In Situ Infrared Spectra for Hydrous Forsterite up to 1243 K: Hydration Effect on Thermodynamic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. FTIR at High Temperature
3. Results and Discussion
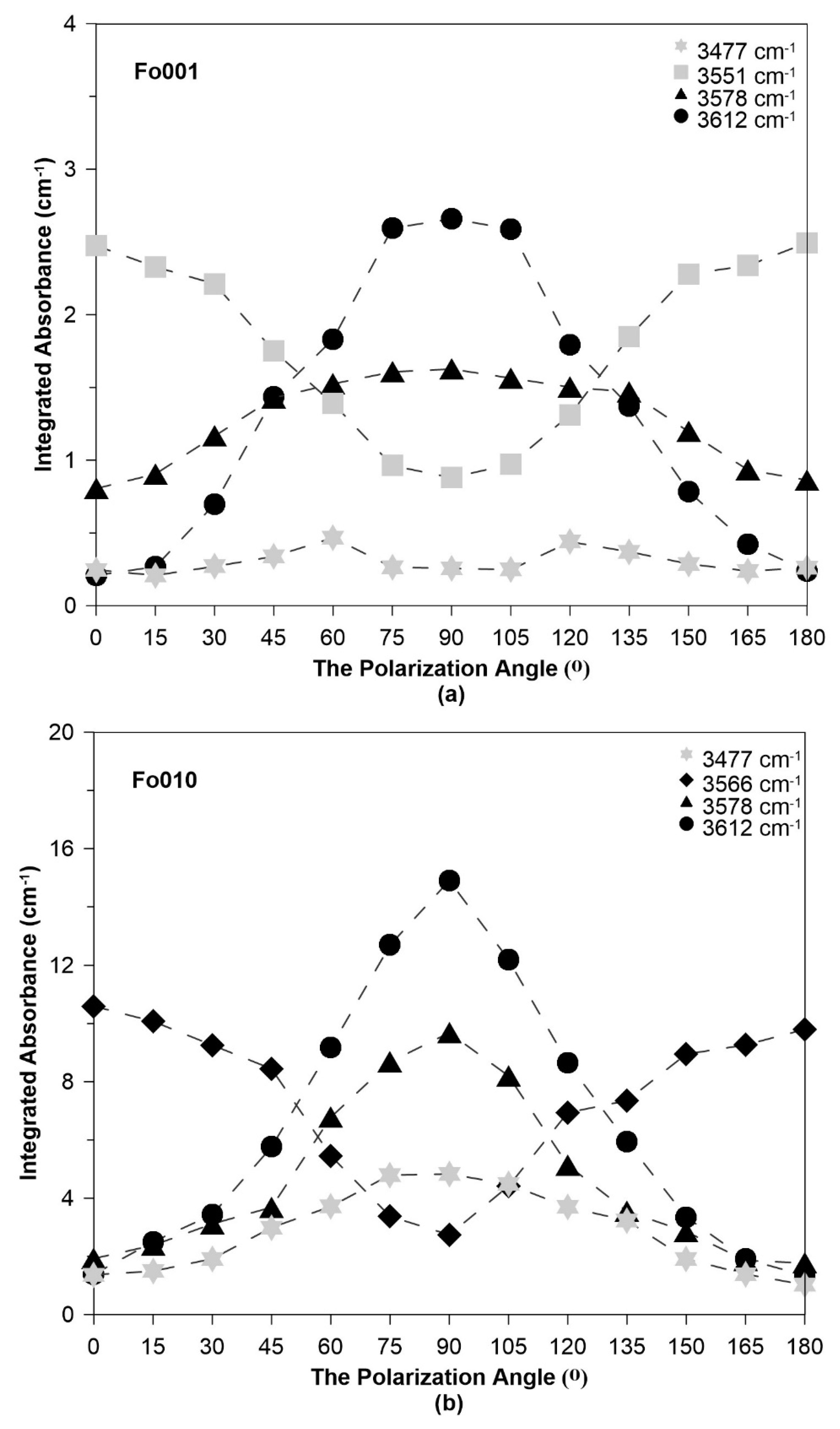
3.1. Polarized FTIR Spectra at Ambient Temperature
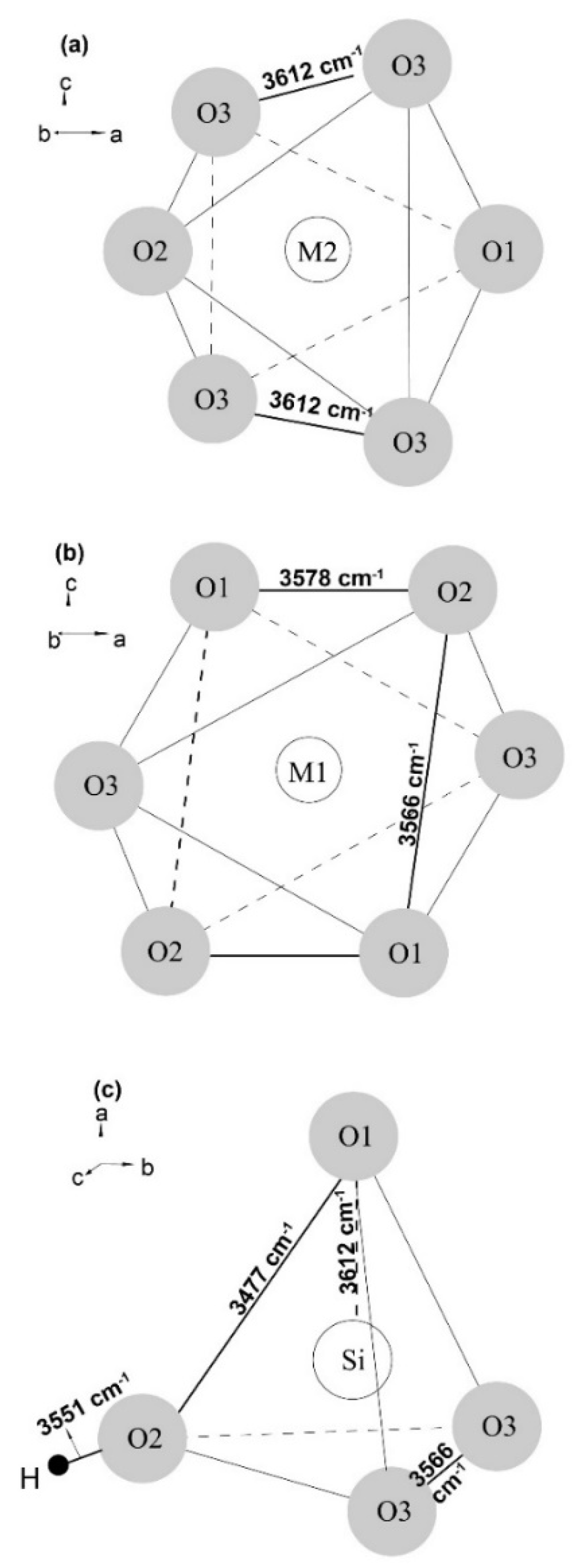
3.2. Hydrogen Substitution Mechanism
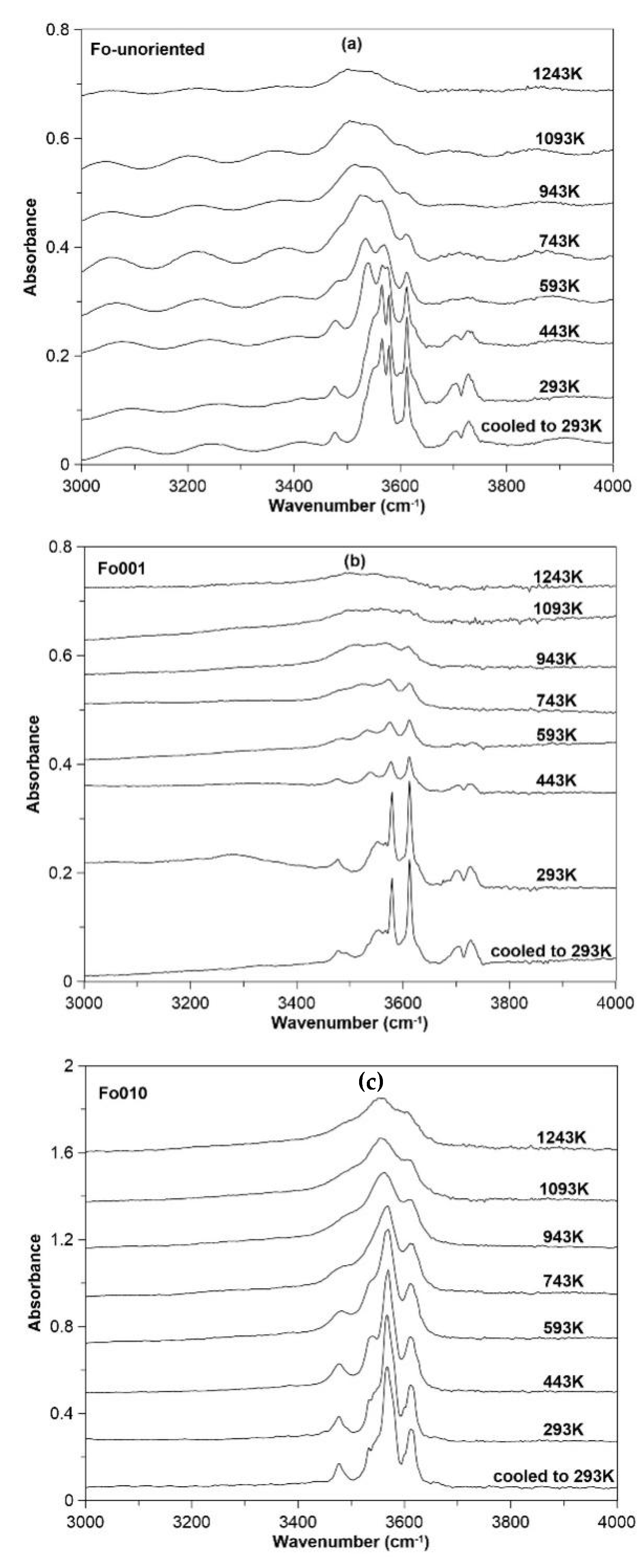
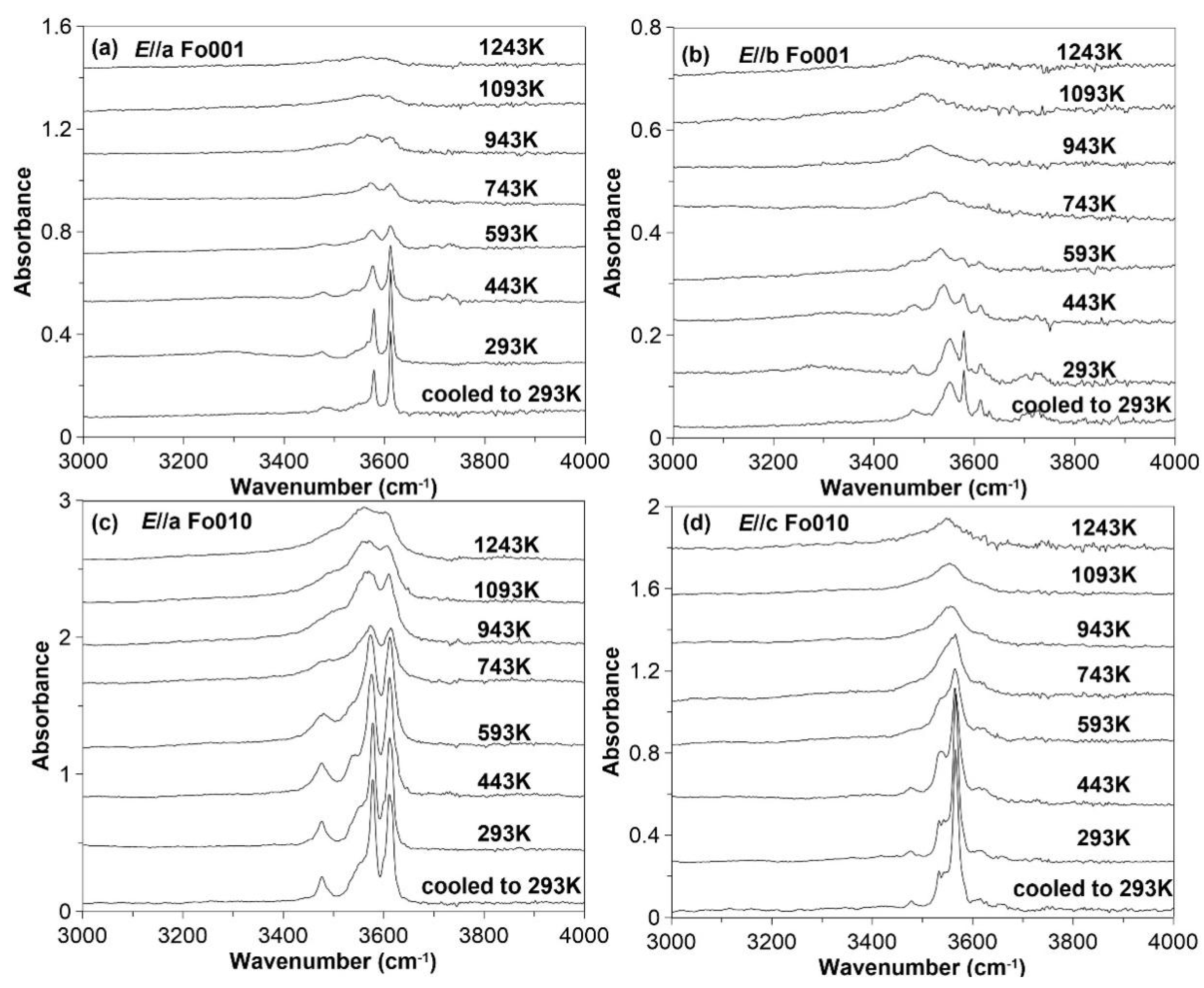
3.3. FTIR Spectroscopy at High Temperature
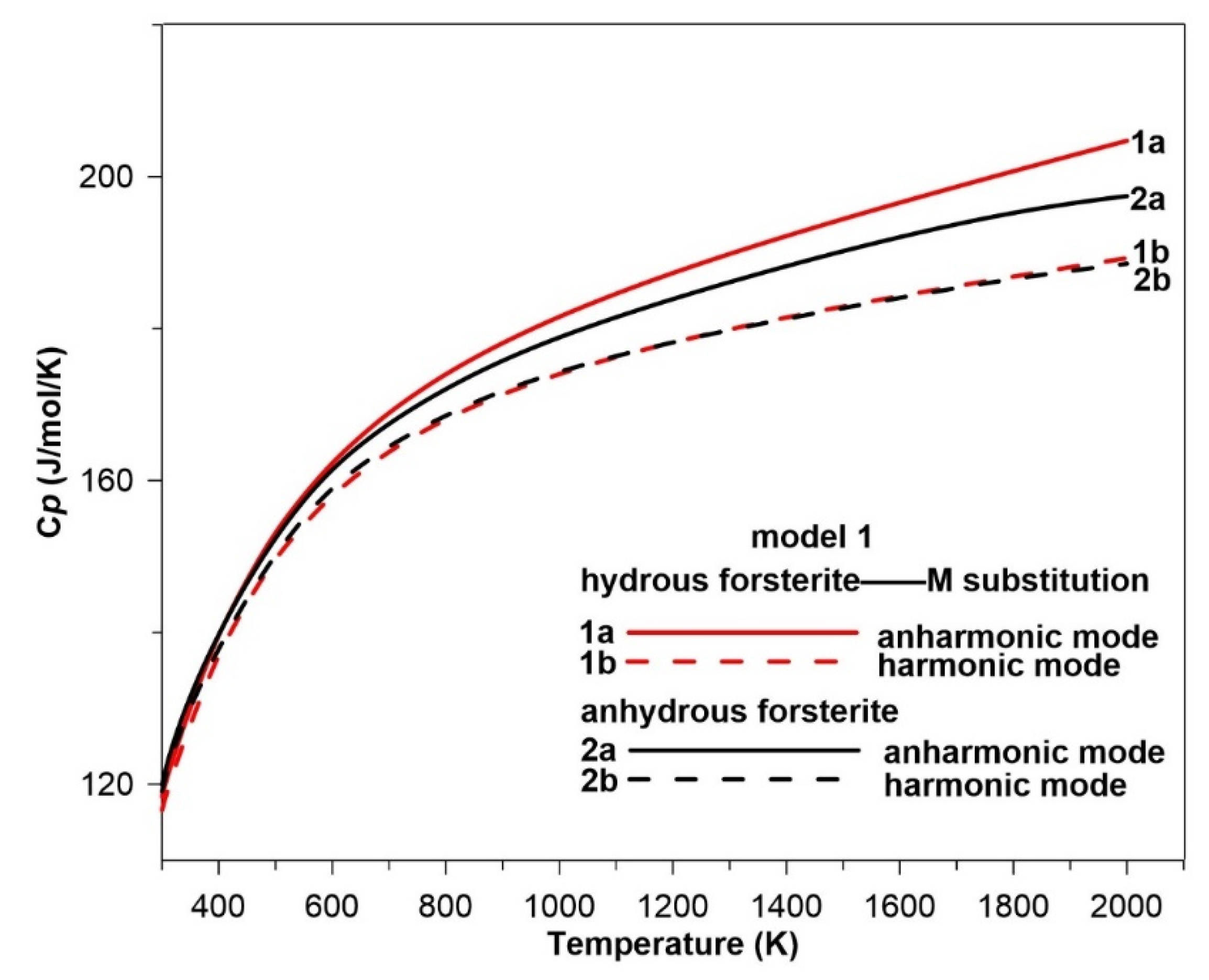
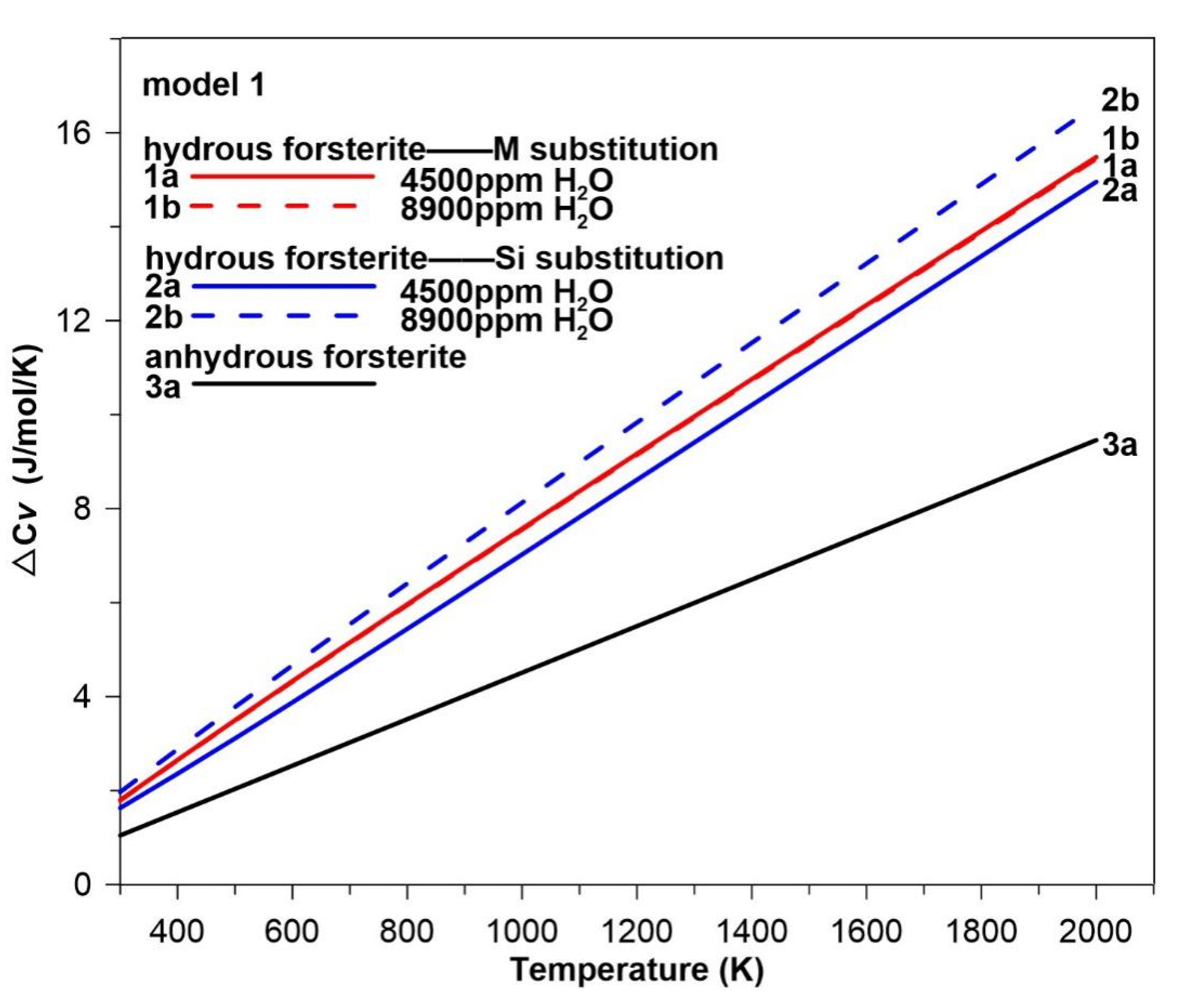
3.4. Hydration Effect on the Intrinsic Anharmonicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kohlstedt, D.L.; Keppler, H.; Rubie, D.C. Solubility of water in the α, β and γ phases of (Mg,Fe)2SiO4. Contrib. Mineral. Petrol. 1996, 123, 345–357. [Google Scholar] [CrossRef]
- Smyth, J.R.; Frost, D.J.; Nestola, F.; Holl, C.M.; Bromiley, G.; Frost, D. Olivine hydration in the deep upper mantle: Effects of temperature and silica activity. Geophys. Res. Lett. 2006, 33, 311–324. [Google Scholar] [CrossRef]
- Litasov, K.D.; Ohtani, E.; Kagi, H.; Jacobsen, S.; Ghosh, S. Temperature dependence and mechanism of hydrogen incorporation in olivine at 12.5–14.0 GPa. Geophys. Res. Lett. 2007, 34, 16314. [Google Scholar] [CrossRef]
- Bali, E.; Bolfan-Casanova, N.; Koga, K.T. Pressure and temperature dependence of H solubility in forsterite: An implication to water activity in the Earth interior. Earth Planet. Sci. Lett. 2008, 268, 354–363. [Google Scholar] [CrossRef]
- Ye, Y.; Schwering, R.A.; Smyth, J.R. Effects of Hydration on Thermal Expansion of Forsterite, Wadsleyite, and Ringwoodite. Am. Mineral. 2009, 94, 899–904. [Google Scholar] [CrossRef]
- Otsuka, K.; Karato, S.-I. Control of the water fugacity at high pressures and temperatures: Applications to the incorporation mechanisms of water in olivine. Phys. Earth Planet. Inter. 2011, 189, 27–33. [Google Scholar] [CrossRef]
- Férot, A.; Bolfan-Casanova, N. Water storage capacity in olivine and pyroxene to 14 GPa: Implications for the water content of the Earth’s upper mantle and nature of seismic discontinuities. Earth Planet. Sci. Lett. 2012, 349–350, 218–230. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Hsieh, W.-P.; Tan, E.; Chen, J. Hydration-reduced lattice thermal conductivity of olivine in Earth’s upper mantle. Proc. Natl. Acad. Sci. USA 2017, 114, 4078–4081. [Google Scholar] [CrossRef]
- Wang, D.; Mookherjee, M.; Xu, Y.; Karato, S.-I. The effect of water on the electrical conductivity of olivine. Nature 2006, 443, 977–980. [Google Scholar] [CrossRef]
- Mei, S.; Kohlstedt, D.L. Influence of water on plastic deformation of olivine aggregates: 1. Diffusion creep regime. J. Geophys. Res. Space Phys. 2000, 105, 21457–21469. [Google Scholar] [CrossRef]
- Costa, F.; Chakraborty, S. The effect of water on Si and O diffusion rates in olivine and implications for transport properties and processes in the upper mantle. Phys. Earth Planet. Inter. 2008, 166, 11–29. [Google Scholar] [CrossRef]
- Hier-Majumder, S.; Anderson, I.M.; Kohlstedt, D.L. Influence of protons on Fe-Mg interdiffusion in olivine. J. Geophys. Res. Space Phys. 2005, 110, 110. [Google Scholar] [CrossRef]
- Fei, H.; Wiedenbeck, M.; Yamazaki, D.; Katsura, T. Small effect of water on upper-mantle rheology based on silicon self-diffusion coefficients. Nature 2013, 498, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.; Wiedenbeck, M.; Yamazaki, D.; Katsura, T. No effect of water on oxygen self-diffusion rate in forsterite. J. Geophys. Res. Solid Earth 2014, 119, 7598–7606. [Google Scholar] [CrossRef]
- Fei, H.; Koizumi, S.; Sakamoto, N.; Hashiguchi, M.; Yurimoto, H.; Marquardt, K.; Miyajima, N.; Katsura, T. Mg lattice diffusion in iron-free olivine and implications to conductivity anomaly in the oceanic asthenosphere. Earth Planet. Sci. Lett. 2018, 484, 204–212. [Google Scholar] [CrossRef]
- Manghnani, M.H.; Hushur, A.; Smyth, J.R.; Nestola, F.; Dera, P.; Sekar, M.; Amulele, G.; Frost, D.J. Compressibility and structural stability of two variably hydrated olivine samples (Fo97Fa3) to 34 GPa by X-ray diffraction and Raman spectroscopy. Am. Miner. 2013, 98, 1972–1979. [Google Scholar] [CrossRef]
- Beran, A.; Putnis, A. A model of the OH positions in olivine, derived from infrared-spectroscopic investigations. Phys. Chem. Miner. 1983, 9, 57–60. [Google Scholar] [CrossRef]
- Bai, Q.; Kohlstedt, D.L. Substantial hydrogen solubility in olivine and implications for water storage in the mantle. Nature 1992, 357, 672–674. [Google Scholar] [CrossRef]
- Bai, Q.; Kohlstedt, D. Effects of chemical environment on the solubility and incorporation mechanism for hydrogen in olivine. Phys. Chem. Miner. 1993, 19, 460–471. [Google Scholar] [CrossRef]
- Demouchy, S.; Mackwell, S. Water diffusion in synthetic iron-free forsterite. Phys. Chem. Miner. 2003, 30, 486–494. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Ginsberg, S.B.; Kohlstedt, D.L. Solubility of hydrogen in olivine: Dependence on temperature and iron content. Contrib. Mineral. Petrol. 2004, 147, 155–161. [Google Scholar] [CrossRef]
- Kohlstedt, D.L. The Role of Water in High-Temperature Rock Deformation. Rev. Mineral. Geochem. 2006, 62, 377–396. [Google Scholar] [CrossRef]
- Deligne, N.I.; Mosenfelder, J.L.; Asimow, P.D.; Rossman, G.R. Hydrogen incorporation in olivine from 2-12 GPa. Am. Miner. 2006, 91, 285–294. [Google Scholar]
- Hushur, A.; Manghnani, M.H.; Smyth, J.R.; Nestola, F.; Frost, D.J. Crystal chemistry of hydrous forsterite and its vibrational properties up to 41 GPa. Am. Miner. 2009, 94, 751–760. [Google Scholar] [CrossRef]
- Karato, S.-I. Theory of isotope diffusion in a material with multiple species and its implications for hydrogen-enhanced electrical conductivity in olivine. Phys. Earth Planet. Inter. 2013, 219, 49–54. [Google Scholar] [CrossRef]
- Karato, S.-I. Some notes on hydrogen-related point defects and their role in the isotope exchange and electrical conductivity in olivine. Phys. Earth Planet. Inter. 2015, 248, 94–98. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Matveev, S.; Ballhaus, C.; Taylor, W.R.; Green, D.H. Effect of Silica Activity on OH− IR Spectra of Olivine: Implications for Low-aSiO2 Mantle Metasomatism. J. Petrol. 2001, 42, 721–729. [Google Scholar]
- Berry, A.J.; Hermann, J.; O’Neill, H.S.; Foran, G.J.; Hermann, J. Fingerprinting the water site in mantle olivine. Geology 2005, 33, 869. [Google Scholar] [CrossRef]
- O’Neill, H.S.; Hermann, J.; Hauri, E.H.; Kovács, I.; O’Neill, H.S. Site-specific infrared O-H absorption coefficients for water substitution into olivine. Am. Miner. 2010, 95, 292–299. [Google Scholar]
- Umemoto, K.; Wentzcovitch, R.M.; Hirschmann, M.M.; Kohlstedt, D.L.; Withers, A.C. A first-principles investigation of hydrous defects and IR frequencies in forsterite: The case for Si vacancies. Am. Miner. 2011, 96, 1475–1479. [Google Scholar] [CrossRef]
- Balan, E.; Ingrin, J.; Delattre, S.; Kovács, I.; Blanchard, M. Theoretical infrared spectrum of OH-defects in forsterite. Eur. J. Mineral. 2011, 23, 285–292. [Google Scholar] [CrossRef]
- Xue, X.; Turner, D.; Loroch, D.; Kanzaki, M. Hydrogen incorporation mechanisms in forsterite: New insights from 1 H and 29 Si NMR spectroscopy and first-principles calculation. Am. Miner. 2017, 102, 519–536. [Google Scholar] [CrossRef]
- Qin, T.; Wentzcovitch, R.; Umemoto, K. Ab initio study of water speciation in forsterite. Am. Miner. 2018, 103, 692–699. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Qi, Z.; Wang, Z.; Smyth, J.R.; Xia, Q. Re-configuration and interaction of hydrogen sites in olivine at high temperature and high pressure. Am. Mineral. 2019, 104, 878–889. [Google Scholar] [CrossRef]
- Padrón-Navarta, J.A.; Hermann, J.; O’Neill, H.S.C. Site-specific hydrogen diffusion rates in forsterite. Earth Planet. Sci. Lett. 2014, 392, 100–112. [Google Scholar] [CrossRef]
- Kohn, S.C.; Brooker, R.A.; Lemaire, C. The effect of silica activity on the incorporation mechanisms of water in synthetic forsterite: A polarised infrared spectroscopic study. Contrib. Mineral. Petrol. 2004, 147, 48–57. [Google Scholar] [CrossRef]
- Berry, A.J.; O’Neill, H.S.; Hermann, J.; Scott, D.R.; Hermann, J. The infrared signature of water associated with trivalent cations in olivine. Earth Planet. Sci. Lett. 2007, 261, 134–142. [Google Scholar] [CrossRef]
- Berry, A.J.; Walker, A.M.; Hermann, J.; O’Neill, H.S.; Foran, G.J.; Gale, J.D.; Hermann, J. Titanium substitution mechanisms in forsterite. Chem. Geol. 2007, 242, 176–186. [Google Scholar] [CrossRef]
- Grant, K.; Brooker, R.; Kohn, S.; Wood, B.; Kohn, S. The effect of oxygen fugacity on hydroxyl concentrations and speciation in olivine: Implications for water solubility in the upper mantle. Earth Planet. Sci. Lett. 2007, 261, 217–229. [Google Scholar] [CrossRef]
- Walker, A.M.; Hermann, J.; Berry, A.J.; O’Neill, H.S.C.; Walker, A.; Berry, A. Three water sites in upper mantle olivine and the role of titanium in the water weakening mechanism. J. Geophys. Res. Space Phys. 2007, 112, 05211. [Google Scholar] [CrossRef]
- Yang, X.-Z.; Keppler, H. In-situ infrared spectra of OH in olivine to 1100 °C. Am. Miner. 2011, 96, 451–454. [Google Scholar] [CrossRef]
- Ingrin, J.; Kovács, I.; Deloule, E.; Balan, E.; Blanchard, M.; Kohn, S.C.; Hermann, J. Identification of hydrogen defects linked to boronsubstitution in synthetic forsterite and natural olivine. Am. Miner. 2014, 99, 2138–2141. [Google Scholar] [CrossRef]
- Padrón Navarta, J.A.; Hermann, J. A subsolidus olivine water solubility equation for the Earth’s upper mantle. JGR Solid Earth 2017, 122, 9862–9880. [Google Scholar] [CrossRef]
- Crépisson, C.; Blanchard, M.; Bureau, H.; Sanloup, C.; Withers, A.C.; Khodja, H.; Surblé, S.; Raepsaet, C.; Béneut, K.; Leroy, C.; et al. Clumped fluoride-hydroxyl defects in forsterite: Implications for the upper-mantle. Earth Planet. Sci. Lett. 2014, 390, 287–295. [Google Scholar] [CrossRef]
- Grant, K.J.; Kohn, S.C.; Brooker, R.A. Solubility and partitioning of water in synthetic forsterite and enstatite in the system MgO–SiO2–H2O ± Al2O3. Contrib. Mineral. Petrol. 2006, 151, 651–664. [Google Scholar] [CrossRef]
- Withers, A.; Hirschmann, M.; Tenner, T. The effect of Fe on olivine H2O storage capacity: Consequences for H2O in the martian mantle. Am. Miner. 2011, 96, 1039–1053. [Google Scholar] [CrossRef]
- Ingrin, J.; Liu, J.; Depecker, C.; Kohn, S.C.; Balan, E.; Grant, K.J. Low-temperature evolution of OH bands in synthetic forsterite, implication for the nature of H defects at high pressure. Phys. Chem. Miner. 2013, 40, 499–510. [Google Scholar] [CrossRef]
- Kudoh, Y.; Kuribayashi, T.; Kagi, H.; Inoue, T. Cation vacancy and possible hydrogen positions in hydrous forsterite, Mg1.985Si0.993H0.06O4, synthesized at 13.5 GPa and 1300 °C. J. Mineral. Petrol. Sci. 2006, 101, 265–269. [Google Scholar] [CrossRef][Green Version]
- Tollan, P.M.E.; Smith, R.; O’Neill, H.S.; Hermann, J. The responses of the four main substitution mechanisms of H in olivine to H2O activity at 1050 °C and 3 GPa. Prog. Earth Planet. Sci. 2017, 4, 200. [Google Scholar] [CrossRef]
- Gillet, P.; Richet, P.; Guyot, F.; Fiquet, G. High-temperature thermodynamic properties of forsterite. J. Geophys. Res. Space Phys. 1991, 96, 11805. [Google Scholar] [CrossRef]
- Liu, D.; Pang, Y.; Ye, Y.; Jin, Z.; Smyth, J.R.; Yang, Y.; Zhang, Z.; Wang, Z. In-situ high-temperature vibrational spectra for synthetic and natural clinohumite: Implications for dense hydrous magnesium silicates in subduction zones. Am. Miner. 2019, 104, 53–63. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Oganov, A.R. Ruby, metals, and MgO as alternative pressure scales: A semiempirical description of shock-wave, ultrasonic, x-ray, and thermochemical data at high temperatures and pressures. Phys. Rev. B. 2007, 75, 024115. [Google Scholar] [CrossRef]
- Dorogokupets, P.; Dymshits, A.; Sokolova, T.; Danilov, B.; Litasov, K. The equations of state of forsterite, wadsleyite, ringwoodite, akimotoite, MgSiO3-perovskite, and postperovskite and phase diagram for the Mg2SiO4 system at pressures of up to 130GPa. Russ. Geol. Geophys. 2015, 56, 172–189. [Google Scholar] [CrossRef]
- Reynard, B.; Caracas, R. D/H isotopic fractionation between brucite Mg(OH)2 and water from first-principles vibrational modeling. Chem. Geol. 2009, 262, 159–168. [Google Scholar] [CrossRef]
- Liu, Q.; Tossell, J.A.; Liu, Y. On the proper use of the Bigeleisen–Mayer equation and corrections to it in the calculation of isotopic fractionation equilibrium constants. Geochim. Cosmochim. Acta 2010, 74, 6965–6983. [Google Scholar] [CrossRef]
- Marcondes, M.L.; Wentzcovitch, R.M.; Assali, L.V. Importance of van der Waals interaction on structural, vibrational, and thermodynamic properties of NaCl. Solid State Commun. 2018, 273, 11–16. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Smyth, J.R.; Liu, J.; Xia, Q. Water effects on the anharmonic properties of forsterite. Am. Miner. 2015, 100, 2185–2190. [Google Scholar] [CrossRef]
- Withers, A.C.; Bureau, H.; Raepsaet, C.; Hirschmann, M.M. Calibration of infrared spectroscopy by elastic recoil detection analysis of H in synthetic olivine. Chem. Geol. 2012, 334, 92–98. [Google Scholar] [CrossRef]
- Nakamoto, K.; Margoshes, M.; Rundle, R.E. Stretching Frequencies as a Function of Distances in Hydrogen Bonds. J. Am. Chem. Soc. 1955, 77, 6480–6486. [Google Scholar] [CrossRef]
- Libowitzky, E. Correlation of O-H stretching frequencies and O–H…O hydrogen bond lengths in minerals. Monatshefte. Fiir. Chemie. 1999, 130, 1047–1059. [Google Scholar]
- Balan, E.; Blanchard, M.; Lazzeri, M.; Ingrin, J. Contribution of interstitial OH groups to the incorporation of water in forsterite. Phys. Chem. Miner. 2014, 41, 105–114. [Google Scholar] [CrossRef]
- Koch-Müller, M.; Matsyuk, S.S.; Rhede, D.; Wirth, R.; Khisina, N. Hydroxyl in mantle olivine xenocrysts from the Udachnaya kimberlite pipe. Phys. Chem. Miner. 2006, 33, 276–287. [Google Scholar] [CrossRef]
- Cynn, H.; Hofmeister, A.M.; Burnley, P.C.; Navrotsky, A. Thermodynamic properties and hydrogen bonding speciation from vibrational spectra of dense hydrous magnesium silicates. Phys. Chem. Miner. 1996, 23, 361–376. [Google Scholar] [CrossRef]
- Holland, T.J.B.; Powell, R. An internally consistent thermodynamic data set for phases of petrological interest. J. Metamorph. Geol. 1998, 163, 309–343. [Google Scholar]
- Okada, T.; Narita, T.; Nagai, T.; Yamanaka, T. Comparative Raman spectroscopic study on ilmenite-type MgSiO3 (akimotoite), MgGeO3, and MgTiO3 (geikielite) at high temperatures and high pressures. Am. Miner. 2008, 93, 39–47. [Google Scholar] [CrossRef]
- Chopelas, A. Single crystal Raman spectra of forsterite, fayalite, and monticellite. Am. Mineral. 1991, 76, 1101–1109. [Google Scholar]
- Hofmeister, A.M. Single-crystal absorption and reflection infrared spectroscopy of forsterite and fayalite. Phys. Chem. Miner. 1987, 14, 499–513. [Google Scholar] [CrossRef]
- Kieffer, S.W. Thermodynamics and lattice vibrations of minerals: 3. Lattice dynamics and an approximation for minerals with application to simple substances and framework silicates. Rev. Geophys. 1979, 17, 35. [Google Scholar] [CrossRef]
- Polyakov, V.B. On anharmonic and pressure corrections to the equilibrium isoto bnpic constants for minerals. Geochim. Cosmochim. Acta. 1988, 62(18), 3077–3085. [Google Scholar] [CrossRef]
- Polyakov, V.; Horita, J.; Cole, D.; Polyakov, V. Pressure effects on the reduced partition function ratio for hydrogen isotopes in water. Geochim. Cosmochim. Acta 2006, 70, 1904–1913. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, F.; Huang, S. Isotope fractionation induced by phase transformation: First-principles investigation for Mg2SiO4. Earth Planet. Sci. Lett. 2015, 409, 339–347. [Google Scholar] [CrossRef]


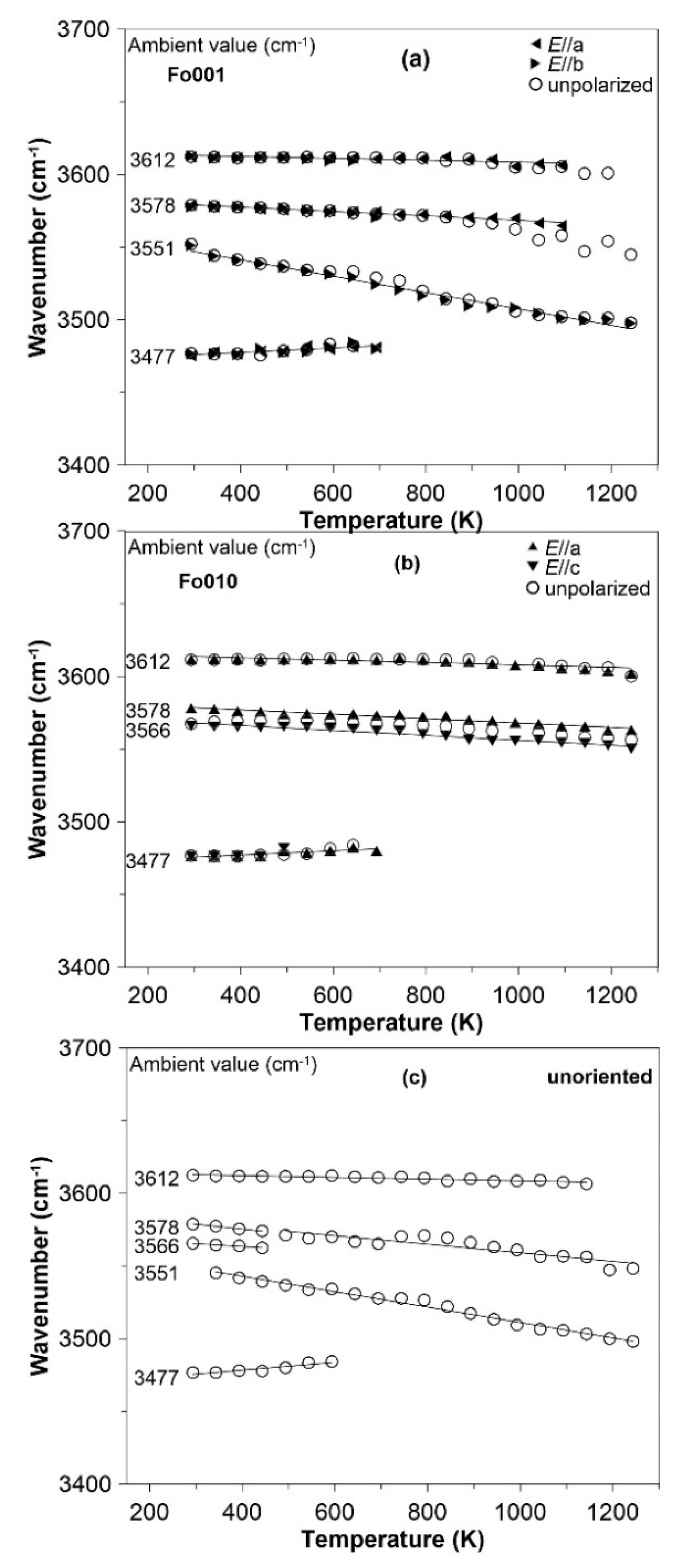
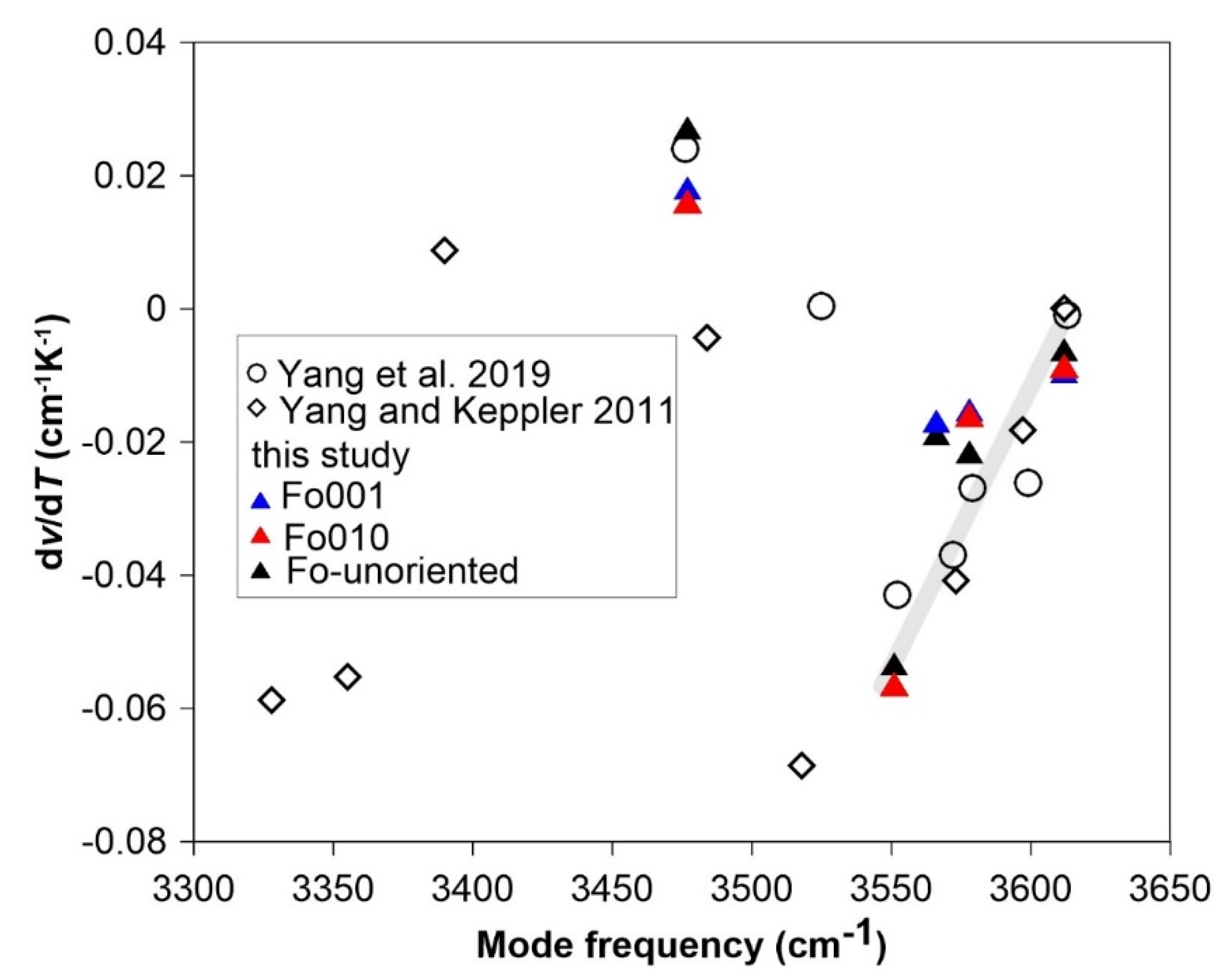
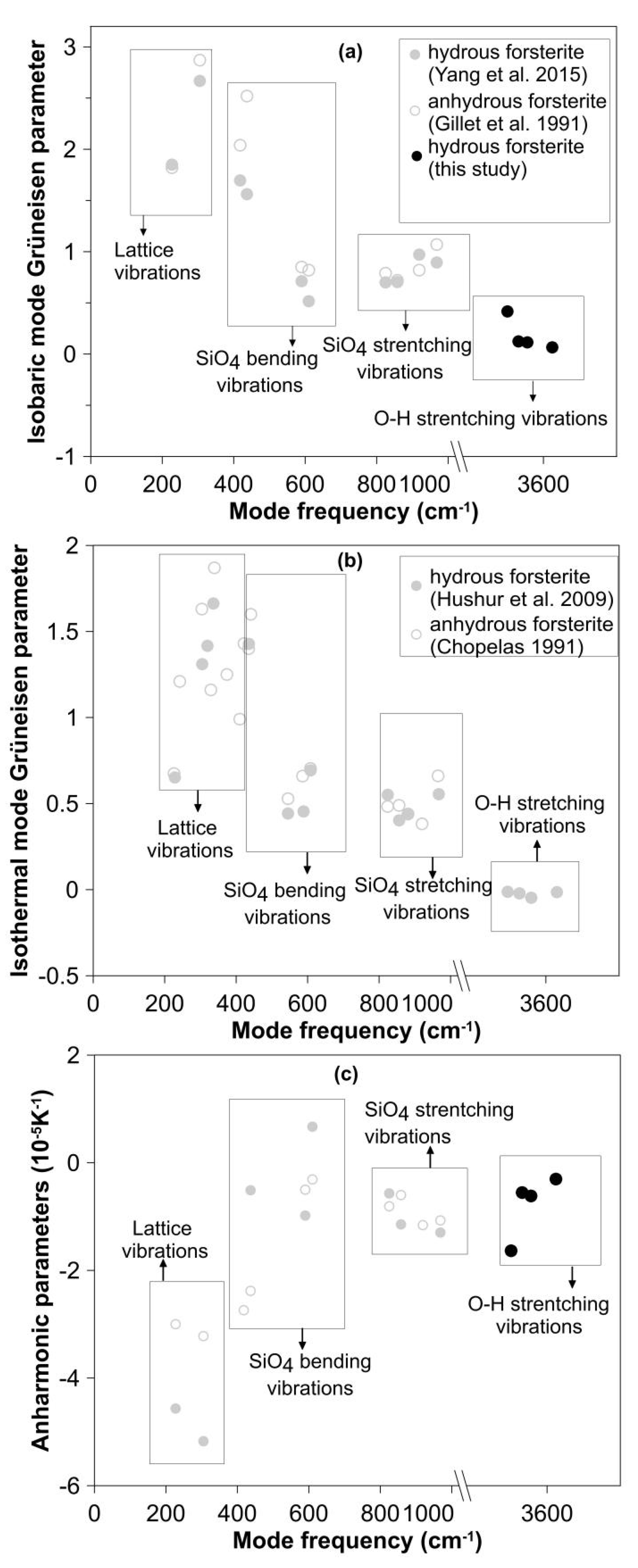
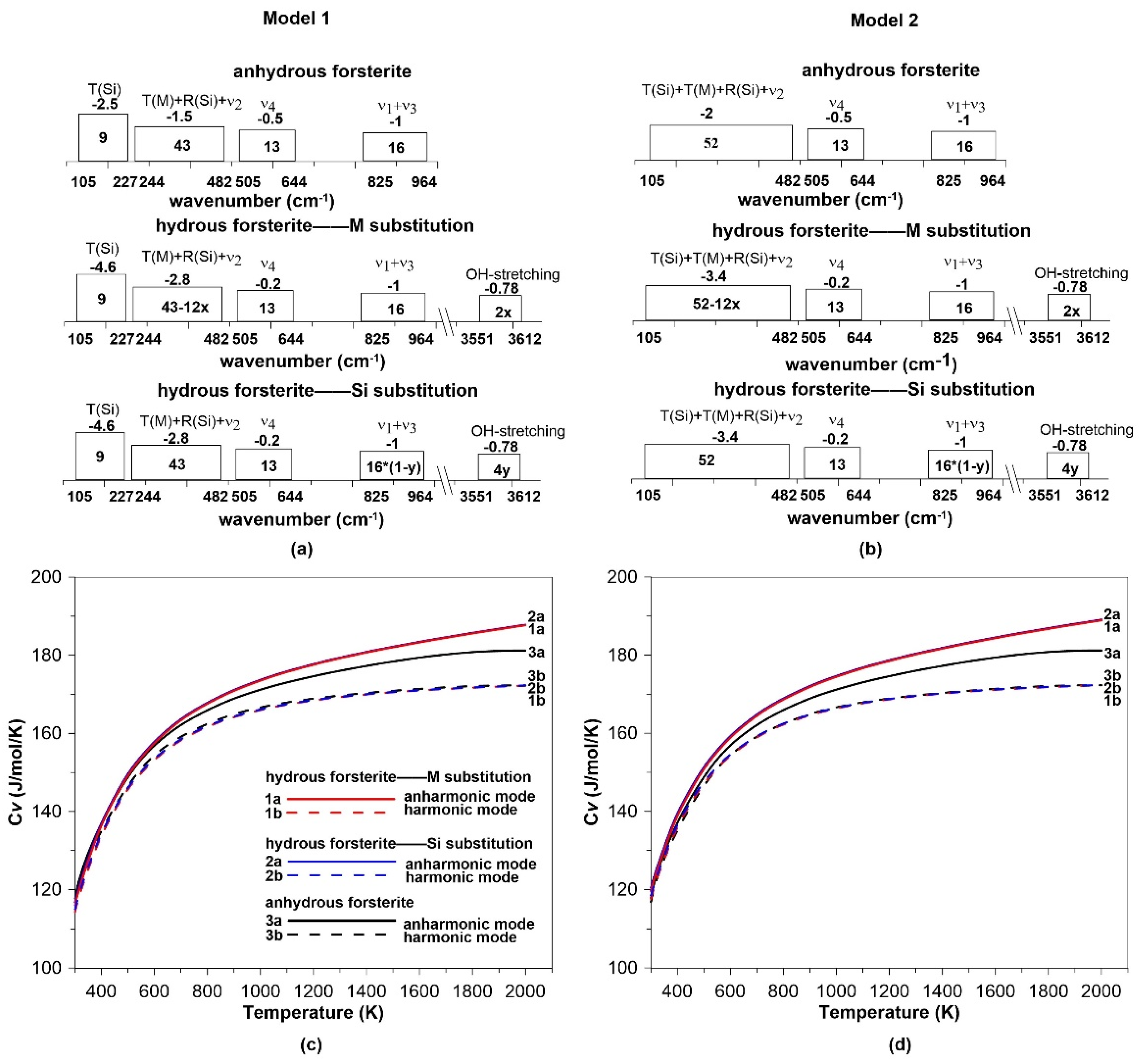
| OH-Band (cm−1) | (∂vi/∂T)P (cm·K)−1 | ||
|---|---|---|---|
| Fo001 | Fo010 | Fo-Unoriented | |
| 3612 | −0.008(6) | −0.009(4) | −0.006(1) |
| 3578 | −0.016(2) | −0.015(2) | −0.021(5) |
| 3566 | −0.016(8) | −0.018(8) | |
| 3551 | −0.056(4) | −0.053(7) | |
| 3477 | 0.016(1) | 0.018(2) | 0.027(2) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Wang, S.; Smyth, J.R.; Zhang, J.; Wang, X.; Zhu, X.; Ye, Y. In Situ Infrared Spectra for Hydrous Forsterite up to 1243 K: Hydration Effect on Thermodynamic Properties. Minerals 2019, 9, 512. https://doi.org/10.3390/min9090512
Liu D, Wang S, Smyth JR, Zhang J, Wang X, Zhu X, Ye Y. In Situ Infrared Spectra for Hydrous Forsterite up to 1243 K: Hydration Effect on Thermodynamic Properties. Minerals. 2019; 9(9):512. https://doi.org/10.3390/min9090512
Chicago/Turabian StyleLiu, Dan, Sha Wang, Joseph R. Smyth, Junfeng Zhang, Xiang Wang, Xi Zhu, and Yu Ye. 2019. "In Situ Infrared Spectra for Hydrous Forsterite up to 1243 K: Hydration Effect on Thermodynamic Properties" Minerals 9, no. 9: 512. https://doi.org/10.3390/min9090512
APA StyleLiu, D., Wang, S., Smyth, J. R., Zhang, J., Wang, X., Zhu, X., & Ye, Y. (2019). In Situ Infrared Spectra for Hydrous Forsterite up to 1243 K: Hydration Effect on Thermodynamic Properties. Minerals, 9(9), 512. https://doi.org/10.3390/min9090512





