacetylation rate >85%) as depressants. The colors and morphologies of chitosan with different molecular weights are shown in
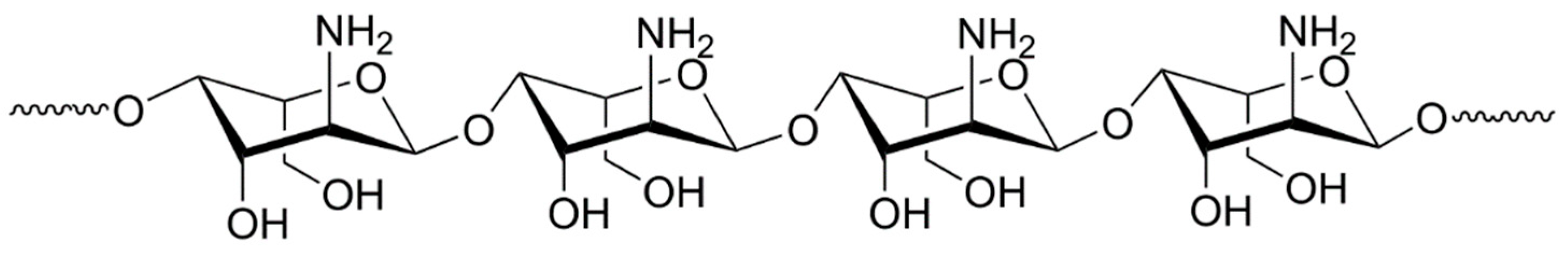
Figure 2, and the structural unit of chitosan is shown in
Figure 3. When the pulp pH was not equal to 8, the pulp condition was adjusted with HCl and NaOH stock solutions (0.1 mol/L). All experiments were carried out in deionized (DI) water with a resistivity of over 18 MΩ × cm.
2.2. Flotation Experiments of Single Mineral and Mixed Minerals
The flotation experiments were carried out in an XFG-type flotation machine [
19,
35,
36,
37,
38] at a spindle speed of 1850 rpm. In each flotation experiment, a single-mineral sample (2.00 g) or mixed-mineral sample (1.00 g pyrite plus 1.00 g galena) was added to the flotation cell (with 40 mL DI water) to obtain the mineral suspension. If necessary, HCl or NaOH stock solution (0.1 mol/L) was added to adjust the pulp pH. Then, chitosan (2−3, 3−6, 10, and 100 kDa), ethyl xanthate, and terpineol with the desired dosage were added in sequence, and floated products were collected for 3 min. Finally, the flotation concentrates and tailings were filtered, dried, and weighed for recovery calculation. At the same time, Fe and Pb grades of flotation concentrates and tailings were assayed. Each flotation experiment was repeated three times to obtain an average value.
2.3. Zeta Potential Measurements
The zeta potential measurement is a common electrochemical test used to detect the reaction process on mineral surfaces and adsorption behavior of flotation reagents [
39]. Zeta potential measurements were conducted at 20 °C, using a Zetasizer Nano series (Malvern, United Kingdom). The mineral suspension was prepared by adding a pyrite or galena sample (20 mg, −5 µm) to a KCl electrolyte solution (0.01 mol/L, 40 mL) in a beaker (100 mL), at a given pH value and chitosan dosage. The mineral suspension was then used for zeta potential measurements after magnetically stirring for 15 min and standing for 5 min. Each zeta potential was tested six times, and the average value was obtained.
2.4. Contact Angle Tests
The contact angle test is a common method to detect the hydrophobic changes of mineral surfaces in the presence or absence of flotation reagents [
40]. The wettability of the prepared pyrite and galena samples in the presence or absence of chitosan with different molecular weights at a dosage of 400 g/t was measured at pH 8. The prepared pyrite or galena sample was first immersed in a beaker (150 mL) containing a given solution (100 mL) and stirred at 20 °C for 30 min. Then, the sample was taken out, gently washed with DI water, and vacuum dried at 50 °C for 10 min, followed by measuring contact angles via the water droplet method [
41]. In each contact angle test, droplets of 3.5 μL DI water were added, and 25−35 s later, when a stable state was reached, the contact angles were calculated based on the shape of the droplets (Windrop++ Software). Under each condition, contact angle tests were carried out three times, to take the average value. After each test, the sample surface was repeatedly washed with DI water and alcohol. Finally, the sample was polished using a 0.05 μm alumina powder solution for the next test.
3. Results
3.1. Single-Mineral Flotation Experiments Results
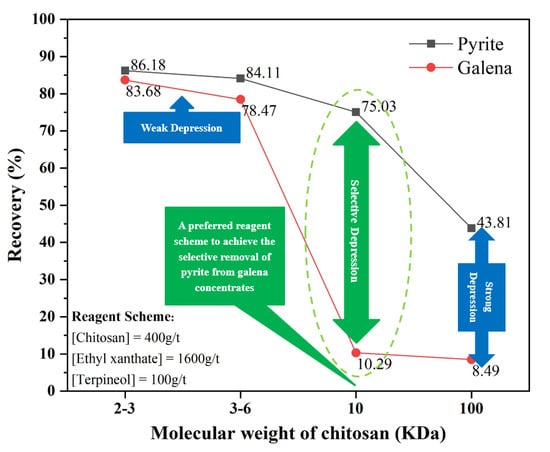
Using different molecular weights of chitosan as depressants, ethyl xanthate as a collector, and terpineol as a frother, the flotation behavior of pyrite and galena was evaluated by the single-mineral flotation, with results shown in
Figure 4. At a certain dosage, with the increase of the chitosan molecular weight, the flotation recoveries of pyrite and galena decrease gradually. The flotation recoveries of pyrite and galena vary slightly with chitosan (2−3 and 3−6 kDa), yet greatly with chitosan (10 and 100 kDa). In particular, the difference in flotation recovery between pyrite and galena using chitosan (10 kDa) is much greater than that using chitosan (100 kDa). Furthermore, in
Figure 4, the arrows point to the optimum molecular weight (10 kDa) and dosage (400 g/t) of chitosan.
At a certain molecular weight of chitosan, recoveries of pyrite and galena decrease gradually with the increase of dosage. Notably, when the chitosan (10 kDa) dosage is increased to 400 g/t, galena recovery drops significantly to 10.29%, whereas the pyrite recovery decreases slightly to 75.02%. Hence, using 400 g/t of chitosan (10 kDa), 1600 g/t of ethyl xanthate, and 100 g/t of terpineol, the maximum recovery difference (64.73%) between pyrite and galena can be achieved. This is the preferred reagent scheme for subsequent experiments.
3.2. Mixed-Mineral Flotation Experiments Results
To further evaluate the flotation separation performance of chitosan with different molecular weights, flotation experiments of mixed pyrite/galena minerals were carried out under the preferred reagent scheme (400 g/t of chitosan with different molecular weights, 1600 g/t of ethyl xanthate, and 100 g/t of terpineol), with the results shown in
Table 1.
Table 1 shows that, when using chitosan with a MW of 2−3 kDa, the metal grades in iron concentrates and lead concentrates are comparable (22.98% Fe and 43.11% Pb in iron concentrates, and 23.50% Fe and 42.14% Pb in lead concentrates). Analogously, chitosan with a MW of 3−6 kDa also shows inferior selectivity (23.43% Fe and 42.28% Pb in iron concentrates, and 22.1% Fe and 44.74% Pb in lead concentrates). These results demonstrate that chitosans with a low molecular weight (2−3 and 3−6 kDa) fail to perform depression selectivity. In contrast, when chitosan (10 and 100 kDa) is used, significant improvements of the Fe grade in iron concentrates and the Pb grade in lead concentrates are obtained. In particular, when chitosan (10 kDa) is used, the Fe grade in iron concentrates and the Pb grade in lead concentrates stand at 39.75% and 66.27%, respectively, which are higher than those of 35.90% and 51.25% when chitosan (100 kDa) is used. In addition, with 400 g/t of chitosan (10 kDa) as a depressant, the maximum recovery difference between Fe and Pb in pyrite or lead concentrates is 62.60%, compared with that of 28.78% with 400 g/t of chitosan (100 kDa).
3.3. Zeta Potential Measurements Results
The zeta potential measurement is conducted to reveal the flotation mechanism [
42,
43,
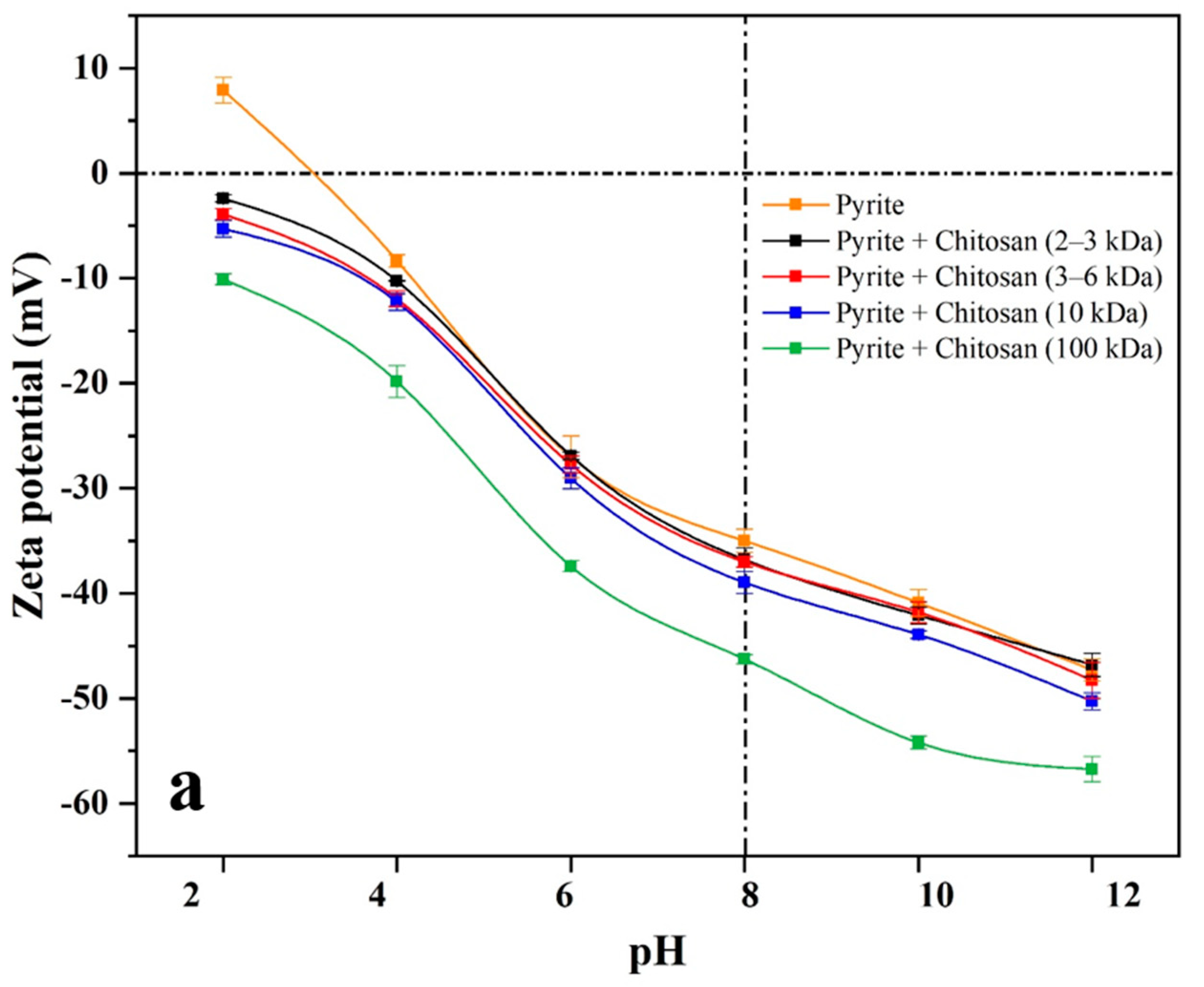
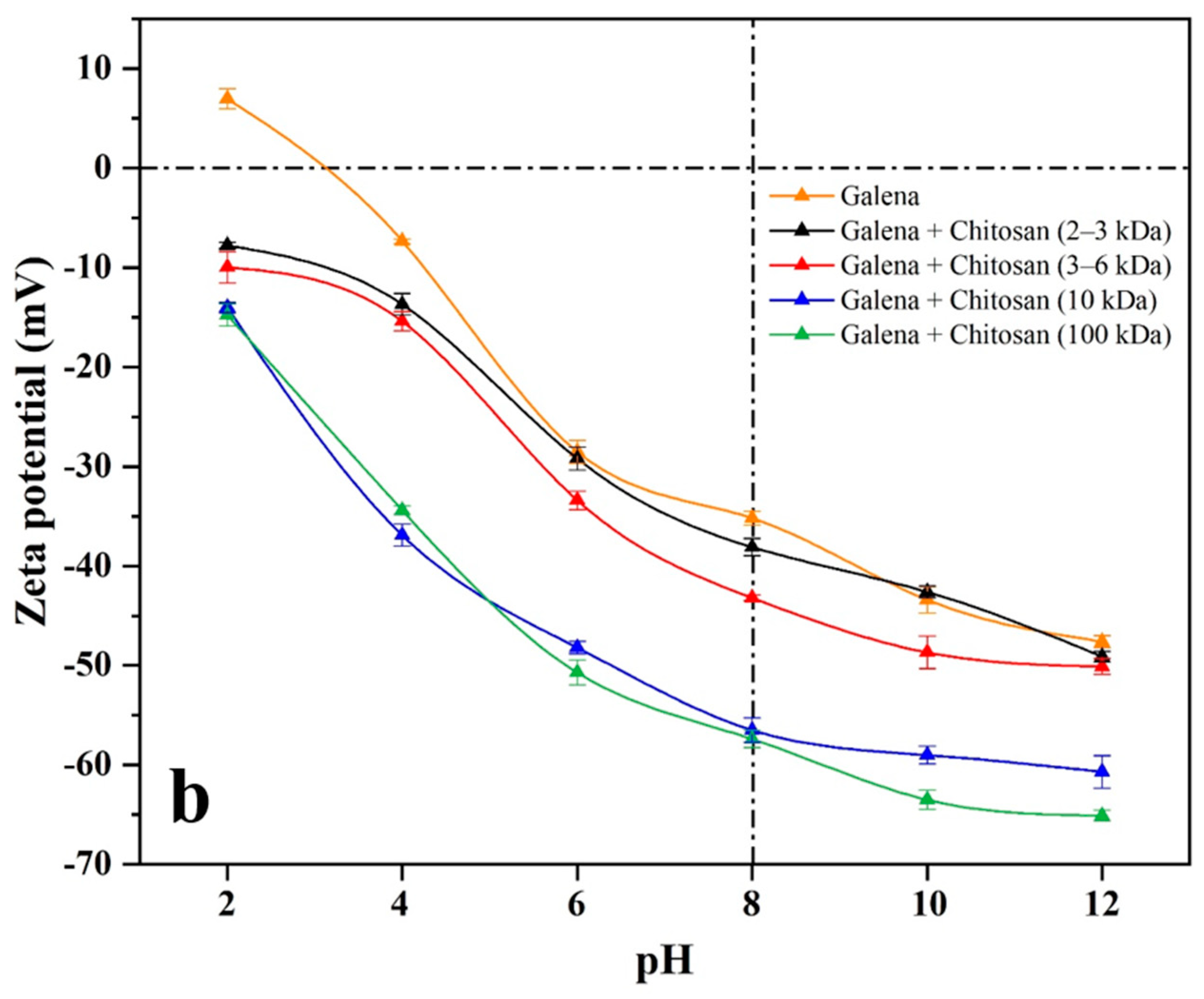
44]. Zeta potentials of pyrite and galena in the presence or absence of 400 g/t of chitosan with different molecular weights were measured under pH 2−12. As shown in
Figure 5, the isoelectric points (IEP) of bare pyrite and galena occurred at about pH 3, which is in line with previous reports [
45,
46,
47].
As can be seen from
Figure 5, compared with zeta potential curves of bare pyrite and galena, the curves of both pyrite and galena with chitosan (2−3 and 3−6 kDa) present a very weak negative shift at pH 4−12. Furthermore, in the presence of chitosan (100 kDa), the zeta potential curves of both pyrite and galena demonstrate a strong negative shift at pH 4−12. However, in the presence of chitosan (10 kDa), the zeta potential curve of galena shows a strong negative shift, while that of pyrite presents a slight one.
At a dosage of 400 g/t and pH 8, and in the presence of chitosan with different molecular weights, the zeta potential changes of pyrite and galena are presented in
Table 2. Compared with that of pyrite and galena, the zeta potential values in the presence of chitosan (2−3 and 3−6 kDa) exhibit a slight negative decrease. In addition, in the presence of chitosan (100 kDa), the zeta potential values of pyrite and galena are significantly reduced by 11.3 and 22.2 mV, respectively. However, in the presence of chitosan (10 kDa), the zeta potential value of galena drops greatly by 21.3 mV at pH 8, while that of pyrite exhibits a negligible diminution by 4.0 mV.
3.4. Contact Angle Tests Results
Establishing the contact angle is a good method for determining the hydrophobicity of a mineral surface and measuring a mineral’s floatability [
48,
49,
50,
51]. To further explore the mechanism of flotation separation, the contact angles of pyrite and galena surfaces in the presence or absence of chitosan with different molecular weights (400 g/t), at pH 8, were tested. As shown in
Figure 6, the contact angle values of pyrite and galena conditioned with DI water stand at 58.8 and 69.3°, respectively. Compared with the results conditioned with DI water, the contact angles of pyrite and galena in the presence of chitosan (2−3 and 3−6 kDa) decrease marginally by 4.5 and 1.7° (chitosan (2−3 kDa)) or 5.7 and 3.4° (chitosan (3–6 kDa)), respectively. The contact angles of pyrite and galena in the presence of chitosan (100 kDa) decrease significantly by 16.1 and 32.1°, respectively. However, in the presence of chitosan (10 kDa), the contact angle of galena reduces sharply by 30.6°, while that of pyrite decreases slightly by 9.5°.
4. Discussion
The single-mineral flotation results demonstrated that, under the proposed reagent scheme (400 of g/t chitosan with different molecular weights, 1600 g/t of ethyl xanthate, and 100 g/t of terpineol), the depression effect of chitosan could be significantly improved with an increase in molecular weight from 2−3 to 100 kDa [
52]. Chitosan (2−3 kDa) and chitosan (3−6 kDa) possess no selective depression effect due to their weak depression abilities for both pyrite and galena. However, both chitosan (10 kDa) and chitosan (100 kDa) have selective depression on galena, and the former has a much better selectivity, which leads to a bigger recovery difference between pyrite and galena. This result means that the separation performance is better by using this reagent scheme. The results of mixed-mineral flotation further confirm that chitosan (2−3 kDa) and chitosan (3−6 kDa) have no separation effect, whereas both chitosan (10 kDa) and chitosan (100 kDa) have a separation effect. The chitosan (100 kDa) obtains a limited separation performance [
29,
53], while chitosan (10 kDa) has a much better performance, obtaining a high-grade lead concentrate with a Pb grade of 66.27% and a Pb recovery of 88.02% from the feed with a Pb grade of 42.91%. The flotation effect is better than other depressants previously reported [
11,
12,
29].
Huang et al [
29]. previously used chitosan to depress pyrite and float galena. In their study, a reagent scheme of 1.25 × 10
−4 mol/L of ethyl xanthate (EX), chitosan (100–300 kDa, 75–85% deacetylation), and pH 4 was employed, which is different from the scheme here (e.g., 5.5 × 10
−4 mol/L of EX, chitosan (100 kDa, 85–98% deacetylation), and pH 8). In addition, the samples used for their flotation experiments were sourced differently from those used in this study. It can be concluded that the chitosan with different molecular weights and deacetylation levels at a different pH can lead to distinctly different flotation performances.
The values of the zeta potential and contact angle on the surfaces of both pyrite and galena change slightly in the presence of chitosan (2−3 kDa) or chitosan (3−6 kDa), indicating a weak adsorption and, as a result, a slight depression for both minerals. In the presence of chitosan (100 kDa), the opposite is the case. Therefore, chitosans (2−3, 3−6, and 100 kDa) exhibit no adsorption selectivity on pyrite and galena, and, therefore, the selective separation cannot be achieved. However, in the presence of chitosan (10 kDa) and at pH 8, the zeta potential (21.3 mV) and contact angle (30.6°) on the galena surface decrease significantly, while the zeta potential (4.0 mV) and contact angle (9.5°) on the pyrite surface only show a slight decrease, indicating that chitosan (10 kDa) is selectively adsorbed on galena rather than pyrite. Therefore, the preferred reagent scheme (400 g/t of chitosan (10 kDa), 1600 g/t of ethyl xanthate, and 100 g/t of terpineol) can be an effective pyrite-removal strategy to upgrade lead concentrates.
5. Conclusions
In this work, the flotation separation performance of chitosan with different molecular weights on pyrite and galena was studied. A novel reagent scheme, i.e., 400 g/t of chitosan (10 kDa) as a depression, 1600 g/t of ethyl xanthate as a collector, and 100 g/t of terpineol as a frother, can achieve the reverse flotation separation of pyrite from galena at pH 8. Zeta potential and contact angle measurements revealed a stronger chitosan (10 kDa) adsorption on the galena surface than on the pyrite surface. Thus, chitosan (10 kDa) has a good industrial application potential to separate pyrite from galena to upgrade lead concentrates.
Author Contributions
Methodology, Z.G.; formal analysis, Y.H. and W.S.; investigation, W.Z. and J.C.; writing—original-draft preparation, W.Z. and J.C.; writing—review and editing, Z.G.
Funding
This research was funded by the National Natural Science Foundation of China (51774328 and 51404300), the Young Elite Scientists Sponsorship Program by CAST (2017QNRC001), the Young Elite Scientists Sponsorship Program by Hunan province of China (2018RS3011), the Natural Science Foundation of Hunan Province of China (2018JJ2520), the Open-End Fund for the Valuable and Precision Instruments of Central South University (CSUZC201806), the Open Fund of Guangdong Provincial Key Laboratory of Development and Comprehensive Utilization of Mineral Resources (2017B030314046), and the National 111 Project (B14034).
Acknowledgments
The authors would like to thank the editors and the anonymous reviewers for their helpful and constructive comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cao, Z.; Chen, X.; Peng, Y. The role of sodium sulfide in the flotation of pyrite depressed in chalcopyrite flotation. Miner. Eng. 2018, 119, 93–98. [Google Scholar] [CrossRef]
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Rasooli, E.; Hajizadeh, H. Depression of pyrite in the flotation of high pyrite low-grade lead-zinc ore using Acidithiobacillus ferrooxidans. Miner. Eng. 2010, 23, 10–16. [Google Scholar] [CrossRef]
- Moslemi, H.; Gharabaghi, M. A review on electrochemical behavior of pyrite in the froth flotation process. J. Ind. Eng. Chem. 2017, 47, 1–18. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96, 143–156. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, C.; Feng, Q.; Chen, Y. Utilization of N-carboxymethyl chitosan as selective depressants for serpentine on the flotation of pyrite. Int. J. Miner. Process. 2017, 163, 45–47. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Sun, W.; Hu, Y.; Cao, J.; Gao, Z. Selective flotation of chalcopyrite from pyrite using diphosphonic acid as collector. Miner. Eng. 2019, 140, 105890. [Google Scholar] [CrossRef]
- Boulton, A.; Fornasiero, D.; Ralston, J. Selective depression of pyrite with polyacrylamide polymers. Int. J. Miner. Process. 2001, 61, 13–22. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Kang, D.; Guo, J. Depression of pyrite in alkaline medium and its subsequent activation by copper. Miner. Eng. 2012, 26, 64–69. [Google Scholar] [CrossRef]
- Liu, G.; Zhong, H.; Dai, T. The separation of Cu/Fe sulfide minerals at slightly alkaline conditions by using ethoxycarbonyl thionocarbamates as collectors: Theory and practice. Miner. Eng. 2006, 19, 1380–1384. [Google Scholar] [CrossRef]
- Chen, X.; Peng, Y.; Bradshaw, D. Effect of regrinding conditions on pyrite flotation in the presence of copper ions. Int. J. Miner. Process. 2013, 125, 129–136. [Google Scholar] [CrossRef]
- Peng, Y.; Grano, S.; Fornasiero, D.; Ralston, J. Control of grinding conditions in the flotation of galena and its separation from pyrite. Int. J. Miner. Process. 2003, 70, 67–82. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, Y.; Wang, T.; Luo, G.; Wang, H.; He, G. The flotation separation of galena and pyrite using serpentine as depressant. Powder Technol. 2019, 342, 486–490. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Sun, N.; Khoso, S.A.; Wang, L.; Sun, W. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 2019, 187, 125–133. [Google Scholar] [CrossRef]
- McFadzean, B.; Mhlanga, S.S.; O’Connor, C.T. The effect of thiol collector mixtures on the flotation of pyrite and galena. Miner. Eng. 2013, 50, 121–129. [Google Scholar] [CrossRef]
- Chai, W.; Huang, Y.; Peng, W.; Han, G.; Cao, Y.; Liu, J. Enhanced separation of pyrite from high-sulfur bauxite using 2-mercaptobenzimidazole as chelate collector: Flotation optimization and interaction mechanisms. Miner. Eng. 2018, 129, 93–101. [Google Scholar] [CrossRef]
- Lin, S.; Liu, R.; Sun, W.; Hu, Y.; Gao, Z.; Han, H. Pyrogallic acid: A novel depressant for bismuthinite in Mo–Bi sulfide ore flotation. Miner. Eng. 2019, 131, 237–240. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Ji, B.; Fan, R.; Liu, R.; Chen, P.; Sun, W.; Hu, Y. Selective Flotation of Cassiterite from Calcite with Salicylhydroxamic Acid Collector and Carboxymethyl Cellulose Depressant. Minerals 2018, 8, 316. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhang, C.; Guan, Q.; Zhang, C. Separation of Molybdenite from Chalcopyrite in the Presence of Novel Depressant 4-Amino-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one. Minerals 2017, 7, 146. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, Y.; Zhu, Y.; Hu, Y.; Sun, W. Selective flotation of calcite from fluorite: A novel reagent schedule. Minerals 2016, 6, 114. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Gao, Y.; Sun, W.; Hu, Y.; Gao, Z. Reverse Flotation Separation of Fluorite from Calcite: A Novel Reagent Scheme. Minerals 2018, 8, 313. [Google Scholar] [CrossRef]
- Ahmadi, M.; Gharabaghi, M.; Abdollahi, H. Effects of type and dosages of organic depressants on pyrite floatability in microflotation system. Adv. Powder Technol. 2018, 29, 3155–3162. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Use of organic polymers in the flotation of polymetallic ores: A review. Miner. Eng. 1999, 12, 341–354. [Google Scholar] [CrossRef]
- Duarte, R.S.; Lima, R.M.F.; Leão, V.A. Effect of inorganic and organic depressants on the cationic flotation and surface charge of rhodonite-rhodochrosite. Rem Rev. Esc. Minas 2015, 68, 463–469. [Google Scholar] [CrossRef]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Guo, Q.; Feng, B.; Zhang, D.; Guo, J. Flotation separation of chalcopyrite from talc using carboxymethyl chitosan as depressant. Physicochem. Probl. Miner. Process. 2017, 53, 1255–1263. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Q. Adsorption of phosphorylated chitosan on mineral surfaces. Colloids Surf. A Physicochem. Eng. Aspects 2013, 436, 656–663. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Zheng, G.; Han, L.; McFadzean, B.; Qian, Z.; Piao, Y.; O’Connor, C. An investigation into the selective separation and adsorption mechanism of a macromolecular depressant in the galena-chalcopyrite system. Miner. Eng. 2019, 134, 291–299. [Google Scholar] [CrossRef]
- McFadzean, B.; Dicks, P.; Groenmeyer, G.; Harris, P.; O’Connor, C. The effect of molecular weight on the adsorption and efficacy of polysaccharide depressants. Miner. Eng. 2011, 24, 463–469. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Selective depression of pyrite with chitosan in Pb–Fe sulfide flotation. Miner. Eng. 2013, 46, 45–51. [Google Scholar] [CrossRef]
- Garcia Vidal, C.A.; Pawlik, M. Molecular weight effects in interactions of guar gum with talc. Int. J. Miner. Process. 2015, 138, 38–43. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Shi, Q.; Zhang, W.; Song, S. Utilization of N-carboxymethyl chitosan as a selective depressant for talc in flotation of chalcopyrite. Physicochem. Probl. Miner. Process. 2019, 55, 108–115. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Sun, W.; Liu, X. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloids Surf. A Physicochem. Eng. Aspects 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Porento, M.; Hirva, P. Theoretical studies on the interaction of anionic collectors with Cu+, Cu2+, Zn2+ and Pb2+ ions. Theor. Chem. Acc. 2002, 107, 200–205. [Google Scholar] [CrossRef]
- Huangfu, Z.; Khoso, S.A.; Sun, W.; Hu, Y.; Chen, C.; Zhang, Q. Utilization of petrochemical by-products as a new frother in flotation separation of molybdenum. J. Clean. Prod. 2018, 204, 501–510. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Hu, Y. Selective flotation of scheelite from calcite: A novel reagent scheme. Int. J. Miner. Process. 2016, 154, 10–15. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Han, H.; Sun, W.; Hu, Y. Improved flotation separation of cassiterite from calcite using a mixture of lead (II) ion/benzohydroxamic acid as collector and carboxymethyl cellulose as depressant. Miner. Eng. 2017, 113, 68–70. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. Pradip Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Niu, X.; Ruan, R.; Xia, L.; Li, L.; Sun, H.; Jia, Y.; Tan, Q. Correlation of surface adsorption and oxidation with a floatability difference of galena and pyrite in high-alkaline lime systems. Langmuir 2018, 34, 2716–2724. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Y.; Sun, W.; Drelich, J.W. Surface-Charge Anisotropy of Scheelite Crystals. Langmuir 2016, 32, 6282–6288. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Luo, X. The effect of quartz on the flotation of pyrite depressed by serpentine. J. Mater. Res. Technol. 2015, 4, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, T.K.; Nguyen, A.V.; Evans, G.M. Heterocoagulation of chalcopyrite and pyrite minerals in flotation separation. Adv. Colloid Interface Sci. 2005, 114, 227–237. [Google Scholar] [CrossRef]
- Santhiya, D.; Subramanian, S.; Rao, K.H.; Natarajan, K.A.; Forssberg, K.S.E. Bio-modulation of galena and sphalerite surfaces using thiobacillus thiooxidans. Int. J. Miner. Process. 2001, 62, 121–141. [Google Scholar] [CrossRef]
- Gao, Z.; Fan, R.; Ralston, J.; Sun, W.; Hu, Y. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing Anisotropic Surface Properties and Surface Forces of Fluorite Crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef]
- Guo, H.; Yen, W. Surface potential and wettability of enargite in potassium amyl xanthate solution. Miner. Eng. 2002, 15, 405–414. [Google Scholar] [CrossRef]
- Yekeler, M.; Ulusoy, U.; Hiçyılmaz, C. Effect of particle shape and roughness of talc mineral ground by different mills on the wettability and floatability. Powder Technol. 2004, 140, 68–78. [Google Scholar] [CrossRef]
- Bicak, O.; Ekmekci, Z.; Bradshaw, D.J.; Harris, P.J. Adsorption of guar gum and CMC on pyrite. Miner. Eng. 2007, 20, 996–1002. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Using chitosan as a selective depressant in the differential flotation of Cu-Pb sulfides. Int. J. Miner. Process. 2012, 106, 8–15. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).